Abstract
Reactive infectious mucosal eruptions (RIME) following Chlamydia pneumoniae infection is a rare and poorly understood clinical presentation that can pose a diagnostic challenge. We report the case of a previously healthy 21-year old male with a cough, fever, numerous penile and mouth ulcers, and severe conjunctivitis. Several differential diagnoses, including Herpes simplex infection, were considered before Chlamydia pneumoniae was established as the causative agent. The patient’s condition improved following treatment with clarithromycin and prednisolone tablets, and he had almost fully recovered at follow-up 10 days after discharge.
1. Introduction
Chlamydia pneumoniae is a known cause of respiratory tract infections with a prevalence of antibodies higher than 50%. In rare cases, mucocutaneous manifestations in relation to the spectrum of erythema multiforme (EM), Stevens–Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN) have been reported secondary to C. pneumoniae infection. It usually presents with symptoms such as maculopapular rash, targetoid lesions, or vesiculobullous lesions, as well as inflammation with or without desquamation of buccal, lip, ocular, and genital mucosa. These conditions are more prevalent in children and young adults, and the disease course is usually mild and has an excellent prognosis. A similar clinical picture has more commonly been reported following Mycoplasma pneumoniae infection and is termed Mycoplasma-induced rash and mucositis (MIRM) to distinguish it from the more severe conditions SJS, TEN, and EM. Expanding the MIRM concept to include C. pneumoniae-induced rash and mucositis (CIRM) was proposed by Mayor-Ibaguren et al. in 2017 [1].
Recently, a pediatric consensus group revised the classifications and proposed the umbrella term reactive infectious mucocutaneous eruption (RIME) to include cases of MIRM-like manifestations secondary to infection other than M. pneumoniae [2].
Here, we present a case of RIME secondary to C. pneumoniae-infection that proved to be clinically challenging due to an initial presentation with mucosal involvement that complicated and delayed obtaining the correct diagnosis and treatment.
2. Case Presentation
A previously healthy 21-year old male was seen in the emergency department due to newly developed mouth ulcers, red and irritated eyes, and a 7-day history of headache, productive cough, and subjective fever. The patient’s symptoms were interpreted as herpes simplex gingivostomatitis, and the patient was started on empiric treatment with acyclovir. The following day, due to the worsening of the patient’s condition, he was referred by his general practitioner to our outpatient clinic for infectious diseases.
The medical history revealed no recent travel history; the patient worked as an electrician and was in a monogamous heterosexual relationship. Examination of the oral cavity revealed numerous painful erosions of both the lips (Figure 1) and oral mucosa and white streaks on the tonsils. Examination of the eyes showed bilateral conjunctival injections (Figure 2). A left-sided 1 cm angular lymph node was palpable. Vitals showed a fever (38.8 °C) as well as tachycardia (103 bpm). Auscultation of the heart and lungs was normal.
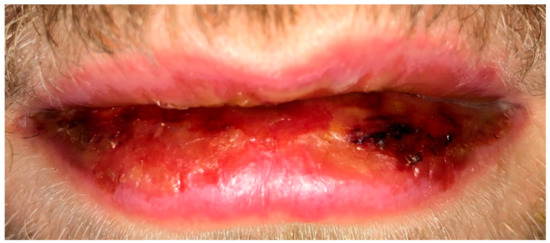
Figure 1.
Initial presentation with erosions of the lips.
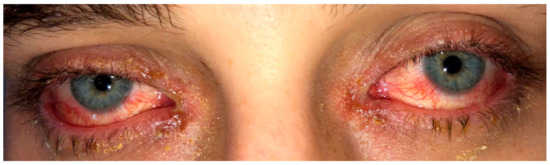
Figure 2.
Initial presentation showing bilateral conjunctival injections.
Due to suspected herpes keratitis, he was referred to the department of ophthalmology for an acute assessment. The ophthalmological slit lamp examination revealed no pseudomembranes or symblepharon formation and the ophthalmologist suspected the patient’s symptoms were caused by adenovirus infection.
Upon his second outpatient visit, his respiratory and oral symptoms, as well as his ability to tolerate oral intake, had worsened to the point that he was subsequently admitted. During inpatient care, two erosions on the glans penis (Figure 3) developed. There were no signs of cutaneous involvement at any point in the disease course.
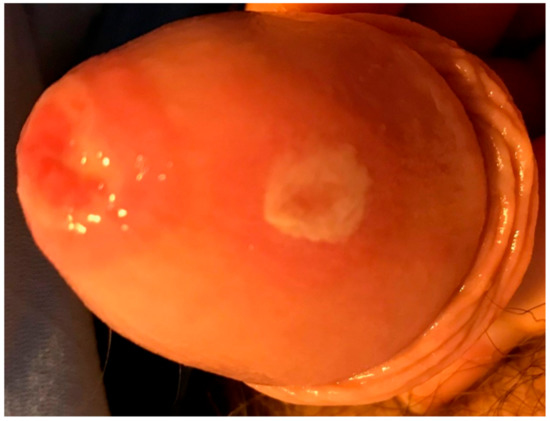
Figure 3.
Erosions on the glans penis developed during inpatient care.
3. Investigations
Blood tests upon the time of admission showed elevated C-reactive protein elevated to 54 mg/L (reference range <10 mg/L), as well a mildly elevated leukocyte count of 8.9 × 109/L (reference range 3.5–8.8 × 109/L) with an elevated neutrophil count of 6.8 × 109/L (reference range 1.6–5.9 × 109/L) and lymphopenia with a lymphocyte count of 0.86 × 109/L (reference range 1.0–3.5 × 109/L).
The patient was screened with a throat swab (polymerase chain reaction (PCR) for influenza A and B, respiratory syncytial virus, human metapneumovirus, parainfluenzavirus types 1–3, adenovirus, and rhino/enterovirus), swabs of the mouth ulcers (PCR for herpes simplex virus 1 and 2, and varicella-zoster virus), and swabs from the conjunctiva (PCR for herpes simplex virus 1 and 2 and adenovirus), all of which came back negative.
A PCR test for M. pneumoniae and C. pneumoniae on a throat swab was positive only for C. pneumoniae. A PCR test on sputum for atypical pneumonia (M. pneumoniae, Legionella species, C. psitacci, and C. pneumoniae) confirmed this finding. C. pneumoniae serology was not performed. Sputum microscopy showed several polymorphonuclear leukocytes but no bacteria or yeasts. Sputum culture revealed no growth of pathogenic bacteria.
A chest X-ray revealed a right-sided lower perihilar infiltrate radiating towards the lower right lobe consistent with atypical pneumonia (Figure 4).
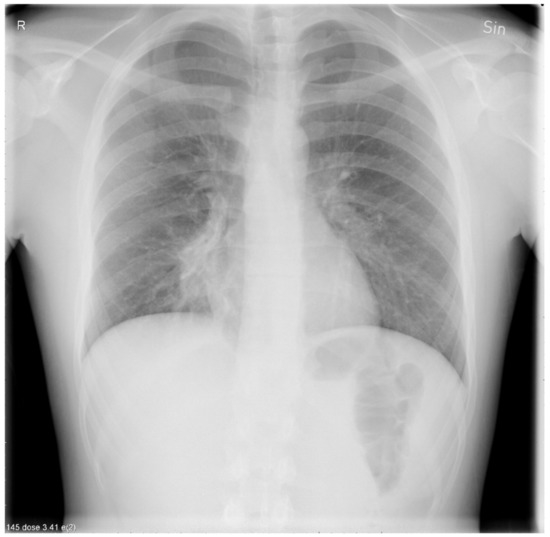
Figure 4.
A chest X-ray showing a right-sided lower perihilar infiltrate radiating towards the lower right lobe.
4. Differential Diagnosis
Involvement of the lips and oral mucosa initially suggested herpes simplex gingivostomatitis, while conjunctival injections were ascribed to adenovirus. Upon negative PCR findings for herpes simplex virus 1 and 2, M. pneumoniae was suspected to account for symptoms from the lower airways and mucous membranes, as seen in MIRM. PCR testing turned out negative for M. pneumoniae and instead proved C. pneumoniae to be the infectious cause of the patient’s symptoms, thus mimicking the symptom complex seen in M. pneumoniae-associated mucositis. Oral mucosal lesions have been reported in patients positive for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) [3,4], however SARS-CoV-2 was not considered in our diagnostic workup since the patient was admitted prior to the COVID-19 pandemic.
5. Treatment
Acyclovir 800 mg × 3 was the first choice of treatment as the cause of the disease was initially suspected to be herpes simplex virus. Only when suspicion fell upon M. pneumoniae the treatment was changed to clarithromycin 500 mg twice daily for 10 days.
The patient underwent a dermatology review, which prompted treatment with prednisolone tablets 25 mg once daily for 7 days.
Paracetamol 1000 mg × 4 daily, ibuprofen 600 mg × 3 daily, and tramadol 50 mg × 1 daily were used as analgesics; lidocaine 2% gel for oral use to lessen his odynophagia and supportive care consisting of intravenous fluids was given during his inpatient stay.
6. Outcome
The patient’s condition improved gradually, and he was discharged after three days. He was then seen at a follow-up visit 10 days after discharge where his clinical condition had improved remarkably. Respiratory symptoms and bilateral conjunctival injection had fully subsided while both the penile erosions and oral sores had subsided substantially, the latter allowing him to tolerate oral intake.
7. Discussion
C. pneumoniae is an obligate intracellular bacterium. The clinical course of infection is most often asymptomatic or mild and self-limiting but may present as an acute lower or upper airway disease such as pharyngitis, sinusitis, bronchitis, or pneumonia. C. pneumoniae has been reported to account for 6%–20% of all community-acquired pneumonia (CAP) [5].
A 2017 review of the literature found a total of 21 reported cases of C. pneumoniae infection with relation to SJS/TEN or EM. Most patients had cutaneous involvement (90.5%) while seven developed mucosal involvement, of which six had oral lesions, five conjunctivitis, and three urogenital lesions. Only two patients had mucositis without cutaneous involvement, which was also the case for our patient [1].
The pathogenesis underlying these mucocutaneous eruptions has not yet been determined. However, skin damage in MIRM may be linked to molecular mimicry or deposition of immune complexes and complement activation [6]. Whether pathogenesis of the mucocutaneous presentations of C. pneumoniae is similar to M. pneumoniae or has unique features is unknown.
Diagnosing RIME in the setting of C. pneumoniae may pose a challenge for clinicians given its rarity and the fact that its clinical presentation is easily misinterpreted, also making it likely that the condition remains underreported. As in our case, clinical suspicion may often fall upon the more frequently encountered MIRM. If laboratory testing for M. pneumoniae turns out negative, C. pneumoniae serology or PCR testing of oropharyngeal swabs or sputum samples in the setting of CAP and mucocutaneous eruptions, in adherence with local clinical guidelines, may be sufficient for determining the diagnosis [7,8].
The literature provides no evidence-based guidelines regarding the treatment of mucocutaneous eruptions following C. pneumoniae infection. Regarding MIRM which also lacks consensus on treatment, but has been studied more comprehensively, supportive care and antibiotic treatment for CAP seem to be generally accepted. Although the condition has a good prognosis and may even be self-limiting, systemic corticosteroids may be necessary to manage mucocutaneous eruptions in some cases. A therapeutic approach of azithromycin and prednisolone 1 mg/kg/day has been suggested in the setting of PCR-confirmed C. pneumoniae infection with mucosal involvement at two or more sites [1].
The clinical presentation of our patient with mucosal but not cutaneous involvement and no history of new medication strays from the typical picture seen in SJS/TEN. This finding supports the use of the term RIME instead of SJS/TEN, which may aid clinicians in determining a more fitting diagnosis and treatment as well as future research into mucocutaneous manifestations following infection.
8. Take Home Messages
- Mucocutaneous eruptions in the younger population often have an infectious cause. Remember to consider C. pneumoniae as it may be an underreported trigger of RIME.
- Most cases can be treated sufficiently with antibiotics, supportive care, and, in some cases, systemic corticosteroids.
- Though the evidence is sparse, the prognosis for mucocutaneous eruptions secondary to C. pneumoniae infection is generally good, and patients seem to recover fully within weeks.
Author Contributions
F.F.L. wrote the report, supervised by A.W. A.R., A.W., and N.W. were responsible for the patient’s care and have approved the report. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge the patient, who generously gave us permission to write this report and share the images.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mayor-Ibarguren, A.; Feito-Rodriguez, M.; González-Ramos, J.; Del Rosal-Rabes, T.; González-Sainz, F.J.; Sánchez-Orta, A.; de Lucas-Laguna, R. Mucositis Secondary to Chlamydia pneumoniae Infection: Expanding the Mycoplasma pneumoniae-Induced Rash and Mucositis Concept. Pediatr. Dermatol. 2017, 34, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Ramien, M.L.; Bahubeshi, A.; Pope, E.; Eichenfield, L.; Lara-Corrales, I.; Nopper, A.J.; Shear, N. Redefining severe cutaneous reactions in children. In Proceedings of the Society for Pediatric Dermatology Annual Meeting, Junior Faculty Forum, Lake Tahoe, CA, USA, 13 July 2018. [Google Scholar]
- Soares, C.D.; Mosqueda-Taylor, A.; de Carvalho, M.G.F.; de Almeida, O.P. Oral vesiculobullous lesions as an early sign of COVID-19: Immunohistochemical detection of SARS-CoV-2 spike protein. Br. J. Dermatol. 2021, 184, e6. [Google Scholar] [CrossRef] [PubMed]
- Cruz Tapia, R.O.; Peraza Labrador, A.J.; Guimaraes, D.M.; Matos Valdez, L.H. Oral mucosal lesions in patients with SARS-CoV-2 infection. Report of four cases. Are they a true sign of COVID-19 disease? Spec. Care Dent. 2020, 40, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Blasi, F.; Tarsia, P.; Aliberti, S. Chlamydophila pneumoniae. Clin. Microbiol. Infect. 2009, 15, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Canavan, T.N.; Mathes, E.F.; Frieden, I.; Shinkai, K. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: A systematic review. J. Am. Acad. Dermatol. 2015, 72, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Contini, C.; Segala, D.; Cultrera, R.; Minichello, V.M.C. Detection of parvovirus B19 and Chlamydophila pneumoniae in a patient with atypical sarcoidosis. Infection 2009, 37, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Dowell, S.F.; Peeling, R.W.; Boman, J.; Carlone, G.M.; Fields, B.S.; Guarner, J.; Hammerschlag, M.R.; Jackson, L.A.; Kuo, C.C.; Maass, M.; et al. Standardizing Chlamydia pneumoniae assays: Recommendations from the Centers for Disease Control and Prevention (USA) and the Laboratory Centre for Disease Control (Canada). Clin. Infect. Dis. 2001, 33, 492–503. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).