Abstract
Metagenomic analysis is the comprehensive study of DNA using clinical specimens of organisms including bacteria, fungi, and viruses. In this study, we investigated the efficacy of metagenomic analysis for diagnosing ocular infections, including 11 keratitis cases, four iridocyclitis cases, and one endophthalmitis case. Corneal scraping, aqueous humor, and vitreous humor, were collected respectively. Ocular specimens were used for bacterial and fungal culture, and PCR for detecting viral DNA. Shotgun metagenomic sequencing for 150 bases of single end was performed by Illumina MiSeq® System. Sequence was retrieved from the database at NCBI using a MegaBLAST search. Since Propionibacterium spp. are commensal bacteria found at the ocular surface, they were excluded from analysis. Six cases (37.5%) were positive for culture or PCR. Metagenome techniques revealed that 9 cases (56.3%) included genomes of organisms that were considered pathogenic in specimens. Five cases (31.3%) possessed genomes of organisms like themselves that were detected by culture and PCR. Six cases (37.5%) were negative for culture, PCR, and metagenome analysis. Moreover, viral pathogens (HSV-1, 2 cases; and VZV, 1 case) were detected by only metagenome analysis. Metagenome analysis using an ocular sample can detect microbial genome comprehensively, and viral pathogens, which were not detected by conventional examination.
1. Introduction
The severity of ocular infection and the response to its treatment depends on the causative agents. If the diagnosis and treatments for ocular infections are delayed, it may become more severe. Thus, rapid detection of pathogens causing an ocular infection is critical to diagnose and determine treatment strategy. In general, culture testing for bacteria and fungi along with direct staining using ocular specimens is the gold standard for detection of pathogens. However, it takes several days to obtain results of a positive culture. Culture tests fail to detect when culture conditions such as medium and temperature are not appropriate for target pathogens. Thus, culture tests are unable to detect all kinds of bacteria and fungi, as sensitivity is low in ocular infection [1,2]. Direct stainings can detect the pathogens preliminarily and quickly, and helps in prescribing an appropriate anti-microbial agent. However, it needs skills to identify pathogens and drug susceptibility. Both, culture test and direct staining are unable to detect viral pathogen such as a herpetic virus. To detect viral pathogens such as herpes simplex virus-1 (HSV-1), varicella-zoster virus (VZV), and cytomegalovirus (CMV), detection of viral DNA is common [3]. Polymerase chain reaction (PCR) can amplify the DNA for a short time and detect pathogens using a small amount of specimen. Thus, PCR for amplification of pathogen DNA has higher sensitivity, when compared with culture testing. Bacterial and fungal DNA have specific gene loci which contain repetition of hypervariable and bacterial conserved regions such as 16S rRNA for bacteria and 18S rRNA for fungi. PCR, which amplifies between conserved regions in 16S rRNA and 18S rRNA can detect all species of bacteria and fungi, respectively [4]. To identify species, it is necessary to analyze the sequence of amplification. Since PCR uses a single pair of primers to amplify one gene locus, a single reaction of PCR detects one target DNA. It is difficult to detect several pathogen DNA (bacteria, fungi, and viruses) using a single reaction. Multiplex PCR uses several pairs of primers in a single tube and can amplify multiple DNA in one reaction. Moreover, in real-time-PCR that can quantitate the amount of DNA-, a fluorescent-tagged probe is widely used. Since we can observe the amplification of DNA in real-time, it takes a short time to identify DNA amplification. Recently, Nakano et al. developed strip PCR, which can detect DNA of the pathogen causing ocular infection, using multiplex and real-time-PCR [5]. It is very effective to detect multiple pathogen DNA simultaneously and diagnose ocular infection. Since it is necessary to know the genetic information to design primers for PCR, it is impossible to detect unknown pathogens.
Since samples of ocular specimens such as corneal scrapings are generally very limited, it is difficult to conduct multiple examinations simultaneously. We should choose one or two effective methods to detect pathogens because each microbial examination has disadvantages such as long detection time, low sensitivity and specificity, and limited pathogen detection. Moreover, conventional methods cannot detect unculturable and unknown pathogens. Thus, we need an ideal method that can comprehensively detect unknown pathogens simultaneously with high sensitivity.
Next-generation sequencing (NGS) is used for gene analysis. The metagenomic analysis is used for analyzing the human genome, human microbiome, such as intestinal microbiome, and environmental microbial communities such as ocean and soil; therefore, it can comprehensively detect pathogens including bacteria, fungi, viruses, and un-culturable organisms, and can be a useful method for diagnosing infectious diseases [6]. Especially, shotgun sequencing can read a fragment of DNA in the sample and assemble DNA without amplification. Thus, shotgun metagenomic analysis (SMA) can detect a range of pathogens (bacterial, viral, fungal, and eukaryotic parasites). Since it can detect various pathogenic DNA in one test, the hypothesis for the pathogen is not needed. SMA has been used for detecting infections or colonization of pathogens in several diseases [6,7,8]. However, little is known about the efficacy of SMA for diagnosis of ocular infections, such as keratitis and iridocyclitis.
In this study, we compared the diagnostic performance of general microbial examinations such as culture test, direct staining, PCR, and SMA for ocular infection, and investigated the usefulness of SMA.
2. Results
2.1. Clinical Diagnosis
Based on the results of microbiological testing, PCR, and clinical records, patients were clinically diagnosed with Cytomegaloviral endotheliitis (two patients), infectious endophthalmitis (one patient), Acanthoamoeba keratitis (two patients), bacterial keratitis (one patient), fungal keratitis (four patients), herpetic keratitis (three patients), noninfectious iridocyclitis (two patients), and Thygeson’s keratitis (Table 1).

Table 1.
Summary of 16 patients with ocular infection.
Reports of Representative Cases
Case 1: The patient was a 78-year-old man with vision disturbances in his right eye. He visited a private clinic and was diagnosed with bullous keratopathy. He was advised Descemet’s stripping automated endothelial keratoplasty (DSAEK). However, he reported a recurrence of visual disturbance in his right eyes. Slit-lamp examination of the right eye revealed stromal edema with keratoprecipitate (Figure 1, top left). Graft rejection or viral endotheliitis was suspected. PCR of the aqueous humor yielded positive results for CMV DNA. He was diagnosed with CMV endotheliitis and treated with 0.5% ganciclovir eye drop. The corneal edema resolved within 1 month. SMA showed 1.6% of the whole genome, which was a different genome than that of humans. The genome of bacteria, eukaryote, and viruses was read in 13.4, 28.9, and 8.5% of root genomics and 49.2% of root genomics were not assigned, hit, or shown in the database. Since the genome of the eukaryote was revealed as another animal genome, it was omitted. Microbiomes which had first and second most genome reads were those of Propionibacterium spp. (2473 reads) and CMV (2283 reads), respectively.
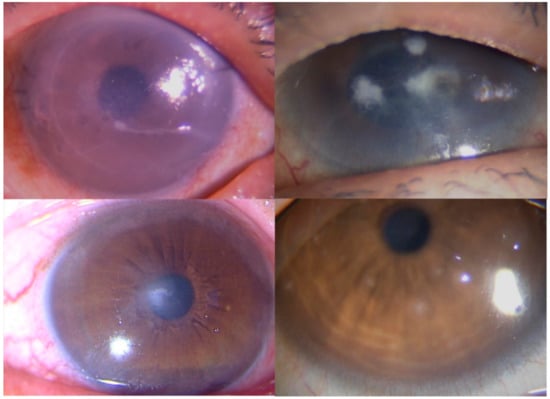
Figure 1.
Images demonstrating keratitis in Cases 1, 11, 13, and 16. (Top left) Slit-lamp photograph showing a corneal edema in Case 1. (Top right) Slit-lamp photograph showing a multiple corneal infiltration in Case 11. (Bottom left) Slit-lamp photograph showing an irregular corneal infiltration in Case 13. (Bottom right) Slit-lamp photograph showing a multiple elevated, white-grey, granular, intraepithelial corneal lesions.
Case 11: The patient was an 80-year-old woman who had been treated with ocular pemphigoid in her right eye and presented multiple corneal infiltrates (Figure 1, top right). Culture tests of corneal scrapings revealed Candida albicans. Thus, we diagnosed the patient with fungal keratitis and treated her with antifungal agents such as topical voriconazole and natamycin. The infiltrates gradually subsided. Shotgun metagenomic testing showed 97.7% (411,413 reads) of the genome, which was a subtracted unanalyzed genome from root genomics, belonged to Candida spp.
Case 13: The patient was a 75-year-old woman who had extensive recurrent herpetic keratitis in her left eye and complained of blurred vision. A slit-lamp examination of the left eye revealed a white stromal infiltrate (Figure 1, bottom left). Culture tests of corneal scrapings revealed Propionibacterium spp., and PCR of herpetic DNA was negative. Shotgun metagenomic testing demonstrated that root genomics which was 0.8% of the whole genome, included 112 reads (0.7% of root genomics) of HSV-1 DNA and 39 reads (0.24% of root genomics) of Propionibacterium spp. DNA as first and second most predominant genomes.
Case 16: A 37-year-old male who complained of corneal irritation was treated with topical steroid. Since steroid did not respond well, he was referred to our hospital. Slit-lamp biomicroscope revealed multiple elevated, white-grey, granular, intraepithelial corneal lesions (Figure 1, bottom right), and Thygeson’s superficial punctate keratitis (TSPK) was diagnosed. PCR of viral DNA using corneal scrapings were negative. We switched to topical 0.1% tacrolimus four times per day. The corneal inflammation and ocular symptoms subsided within 2 weeks. Shotgun metagenomic testing demonstrated a total of 1,605,632 reads, which were similar to the human genome, and excluded genome of microorganisms.
2.2. Summary of SMA
SMA showed extracted DNA included 2.09 ± 0.69 × 106 genome reads, including that of the human genome. (Table 2) Reads with human genome subtracted were 1.06 ± 3.19 × 105 and 5.2 ± 14.4% of the original reads.

Table 2.
Summary of SMA results in 16 cases.
The rate of microbial or the unclassified genomes due to no-hit, no-assign, and not shown in the database, in root genomics of each case is shown in Figure 2A. There were higher rates of unclassified and eukaryotic genome, with the exception of Case 8. The rate of the viral genome was small. Since eukaryotic genome included several genomes of mammalian cells, which were not related to the pathogen, the only fungal genome was used for analysis. The rate of the bacterial, viral, and fungal genome was compared among cases. (Figure 2B) Since there were very few bacterial genomes in Cases 3, 4, 12, and 16, they did not include pathogenic genome. Cases 1, 9, 13, and 15 included viral genome, and Cases 9, 10, and 11 had a high proportion of the fungal genome. The relative abundance of microbial species detected in each case is shown in Figure 3. Eight cases included >10% genome of Propionibacterium species, and Cases 8, 10, and 11 had >90% single genome of Staphylococcus (Case 8) and Candida species (Cases 10 and 11). Viral genome was detected in Cases 1 (CMV), 9 (HSV-1), 13 (HSV-1), and 15 (VZV). Several cases had >50% other genes, which included multiple genomes with few reads (<5%).
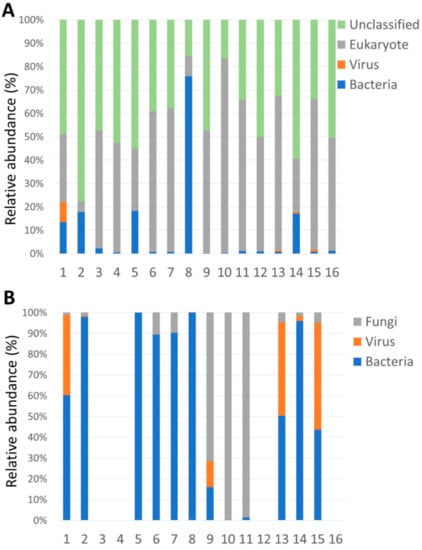
Figure 2.
Rate of reads in specimens (A) Reads after genomic subtraction of human genome (B) Reads after genomic subtraction of unclassified and eukaryotic genome.
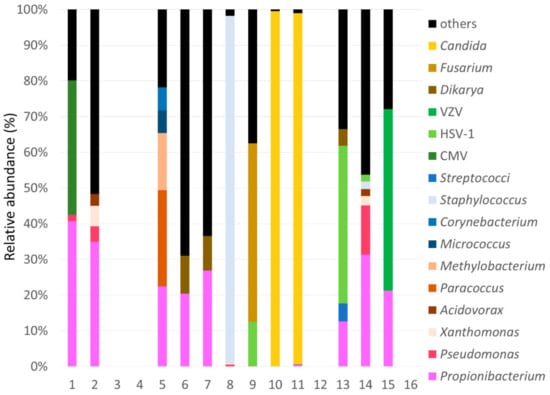
Figure 3.
Rate of microbial reads in each case.
2.3. Comparison between SMA and Conventional Examination
From results of SMA, the causative pathogen in each case was defined as pathogen and probable pathogen. Pathogen was defined when pathogenic genome was occupied >50% of the microbial genome. Probable pathogen was defined as follow; 1: pathogenic genome other than Propionibacterium spp. occupied >20% of the microbial genome. 2: detection of the viral genome. The list of pathogens detected by SMA is shown in Table 2. Detected pathogens were Staphylococcus spp. (Case 8), Fusarium spp. (Case 9), Candida spp. (Cases 10 and 11), and VZV (Case 15) in keratitis, and they were also detected by culture, except for Case 15. Probable pathogens were CMV (Case 1) in endotheliitis, Paracoccus spp. (Case 5) in endotheliitis, and HSV-1 (Cases 9, 13, and 14) in keratitis. Any predominant pathogen was not detected in Cases 2, 6, and 7. Both Fusarium spp. and HSV-1 were detected in Case 9. Comparison of SMA with conventional examinations was performed in results of detecting pathogens. (Table 3) Since Propionibacterium spp.is commensal bacteria at the ocular surface, they have not considered a pathogen in Cases 7 and 12. The positive rate of SMA and conventional examination was 56.3% and 37.5%, respectively. Identification of pathogens, which were positive in both examinations was the same. Four cases (Case 5; Paracoccus spp., Cases 13 and 14; HSV-1, Case 15; VZV) were positive for only SMA. In nine cases (56.3%), results of SMA corresponds with the clinical diagnosis of ocular infection.

Table 3.
Comparison of shotgun metagenomic testing with conventional methods for ocular infection.
3. Discussion
Pathogen detection using NGS is expected in the ophthalmology field because it is a hypothesis-free approach that can detect pathogens from a single specimen in one test [6,9]. This technology can routinely detect a range of pathogens (bacterial, viral, fungal, and eukaryotic parasites), and there is no need to consider what pathogens are probable present and select examination. Several studies have already demonstrated metagenomic DNA. Sequencing was useful to detect the pathogen in intraocular infection and uveitis [10,11,12]. Lee et al. demonstrated that torque teno virus was identified in culture-negative endophthalmitis by a deep-sequencing method (biome representational in silico karyotyping [BRiSK]), similarly metagenomic deep sequencing identified fungi, parasites, DNA and RNA viruses in small volumes of intraocular fluid samples [11,12]. Metagenomic analysis using NGS can be useful to detect pathogens for ocular infection, especially intraocular infection because there is little contamination of microfloral bacteria to the intraocular lesion. In this study, five ocular fluids collected from endotheliitis, iridocyclitis, and endophthalmitis, were examined by metagenomic analysis. Two cases were positive for metagenomic analysis (CMV and Paracoccus spp.) Corneal endotheliitis is defined as inflammation of the corneal endothelium accompanied by the progressive destruction of the corneal endothelium. Several viruses including HSV-1 and CMV can be pathogens associated with corneal endotheliitis [13]. It is critical to detect viral DNA from the aqueous humor of the patient, and the PCR method is a strong tool for the diagnosis of corneal endotheliitis. Metagenomic analysis can detect reads of CMV from a sample in which CMV-DNA was detected using PCR. Thus, metagenomic analysis can be useful to detect viral genes in endotheliitis or iridocyclitis. However, one case (Case 2) was positive for CMV-DNA using PCR method but not metagenomic analysis. Thus, the PCR method is the gold standard for viral detection in viral intraocular infection. Since several studies reported, other viruses related to intraocular inflammation such as endotheliitis, iridocyclitis, and uveitis [14,15], hypothesis-free approaches such as metagenomic analysis can be useful to detect pathogens of viral intraocular infection. Moreover, Paracoccus spp. was detected from endophthalmitis via metagenomic analysis. It is classified within the genus Rhodobacteraceae, and obligate aerobic, nonfermenting, gram-negative cocci, diplococci, or coccobacilli. Paracoccus yeei were detected in intraocular fluid specimens in uveitis and corneal graft rejection [16,17]. Thus Paracoccus spp. may be intraocular inflammation. Since metagenomic analysis could detect bacteria which were difficult to culture, it is useful to detect pathogens causing intraocular infection. Although intraocular fluid was collected under sterile conditions, the gene of Propionibacterium spp. which are considered not only pathogen but also microflora of ocular surface were detected by metagenomic analysis. One possibility is the contamination of microflora and another is that Propionibacterium spp. can have some role in intraocular inflammation. Thus far, gene or antigen of Propionibacterium spp. were widely detected in uveitis, especially ocular sarcoidosis [18,19,20]. Further investigations about the gene of Propionibacterium spp. detected in ocular fluid are needed.
There are few investigations related to the usage of metagenomic analysis for the detection of the pathogen causing ocular surface infection such as keratitis. Li et al. demonstrated that NGS can identify various pathogens in formalin-fixed corneal specimens [21]. They also showed that 96% of the reads detected with NGS were classified as human gene, and 1.7% represented microbial sequences, and 2.4% cannot be classified, and various human gene reads were included. Compared with our study, there were more reads detected by NGS. Since they used formalin-fixed corneal specimens, several specimens were more than corneal scraping. A lot of reads were unclassified in our study because corneal specimens might be influenced by contamination of other genes. Moreover, unclassified reads generally include a lot of repeated sequences. When the database used for analysis is improved, unclassified genes may be reduced. Our study revealed that cases of fungal keratitis were positive for both conventional culture and SMA. A lot of fungal genes were detected by metagenomic analysis, and it was easy to consider fungi as causative agents (Candida spp. and Fusarium spp.). Like cases of fungal keratitis, the high rate of Staphylococcus spp. was detected from a case of bacterial keratitis. When low reads of the bacterial gene such as Propionibacterium spp. were included in specimens, it was very difficult to distinguish between pathogenic or non-pathogenic agents. Propionibacterium spp. are generally microflora of ocular surface. In this study, almost samples included genes of Propionibacterium spp. Moreover, it was little known if it was true that the low rate of a bacterial gene in SMA may indicate that it is microflora and non-pathogenic. Further investigation about identification of the real causative agent in SMA should be needed.
The herpetic virus was detected in several cases of SMA. From one case, HSV-1 and Fusarium spp. were detected by SMA, and it presented with double infection. Mixed corneal infection of fungi, bacteria and herpetic virus was reported [22]. Thus, SMA can be useful to detect multiple pathogenic agents and diagnose mixed infection. Interestingly, metagenomic analysis but not PCR detected HSV-1 or VZV in three cases. PCR can amplify only part designed, meanwhile metagenomic analysis can detect all gene existed in specimens. Thus, SMA can be effective to detect viral infection in the cornea.
We used this technique for the case of TSPK. TSPK is characterized by multiple elevated, white-grey, granular, intraepithelial corneal lesions and treated with steroid agents [23]. Although the etiology of TSPK is still unclear, Thygeson considered it as a viral etiology [24]. However, several studies demonstrated PCR was negative for viral DNA in cases of TSPK [25,26]. Not only PCR but also metagenomic analysis cannot detect viral DNA in TSPK, thus, etiology of TSPK may not be related to viral infection. Metagenomic analysis can be useful to confirm the existence of pathogens in ocular surface disease with unknown etiology.
The results of our study should be interpreted with caution because of some limitations. To begin with, SMA showed various non-classified and eukaryotic genes which were not related to the infection. And SMA cannot detect RNA viruses. Contamination of genes should be considered in this technique. The results showed huge variations in the number of SMA reads among samples because size of samples such as corneal scraping was various. We need to use quantitively corneal samples. As mentioned before, the improvement of the database used for analysis can increase the accuracy of results. Also, it was difficult to determine if the organism detected in the metagenomic analysis was the pathogenic or nonpathogenic agent. We have not examined SMA against control sample (healthy corneal scrapings), and it is very difficult to set the detection cutoff threshold. In future, we will correct data of control samples and analyze SMA data for non-pathogenic agents on ocular surface. Hence a cut-off value of reads must be set to determine pathogenic or non-pathogenic agent. The balance the sensitivity and specificity of pathogen identification should be very important. High sensitivity could pick up genes of not only pathogenic but also non-pathogenic agents, and reduce specificity of SMA for detection of pathogenic agents. Further investigations are needed to confirm that this definition was consistent to all cases of ocular infection. Additionally, it took a long time to obtain the results of metagenomic analysis and high cost. To use as clinical setting, less time and lower costs are needed.
In this study, SMA could detect various pathogenic DNA in one test. Comparing with other examinations such as culture and PCR, it could give us a lot of information. However, it was sometimes difficult to consider real pathogen from SMA data. In order to use SMA for detection pathogen causing ocular infection in clinical setting, database of SMA should be improved, and lower cost and shorter time for analyzing should be necessary.
4. Materials and Methods
This single-centre, prospective study was approved by the Ethics Committee of Faculty of Medicine, Toho University School of Medicine (No. 27019) and conformed to the tenets of the Declaration of Helsinki. Informed consent was obtained from patients after explaining the nature and possible consequences of the study. The clinical records of patients who were treated with an ocular infection such as infectious keratitis, iridocyclitis, and endophthalmitis at Toho University Omori Medical Center were collected from 1 January 2016 to 31 December 2018. Ocular specimens were divided into 2–3 samples equally, from which one or two samples were used for microbiological examination or PCR and the remaining samples were stocked at –80 °C for use in SMA. Final clinical diagnoses were based on microbiological tests, detection of viral DNA using PCR in the cornea and aqueous humor, treatment response, and requirement for surgical intervention.
In total, 16 cases of ocular infections (11 keratitis cases, 2 iridocyclitis cases, 2 endotheliitis cases, and 1 endophthalmitis case) were evaluated in this study. The age of the patients ranged from 14 to 91 years (mean, 54 ± 25.2 years). In total there were six male patients and ten female patients.
4.1. Microbiological Examination
Cultures were procured from a Japanese company specializing in laboratory testing services (Handai biken, Inc., Osaka, Japan). In infectious keratitis, corneal scrapings were cultured on sheep blood agar and chocolate agar plates and were incubated at 35 °C. Bacterial identification was performed by gram staining and biochemical testing.
4.2. PCR
Detection of viral DNA in the cornea and aqueous humor were outsourced to a Japanese company specializing in laboratory testing services (SRL, Inc., Tokyo, Japan). DNA extraction and viral PCR for HSV-1, VZV, and CMV were performed.
4.3. Shotgun Metagenomic Sequencing Analysis
The corneal scraping in 100 μL of saline, aqueous humor, or vitreous humor was stocked at –80 °C before SMA. SMA was performed at the Department of Microbiology and Infectious Disease, Toho University Faculty of Medicine [27]. Stocked samples were treated with achromopeptidase, and nucleotides were extracted using a Recover All Total Nucleic Acid Isolation Kit for FFPE (Life Technologies, Thermo Fisher Scientific Inc., Waltham, MA, USA). The next-generation sequencer, Nextra XT DNA Library Preparation Kit (Illumina, Inc., San Diego, CA, USA) was used for the analysis of extracted DNA and a shotgun sequencing library was prepared. Shotgun metagenomic sequencing for 150 bases of the single end was performed using a MiSeq platform (Illumina, Inc., San Diego, CA, USA). Skewer (version 0.1.126) was used for trimming adapter sequence to less than the Phred quality score (Q)15 for low-quality sequences. Human genome sequences were subtracted using the Burrows-Wheeler Aligner (BWA) with “MEM” option with the human genome GRCh37.p13 (GenBank assembly accession: GCA_000001405.14) as a mapping reference and SAM tools (version 1.3) [28,29]. Reads with human genome subtracted were analyzed by a MEGABLAST search against the GenBank nt and WGS databases (ftp://ftp.ncbi.nlm.nih.gov/blast/db/) (the date of last access: 1 June 2019) downloaded in May 2016, followed by metagenomic browser MEGAN5 (http://ab.inf.uni-tuebingen.de/software/megan5) (the date of last access: 1 June 2019) [30]. Human genome was subtracted from the whole genome, and the subtracted genome was considered the microbial genome (bacterial, eukaryotic, and viral genome).
5. Conclusions
We used SMA for detection of the pathogen in ocular infection. It was useful to detect pathogens such as fungi, bacteria, and especially viruses. Improvement of database and determination of cut-off value reads for pathogens is needed for further development and dissemination of this technology in ocular infection.
Author Contributions
Conceptualization, T.S. and Y.H.; methodology, Y.O. and K.A.; formal analysis, K.A.; investigation, T.K.; resources, Y.I., K.T. and Y.H.; data curation, T.K.; writing—original draft preparation, T.K. and T.S.; writing—review and editing, Y.I. and Y.H.; visualization, T.S.; supervision, K.T. and Y.H.; project administration, Y.H.; funding acquisition, Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Japan Society for the Promotion of Science KAKENHI Grant (19K09961), and by Health and Labor Sciences Research Grant (20FC1032).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Faculty of Medicine, Toho University School of Medicine (protocol code No. 27019 and date of approval; 17 September 2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
We thank the Department of Clinical Laboratory, Toho University Omori Medical Center, for their valuable technical assistance with clinical examinations.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| PCR | Polymerase chain reaction |
| HSV | Herpes simplex virus |
| VZV | Varicella-zoster virus |
| CMV | Cytomegalovirus |
| NGS | Next-generation sequencing |
| SMA | Shotgun metagenomic analysis |
| DSAEK | Descemet’s stripping automated endothelial keratoplasty |
| TSPK | Thygeson’s superficial punctate keratitis |
| AH | Aqueous humor |
| CS | Corneal scraping |
| BRiSK | Biome representational in silico karyotyping |
References
- Kratz, A.; Levy, J.; Klemperer, I.; Lifshitz, T. Broth cultures yield vs traditional approach in the workup of infectious keratitis. Eye 2006, 20, 215–220. [Google Scholar] [CrossRef]
- Pakzad-Vaezi, K.; Levasseur, S.D.; Schendel, S.; Mark, S.; Mathias, R.; Roscoe, D.; Holland, S.P. The corneal ulcer one-touch study: A simplified microbiological specimen collection method. Am. J. Ophthalmol. 2015, 159, 37–43. [Google Scholar] [CrossRef]
- Inoue, T.; Ohashi, Y. Utility of real-time PCR analysis for appropriate diagnosis for keratitis. Cornea 2013, 32 (Suppl 1), S71–S76. [Google Scholar] [CrossRef]
- Knox, C.M.; Cevellos, V.; Dean, D. 16S ribosomal DNA typing for identification of pathogens in patients with bacterial keratitis. J. Clin. Microbiol. 1998, 36, 3492–3496. [Google Scholar] [CrossRef]
- Nakano, S.; Sugita, S.; Tomaru, Y.; Hono, A.; Nakamuro, T.; Kubota, T.; Takase, H.; Mochizuki, M.; Takahashi, M.; Shimizu, N. Establishment of Multiplex Solid-Phase Strip PCR Test for Detection of 24 Ocular Infectious Disease Pathogens. Invest. Ophthalmol. Vis. Sci. 2017, 58, 1553–1559. [Google Scholar] [CrossRef]
- Allcock, R.J.N.; Jennison, A.V.; Warrilow, D. Towards a Universal Molecular Microbiological Test. J. Clin. Microbiol. 2017, 55, 3175–3182. [Google Scholar] [CrossRef][Green Version]
- Street, T.L.; Sanderson, N.D.; Atkins, B.L.; Brent, A.J.; Cole, K.; Foster, D.; McNally, M.A.; Oakley, S.; Peto, L.; Taylor, A.; et al. Molecular Diagnosis of Orthopedic-Device-Related Infection Directly from Sonication Fluid by Metagenomic Sequencing. J. Clin. Microbiol. 2017, 55, 2334–2347. [Google Scholar] [CrossRef]
- Wilson, M.R.; Suan, D.; Duggins, A.; Schubert, R.D.; Khan, L.M.; Sample, H.A.; Zorn, K.C.; Rodrigues Hoffman, A.; Blick, A.; Shingde, M.; et al. A novel cause of chronic viral meningoencephalitis: Cache Valley virus. Ann. Neurol. 2017, 82, 105–114. [Google Scholar] [CrossRef]
- Doan, T.; Pinsky, B.A. Current and future molecular diagnostics for ocular infectious diseases. Curr. Opin. Ophthalmol. 2016, 27, 561–567. [Google Scholar] [CrossRef]
- Doan, T.; Acharya, N.R.; Pinsky, B.A.; Sahoo, M.K.; Chow, E.D.; Banaei, N.; Budvytiene, I.; Cevallos, V.; Zhong, L.; Zhou, Z.; et al. Metagenomic DNA Sequencing for the Diagnosis of Intraocular Infections. Ophthalmology 2017, 124, 1247–1248. [Google Scholar] [CrossRef]
- Doan, T.; Wilson, M.R.; Crawford, E.D.; Chow, E.D.; Khan, L.M.; Knopp, K.A.; O’Donovan, B.D.; Xia, D.; Hacker, J.K.; Stewart, J.M.; et al. Illuminating uveitis: Metagenomic deep sequencing identifies common and rare pathogens. Genome Med. 2016, 8, 90. [Google Scholar] [CrossRef]
- Lee, A.Y.; Akileswaran, L.; Tibbetts, M.D.; Garg, S.J.; van Gelder, R.N. Identification of torque teno virus in culture-negative endophthalmitis by representational deep DNA sequencing. Ophthalmology 2015, 122, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Ohashi, Y. Corneal endotheliitis. Semin Ophthalmol. 2008, 23, 235–240. [Google Scholar] [CrossRef]
- Gonzales, J.A.; Hinterwirth, A.; Shantha, J.; Wang, K.; Zhong, L.; Cummings, S.L.; Qian, Y.; Wilson, M.R.; Acharya, N.R.; Doan, T. Association of Ocular Inflammation and Rubella Virus Persistence. JAMA Ophthalmol. 2019, 137, 435–438. [Google Scholar] [CrossRef]
- De Paula Freitas, B.; Ventura, C.V.; Maia, M.; Belfort, R., Jr. Zika virus and the eye. Curr. Opin. Ophthalmol. 2017, 28, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Drancourt, M.; Berger, P.; Terrada, C.; Bodaghi, B.; Conrath, J.; Raoult, D.; LeHoang, P. High prevalence of fastidious bacteria in 1520 cases of uveitis of unknown etiology. Medicine 2008, 87, 167–176. [Google Scholar] [CrossRef]
- Kanis, M.J.; Oosterheert, J.J.; Lin, S.; Boel, C.H.; Ekkelenkamp, M.B. Corneal graft rejection complicated by Paracoccus yeei infection in a patient who had undergone a penetrating keratoplasty. J. Clin. Microbiol. 2010, 48, 323–325. [Google Scholar] [CrossRef]
- Goto, H.; Usui, Y.; Umazume, A.; Uchida, K.; Eishi, Y. Propionibacterium acnes as a possible pathogen of granuloma in patients with ocular sarcoidosis. Br. J. Ophthalmol. 2017, 101, 1510–1513. [Google Scholar] [CrossRef]
- Nagata, K.; Eishi, Y.; Uchida, K.; Yoneda, K.; Hatanaka, H.; Yasuhara, T.; Nagata, M.; Sotozono, C.; Kinoshita, S. Immunohistochemical Detection of Propionibacterium acnes in the Retinal Granulomas in Patients with Ocular Sarcoidosis. Sci. Rep. 2017, 7, 15226. [Google Scholar] [CrossRef]
- Yasuhara, T.; Tada, R.; Nakano, Y.; Tei, M.; Mochida, C.; Kamei, M.; Kinoshita, S. The presence of Propionibacterium spp. in the vitreous fluid of uveitis patients with sarcoidosis. Acta Ophthalmol. Scand. 2005, 83, 364–369. [Google Scholar] [CrossRef]
- Li, Z.; Breitwieser, F.P.; Lu, J.; Jun, A.S.; Asnaghi, L.; Salzberg, S.L.; Eberhart, C.G. Identifying Corneal Infections in Formalin-Fixed Specimens Using Next Generation Sequencing. Invest Ophthalmol. Vis. Sci. 2018, 59, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Boisjoly, H.M.; Pavan-Langston, D.; Kenyon, K.R.; Baker, A.S. Superinfections in herpes simplex keratitis. Am. J. Ophthalmol. 1983, 96, 354–361. [Google Scholar] [CrossRef]
- Thygeson, P. Superficial punctate keratitis. J. Am. Med. Assoc. 1950, 144, 1544–1549. [Google Scholar] [CrossRef] [PubMed]
- Thygeson, P. Clinical and laboratory observations on superficial punctate keratitis. Am. J. Ophthalmol. 1966, 61, 1344–1349. [Google Scholar] [CrossRef]
- Connell, P.P.; O’Reilly, J.; Coughlan, S.; Collum, L.M.; Power, W.J. The role of common viral ocular pathogens in Thygeson’s superficial punctate keratitis. Br. J. Ophthalmol. 2007, 91, 1038–1041. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reinhard, T.; Roggendorf, M.; Fengler, I.; Sundmacher, R. PCR for varicella zoster virus genome negative in corneal epithelial cells of patients with Thygeson’s superficial punctate keratitis. Eye 2004, 18, 304–305. [Google Scholar] [CrossRef]
- Fukui, Y.; Aoki, K.; Okuma, S.; Sato, T.; Ishii, Y.; Tateda, K. Metagenomic analysis for detecting pathogens in culture-negative infective endocarditis. J. Infect. Chemother. 2015, 21, 882–884. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. Genome Project Data Processing, S. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Huson, D.H.; Mitra, S.; Ruscheweyh, H.J.; Weber, N.; Schuster, S.C. Integrative analysis of environmental sequences using MEGAN4. Genome Res. 2011, 21, 1552–1560. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).