Abstract
Spindle cell carcinoma (SCSCC) with osteoid and/or cartilage formation in the head and neck is rare; only one case was reported in the tongue. Herein, we report an SCSCC with osteoid and cartilage formation of the tongue developed in an 85-year-old man, and then review the report.
1. Introduction
Spindle cell carcinoma (SCSCC) of the head and neck is a rare variant of squamous cell carcinoma. It is characterized by a malignant spindle and/or pleomorphic cells with a sarcomatous appearance. Rarely, osteoid and/or cartilage formation resembling osteosarcoma and/or chondrosarcoma may be observed; only one case has been reported in the tongue [1]. Herein, we report the second case of SCSCC with osteoid and cartilage formation in the tongue, along with a review of the literature.
2. Case Report
An 85-year-old man with a previous medical history of tongue cancer and received radiation therapy 13 years ago noticed a mass in the body of the tongue within the radiation field. He visited our hospital, and malignancy was suspected. On clinical examination, hard raised and polypoid mass with the bumpy surface was found (Figure 1). The tumor occupied more than half of the tongue, and the midline of the tongue was displaced to the right. There was no cervical lymph node enlargement on computed tomography (CT) (Figure 2). The volume reduction surgery was performed, and macroscopically, the excised tumor was a solid mass, 5.6 × 4.2 × 2.2 cm in size (Figure 3). The cut surface of the tumor was white to yellowish-brown and brown near the surface. Histologically, the surface of the tumor was ulcerated, and the tumor showed proliferation of predominant spindle cells and a few atypical epithelial cells (Figure 4). The spindle cells with pleomorphic nuclei, was continuous with epithelial cells (Figure 5). Immunohistochemically, the spindle cells were negative for cytokeratin AE1/AE3 and p63, but epithelial cells were positive for both of them. Both spindle and epithelial cells were positive for p53. The foci of osteoid and bone formation were found in the spindle cell component and showed osteosarcomatous appearance (Figure 6). In addition, there were the atypical round-to-oval cells with a large nucleus and binuclear in lacunae with cartilage formation. It showed a chondorosarcomatous appearance. The diagnosis of SCSCC with osteoid and cartilage formation was made. After the operation, no recurrence was observed at the five-month follow-up.
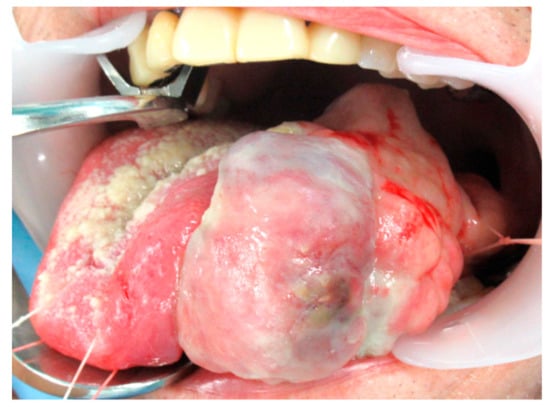
Figure 1.
Gross findings of the tumor. The tumor was an exophytic, polypoid mass in the body of the tongue.
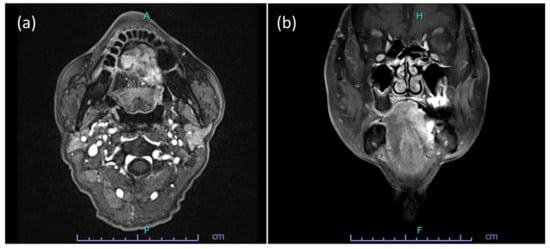
Figure 2.
Radiographic findings of the tumor. (a,b) The tumor localized in the body of the tongue and occupied more than half of the tongue. The cervical lymph node is not enlarged.
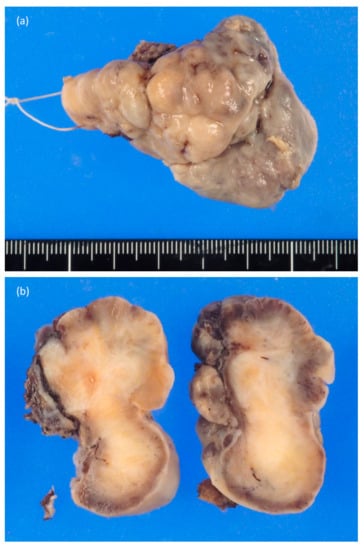
Figure 3.
Gross findings of the resected material. (a) The tumor was raised mass with a bumpy surface. (b) The cut surface of the tumor was solid, white to yellowish-brown, and brown near the surface.
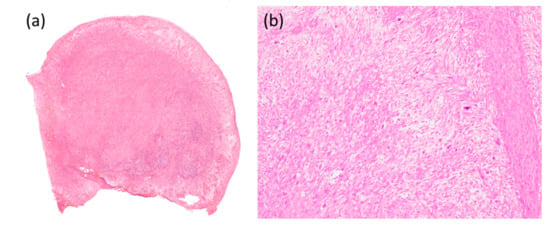
Figure 4.
Histological findings of the tumor. (a) The tumor showed an exophytic mass with an ulcerated surface. (b) The tumor was mainly composed of spindle cells and a few atypical epithelial cells (100×).
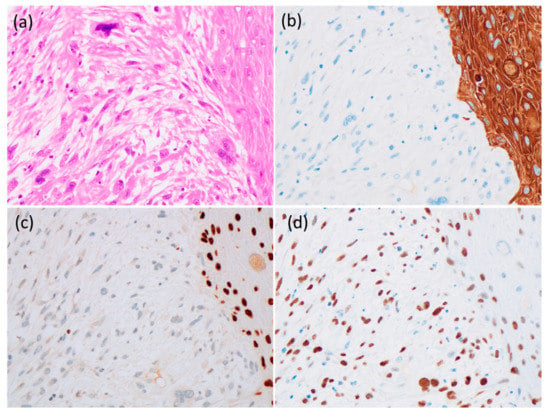
Figure 5.
Histological and immunohistochemical finding of the tumor. (a) The spindle cells with nuclear pleomorphism had continuity with atypical epithelial cells (400×). (b) The spindle cells were negative for cytokeratin AE1/AE3 (400×), but atypical epithelial cells were positive. (c) The spindle cells were negative for p63 (400×), but atypical epithelial cells were positive. (d) Both the spindle and atypical epithelial cells were positive for p53 (400×).
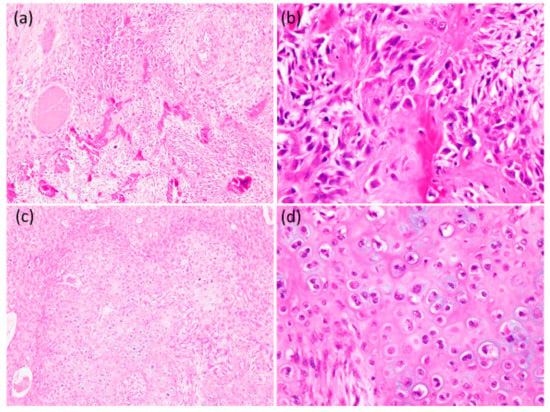
Figure 6.
Histological findings of the tumor. (a) There were foci of osteoid and irregular trabeculae formation (100×). (b) It showed a proliferation of short-spindle and polygonal-shaped atypical cells with hyperchromatic nuclei (400×). (c) The foci of lobules of cartilage formation were found (100×). (d) Increased cellularity and atypical round-to-oval cells in lacunae were noted (400×).
3. Discussion
SCSCC of the head and neck is a rare neoplasm. The existence of matrix production in SCSCC is uncommon.
In molecular analyses, two hypotheses have been proposed to explain tumor development in SCSCC, namely, that a monoclonal origin from a stem cell gives rise to both components and that there is an independent multiclonal origin for each of the mesenchymal and the squamous components [2,3,4,5]. Immunohistochemically, the p53 stain was almost the same in the paired epithelial and spindle cells of SCSCC in the upper respiratory tract [6]. In the present case, both spindle and epithelial cells showed positive for p53; this may support that both components were monoclonal origin.
We reviewed the cases of SCSCC with osteoid and/or cartilage formation, including carcinosarcoma that is a synonym of spindle cell carcinoma. In oral mucosa, only six cases of SCSCC and carcinosarcoma that had osteoid and/or cartilage formation were reported; this is the second case arising in the tongue (Table 1). The most common site of SCSCC and carcinosarcoma with osteoid and/or cartilage formation in oral mucosa was alveolar ridge or gingiva; Vishal et al. described the first carcinosarcoma case in the tongue, which showed predominant osteosarcomatous component with the foci of cartilage, 8.0 × 5.0 cm in size [1]. In the present case, the spindle cell component was predominant in the tumor. Osteoid and cartilage formation were in the spindle cell component of the tumor and in the limited area, 5.6 × 4.2 cm in size. There may be a correlation between the production of bone and/or cartilage matrix and the size of the lesion, but the cases of SCSCC in oral is limited; more cases are needed.

Table 1.
Summary of previous and current reports of spindle cell carcinoma (SCSCC) and carcinosarcoma with osteoid and cartilage formation in the oral mucosa.
SCSCC with matrix production has been mainly reported in the larynx. Thompson et al. described that 7% of SCSCC in the larynx showed bone and/or cartilage formation [7]. The patient of the present case had a history of radiation therapy; some reports imply the formation of bone and/or cartilage in laryngeal SCSCC is related to radiation therapy [8,9]. However, the mechanism of induction of matrix production is still unclear [8,9].
The presence of bone and/or cartilage formation in laryngeal SCSCC did not yield a worse patient outcome when compared with patients who did not have the histologic feature [7]. In oral mucosa, as follow-up information of SCSCC with matrix production is limited, the prognostic impact of the matrix production component is unclear.
In conclusion, we report a rare case of SCSCC with osteoid and cartilage formation in the tongue. Only six cases of SCSCC and carcinosarcoma in oral mucosa were reported; there was little information about clinicopathological features. To characterize clinicopathological features, the study of additional cases is necessary.
Author Contributions
S.O.: pathology fellow responsible for working up the case. Write-up of the manuscript and final submission. H.Y.: pathology professor responsible for the interpretation and final diagnosis of case. H.K., K.T. (Kiyofumi Takabatake), and K.N. (Keisuke Nakano)., T.M.: Oral pathology assistant professor responsible for interpretation, review, and editing of final manuscript. T.T., K.T. (Kohei Taniguchi), and K.N. (Kenji Nishida): pathology assistant professor contributed to the data collection. H.N.: oral pathology professor, consultant during the interpretation and getting to the final diagnosis. T.Y.: pathology professor, reviewed manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
None of the authors have any conflicts of interest to declare.
References
- Vishal, R.; Aleena, J.; Sudha, M.; Chandra, S.R. Carcinosarcoma of Tongue with Predominant Osteosarcomatous Component. Indian J. Surg. Oncol. 2018, 9, 609–612. [Google Scholar]
- Huszar, M.; Herczeg, E.; Lieberman, Y. Distinctive immunofluorescence labeling of epithelial and mesenchymal elements of carcinosarcoma with antibodies specific for different intermediate filaments. Hum. Pathol. 1984, 15, 532–538. [Google Scholar] [CrossRef]
- Mathieu, M.C.; Micheau, C.; Caillaud, J.M.; Bosq, J.; Carlu, C. Immunohistologic marking of epithelial antigens in sarcomatoid carcinomas of the upper aerodigestive tracts. Ann. Pathol. 1986, 6, 313–322. [Google Scholar] [PubMed]
- Thompson, L.; Chang, B.; Barsky, S.H. Monoclonal origins of malignant mixed tumors (carcinosarcomas): Evidence for a divergent histogenesis. Am. J. Surg. Pathol. 1996, 20, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.R.; Sturgis, E.M.; Rosenthal, D.I.; Luna, M.A.; Batsakis, J.G.; El-Naggar, A.K. Sarcomatoid carcinoma of the head and neck: Molecular evidence for evolution and progression from conventional squamous cell carcinomas. Am. J. Surg. Pathol. 2003, 27, 1216–1220. [Google Scholar] [CrossRef] [PubMed]
- Ansari-Lari, M.A.; Hoque, M.O.; Califano, J.; Wsetra, W.H. Immunohistochemical p53 Expression Patterns in Sarcomatoid Carcinomas of the Upper Respiratory Tract. Am. J. Surg. Pathol. 2002, 26, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.D.R.; Wieneke, J.A.; Miettinen, M.; Dennis, K.H. Spindle Cell (Sarcomatoid) Carcinomas of the Larynx. A Clinicopathologic Study of 187 Cases. Am. J. Surg. Pathol. 2002, 26, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.E.; Olsen, K.D.; Sebo, T.J. Spindle cell carcinoma of the larynx: Review of 26 cases including DNA content and immunohistochemistry. Hum. Pathol. 1997, 28, 664–673. [Google Scholar] [CrossRef]
- Lambert, P.R.; Ward, P.H.; Berci, G. Pseudosarcoma of the larynx: A comprehensive analysis. Arch. Otolaryngol. 1980, 106, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Gary, L.E.; Russell, L.C. Spindle Cell Carcinoma of the Oral Cavity. A Clinicopathologic Assessment of Fifty-Nine Cases. Oral Surg. Oral Med. Oral Pathol. 1980, 50, 523–533. [Google Scholar]
- Katase, N.; Tamamura, R.; Gunduz, M.; Murakami, J.; Asaumi, J.; Tsukamoto, G.; Sasaki, A.; Nagatsuka, H. A spindle cell carcinoma presenting with osseous metaplasia in the gingiva: A case report with immunohistochemical analysis. Head Face Med. 2008, 4, 1–6. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).