Abstract
Pediatric ependymoma (EPN) is the third most common central nervous system (CNS) tumor, with 90% arising intracranially. Management typically involves maximal surgical resection and radiotherapy, but patients’ outcome is poor. Moreover, there are only a few therapeutical options available for recurrent or refractory disease. In this report, we present the case of a 7-year-old girl with relapsed refractory multifocal grade III EPN who failed conventional treatments and experienced a stable and durable response to the immune checkpoint inhibitor (ICPI) nivolumab in association with the mammalian target of rapamycin (m-TOR) inhibitor sirolimus. This experimental therapy was targeted on immune phenotypical analyses of the patient’s last relapse tumor sample, and this procedure should be routinely done to find new possible therapeutical approaches in recurrent solid tumors.
1. Introduction
Ependymoma (EPN) is the third most common brain tumor in children, accounting for 6–10% of all intracranial tumors [1,2]. Standard treatment consists of surgery, followed by post-operative radiation therapy to the tumor bed [3]. The role of post-surgery chemotherapy in this entity is still debated and under investigation in international cooperative studies. Recently, large-scale genomic and epigenetic studies have revealed distinct molecular subgroups associated with different prognosis [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19]. In particular, some data indicate that the RELA gene fusion subtype (ST-EPN-RELA) is associated with a worse outcome, whereas the YAP1 subtype (ST-EPN-YAP1) is very rare and correlates with better survival [20]. However, another important obstacle is the paucity of reports that have included a large single or multi-center EPN pediatric population with a long-term follow-up. More previous studies report a 3- or 5-year follow-up [21,22], and those that report longer-term outcomes include relatively small numbers of patients [13,23,24,25,26,27,28]. Complete resection, when possible, has been otherwise associated with a better outcome [29]. With these preliminary remarks, the survival rate is less than 20% in high-risk cases, so new therapeutic strategies are urgently needed [30,31]. Immunotherapy with ICPI acting through the programmed cell-death-1 (PD-1) pathway has been demonstrated to be effective in some chemotherapy-resistant adult cancers, also arising interest in the pediatric setting [32,33]. To date, it is standard practice in some centers to analyze tumor molecular and genetic characteristics to find suitable markers as the target of new drugs [34,35]. The PD-1/PD-L1 pathway is, for example, well known in glioma [36,37] and glioblastoma [38] pathogenesis and ICPI therapy has been successfully applied in selected cases [32,39,40], while few data are available on EPN, even if some evidences indicate a role in tumor escape mechanisms [39].
We report the case of a child affected by relapsed refractory EPN who maintained stable disease for 12 months with a combination of nivolumab and sirolimus therapy.
2. Case Report
A 7-year-old female patient from Morocco came to our attention with a diagnosis of relapsed grade III EPN 4 years from the primary diagnosis, which was made in her origin country, done after seizure appearance. The tumor was localized in the left frontal lobe and was completely removed by surgery. From December 2016 to February 2017, the child received three courses of adjuvant chemotherapy with cisplatin and etoposide. In March 2017, a relapse occurred in the primary tumor site. It was treated with surgery and three courses of chemotherapy with ifosfamide and etoposide. Complete remission was obtained. In October 2017, a second relapse occurred again in the left frontal lobe, which was treated with surgery and radiotherapy (59.4 Gy). Complete remission was achieved. In April 2019, a third relapse was diagnosed, and the patient was considered incurable in her origin country. For this reason, she was referred to our Center, where a full CNS magnetic resonance imaging (MRI) evaluation documented a multifocal disease both in the brain and in the spinal cord. CNS fluid analysis was negative for leptomeningeal dissemination. We decided to treat her with oral etoposide and dexamethasone. A follow-up MRI done in October 2019 showed a disease progression, but clinical conditions were still good, and we did not modify the current treatment. In December 2019, the patient was admitted to the hospital due to worsening general clinical conditions, which moved rapidly to a comatose state. We administered anti-edema treatment with high-dose dexamethasone and 20% mannitol, with an improvement of the clinical feature. The MRI showed an ulterior disease progression. For this reason, we decided to substitute etoposide with temozolomide. In January 2020, the patient was again admitted to the hospital because of neurological symptoms (headache, vomiting, and hyposthenia of the right part of the body); anti-edema treatment was done and then radiotherapy on the left frontal lesion. We obtained histological material from the last surgery done in her origin country. Diagnoses of anaplastic ependymoma with RELA gene expression were confirmed. The immune phenotype analyses on paraffine tumor sample revealed: p65+, LICAM+, OLIG2-, p53+, GFAP+, EMA+, Synaptophysine+, ATRX+, m-TOR+, PD-L1+ (20%), and PD-1-. Based on these findings, in February 2020, we decided to start targeted therapy with orally dispensed sirolimus 2 mg/Kg/day every day and IV nivolumab 3 mg/Kg every 2 weeks. Sirolimus dosage was modulated on patient plasmatic concentration. Local Ethics Committee approved the nivolumab off-label use, and written informed consent was signed by the parents, according to the Declaration of Helsinki. Treatment was continued for 1 year until disease progression. During this period, the patient experienced a varicella-zoster virus (VZV) infection, which required IV acyclovir, and dexamethasone was definitively withdrawn (July 2020). In April and August of 2020, control MRI showed substantially stable disease in both the brain and the spinal cord (Figure 1). At one-year follow-up, the patient had disease progression; however, her current clinical conditions are good and neurological assessment completely negative.
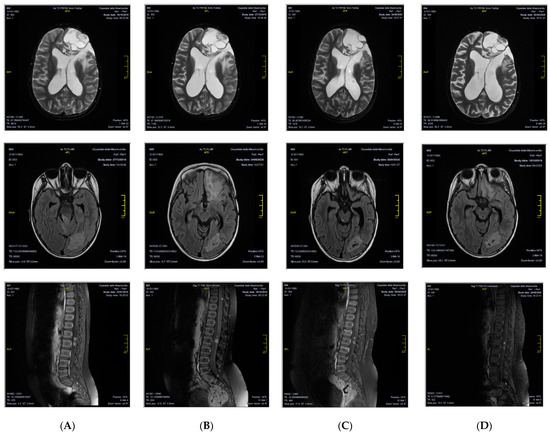
Figure 1.
CNS MRI patient evaluation during therapy with nivolumab and sirolimus, showing a substantially stable disease in the brain and in the spinal cord at different time points. (A) January 2020; (B) April 2020; (C) August 2020; (D) January 2021.
3. Discussion
Available therapies for EPN are to date insufficient to prevent relapse in almost half of the cases [31,41]. Recurrences occur prevalently after a median of 2 years from diagnosis, but sometimes they could happen many years later. In these cases, relapses could be multiple and cause important morbidity, such as in our case report. Moreover, recurrent/refractory EPN has very few therapeutic chances, especially in the pediatric population [31,40,41]. Recently, proton-beam radiation therapy has been proposed with the aim to reduce the long-term toxicity, but it did not improve outcomes [42]. CAR-T cell therapy is otherwise promising but still under investigation [43], so actual approaches are insufficient in these cases. Recent studies on genomic alterations and molecular biology underlying solid tumors expanded our understanding of their development and progression, offering great opportunities for large-scale therapeutical trials [32]. In particular, immunotherapy has demonstrated remarkable clinical efficacy in adult patients with refractory solid tumors such as melanoma, non-small-cell lung cancer or renal cell carcinoma, with optimal results also in advanced diseases [44,45,46]. Regarding pediatric cancers, PD-L1 expression was demonstrated in soft-tissue sarcomas, osteosarcoma, neuroblastoma, and brain tumors such as gliomas or glioblastomas [47,48,49,50], but to date, there have been only a few reports on pediatric cancer patients treated with agents targeting the PD-1 pathway, and most of them involving genetically unselected patients. Unfortunately, the majority of these studies report disappointing outcomes, despite preclinical data suggesting better results [47,48,49,50]. Very few data are otherwise available about EPN.
In a reported pediatric brain tumor series, one EPN case was treated with two courses of nivolumab and had disease progression in about 6 weeks, but retrospective analyses showed PD-L1 negativity [51].
In another reported series, one child with EPN was treated with nivolumab plus ipilimumab, obtaining durable stable disease for 18 months until disease progression. In this case, PD-L1 positivity was checked by immune histochemistry before treatment and was considered significant >1% [52].
On the other side, the hyper-activation of the downstream mammalian target of rapamycin (m-TOR) pathway is frequently observed in pediatric brain tumors, particularly low-grade gliomas, and sirolimus and its analogues temsirolimus or everolimus are currently used in this entity treatment [53,54,55].
In this report, we described for the first time the association therapy with nivolumab plus sirolimus in a child affected by refractory anaplastic EPN and obtained a stable disease. Up-regulation of immune checkpoints in brain tumor cells has resulted in high interest for ICPI therapy in these nosological entities. Our experience, as well as others additionally reported, showed that, among pediatric patients with recurrent/refractory brain tumors who failed standard treatment, nivolumab was well tolerated without any serious adverse event due to autoimmune side effects, including transaminitis, pancreatitis, colitis, vitiligo, and hepatitis [51,52]. In our report, the patient had no toxicity, developed only one infectious episode (VZV reactivation) and had a very good quality of life without hospitalization. Therapy was targeted on the immune phenotype evaluation of the last relapse sample and was safe and effective in a 12-month overall treatment. The effects of PD-L1 expression in pediatric brain tumors have been reported, but nivolumab was generally discontinued in median <6 weeks because of disease progression [51]. In our case, the response was maintained for 1 year, and the patient remained clinically stable. PD-L1 expression status is needed to demonstrate nivolumab’s efficacy in the management of recurrent/refractory brain tumors. Furthermore, this study confirms the not completely satisfying outcome of these cases, even if a good expression of PD-L1 (20%) and m-TOR (99%) in the tumor sample was observed. Some evidences indicate that the combination therapy of nivolumab with the CTLA-4 inhibitor ipilimumab or other drugs could increase the response rate [32,52]. PD-L1 is variably expressed in pediatric cancer and does not always correlate with drug efficacy and outcome. Despite these considerations, routine collection of tumor samples, in particular relapsed ones, could be very useful in identifying new therapeutic strategies and should be applied in larger pediatric studies.
In conclusion, nivolumab plus sirolimus was well-tolerated in our patient with recurrent/refractory EPN, and obtained stable disease. To obtain a better and more durable response, we suggest ICPI therapy be limited to those with elevated PD-L1 expression upon these findings.
Author Contributions
K.P. had the idea and wrote the paper. A.M. did molecular and immune-histochemistry analyses and critically contributed to the paper. M.L. did radiotherapy treatment. F.A., I.C., C.C., G.M.I.G., M.S.M. and E.M. took care of the patient and helped in collecting data. M.C. supervised all the research and gave scientific contributions. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by Regione Umbria Ethics Committee, protocol code 18141/20/PUC, 30 January 2020.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Goldwein, J.W.; Leahy, J.M.; Packer, R.J.; Sutton, L.N.; Curran, W.J.; Rorke, L.B.; Schut, L.; Littman, P.S.; D’Angio, G.J. Intracranial ependymoma in children. Int. J. Radiat. Oncol. Biol. Phys. 1990, 19, 1497–1502. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenbergerer, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Merchant, T.E.; Fouladi, M. Ependymoma: New therapeutic approaches including radiation and chemiotherapy. J. Neurooncol. 2005, 75, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Dubuc, A.M.; Northcott, P.A.; Mack, S.; Witt, H.; Pfister, S.; Taylor, M.D. The genetics of pediatric brain tumors. Curr. Neurol. Neurosci. Rep. 2010, 10, 215–223. [Google Scholar] [CrossRef]
- Gajjar, A.; Pfister, S.M.; Taylor, M.D.; Gilbertson, R.J. Molecu- lar insights into pediatric brain tumors have the potential to trans- form therapy. Clin. Cancer Res. 2014, 20, 5630–5640. [Google Scholar] [CrossRef]
- Koos, B.; Bender, S.; Witt, H.; Mertsch, S.; Felsberg, J.; Beschorner, R.; Korshunov, A.; Riesmeier, B.; Pfister, S.; Paulus, W.; et al. The transcription factor evi-1 is overexpressed, promotes proliferation, and is prognostically unfavorable in infratentorial ependymomas. Clin. Cancer Res. 2010, 17, 3631–3637. [Google Scholar] [CrossRef]
- Mack, S.C.; Witt, H.; Wang, X.; Milde, T.; Yao, Y.; Bertrand, K.C.; Korshunov, A.; Pfister, S.M.; Taylor, M.D. Emerging insights into the ependymoma epigenome. Brain Pathol. 2013, 23, 206–209. [Google Scholar] [CrossRef]
- Mack, S.C.; Witt, H.; Piro, R.M.; Gu, L.; Zuyderduyn, S.; Stütz, A.M.; Wang, X.; Gallo, M.; Garzia, L. Epigenomic alterations define lethal CIMP-positive ependymomas of infancy. Nature 2014, 506, 445–450. [Google Scholar] [CrossRef]
- Mendrzyk, F.; Korshunov, A.; Benner, A.; Toedt, G.; Pfister, S.; Radl-wimmer, B.; Lichter, P. Identification of gains on 1q and epidermal growth factor receptor overexpression as independent prognostic markers in intracranial ependymoma. Clin. Cancer Res. 2006, 12, 2070–2079. [Google Scholar] [CrossRef] [PubMed]
- Pajtler, K.W.; Witt, H.; Sill, M.; Jones, D.T.; Hovestadt, V.; Kratochwil, F.; Wani, K.; Tatevossian, R.; Punchihewa, C. Molecular classifi- cation of ependymal tumors across all CNS compartments, his-topathological grades, and age groups. Cancer Cell 2015, 27, 728–743. [Google Scholar] [CrossRef]
- Pajtler, K.W.; Pfister, S.M.; Kool, M. Molecular dissection of ependymomas. Oncoscience 2015, 2, 827–828. [Google Scholar] [CrossRef] [PubMed]
- Pfister, S.; Hartmann, C.; Korshunov, A. Histology and molecular pathology of pediatric brain tumors. J. Child Neurol. 2009, 24, 1375–1386. [Google Scholar] [CrossRef]
- Ramaswamy, V.; Hielscher, T.; Mack, S.C.; Lassaletta, A.; Lin, T.; Pajtler, K.W.; Jones, D.T.; Luu, B.; Cavalli, F.M. Therapeutic impact of cytoreductive surgery and irradiation of posterior fossa ependymoma in the molecular era: A retrospective multicohort analysis. J. Clin. Oncol. 2016, 34, 2468–2477. [Google Scholar] [CrossRef]
- Witt, H.; Mack, S.C.; Ryzhova, M.; Bender, S.; Sill, M.; Isserlin, R.; Benner, A.; Hielscher, T.; Milde, T.; Remke, M.; et al. Delineation of two clinically and molecularly distinct subgroups of posterior fossa ependymoma. Cancer Cell 2011, 20, 143–157. [Google Scholar] [CrossRef]
- Witt, H.; Korshunov, A.; Pfister, S.M.; Milde, T. Molecular approaches to ependymoma: The next step(s). Curr. Opin. Neurol. 2012, 25, 745–750. [Google Scholar] [CrossRef]
- Johnson, R.A.; Wright, K.D.; Poppleton, H.; Mohankumar, K.M.; Finkelstein, D.; Pounds, S.B.; Rand, V.; Leary, S.E.; White, E.; Eden, C.; et al. Cross-species genomics matches driver mutations and cell compartments to model ependymoma. Nature 2010, 466, 632–636. [Google Scholar] [CrossRef]
- Kilday, J.P.; Mitra, B.; Domerg, C.; Ward, J.; Andreiuolo, F.; Osteso- Ibanez, T.; Mauguen, A.; Varlet, P.; Le Deley, M.C.; Lowe, J.; et al. Copy number gain of 1q25 predicts poor progression-free survival for pediatric intracranial ependymomas and enables patient risk stratification: A prospective European clinical trial cohort analysis on behalf of the Children’s Cancer Leukaemia Group CCLG), Societe Francaise d’Oncologie Pediatrique (SFOP), and International Society for Pediatric Oncology (SIOP). Clin. Cancer Res. 2012, 18, 2001–2011. [Google Scholar] [PubMed]
- Wani, K.; Armstrong, T.S.; Vera-Bolanos, E.; Raghunathan, A.; Ellison, D.; Gilbertson, R.; Vaillant, B.; Goldman, S.; Packer, R.J.; Fouladi, M.; et al. Network CER. A prognostic gene expression signature in infratentorial ependymoma. Acta Neuropathol. 2012, 123, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, R.J.; Bentley, L.; Hernan, R.; Junttila, T.T.; Frank, A.J.; Haapasalo, H.; Connelly, M.; Wetmore, C.; Curran, T.; Elenius, K.; et al. ERBB receptor signaling promotes ependymoma cell proliferation and represents a potential novel therapeutic target for this disease. Clin. Cancer Res. 2002, 8, 3054–3064. [Google Scholar] [PubMed]
- Massimino, M.; Barretta, F.; Modena, P.; Witt, H.; Minasi, S.; Pfister, S.M.; Pajtler, K.W.; Antonelli, M.; Gandola, L.; Garrè, M.L.; et al. The AIEOP 2nd series of children and adolescent intracranial ependymoma. An integrated molecular and clinical characterization with a long-term follow-up. Neuro Oncol. 2020, 23, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Robertson, P.L.; Zelter, P.M.; Boyett, J.M.; Rorke, L.B.; Allen, J.C.; Geyer, J.R.; Stanley, P.; Li, H.; Albright, A.L.; McGuire-Cullen, P.; et al. Survival and prognostic factors following radiation therapy and chemotherapy for ependymomas in children: A report of the Children’s Cancer Group. J. Neurosurg. 1998, 88, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, S.M.; Sethi, R.; Lavally, B.; Yeap, B.Y.; Marcus, K.J.; Caruso, P.; Pulsifer, M.; Huang, M.; Ebb, D.; Tarbell, N.J.; et al. Proton radiotherapy for pediatric central nervous system ependymoma: Clinical outcomes for 70 patients. Neuro Oncol. 2013, 15, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Mansur, D.B.; Perry, A.; Rajaran, V.; Michalski, J.M.; Park, T.S.; Leonard, J.R.; Luchtman-Jones, L.; Rich, K.M.; Grigsby, P.W.; Lockett, M.A.; et al. Postoperative radiation therapy for grade II and III intracranial ependymoma. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.K.; Sall, W.F.; Maity, A.; Tochner, Z.A.; Janss, A.J.; Belasco, J.B.; Rorke-Adams, L.B.; Phillips, P.C.; Sutton, L.N.; Fisher, M.J. Childhood intracranial ependymoma: Twenty-year experience from a single institution. Cancer 2007, 100, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Horn, B.; Heideman, R.; Geyer, R.; Pollack, I.; Parker, R.; Goldwein, J.; Tomitta, T.; Schomberg, P.; Ater, J.; Luchtman-Jones, L.; et al. A multi-institutional retrospective study of intracranial ependymoma in children: Identification of risk factors. J. Pediatr. Hematol. Oncol. 1999, 21, 203–211. [Google Scholar] [CrossRef]
- Merchant, T.E.; Li, C.; Xiong, X.; Kun, L.E.; Boop, F.A.; Sanford, R.A. Conformal radiotherapy after surgery for paediatric ependymoma: A prospective study. Lancet Oncol. 2009, 10, 258–266. [Google Scholar] [CrossRef]
- Oya, N.; Shimaboto, Y.; Nagata, Y.; Negoro, Y.; Hiraoka, M. Postoperative radiotherapy for intracranial ependymoma: Analyses of prognostic factors and patterns of failure. J. Neurooncol. 2002, 56, 87–94. [Google Scholar] [CrossRef]
- Van Veelen-Vincent, M.L.; Pierre-Khan, A.; Kalifa, C.; Sainte-Rose, C.; Zerah, M.; Thorne, J.; Renier, D. Ependymoma in childhood: Prognostic factors, extent of surgery, and adjuvant therapy. J. Neurosurg. 2002, 97, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Goldwein, J.W.; Glauser, T.A.; Packer, R.J.; Finlay, J.L.; Sutton, L.N.; Curran, W.J.; Laehy, J.M.; Rorke, L.B.; Shut, L.; D’Angio, G.I. Recurrent intracranial ependymoma in children, survival, pattern of failure, and prognostic factors. Cancer 1990, 66, 557–563. [Google Scholar] [CrossRef]
- McLendon, R.E.; Lipp, E.; Satterfield, D.; Ehinger, M.; Austin, A.; Fleming, D.; Perkinson, K.; Lefaivre, M.; Zagzag, D.; Wiener, B.; et al. Prognostic marker analyses in pediatric intracranial ependymoma. J. Neurooncol. 2015, 122, 255–261. [Google Scholar] [CrossRef]
- Marinoff, A.M.; Clement, M.; Guo, D.; Snuderl, M.; Wright, K.D.; Manley, P.E.; Al-Sayegh, H.; Sinai, C.E.; Ulrich, N.J.; Marcus, K.; et al. Rethinking childhood ependymoma: A retrospective, multi-center analyses reveals poor long-term survival. J. Neurooncol. 2017, 135, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Wagner, L.M.; Adams, V.R. Targeting the PD-1 pathway in pediatric solid tumors and brain tumors. Onco Targets Ther. 2017, 10, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Kline, C.N.; Joseph, N.M.; Grenert, J.P.; van Ziffle, J.; Talevich, E.; Onodera, C.; Aboian, M.; Cha, S.; Raleigh, D.R.; Braunstein, S.; et al. Targeted next-generation sequencing of pediatric neuro-oncology patients improve diagnosis, identifies pathogenic germline mutations, and directed targeted therapy. Neuro-Oncology 2017, 19, 699–709. [Google Scholar] [PubMed]
- Van Tiburg, C.M.; Witt, R.; Heiss, M.; Paitler, K.W.; Plass, C.; Poschke, I.; Platten, M.; Harting, I.; Sedlazek, O.; Freitag, A.; et al. INFORM2 NivEnt: The first trial of the INFORM2 biomarker driven phase I/II trial series: The combination of nivolumab and etinostat in children and adolescents with refractory high-risk malignancies. BMC Cancer 2020, 20, 523. [Google Scholar] [CrossRef]
- De, B.; Khakoo, Y.; Souweidane, M.M.; Dunkel, I.J.; Patel, S.H.; Gilheeney, S.W.; De Braganca, K.C.; Karajannis, M.A.; Wolden, S.L. Patterns of relapsed for children with localized intracranial ependymoma. J. Neurooncol. 2018, 138, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Kline, C.; Liu, S.J.; Duriseti, S.; Banerjee, A.; Nicolaides, T.; Raber, S.; Gupta, N.; Haas-Kogan, D.; Braunstein, S.; Mueller, S. Reirradiation and PD-1 inhibition with nivolumab for the treatment of recurrent diffuse intrinsic pontine glioma: A single-institution experience. J. Neuro-Oncol. 2018, 140, 629–638. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, X.; Gao, L.; Wang, Y.; Guo, Y.; Xing, B.; Ma, W. Classification of pediatric gliomas based on immunological profiling: Implications for immunotherapy strategies. Mol. Ther. Oncolytics 2020, 20, 34–47. [Google Scholar] [CrossRef]
- Al Harbi, M.; Mobark, N.A.; Al Mubarak, L.; Alielaify, R.; Al Saeed, M.; Almutairi, A.; Aqubaishi, F.; Emarat Hussain, M.; Balbaid, A.A.O.; Marie, A.S.; et al. Durable response to nivolumab in pediatric patients with refractory glioblastoma and constitutional biallelic mismatched repair deficiency. Oncologist 2018, 23, 1401–1406. [Google Scholar] [CrossRef]
- Witt, D.A.; Donson, A.M.; Amani, V.; Moreira, D.C.; Sanford, B.; Hoffman, L.M.; Handler, M.H.; Mulchay Levy, J.M.; Jones, K.L.; Nellan, A.; et al. Specific expression of PD-L1 in RELA-fusion supratentorial ependymoma: Implications for PD-1 targeted therapy. Pediatr. Blood Cancer 2018, 65, e26960. [Google Scholar] [CrossRef]
- Ottensmeier, H.; Schlegel, P.G.; Eyrich, M.; Wolff, J.E.; Juhnke, B.-O.; Von Hoff, K.; Frahsek, S.; Schmidt, R.; Faldum, A.; Fleischhack, G.; et al. Treatment of children under 4 years of age with medulloblastoma and ependymoma in the HIT2000/HIT-REZ 2005 trials: Neuropsychological outcome 5 years after treatment. PLoS ONE 2020, 15, e0227693. [Google Scholar] [CrossRef]
- Ritzmann, T.A.; Rogers, H.A.; Paine, S.M.L.; Storer, L.C.D.; Jacques, T.S.; Chapman, R.J.; Ellison, D.; Donson, A.M.; Foreman, N.K.; Grundy, R.G. A retrospective analysis of recurrent pediatric ependymoma reveals extremely poor survival and ineffectiveness of current treatments across central nervous system locations and molecular subgroups. Pediatr. Blood Cancer 2020, 67, e28426. [Google Scholar] [CrossRef]
- Sato, M.; Gunther, J.R.; Mahajan, A.; Jo, E.; Paulino, A.C.; Adesina, A.M.; Jones, J.Y.; Ketonen, L.M.; Su, J.M.; Ocku, M.F.; et al. Progression-free survival of children with localized ependymoma treated with intensity-modulated radiation therapy or proton-beam radiation therapy. Cancer 2017, 123, 2570–2578. [Google Scholar] [CrossRef] [PubMed]
- Donovan, L.K.; Delaidelli, A.; Joseph, S.K.; Bielamowicz, K.; Fousek, K.; Holgado, B.L.; Manno, A.; Srikanthan, D.; Gad, A.Z.; van Ommeren, R.; et al. Locoregional delivery of CAR T cells to the cerebrospinal fluid for treatment of metastatic medulloblastoma and ependymoma. Nat. Med. 2020, 26, 720–731. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Aren Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthelemy, P.; Porta, C.; George, S.; et al. CheckMate 214 Investigators. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Brahmer, J. Nivolumab in nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 2016, 374, 493–494. [Google Scholar]
- Paydas, S.; Bagir, E.K.; Deveci, M.A.; Gonlusen, G. Clinical and prognostic significance of PD-1 and PD-L1 expression in sarcomas. Med. Oncol. 2016, 33, 93. [Google Scholar] [CrossRef]
- Burgess, M.A.; Crowley, J.; Reinke, D.K.; Riedel, R.F.; George, S.; Movva, S.; van Tine, B.A.; Davis, L.E.; Schuetze, S.; Hu, J.; et al. SARC 028: A phase II study of the anti-PD1 antibody pembrolizumab (P) in patients (Pts) with advanced sarcomas. J. Clin. Oncol. 2015, 33 (Suppl. 15), TPS10578. [Google Scholar] [CrossRef]
- Dondero, A.; Pastorino, F.; Della Chiesa, M.; Corrias, M.V.; Morandi, F.; Pistoia, V.; Olive, D.; Bellora, F.; Locatelli, F.; Castellano, A.; et al. PD-L1 expression in metastatic neuroblastoma as an additional mechanism for limiting immune surveillance. Oncoimmunology 2016, 5, e1064578. [Google Scholar] [CrossRef]
- Majzner, R.G.; Martinez, D.; Pawel, B.; Santi, M.; Sorensen, P.H.B.; Mackall, C.L.; Maris, J.M. Assessment of PD-L1 expression and tumor associated immune cells in pediatric cancer tissues. J. Clin. Oncol. 2016, 34 (Suppl. 15), 11542. [Google Scholar] [CrossRef]
- Gorsi, H.S.; Malicki, D.M.; Barsan, V.; Tumblin, M.; Yeh-Nayre, L.; Milburn, M.; Elster, J.D.; Crawford, J.R. Nivolumab in the Treatment of Recurrent or Refractory Pediatric Brain Tumors: A Single Institutional Experience. J. Pediatr. Hematol. Oncol. 2018, 41, e235–e241. [Google Scholar] [CrossRef] [PubMed]
- Cacciotti, C.; Choi, J.; Alexandrescu, S.; Zimmerman, M.A.; Cooney, T.M.; Chordas, C.; Clymer, J.; Chi, S.; Yeo, K.K. Immune checkpoint inhibition for pediatric patients with recurrent/refractory CNS tumors: A single institution experience. J. Neuro-Oncol. 2020, 149, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Piha-Paul, S.A.; Shin, S.J.; Vats, T.; Guha-Thakurta, N.; Aaron, J.; Rytting, M.; Kleinerman, E.; Kurzrock, R. Pediatric patients with refractory central nervous system tumors: Experiences of a clinical trial combining Bevacizumab and Everolimus. Cancer Res. 2014, 34, 1939–1946. [Google Scholar]
- Cacchione, A.; Lodi, M.; Carai, A.; Miele, E.; Tartaglia, M.; Megaro, G.; Del Baldo, G.; Alessi, I.; Colafati, G.S.; Carboni, A.; et al. Upfront treatment with mTOR inhibitor everolimus in pediatric low-grade gliomas: A single-center experience. Int. J. Cancer 2020, 15. [Google Scholar] [CrossRef]
- Becher, O.J.; Millard, N.E.; Modak, S.; Kushner, B.H.; Haque, S.; Spasojevic, I.; Trippett, T.M.; Gilheeney, S.W.; Khakoo, Y.; Lyden, D.C.; et al. A phase I study of single-agent perifosine for recurrent or refractory pediatric CNS and solid tumors. PLoS ONE 2017, 12, e0178593. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).