Abstract
Background: This study examined the impact of apolipoprotein ɛ4 (APOEɛ4) allele frequency and sex on the phenotype of Alzheimer’s disease (AD). Methods: This post hoc study evaluated the baseline characteristics, cerebrospinal fluid (CSF) and neuroimaging biomarkers, and cognition scores collected from 45 patients aged 50–74 years with CSF-biomarker-confirmed mild cognitive impairment or mild dementia due to AD from clinical trial NCT03186989. Results: A phenotypic spectrum was observed from a predominant amyloid and limbic–amnestic phenotype in male APOEɛ4 homozygotes to a predominantly tau, limbic-sparing, and multidomain cognitive impairment phenotype in female APOEɛ4 noncarriers. Amyloid pathology was inversely correlated with tau pathophysiology, glial activation, and synaptic injury, with the strongest associations observed in male APOEɛ4 carriers. Tau pathophysiology was correlated with glial activation, synaptic injury, and neuroaxonal damage, with the strongest correlation observed in female APOEɛ4 noncarriers. Conclusions: These data support the hypothesis that functional glial activation is influenced by apoE isoform and sex and might explain much of the biological and clinical heterogeneity in early clinical AD in those aged 50–74 years. Conclusions are limited because of the retrospective nature and small sample size. Trial Registration: Clinical Trial NCT03186989.
1. Introduction
The APOEɛ4 gene variant is the major genetic risk factor for sporadic AD, mainly exerting its pathogenic effects via altered glial activation and amyloid pathology accumulation [1,2]. In preclinical models, transcriptional profiles of astroglial and microglial and interactions with amyloid plaques are influenced by sex and the presence of APOEɛ4 alleles [1,2,3,4,5,6,7,8,9,10,11,12,13]. Functional levels of glial activation may balance the cellular needs to stimulate amyloid-beta (Aβ) clearance and limit both synaptic engulfment and the spread of tau pathology [14,15,16,17]. Glial activation is associated with the spread of tau and synaptic pathology [16,18,19]. In APOEɛ4 noncarriers, females are more likely to develop overactivated microglia with age [5], and have higher levels of CSF tau pathology in both prodromal disease and mild AD compared to males [6]. In females with AD, glial activation is generally higher [7] and cortical microglial activation is more important for AD progression [8]. In contrast, functional glial responses in APOEɛ4 carriers are deficient and favor the accumulation of amyloid pathology [9]. This deficiency implicates altered lipid dynamics, altered cholinergic and immune checkpoint signaling, and may also be a self-reinforcing consequence of Aβ accumulation.
Poor lipidation of apoE4 impairs its ability to opsonize extracellular debris such as fibrillar Aβ42 and myelin breakdown products, which impairs microglial recognition and clearance responses [10,11]. Rather than clearing debris effectively, APOEɛ4 microglia show phagocytic and endolysosomal dysfunction, become senescent, upregulate pro-inflammatory response genes, and downregulate genes responsible for migration and phagocytosis [13,20]. In a recent study in an aged amyloid mouse model, animals with APOEɛ4 had more microglia in a functionally impaired and exhausted-like state compared to animals with APOEɛ3, and these microglia were more prevalent in males [12]. Like ‘overactive’ glia, these functionally underactive glia produce proinflammatory cytokines [21]; therefore, it is critical that biomarkers of glial activation represent functional activation of glia and not inflammation [22].
ApoE4 is associated with decreased intracellular exchange of fatty acids and cholesterol between neurons and astrocytes with reduced fatty acid β-oxidation, lipid droplet accumulation, increased neuronal cholesterol synthesis, and impaired abilities to maintain myelin and cell membranes [23]. Fatty-acid fueled bioenergetics improve microglial migration, phagocytic ability, and lysosomal degradation [24,25], whereas the loss of these functions is associated with lipid droplet formation that is markedly exacerbated in APOEɛ4 carriers and by proximity to Aβ plaques [26]. In addition, altered lipid composition and fluidity of the cell membrane alters the stability and function of membrane receptors, including alpha 7 nicotinic acetylcholine (ACh) receptors (α7-nAChRs) [27,28].
The cholinergic system acts to control glial reactivity and function via rapid focal synaptic signaling and slow diffuse extracellular signaling though α7-nAChRs [29]. Aβ signals through α7-nAChRs in a concentration- and aggregation-dependent manner. This results in downstream effects on the extracellular fluid equilibrium between the breakdown and synthesis of ACh via changes to the ACh-hydrolyzing capacity of cholinesterases [30] and choline acetyltransferase (ChAT) activity [31]. ApoE forms soluble and highly stable complexes with Aβ and cholinesterase enzymes. Depending on the availability of Aβ, these complexes can fluctuate between slow and ultrafast ACh hydrolysis [32]. A reduction in glial activation and cholinesterase activity is seen in APOEɛ4 carriers, particularly in individuals with known gene variants that encode cholinesterase enzymes with lower activity [32,33]. These individuals accumulate Aβ earlier with a younger age at onset of AD [34]. Amnestic symptoms parallel the progressive denervation of basal forebrain corticolimbic cholinergic neuronal projections. This denervation removes the cholinergic ‘brake’ on glial activation in the medial temporal lobe (MTL) and other cortical regions, allowing for the spread of tau and neurodegenerative pathology, and transition into AD [34,35].
This study sought to examine the early clinical AD phenotypic spectrum with respect to APOEɛ4 genotype and sex—more specifically, how this continuum may be altered by levels of functional glial activation, indexed by CSF chitinase-3-like protein 1 (YKL-40) (Table S2; [22]). The study analyzed cross-sectional demographic, CSF biomarker, neuroimaging and cognitive domain assessments from patients aged 50–74 years who were diagnosed with early clinical AD. The association between hippocampal neuritic plaques and neurofibrillary tangles with dementia has been shown to be stronger in younger older persons (75 years old, odds ratio 10.19 and 8.61, respectively) than in older individuals (95 years old, odds ratio 1.42 and 2.11, respectively) [36]. The younger age of participants may limit the likelihood of other age-related pathologies, allowing for a clearer examination of the influence of genotype and sex on the phenotype of early clinical AD. A companion article using the same clinical trial data and methods examined the effects of combined BCHE-K and APOEɛ4 genotypes and has already been published [34].
2. Materials and Methods
2.1. Clinical Trial
Baseline samples obtained from Clinical Trial NCT03186989 were assessed for biomarkers in a retrospective study. The trial was conducted between August 2017 and February 2020 at 12 centers in Canada, the UK, Finland, the Netherlands, Germany, and Sweden. The trial was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki and the Good Clinical Practice Guidelines of the International Council for Harmonisation. The clinical study [37] was approved by relevant ethics committees (Table S1). Written informed consent was provided by the participants.
2.2. Study Eligibility
Eligible study participants were between the ages of 50 and 74; had probable early clinical AD (amnestic or non-amnestic), defined by a Mini-Mental State Examination score (MMSE) [38] of 20–27, inclusive, and either a Clinical Dementia Rating [39] Overall Global Score of 1, or a Global Score of 0.5 with a Memory Score of 1; a CSF pattern of low Aβ1–42 (≤1200 pg/mL), elevated total-tau (>200 pg/mL) and p-tau (>18 pg/mL) and a total-tau to Aβ1–42 ratio > 0.28; and a diagnosis of probable AD based on National Institute of Aging-Alzheimer’s Association (NIA-AA) criteria [40]. Exclusion criteria included any condition preventing participation in writing tasks, MRI or lumbar puncture (LP); significant risk of suicide, major depression, psychosis, confusional state or violent behavior; clinically significant laboratory, vital sign or electrocardiogram finding; and medical history of brain or spinal disease that would be expected to interfere with CSF circulation.
2.3. Data Collection and Processing
CSF collected from participants at the baseline of an interventional study was analyzed for markers of amyloid accumulation (inversely indexed by Aβ42), tau pathophysiology (p-tau181), neuroaxonal degeneration (neurofilament light chain, NfL), synaptic injury (neurogranin, Ng), and glial activation (YKL-40) (Table S2). YKL-40 is a glycoprotein secreted by activated glial cells, particularly reactive astrocytes [41]. NfL is an axonal protein that is released into the CSF following damage to large-caliber myelinated axons and is used as a biomarker for neuroaxonal damage, regardless of the cause [42,43]. Ng is a post-synaptic protein that is enriched in dendritic spines and expressed primarily in the cortex and hippocampus and is a biomarker of synaptic loss in AD [43,44]. Participants were characterized for common variants of APOE (i.e., APOEɛ4, APOEɛ3, and APOEɛ2) and butyrylcholinesterase (BCHE) (i.e., BCHE-K). Mutations associated with dominantly inherited AD were also assessed (i.e., APP, PSEN1, and PSEN2).
The study required three-dimensional T1-weighted structural magnetic resonance imaging (MRI) scans of the head with volumetric analyses calculated using VivoQuantTM by Invicro, 119 Fourth Avenue, Needham, MA 02494, USA, which includes preprocessing and multi-atlas segmentation modules, followed by visual inspection and manual editing, if needed [45]. The mean baseline ventricular volume and hippocampal volume were expressed as a percentage of the total intracranial volume (% ICV). Baseline Mini-Mental State Examination (MMSE) and Repeatable Battery for the Assessment of Neurological Status (RBANS) scores were also reported.
2.4. Statistical Analysis
Patient baseline characteristics were analyzed according to genotype (e.g., APOEɛ4 noncarrier, heterozygous, or homozygous), sex (e.g., male, female), and combined genotype and sex (e.g., male or female, APOEɛ4 carrier or noncarrier). Quantitative assessments were summarized using descriptive statistics, including number of patients, mean, and standard deviation. Qualitative assessments were summarized using frequency counts and percentages. The exact test was used to examine the Hardy–Weinberg equilibrium (HWE) in the distribution of APOEɛ4 alleles in the study population. All exact tests were performed using the R package “Hardy Weinberg” [46].
Analysis of variance (ANOVA) and analysis of covariance (ANCOVA) were used to test whether the burden of amyloid pathology differed across APOEɛ4 homozygotes, heterozygotes, and noncarriers. When the ANCOVA model was applied, the model included BCHE-K carrier status and sex as factors and the baseline MMSE total score as a covariate. Prior to performing ANOVA and ANCOVA, the normality assumption of residuals was tested using the Kolmogorov–Smirnov test. If significant departures from normality were observed, the Wilcoxon Rank Sum test was applied. Both ANOVA and ANCOVA were applied to test baseline CSF markers across two or more genotype groups. When ANCOVA was applied, age at baseline was included as an additional covariate in the model. If the normality assumption was not satisfied, both ANOVA and ANCOVA models were fitted to the log-transformed data. Box plots were used to visualize data by group.
Relationships between CSF Aβ42, CSF p-tau181, and other CSF biomarkers, as well as brain volumes, were explored in the overall population and in each genotype group in a simple linear correlation analysis with a Pearson correlation coefficient. The squared Pearson correlation coefficient (R2) and p value were provided in the correlation analysis. Correlation coefficients were defined as follows: 0.81 ≤ R2 < 1 as strong; 0.49 ≤ R2 < 0.81 as moderately strong; 0.25 ≤ R2 < 0.49 as moderate; 0.09 ≤ R2 < 0.25 as weak; and R2 < 0.09 as negligible. Scatterplots with a simple linear regression line were produced to depict the relationships between two quantitative variables.
Multiple regression analysis was also utilized to assess the functional relationships between biomarkers of interest. This model was applied with CSF Aβ42 or CSF p-tau181 as the response variable, and APOEɛ4, sex, and the biomarker of interest (i.e., CSF p-tau181 or Aβ42, NfL, Ng, YKL-40; hippocampal volume, ventricular volume) as independent variables. This determined the strength of association of CSF Aβ42 or CSF p-tau with parameters of interest, in conjunction with the other independent variables included in the model.
3. Results
3.1. Patient Cohort
A total of 102 participants were assessed for eligibility, 56 were excluded based on the exclusion criteria, and 1 enrolled participant did not have genetic test results [37]. The study sample included 45 participants with a mean age of 65.8 years and a mean baseline MMSE total score of 23.6 (Table S3). The APOEɛ4 allele was carried by 33 (73%) participants; 10 (22%) were homozygotes and 23 (51%) were heterozygotes (Table S3). All APOEɛ4 noncarriers were homozygous for APOEɛ3, except for one that was homozygous for APOEɛ2/ɛ3. The distribution of APOE genotypes (HWE exact p value = 1) was consistent with Hardy–Weinberg equilibrium. Participant baseline characteristics were summarized according to APOEɛ4 genotype and sex (Tables S3 and S4) and have also been described previously [37]. The impact of BCHE-K allelic status on the phenotype of early AD in APOEε4 carriers was also assessed and reported [34].
3.2. Age at Diagnosis and APOEɛ4 Allele Status
APOEɛ4-allele-frequency-dependent effects on the risk of onset of AD are highest between 60 and 70 years of age but begin to wane after 70 years of age and are especially reduced after 80 years of age [47,48,49]. Therefore, in this sample of early AD patients aged 50–74 years, the effects of APOEɛ4 on the AD phenotype may be maximal. In the overall population, the mean age at diagnosis of AD was 63.3 years in males and 65.7 years in females. This contrasted with female APOEɛ4 noncarriers, whose mean age at diagnosis was 10.7 years later than male APOEɛ4 noncarriers (Figure 1a; 70.4 years in females vs. 59.7 years in males, p = 0.009, ANOVA) and with nearly fivefold less time from age at diagnosis of AD to study baseline (Figure 1b; 4.8 months in females vs. 21.6 months in males). The restricted age range for study entry may have been at least partly responsible for the lack of any significant APOEɛ4-allele-frequency-dependent reductions in age at diagnosis of AD or age at baseline in the overall population, although a trend was observed in females between increasing allele frequency and decreased age (Figure 1a). Additionally, female APOEɛ4 carriers spent a mean of 0.8 years between AD diagnosis and study baseline, whereas male carriers spent 2.0 years (Figure 1b), which is consistent with prior studies showing that female APOEɛ4 carriers less than 75 years of age have a greater risk for AD and a more rapid transition from MCI to AD, especially in heterozygotes [50,51,52]. In APOEɛ4 homozygotes less than 75 years, the risk for AD may be similar between males and females [53].
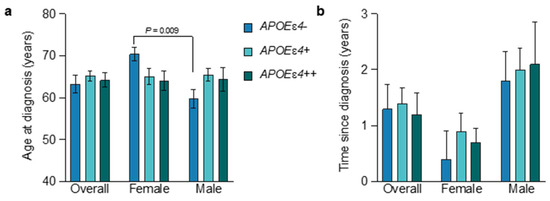
Figure 1.
Age at diagnosis and time since diagnosis by APOEe4 status and sex. The mean (±SEM) was calculated for the age at diagnosis (a) and time since diagnosis (b) for all subgroups. Subgroups included the overall population, females, and males, subdivided according to APOEɛ4 genotype (blue, APOEɛ4 noncarriers; light blue, APOEɛ4 heterozygotes; green, APOEɛ4 homozygotes). Subgroups and their labels are consistent throughout all figures. Female participants of all genotypes displayed a shorter time since diagnosis than males. See Tables S3 and S4 for more details.
3.3. APOEɛ4-Allele-Frequency-Dependent Changes on CSF Markers
APOEɛ4-allele-frequency-dependent increases in amyloid pathology were observed, indexed inversely by CSF Aβ42 (Figure 2a). The level of amyloid pathology was significantly different across APOEɛ4 noncarriers, heterozygotes, and homozygotes from the overall population (Figure 2a; CSF Aβ42 of 799.2 pg/mL vs. 700.2 pg/mL vs. 580.7 pg/mL, respectively; p = 0.018 ANOVA, p = 0.024 ANCOVA). When the population was subdivided by males and females, the APOEɛ4-allele-frequency-dependent increase in amyloid pathology followed the same trend, but did not reach significance. Levels of amyloid pathology were similar between male and female APOEɛ4 homozygotes (CSF Aβ42 582.1 vs. 579.7 pg/mL, respectively) and heterozygotes (CSF Aβ42 699.5 vs. 700.8 pg/mL, respectively). In contrast, in APOEɛ4 non-carriers, CSF Aβ42 levels were lower in males, indicating higher levels of amyloid pathology (Figure 2a). Higher levels of amyloid pathology are compatible with accumulation beginning at an earlier age in association with APOEɛ4 allele frequency, with higher levels of amyloid accumulation required in APOEɛ4/ɛ4 > APOEɛ3/ɛ4 > APOEɛ3/ɛ3 to induce symptoms [54].
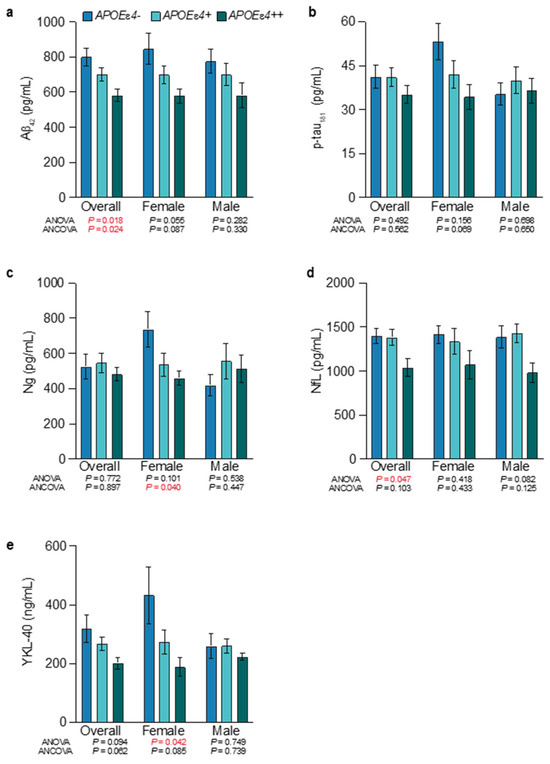
Figure 2.
Levels of amyloid pathology, tau pathophysiology, glial activation, and synaptic injury by APOEε4 allele frequency and sex. The mean and standard error of the mean for CSF Aβ42 (a), CSF p-tau181 (b), CSF Ng (c), CSF NfL (d), and CSF YKL-40 (e). Females showed significant APOEɛ4-allele-frequency-dependent relationships for synaptic injury (CSF Ng) and glial activation (CSF YKL-40), and CSF Aβ42 and CSF p-tau181 showed a trend towards significance. See Tables S3 and S4 for more details. Significant p values (p > 0.05) are shown in red.
No significant differences were observed between groups for p-tau181. However, APOEɛ4 homozygotes showed the lowest levels of tau pathology, and in female APOEɛ4 homozygotes, this was most pronounced when compared to female APOEɛ4 noncarriers (Figure 2b; CSF p-tau181 of 34.4 pg/mL vs. 53.3 pg/mL). In addition to tau pathology, synaptic injury was also lowest in APOEɛ4 homozygotes, and significantly different across female noncarriers, heterozygotes, and homozygotes (Figure 2c; CSF Ng of 737.3 pg/mL vs. 536.3 pg/mL vs. 460.5 pg/mL, respectively; p = 0.101 ANOVA, p = 0.040 ANCOVA). Males showed the opposite trend, where noncarriers exhibited the lowest levels of synaptic injury. Neuroaxonal injury was also significantly different across noncarriers, heterozygotes, and homozygotes (Figure 2d; CSF NfL of 1398.9 pg/mL vs. 1383.1 pg/mL vs. 1038.5 pg/mL, respectively; p = 0.047 ANOVA, p = 0.103 ANCOVA) and followed the same trend in males and females.
Glial activation was highest in noncarriers and lowest in APOEɛ4 homozygotes (Figure 2e). This was especially pronounced in females that showed significant differences across noncarriers, heterozygotes, and homozygotes (CSF YKL-40 of 433.2 ng/mL vs. 273.3 ng/mL vs. 187.9 ng/mL, respectively; p = 0.042 ANOVA, p = 0.085 ANCOVA). Overall, APOEɛ4 homozygotes had the highest levels of amyloid pathology and the lowest levels of tau pathophysiology, neuroaxonal damage, postsynaptic injury, and glial activation (Figure 2). This finding was most pronounced in female APOEɛ4 homozygotes.
3.4. Amyloid Accumulation, Hypofunctional Glia-Mediated Clearance, and Neurodegenerative Pathology
As reported previously [34], multiple regression analyses of the overall population identified that amyloid accumulation (inversely indexed by CSF Aβ42) was associated with APOEɛ4 carrier status (p < 0.029), a larger total brain ventricle volume (p < 0.021), less synaptic injury (CSF Ng, p < 0.001), and less tau pathophysiology (CSF p-tau181, p = 0.005). There was also a trend towards less glial activation (CSF YKL-40, p = 0.097). In the overall population, simple linear correlation analyses (Figure S1) with amyloid pathology showed weak-to-moderate inverse correlations with tau pathophysiology (R2 = 0.18, p = 0.004), synaptic injury (R2 = 0.26, p <.001), and glial activation (R2 = 0.11, p = 0.028) (Figure 3a–c). These inverse correlations were absent in APOEɛ4 noncarriers but were strengthened in APOEɛ4 carriers and in males, and in male APOEɛ4 carriers, they were moderate (Figure 3a–c). Amyloid pathology also had a weak inverse correlation with synaptic injury in females (R2 = 0.24, p = 0.020), which strengthened to a moderate inverse correlation with in female APOEɛ4 carriers (R2 = 0.33, p = 0.013) (Figure 3b). Amyloid pathology and neuroaxonal damage were not significantly correlated in any subgrouping (Figure 3d).
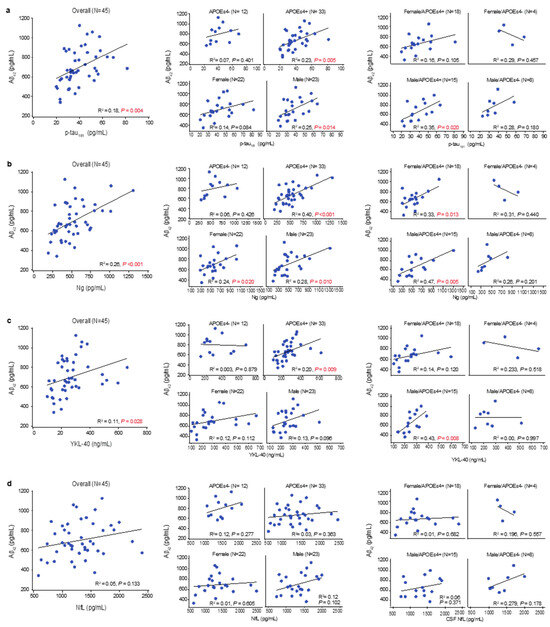
Figure 3.
Linear relationships between levels of CSF Aβ42 and other CSF markers. Multiple regression identified an association between CSF Aβ42 and CSF levels of p-tau181, CSF Ng, CSF NfL, and CSF YKL-40. These associations were fit to simple linear regressions to obtain R-squared and p values; significant p values (p > 0.05) are shown in red. (a) CSF Aβ42 (lower levels indicate more amyloid pathology) showed a positive correlation with CSF p-tau181 (higher levels indicate more tau pathophysiology) in the overall population, especially in APOEε4 carriers. When stratified by sex, male APOEε4 carriers showed the strongest correlation, indicating that higher levels of amyloid pathology were associated with less tau pathology. (b) CSF Aβ42 showed a positive correlation with Ng (higher levels indicate more synaptic injury) in the overall population, especially in APOEε4 carriers. When stratified by sex, both male and female APOEε4 carriers exhibited a strong correlation. (c) CSF Aβ42 showed a positive correlation with YKL-40 (higher levels indicate more glial activation) in the overall population, especially in APOEε4 carriers. When further stratified by sex, male APOEε4 carriers exhibited the strongest correlation, indicating that lower levels of glial activation were associated with more amyloid pathology. (d) CSF Aβ42 showed no correlations with NfL (higher levels indicate more axonal injury).
3.5. Tau Pathophysiology, Neurodegenerative Pathology, and Glial Activation
As reported previously [34], multiple regression analysis demonstrated that tau pathophysiology was associated with greater neuroaxonal damage (CSF NfL, p = 0.002), more synaptic injury (CSF Ng, p < 0.001), and higher levels of glial activation (CSF YKL-40, p = 0.01). In simple linear regressions (Figure S1), tau pathophysiology was moderately correlated with neuroaxonal damage in the overall population (R2 = 0.27, p < 0.001) and in APOEɛ4 carriers (R2 = 0.34, p < 0.001) (Figure 4a). In nearly all subgroups, tau pathophysiology showed moderately strong and strong correlations with synaptic injury (Figure 4b). Tau pathophysiology was weakly correlated with glial activation in the overall population (R2 = 0.18, p = 0.004) and moderately correlated in APOEɛ4 noncarriers (R2 = 0.37, p = 0.035) (Figure 4c).
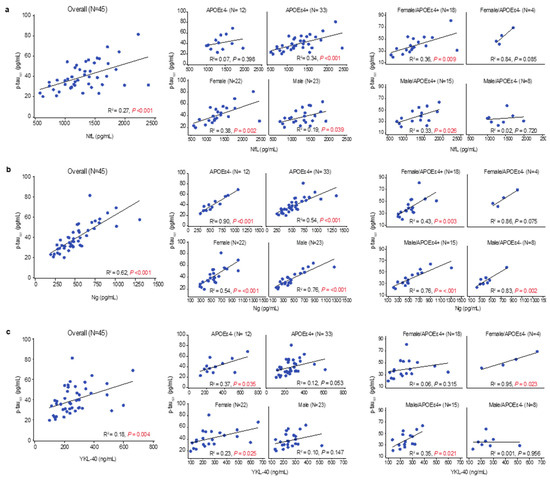
Figure 4.
Linear relationships between levels of CSF p-tau181 and other CSF markers. Multiple regression identified an association between CSF p-tau181 and CSF levels of neurofilament light chain (NfL), Ng, and YKL-40. These associations were fit to simple linear regressions to obtain R-squared and p values; significant p values (p > 0.05) are shown in red. (a) CSF p-tau181 showed a positive correlation with CSF NfL (higher levels indicate more neuroaxonal injury) in the overall population, which was driven by APOEε4 carriers. When stratified by sex, both male and female APOEε4 carriers showed a positive correlation. (b) CSF p-tau181 showed a positive correlation with Ng (higher levels indicate more synaptic injury) that was moderately strong in the overall population and APOEε4 noncarriers and strong in APOEε4 carriers. This relationship was evident in both males and females, independent of APOEε4 status. (c) CSF p-tau181 showed a positive correlation with YKL-40 (higher levels indicate more glial activation) in the overall population that was stronger in APOEε4 noncarriers than carriers. When stratified by sex, a positive correlation was present in male APOEε4 carriers and female APOEε4 noncarriers.
Tau pathophysiology and neuroaxonal damage showed a weak correlation in males (R2 = 0.19, p = 0.039) and a moderate correlation in females (R2 = 0.38, p = 0.002) (Figure 4a). Tau pathophysiology showed a moderately strong correlation with synaptic injury in both males (R2 = 0.76, p = 0.001) and females (R2 = 0.54, p < 0.001) (Figure 4b) and a weak correlation with glial activation in females (R2 = 0.23, p = 0.025) (Figure 4c). Notably, correlation of neuroaxonal damage with synaptic injury was significant, but weak in the overall population (R2 = 0.13, p = 0.016), as well as in APOEɛ4 carriers (R2 = 0.14, p = 0.033) (Figure S1). In female APOEɛ4 noncarriers, correlations between tau and glial activation were significant and strong (R2 = 0.95, p = 0.023), and correlations between tau and neuroaxonal damage or synaptic injury were strong but did not achieve statistical significance. Conversely, in male APOEɛ4 noncarriers, correlations of tau with glial activation and neuroaxonal damage were absent (Figure 4a,c), and correlation with synaptic injury was strong but lacked statistical significance (Figure 4b). In female APOEɛ4 carriers, correlations with tau pathophysiology were absent for glial activation (Figure 4c) and moderate for neuroaxonal damage (R2 = 0.36, p = 0.009) and synaptic injury (R2 = 0.43, p = 0.003) (Figure 4a,b). In male APOEɛ4 carriers, tau pathophysiology was moderately correlated with glial activation (R2 = 0.35, p = 0.021) and neuroaxonal damage (R2 = 0.33, p = 0.026) (Figure 4a,c), and showed moderately strong correlations with synaptic injury (R2 = 0.76, p < 0.001) (Figure 4b).
The most compelling associations of tau pathophysiology with glial activation and neurodegenerative pathology were in female APOEɛ4 noncarriers and male APOEɛ4 carriers. Higher CSF p-tau181 levels are typically seen in female APOEɛ4 carriers with preclinical and prodromal AD but not in mild or later dementia stages [6]. In APOEε4 heterozygotes, females may be able to leverage a non-APOEε4 allele to achieve higher levels of glial activation and increased tau pathology. Increasing levels of tau pathophysiology in male APOEɛ4 carriers with early AD may reflect more advanced degeneration of the basal forebrain corticolimbic cholinergic neurons, which is indexed by greater limbic–amnestic features and is associated with removal of the cholinergic ‘brake’ on glial function in the MTL followed by wider spread of cortical tau and synaptic pathology. In early AD, spreading cholinergic-denervation-induced cortical-glial-activation-mediated tau and neurodegenerative pathology may be most evident in male APOEɛ4 homozygotes [35].
3.6. Sex and APOEɛ4 Allele Influences on Cognition
Overall, APOEɛ4 carriers had a more temporo-limbic (hippocampal atrophy > ventricular expansion) and amnestic (memory > visuospatial impairment) phenotype relative to APOEɛ4 noncarriers (Figure 5 and Figure 6), as described previously [55,56]. In APOEɛ4 homozygotes, male participants exhibited more of a limbic–amnestic phenotype than females, as evidenced by slightly greater amnestic deficits (Figure 5b,e; MMSE memory 4.0 vs. 4.2; RBANS delayed memory 48 vs. 56; for males vs. females) and hippocampal atrophy (Figure 6a; 0.22 vs. 0.26%ICV; for males vs. females). In APOEɛ4 heterozygotes, numerically greater amnestic deficits were also observed in male participants (Figure 5b,e; MMSE memory 3.8 vs. 4.3; RBANS delayed memory 46.9 vs. 49.0). These apparent sex differences in APOEɛ4 homozygotes and heterozygotes were not reflected in amyloid accumulation (Figure 2a), tau pathophysiology (Figure 2b), or in the mean age at diagnosis of AD (64.4 vs. 64.0 years in homozygotes; and 65.5 years vs. 65.0 years in heterozygotes) (Figure 1a).
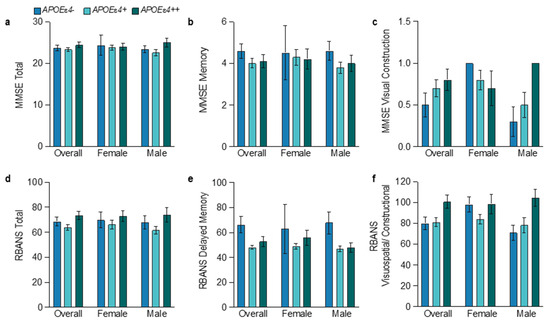
Figure 5.
Baseline cognition test scores by APOEε4 status and sex. The mean and standard error of the mean for baseline cognition tests and two of their individual components: for the MMSE total (a), memory (b), and visual construction (c); for the RBANS total (d), delayed memory (e), and visuospatial/constructional (f). Both tests followed the same trends between genotypes with the least overall cognitive and visuospatial deficits in APOEε4 homozygotes and the greatest amnestic deficits in APOEε4 carriers. See Tables S3 and S4 for more details.
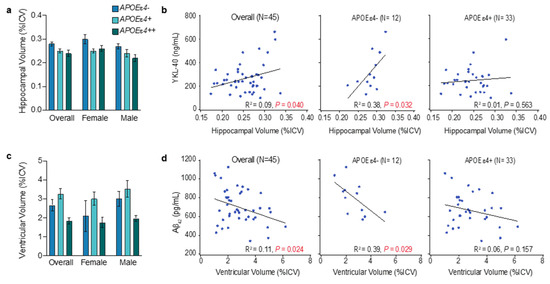
Figure 6.
Observations for hippocampal and ventricular volume by APOEe4 status. The mean and standard error of the mean for hippocampal volume (a) or ventricular volume (c) (as a percentage of total intracranial volume (%ICV)). R2 and p values were obtained by fitting simple linear regressions (b,d); significant p values (p > 0.05) are shown in red. CSF YKL-40 correlated with hippocampal volume in the overall population and APOEε4 noncarriers (b). CSF Aβ42 correlated with ventricular volume in the overall population and in APOEε4 noncarriers (d). See Tables S3 and S4 for more details.
Overall, the limbic–amnestic phenotype was more prominent in male APOEɛ4 carriers (Figure 5 and Figure 6), suggesting that there is earlier and greater degeneration of the basal forebrain corticolimbic cholinergic projection system in males. Thus, in early-stage AD, male APOEɛ4 homozygotes may have developed slightly higher levels of glial activation and tau pathophysiology than females because of greater removal of the corticolimbic cholinergic “brake” on glial activation in the MTL and other cortical areas. In male APOEɛ4 carriers, tau pathophysiology correlated moderately with glial activation (R2 = 0.35, p = 0.021), but this correlation was absent in females (Figure 4c). In male APOEɛ4 carriers with early AD below the age of 75 years, this could indicate that increases in tau pathology are more dependent on limbic and cortical activation of glial due to corticolimbic cholinergic denervation that is indexed by the limbic–amnestic phenotype. Thus, in early AD, APOEɛ4 homozygotes may still have the lowest levels of tau pathophysiology, and males and females may be on slightly different journeys to end-stage disease where APOEɛ4 homozygotes will rapidly evolve the greatest burden of tau pathology as they progress through the dementia disease stage continuum [57].
3.7. Brain Volumes in APOEɛ4 Noncarriers
Neither amyloid nor tau were associated with hippocampal volume. However, the total hippocampal volume showed a weak association with glial activation in simple linear correlation analysis (Figure 6b): lower glial activation correlated with more hippocampal atrophy. This association was moderate in APOEɛ4 noncarriers (R2 = 0.38, p = 0.032) and absent in APOEɛ4 carriers (Figure 6b). This finding aligns with the putative role of increased glial activation in APOEɛ4 noncarriers, where increased glial activation limits the accumulation of amyloid pathology and is associated with more intact basal forebrain corticolimbic cholinergic projections. Thus, in early AD aged less than 75 years, the absence of apoE4 results in less tau and neurodegenerative pathology in the MTL [56].
Total brain ventricle size showed a weak positive association with amyloid burden in both multiple and simple linear (R2 = 0.11, p = 0.024) correlation analyses (Figure 6d). This aligns with weak or equivocal associations between amyloid burden and ventricular expansion or whole-brain atrophy in previous studies [58]. The association between ventricle size and amyloid burden was moderate in APOEɛ4 noncarriers (R2 = 0.39, p = 0.029) and absent in APOEɛ4 carriers, suggesting that ventricular expansion, and presumably whole-brain atrophy, may be more evident in APOEɛ4 noncarriers with higher levels of amyloid pathology than in APOEɛ4 carriers. Amyloid pathology is generally lower in APOEɛ4 non-carriers, but its presence may be required to trigger the spread of tau and neurodegenerative pathology. Tau pathophysiology was not correlated with ventricular size.
3.8. APOEɛ4 Heterozygotes Exhibit Influences of Both Alleles
Relative to homozygotes, APOEɛ4 heterozygotes had a less extreme limbic–amnestic phenotype, less amyloid, more tau and neurodegenerative pathology, more widespread brain atrophy changes, and higher levels of glial activation (Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6). Relative to noncarriers, heterozygotes also had more amyloid, similar levels of tau and neurodegenerative pathology, and less glial activation. Thus, APOEɛ4 heterozygotes demonstrate clear influences of both the APOEɛ4 allele on amyloid accumulation and the non-APOEɛ4 allele on a more permissive environment for tau spreading. With respect to the limbic–amnestic phenotype, male heterozygotes appear slightly closer to that of all APOEɛ4 homozygotes, whereas the phenotype of female heterozygotes is slightly closer to all APOEɛ4 noncarriers.
3.9. Hyperfunctional Glia in Female APOEɛ4 Noncarriers
Post hoc analysis of APOEɛ4 noncarriers requires cautious interpretation because of the small number of participants and the preponderance of male (n = 8) relative to female (n = 4) participants. Confirmation is required in prospective analyses of larger populations. Nonetheless, the subsequent results align well with those of a prodromal AD cohort [35,52].
Despite the highest burdens of glial activation, tau pathophysiology and synaptic injury (Figure 2) and fivefold less time from age at diagnosis of AD to study baseline (Figure 1b), the mean age at diagnosis of AD in female APOEɛ4 noncarriers was 10.7 years later than in male noncarriers (Figure 1a). Male APOEε4 noncarriers had slightly greater amyloid accumulation at baseline, but their levels of glial activation, tau pathophysiology, and synaptic injury were markedly lower than in female noncarriers and do not explain their younger age at diagnosis of AD. Interestingly, in a 3–4 year study of individuals with prodromal AD, male APOEɛ4 noncarriers exhibited the slowest decline in brain volumes and the lowest rate of transition to dementia of all the sex and genotype groups [52]. Overall, cognitive impairment in APOEɛ4 noncarriers was similar in male and female participants, and both had relatively low amnestic deficits. However, males had more dysexecutive clinical features than females, demonstrated by greater deficits in visuospatial/constructional (Figure 5c,f; MMSE visual construction, 0.3 vs. 1.0; RBANS visuospatial/constructional, 70.9 vs. 98.0) and attentional (MMSE attention/calculation, 2.8 and 3.8; RBANS attention, 71.4 and 81.0, respectively) assessments. Thus, both sexes had relatively non-amnestic cognitive deficits, but female participants exhibited less visuospatial/constructional and attentional impairment, had delayed AD onset (a decade later), and spent less time in the mild AD stage since diagnosis.
In the current study, the highest levels of glial activation, tau and synaptic pathology, and the lowest levels of amyloid pathology were evidenced in female APOEɛ4 noncarriers (Figure 2b,c,e). In addition, females, but not males, showed inverse APOEɛ4-allele-frequency-dependent relationships with glial activation, tau pathophysiology and synaptic injury (Figure 2). In APOEɛ4 noncarriers, tau pathophysiology was strongly correlated with synaptic pathology in both males and females, but correlations with glial activation and neuroaxonal damage were only strong in females and absent in males (Figure 4a–c). For example, correlations of glial activation with tau pathophysiology were weak in the overall population (R2 = 0.18, p = 0.004) and strong in female APOEɛ4 noncarriers (R2 = 0.95, p = 0.023) (Figure 4c). Correlations of synaptic injury with tau pathophysiology were moderately strong in the overall population (R2 = 0.62, p < 0.001) and strong in female APOEɛ4 noncarriers (R2 = 0.86, p = 0.075) (Figure 4b). Correlations of neuroaxonal injury with tau pathophysiology were moderately strong in the overall population (R2 = 0.27, p < 0.001) and strong in female APOEɛ4 noncarriers (R2 = 0.84, p = 0.085) (Figure 4a).
4. Discussion
Genetic variation has a clear influence on the phenotype of early clinical AD (i.e., with prodromal or mild dementia) in individuals aged 50–74 years. Amyloid or tau pathology on their own may not pose a substantial risk for neurodegenerative brain pathology, but their co-occurrence may index major risk [59,60,61]. In APOEɛ4 homozygotes, deficient glial clearance mechanisms result in early amyloid pathology; however, substantial neurodegeneration and symptoms may only begin when tau pathology spreads. In contrast, in female APOEɛ4 noncarriers, more functionally active glia may initially limit amyloid pathology, but amyloid accumulation eventually reaches a minimum threshold to trigger tau pathology, resulting in accelerated replication and spread of tau and synaptic pathology. When viewed together, end-stage AD pathology looks similar and does not reflect the divergent pathways taken to get there [57]. Through the examination of younger elderly, this study demonstrates that genotype and sex can differentiate phenotypic extremes in early clinical AD.
The amyloid cascade hypothesis of AD implies that parenchymal amyloid positivity at an earlier age should drive secondary effector tau and synaptic pathology, and present with an earlier age at onset of clinical AD [62]. Although tau pathology and synaptic loss may be necessary to produce clinical impairments, in APOEɛ4 carriers in the current study, and particularly in males, amyloid pathology was inversely correlated with tau pathophysiology, glial activation and synaptic injury (Figure 3). APOEɛ4-allele-frequency-dependent reductions in functional glial activation were associated with putatively lower Aβ clearance and accumulations of amyloid pathology, but also limited tau pathophysiology and synaptic injury (Figure 7A). APOEɛ4 homozygotes had the highest amyloid and the lowest tau and neurodegenerative pathology (Figure 2). An explanation for these findings could be that tau and neurodegenerative pathology localized in the MTL and unable to strongly demonstrate its presence in CSF may transition APOEɛ4 homozygotes from preclinical to clinical AD at an earlier age despite having less neurodegenerative and tau pathology (Figure 2) [56,63]. For example, in younger elderly APOEɛ4 carriers with global amyloid pathology, tau-PET imaging has shown that tau pathology is more severe with a focal MTL distribution [56,64], which is associated with more hippocampal atrophy and less global cerebral atrophy.
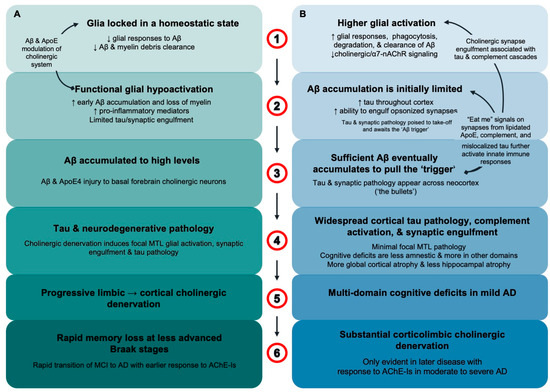
Figure 7.
Glial activation differentiates APOEε4 homozygotes from APOEε4 noncarriers in early AD. (A) In APOEε4 homozygotes aged < 75 years, ApoE4 locks glia in a homeostatic state with decreased responsiveness to extracellular debris including Aβ fibrils (1). “Functionally underactive” glia result in earlier and greater amyloid accumulation but limited synaptic engulfment and loss (2). Aβ accumulates to high levels and, along with ApoE4, induces degeneration of basal forebrain corticolimbic cholinergic neurons (3). In prodromal AD, limbic cholinergic denervation removes the cholinergic “brake” on glia to increase glial activation and tau and neurodegenerative pathology in the MTL. This is followed by the spread of tau and neurodegenerative pathology as cholinergic denervation and glial activation progresses to other cortical regions (4) and is paralleled by the emergence of a rapidly progressing limbic–amnestic phenotype (5). As corticolimbic cholinergic denervation, glial activation, tau pathology and neurodegeneration spread, there is rapid progression from prodromal to dementia stages of AD with an uncharacteristically good response to AChE-Is in mild AD (6). (B) In contrast, female APOE4 noncarriers usually aged > 75 years have higher levels of glial activation, with induction of microglia response genes that include APOE (1). This results in chronically “functionally over-active” glia with increased phagocytic and degradative functions that limit Aβ pathology (2). Higher levels of glial activation result in lower levels of amyloid accumulation, but once sufficient Aβ fibrils are present, tau pathology and synaptic engulfment accelerate, and these pathologies spread across the neocortex (3). Generalized cortical tau pathology and synaptic injury index glia tilting through balanced-functionality thresholds to initiate excessive inflammatory and complement system cascades. The lack of focal MTL tau and neurodegenerative pathology means relative sparing of amnestic deficits and hippocampal atrophy, and cortical atrophy changes are more global (4). Multidomain cognitive deficits rapidly transition patients from prodromal to mild AD (5). Corticolimbic cholinergic denervation only becomes apparent in the moderate and severe stages of AD where robust AChE-I treatment effects are also observed (6).
In preclinical AD, the greatest loss of basal forebrain volume is exhibited by APOEɛ4 carriers [65]. The degeneration of basal forebrain cholinergic neurons that project into cortical structures, specifically the MTL, precedes and predicts longitudinal MTL degeneration [66]. The ascending neuronal projections of the basal forebrain cholinergic system may be especially vulnerable to the combination of apoE4-mediated glial hypofunction and deficient lipid delivery with high levels of Aβ and tau pathology [67,68,69]. Corticolimbic cholinergic denervation has been observed at early stages of AD [70], and breakdown of this pathway is inextricably linked with amnestic deficits [71]. Aβ and apoE4 also influence synaptic and extracellular ACh levels [33,72].
In APOEɛ4 homozygotes aged 50–74 years, parenchymal amyloid pathology may accumulate to high levels before the combined effects of apoE4, Aβ and tau pathology induce sufficient degeneration of basal forebrain corticolimbic cholinergic neurons to release the ‘brake’ on glial activation. This release may be indexed by rising levels of the putative glial master regulator, YKL-40, in the CSF. Glial activation, tau, and neurodegenerative pathology first appear in the MTL and then spread to involve other cortical areas as cholinergic denervation becomes more widespread (Figure 7A). Across the aging and AD spectrum, APOEɛ4 carriers present increased microglial activation in early Braak stage regions within the MTL compared to noncarriers, which mediates Aβ-independent effects of APOEɛ4 on tau accumulation [73]. The rapidly progressing limbic–amnestic phenotype indexes the spreading of pathology that is responsible for ushering APOEɛ4 carriers through prodromal stages of disease and over the dementia threshold, with a response to acetylcholinesterase inhibitors (AChE-Is) that is apparent in the mild stage of AD [74]. Requirements for corticolimbic cholinergic denervation may include apoE4, low levels of functional glial activation that limit Aβ clearance, and high levels of Aβ accumulation. Importantly, in many and possibly most APOEɛ4 carriers, an accumulated high amyloid burden inducing damage to basal forebrain neurons and cholinergic denervation may not be necessary to induce cortical glial activation and tau spread if levels of glial activation are elevated for other reasons, such as the expression of non-APOEɛ4 alleles, in females, and in patients over the age of about 75 years [75]. Thus, the phenomenon of high levels of accumulated Aβ inversely related to tau pathology, glial activation and synaptic injury may only be clearly evidenced in younger elderly with less age-related elevation of glial activation.
Further lowering of functional glial activation in APOEɛ4 carriers may induce amyloid accumulation at younger ages, further lower the age at onset of AD, and hasten progression from prodromal to dementia AD, especially in those below the age of 75 years [34,35,76,77]. In contrast, factors that increase glial activation, such as aging, may explain why APOEɛ4 effects on risk for AD may be maximal below the age of 70 years and largely absent above the age of 85 years [47,48,49]. APOEɛ4 carriers with early AD and a mean age of 70 years show APOEɛ4-allele-frequency-dependent accelerated progression of hippocampal atrophy and less global cerebral atrophy, but in individuals more advanced in age or disease progression, brain volume atrophy does not differ by genotype [78,79,80]. In younger APOEɛ4 homozygotes, disseminated neocortical tau pathology and widespread synaptic injury may be initially limited (Figure 7A), but as corticolimbic cholinergic denervation progresses from MTL structures to involve other cortical regions, the spread of tau pathology and synaptic degeneration is facilitated and may become more rapid than in APOEɛ4 noncarriers. In the current study, tau pathophysiology in APOEɛ4 carriers had at least moderately strong correlations with synaptic injury and neuroaxonal injury, suggesting that once tau pathology is triggered, it is associated with neurodegeneration (Figure 4). In male APOEɛ4 carriers, moderate to moderately strong correlations of tau pathophysiology with glial activation, synaptic injury, and neuroaxonal damage suggest that increases in glial activation may be particularly associated with tau and neurodegenerative pathology in male APOEɛ4 carriers with early clinical AD (Figure 4).
APOEɛ4-allele-frequency-dependent reduction in functional glial activation in early clinical AD is most evident below the age of 75 years, which contrasts with potential over-activation of glia in female noncarriers that is most evident greater than 75 years [35]. In the current study in patients aged 50–74 years, female APOEɛ4 noncarriers also had the highest levels of functional glial activation (Figure 2e). More effective clearance of Aβ may limit amyloid pathology and avoid substantial accumulation of amyloid in younger elderly individuals; however, female noncarriers are at increasing risk of developing dementia as they age [35]. Female noncarriers may be vulnerable because even relatively low levels of amyloid pathology can trigger tau and synaptic pathology to spread rapidly (Figure 7B). Progression to clinical AD in female APOEɛ4 noncarriers may likely only occur after amyloid accumulation eventually reaches the minimum “threshold” to engage the secondary effector tauopathy mechanism when the replication and spread of tau pathology is readily facilitated [62]. The substantially greater burden of tau pathology in female APOEɛ4 noncarriers, indexed in current study by CSF p-tau181, likely reflects a more diffuse distribution of neocortical tau pathology facilitated by an interaction between sufficient accumulated toxic species of Aβ and a permissive state of glial activation (Figure 2, Figure 3 and Figure 4) [18,19]. Thus, the rapid spread/replication of tau in the cerebral cortex of female APOEɛ4 noncarriers is strongly associated with higher levels of glial activation, synaptic and neuroaxonal degeneration, and an early clinical AD phenotype of multidomain cognitive impairment with relative preservation of corticolimbic cholinergic innervation, hippocampal volume, and memory function. In APOEɛ4 noncarriers, a significant response to AChE-Is is generally only seen in moderate and severe stages of AD (Figure 7B) [35,74]. Female APOEɛ4 noncarriers in the current study align with a subtype found in 30% of AD patients in a large-scale CSF mass spectrometry proteomic analysis that was characterized by overactive glia [81]. This subtype was generally slightly older and characterized by increased CSF t-tau and p-tau181; higher levels of synaptic injury; relatively widespread cortical atrophy; and increased specific microglial proteins involved in detecting and engulfing amyloid plaque and in innate immune activation, including ApoE and complement complex proteins such as complement component C1q.
In the small population of APOEɛ4 noncarriers in the current study, sex had a major effect on age at diagnosis of clinical AD, with a significantly earlier mean age in male relative to female participants of 10.7 years. Interestingly, although AD may affect females with more intensity, more rapid brain atrophy, and faster cognitive decline than in males, it does not appear to do so earlier [82,83]. The higher prevalence of AD in females is mostly driven by a divergence of prevalence rates in males and females with increasing age [84,85]. There is an inflection point near 75 years of age where it is difficult to detect significant differences by sex below 75 years, and incidence rates become more divergent above 75 years [86]. Most AD below the age of 75 years may be in APOEɛ4 carriers, with a moderately increased risk in female APOEɛ4 heterozygotes [53]. Moreover, in APOE4 carriers, the risk for AD below the age of 75 years is greater in females, but over the age of 75 years, the risk for AD may be greater in males [47]. On the other hand, in APOEɛ4 noncarriers over the age of 75 years, the overall prevalence rates for AD may be substantially higher in females than in males [52]. Cortical microglial activation may be higher with age and disproportionately more important for AD progression in females [8]. For example, a prior study using neuropathological indicators in human tissue identified that microglial activation in females was associated with both amyloid-mediated increases in tau pathology (~50%) and direct induction of tau pathology (~50%), whereas in males, microglial activation was predominantly associated with direct induction of tau pathology (~74%) [8]. In APOE4 noncarriers, greater CSF tau pathology emerges in females relative to males in prodromal disease, and this difference is also present in mild AD [6]. In the current study in patients with early AD, tau pathophysiology was substantially greater in female APOE4 noncarriers (Figure 2b). Thus, while female APOEɛ4 noncarriers may be relatively resilient at younger ages, their risk for AD may increase with age becoming particularly divergent from males over the age of 75 years [86]. For example, in a 4-year longitudinal study in patients with prodromal AD, 40% of female APOEɛ4 noncarriers aged 75 years or more transitioned to dementia compared to only 21% of male noncarriers [52].
Limitations of this current study include its retrospective nature, its small size, and that participants were largely of European ancestry. In addition, discerning clinical phenotypes on a variable background of AChE-I therapy (Tables S3 and S4) may be problematic because APOEɛ4 carriers are more responsive to AChE-I treatment in the mild stage of AD; therefore, attention, processing speed, and amnestic deficits may have been partly obscured [74].
5. Conclusions
In early clinical AD in individuals aged 50–75 years, increased APOEɛ4 allele frequency is associated with decreased functional activation of glia. This allele frequency-dependent effect was evident in females, but not in males. Male and female APOEε4 homozygotes had the lowest levels of glial activation, while female APOEɛ4 noncarriers, and to a lesser extent female APOEɛ4 heterozygotes, had the highest levels of glial activation. These age, genotype, and sex influences on levels of glial activation may be important determinants in the mix and timing of amyloid and tau pathology, neurodegeneration, denervation of the corticolimbic cholinergic system, and clinical features of early clinical AD. They may explain much of the phenotypic heterogeneity in early clinical AD populations aged below 75 years. In individuals aged 50–74 years, assessing APOE genotype in those developing AD may provide valuable information that could guide treatment decisions and prognosis, including age at onset and disease course. Along with ethical and practical considerations, larger, longitudinal, and prospective studies are required to determine the prognostic and clinical management insights of APOE4 genotype testing by sex and age.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/neuroglia5030022/s1: Table S1: Ethics committees that approved the clinical study; Table S2: Description of CSF biomarkers of AD pathology, glial activation, neuroaxonal damage, and synaptic injury; Table S3: Mild AD phenotype across genotype groups defined by APOE4 allele frequency; Table S4: Mild AD phenotype in APOE4 carriers by APOE4 allele frequency and sex; Figure S1: R squared linear regression results across all comparisons.
Author Contributions
Conceptualization, R.M.L. and T.D.-S.; methodology, R.M.L. and T.D.-S.; software, D.L.; validation, D.L.; formal analysis, D.L.; data curation, D.L.; writing—original draft preparation, R.M.L.; writing—review and editing, R.M.L., D.L. and T.D.-S.; visualization, D.L.; supervision, R.M.L.; project administration, R.M.L.; funding acquisition, R.M.L. All authors have read and agreed to the published version of the manuscript.
Funding
The clinical trial from which these baseline results were obtained was funded by Biogen. The preparation of this manuscript was funded by Ionis Pharmaceuticals.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board or Ethics Committee of multiple institutions; the committee name/institution, principal investigator, and reference number are provided in Table S1.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We thank the participants and their companions who participated in the study; the sites, and study team from Ionis for executing the study; and Gwendolyn Kaeser, who created the figures and edited and styled the manuscript per journal requirements.
Conflicts of Interest
R.M.L. and D.L. are employees of, and holders of stock/stock options in, Ionis Pharmaceuticals Inc. T.D.-S. has no conflicts of interest.
References
- Yamazaki, Y.; Zhao, N.; Caulfield, T.R.; Liu, C.C.; Bu, G. Apolipoprotein E and Alzheimer disease: Pathobiology and targeting strategies. Nat. Rev. Neurol. 2019, 15, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Koutsodendris, N.; Nelson, M.R.; Rao, A.; Huang, Y. Apolipoprotein E and Alzheimer’s Disease: Findings, Hypotheses, and Potential Mechanisms. Annu. Rev. Pathol. 2022, 17, 73–99. [Google Scholar] [CrossRef] [PubMed]
- Moser, V.A.; Workman, M.J.; Hurwitz, S.J.; Lipman, R.M.; Pike, C.J.; Svendsen, C.N. Microglial transcription profiles in mouse and human are driven by APOE4 and sex. iScience 2021, 24, 103238. [Google Scholar] [CrossRef] [PubMed]
- Stephen, T.L.; Breningstall, B.; Suresh, S.; McGill, C.J.; Pike, C.J. APOE genotype and biological sex regulate astroglial interactions with amyloid plaques in Alzheimer’s disease mice. J. Neuroinflamm. 2022, 19, 286. [Google Scholar] [CrossRef]
- Sala Frigerio, C.; Wolfs, L.; Fattorelli, N.; Thrupp, N.; Voytyuk, I.; Schmidt, I.; Mancuso, R.; Chen, W.T.; Woodbury, M.E.; Srivastava, G.; et al. The Major Risk Factors for Alzheimer’s Disease: Age, Sex, and Genes Modulate the Microglia Response to Abeta Plaques. Cell Rep. 2019, 27, 1293–1306.e6. [Google Scholar] [CrossRef]
- Mofrad, R.B.; Tijms, B.M.; Scheltens, P.; Barkhof, F.; van der Flier, W.M.; Sikkes, S.A.; Teunissen, C.E. Sex differences in CSF biomarkers vary by Alzheimer disease stage and APOE ε4 genotype. Neurology 2020, 95, e2378–e2388. [Google Scholar]
- Berchtold, N.C.; Cribbs, D.H.; Coleman, P.D.; Rogers, J.; Head, E.; Kim, R.; Beach, T.; Miller, C.; Troncoso, J.; Trojanowski, J.Q.; et al. Gene expression changes in the course of normal brain aging are sexually dimorphic. Proc. Natl. Acad. Sci. USA 2008, 105, 15605–15610. [Google Scholar] [CrossRef]
- Casaletto, K.B.; Nichols, E.; Aslanyan, V.; Simone, S.M.; Rabin, J.S.; La Joie, R.; Brickman, A.M.; Dams-O’Connor, K.; Palta, P.; Kumar, R.G.; et al. Sex-specific effects of microglial activation on Alzheimer’s disease proteinopathy in older adults. Brain 2022, 145, 3536–3545. [Google Scholar] [CrossRef]
- Yin, Z.; Rosenzweig, N.; Kleemann, K.L.; Zhang, X.; Brandao, W.; Margeta, M.A.; Schroeder, C.; Sivanathan, K.N.; Silveira, S.; Gauthier, C.; et al. APOE4 impairs the microglial response in Alzheimer’s disease by inducing TGFbeta-mediated checkpoints. Nat. Immunol. 2023, 24, 1839–1853. [Google Scholar] [CrossRef]
- Machlovi, S.I.; Neuner, S.M.; Hemmer, B.M.; Khan, R.; Liu, Y.; Huang, M.; Zhu, J.D.; Castellano, J.M.; Cai, D.; Marcora, E.; et al. APOE4 confers transcriptomic and functional alterations to primary mouse microglia. Neurobiol. Dis. 2022, 164, 105615. [Google Scholar] [CrossRef]
- Podleśny-Drabiniok, A.; Marcora, E.; Goate, A.M. Microglial Phagocytosis: A Disease-Associated Process Emerging from Alzheimer’s Disease Genetics. Trends Neurosci. 2020, 43, 965–979. [Google Scholar] [CrossRef] [PubMed]
- Millet, A.; Ledo, J.H.; Tavazoie, S.F. An exhausted-like microglial population accumulates in aged and APOE4 genotype Alzheimer’s brains. Immunity 2024, 57, 153–170.e6. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Seo, J.; Gao, F.; Feldman, H.M.; Wen, H.L.; Penney, J.; Cam, H.P.; Gjoneska, E.; Raja, W.K.; Cheng, J.; et al. APOE4 Causes Widespread Molecular and Cellular Alterations Associated with Alzheimer’s Disease Phenotypes in Human iPSC-Derived Brain Cell Types. Neuron 2018, 98, 1141–1154.e7. [Google Scholar] [CrossRef]
- Guillot-Sestier, M.V.; Doty, K.R.; Gate, D.; Rodriguez, J.; Jr Leung, B.P.; Rezai-Zadeh, K.; Town, T. Il10 deficiency rebalances innate immunity to mitigate Alzheimer-like pathology. Neuron 2015, 85, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Calvet, M.; Morenas-Rodriguez, E.; Kleinberger, G.; Schlepckow, K.; Araque Caballero, M.A.; Franzmeier, N.; Capell, A.; Fellerer, K.; Nuscher, B.; Eren, E.; et al. Early increase of CSF sTREM2 in Alzheimer’s disease is associated with tau related-neurodegeneration but not with amyloid-beta pathology. Mol. Neurodegener. 2019, 14, 1. [Google Scholar] [CrossRef]
- Jain, N.; Lewis, C.A.; Ulrich, J.D.; Holtzman, D.M. Chronic TREM2 activation exacerbates Abeta-associated tau seeding and spreading. J. Exp. Med. 2023, 220, e20220654. [Google Scholar] [CrossRef]
- Gratuze, M.; Leyns, C.E.; Sauerbeck, A.D.; St-Pierre, M.K.; Xiong, M.; Kim, N.; Serrano, J.R.; Tremblay, M.E.; Kummer, T.T.; Colonna, M.; et al. Impact of TREM2R47H variant on tau pathology-induced gliosis and neurodegeneration. J. Clin. Investig. 2020, 130, 4954–4968. [Google Scholar] [CrossRef]
- Pascoal, T.A.; Benedet, A.L.; Ashton, N.J.; Kang, M.S.; Therriault, J.; Chamoun, M.; Savard, M.; Lussier, F.Z.; Tissot, C.; Karikari, T.K.; et al. Microglial activation and tau propagate jointly across Braak stages. Nat. Med. 2021, 27, 1592–1599. [Google Scholar] [CrossRef]
- Bellaver, B.; Povala, G.; Ferreira, P.C.L.; Ferrari-Souza, J.P.; Leffa, D.T.; Lussier, F.Z.; Benedet, A.L.; Ashton, N.J.; Triana-Baltzer, G.; Kolb, H.C.; et al. Astrocyte reactivity influences amyloid-beta effects on tau pathology in preclinical Alzheimer’s disease. Nat. Med. 2023, 29, 1775–1781. [Google Scholar] [CrossRef]
- Safaiyan, S.; Kannaiyan, N.; Snaidero, N.; Brioschi, S.; Biber, K.; Yona, S.; Edinger, A.L.; Jung, S.; Rossner, M.J.; Simons, M. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat. Neurosci. 2016, 19, 995–998. [Google Scholar] [CrossRef]
- Hu, Y.; Fryatt, G.L.; Ghorbani, M.; Obst, J.; Menassa, D.A.; Martin-Estebane, M.; Muntslag, T.A.O.; Olmos-Alonso, A.; Guerrero-Carrasco, M.; Thomas, D.; et al. Replicative senescence dictates the emergence of disease-associated microglia and contributes to Abeta pathology. Cell Rep. 2021, 35, 109228. [Google Scholar] [CrossRef] [PubMed]
- Connolly, K.; Lehoux, M.; O’Rourke, R.; Assetta, B.; Erdemir, G.A.; Elias, J.A.; Lee, C.G.; Huang, Y.A. Potential role of chitinase-3-like protein 1 (CHI3L1/YKL-40) in neurodegeneration and Alzheimer’s disease. Alzheimer’s Dement. 2023, 19, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Yin, F. Lipid metabolism and Alzheimer’s disease: Clinical evidence, mechanistic link and therapeutic promise. FEBS J. 2023, 290, 1420–1453. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Yuan, Z.; Pan, R.; Su, X.; Wang, H.; Xue, J.; Zhuang, K.; Gao, J.; Chen, Z.; Lin, H.; et al. Microglial hexokinase 2 deficiency increases ATP generation through lipid metabolism leading to beta-amyloid clearance. Nat. Metab. 2022, 4, 1287–1305. [Google Scholar] [CrossRef]
- Choi, H.; Mook-Jung, I. Lipid fuel for hungry-angry microglia. Nat. Metab. 2022, 4, 1223–1224. [Google Scholar] [CrossRef]
- Prakash, P.; Manchanda, P.; Paouri, E.; Bisht, K.; Sharma, K.; Wijewardhane, P.R.; Randolph, C.E.; Clark, M.G.; Fine, J.; Thayer, E.A.; et al. Amyloid beta Induces Lipid Droplet-Mediated Microglial Dysfunction in Alzheimer’s Disease. bioRxiv 2023. 2023.06.04.543525. [Google Scholar] [CrossRef]
- Baenziger, J.E.; Morris, M.L.; Darsaut, T.E.; Ryan, S.E. Effect of membrane lipid composition on the conformational equilibria of the nicotinic acetylcholine receptor. J. Biol. Chem. 2000, 275, 777–784. [Google Scholar] [CrossRef]
- Barrantes, F.J. Cholesterol effects on nicotinic acetylcholine receptor: Cellular aspects. Subcell. Biochem. 2010, 51, 467–487. [Google Scholar]
- Benfante, R.; Di Lascio, S.; Cardani, S.; Fornasari, D. Acetylcholinesterase inhibitors targeting the cholinergic anti-inflammatory pathway: A new therapeutic perspective in aging-related disorders. Aging Clin. Exp. Res. 2021, 33, 823–834. [Google Scholar] [CrossRef]
- Kumar, R.; Nordberg, A.; Darreh-Shori, T. Amyloid-beta peptides act as allosteric modulators of cholinergic signalling through formation of soluble BAbetaACs. Brain 2016, 139 Pt 1, 174–192. [Google Scholar] [CrossRef]
- Baidya, A.T.; Kumar, A.; Kumar, R.; Darreh-Shori, T. Allosteric Binding Sites of Abeta Peptides on the Acetylcholine Synthesizing Enzyme ChAT as Deduced by In Silico Molecular Modeling. Int. J. Mol. Sci. 2022, 23, 6073. [Google Scholar] [CrossRef] [PubMed]
- Darreh-Shori, T.; Siawesh, M.; Mousavi, M.; Andreasen, N.; Nordberg, A. Apolipoprotein epsilon4 modulates phenotype of butyrylcholinesterase in CSF of patients with Alzheimer’s disease. J. Alzheimer’s Dis. 2012, 28, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Darreh-Shori, T.; Vijayaraghavan, S.; Aeinehband, S.; Piehl, F.; Lindblom, R.P.; Nilsson, B.; Ekdahl, K.N.; Langstrom, B.; Almkvist, O.; Nordberg, A. Functional variability in butyrylcholinesterase activity regulates intrathecal cytokine and astroglial biomarker profiles in patients with Alzheimer’s disease. Neurobiol. Aging 2013, 34, 2465–2481. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.M.; Darreh-Shori, T.; Junge, C.; Li, D.; Yang, Q.; Edwards, A.L.; Graham, D.L.; Moore, K.; Mummery, C.J. Onset of Alzheimer disease in apolipoprotein varepsilon4 carriers is earlier in butyrylcholinesterase K variant carriers. BMC Neurol. 2024, 24, 116. [Google Scholar] [CrossRef]
- Lane, R.M.; Darreh-Shori, T. Understanding the beneficial and detrimental effects of donepezil and rivastigmine to improve their therapeutic value. J. Alzheimer’s Dis. 2015, 44, 1039–1062. [Google Scholar] [CrossRef]
- Savva, G.M.; Wharton, S.B.; Ince, P.G.; Forster, G.; Matthews, F.E.; Brayne, C.; Medical Research Council Cognitive Function and Ageing Study. Age, neuropathology, and dementia. N. Engl. J. Med. 2009, 360, 2302–2309. [Google Scholar] [CrossRef]
- Mummery, C.J.; Borjesson-Hanson, A.; Blackburn, D.J.; Vijverberg, E.G.B.; De Deyn, P.P.; Ducharme, S.; Jonsson, M.; Schneider, A.; Rinne, J.O.; Ludolph, A.C.; et al. Tau-targeting antisense oligonucleotide MAPT(Rx) in mild Alzheimer’s disease: A phase 1b, randomized, placebo-controlled trial. Nat. Med. 2023, 29, 1437–1447. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Morris, J.C.; Ernesto, C.; Schafer, K.; Coats, M.; Leon, S.; Sano, M.; Thal, L.J.; Woodbury, P. Clinical dementia rating training and reliability in multicenter studies: The Alzheimer’s Disease Cooperative Study experience. Neurology 1997, 48, 1508–1510. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- Zeng, X.; Cheung, S.K.K.; Shi, M.; Or, P.M.Y.; Li, Z.; Liu, J.Y.H.; Ho, W.L.H.; Liu, T.; Lu, K.; Rudd, J.A.; et al. Astrocyte-specific knockout of YKL-40/Chi3l1 reduces Aβ burden and restores memory functions in 5xFAD mice. J. Neuroinflamm. 2023, 20, 290. [Google Scholar] [CrossRef] [PubMed]
- Budelier, M.M.; He, Y.; Barthelemy, N.R.; Jiang, H.; Li, Y.; Park, E.; Henson, R.L.; Schindler, S.E.; Holtzman, D.M.; Bateman, R.J. A map of neurofilament light chain species in brain and cerebrospinal fluid and alterations in Alzheimer’s disease. Brain Commun. 2022, 4, fcac045. [Google Scholar] [CrossRef]
- Arvidsson Rådestig, M.; Skoog, I.; Skillbäck, T.; Zetterberg, H.; Kern, J.; Zettergren, A.; Andreasson, U.; Wetterberg, H.; Kern, S.; Blennow, K. Cerebrospinal fluid biomarkers of axonal and synaptic degeneration in a population-based sample. Alzheimer’s Res. Ther. 2023, 15, 44. [Google Scholar] [CrossRef]
- Tarawneh, R.; D’Angelo, G.; Crimmins, D.; Herries, E.; Griest, T.; Fagan, A.M.; Zipfel, G.J.; Ladenson, J.H.; Morris, J.C.; Holtzman, D.M. Diagnostic and Prognostic Utility of the Synaptic Marker Neurogranin in Alzheimer Disease. JAMA Neurol. 2016, 73, 561–571. [Google Scholar] [CrossRef]
- Wang, X.; Ghayoor, A.; Novicki, A.; Holmes, S.; Seibyl, J.; Hesterman, J. [P4–266]: Application of a Multi-Atlas Segmentation Tool to Hippocampus, Ventricle and Whole Brain Segmentation. Alzheimer’s Dement. 2017, 13, P1385–P1386. [Google Scholar] [CrossRef]
- Graffelman, J. Exploring diallelic genetic markers: The HardyWeinberg package. J. Stat. Softw. 2015, 64, 1–23. [Google Scholar] [CrossRef]
- Huang, L.C.; Lee, M.Y.; Chien, C.F.; Chang, Y.P.; Li, K.Y.; Yang, Y.H. Age and sex differences in the association between APOE genotype and Alzheimer’s disease in a Taiwan Chinese population. Front. Aging Neurosci 2023, 15, 1246592. [Google Scholar] [CrossRef] [PubMed]
- Bellou, E.; Baker, E.; Leonenko, G.; Bracher-Smith, M.; Daunt, P.; Menzies, G.; Williams, J.; Escott-Price, V.; Alzheimer’s Disease Neuroimaging Initiative. Age-dependent effect of APOE and polygenic component on Alzheimer’s disease. Neurobiol Aging 2020, 93, 69–77. [Google Scholar] [CrossRef]
- Whitwell, J.L.; Tosakulwong, N.; Weigand, S.D.; Graff-Radford, J.; Ertekin-Taner, N.; Machulda, M.M.; Duffy, J.R.; Schwarz, C.G.; Senjem, M.L.; Jack, C.R.; et al. Relationship of APOE, age at onset, amyloid and clinical phenotype in Alzheimer disease. Neurobiol. Aging 2021, 108, 90–98. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, W.; Ye, T.; Lin, X.; Zhang, J.; Alzheimer’s Disease Neuroimaging Initiative. Sex Difference in the Association of APOE4 with Memory Decline in Mild Cognitive Impairment. J. Alzheimer’s Dis. 2019, 69, 1161–1169. [Google Scholar] [CrossRef]
- Neu, S.C.; Pa, J.; Kukull, W.; Beekly, D.; Kuzma, A.; Gangadharan, P.; Wang, L.S.; Romero, K.; Arneric, S.P.; Redolfi, A.; et al. Apolipoprotein E Genotype and Sex Risk Factors for Alzheimer Disease: A Meta-analysis. JAMA Neurol. 2017, 74, 1178–1189. [Google Scholar] [CrossRef]
- Lane, R.M.; He, Y. Butyrylcholinesterase genotype and gender influence Alzheimer’s disease phenotype. Alzheimer’s Dement. 2013, 9, e1–e73. [Google Scholar] [CrossRef] [PubMed]
- Farrer, L.A.; Cupples, L.A.; Haines, J.L.; Hyman, B.; Kukull, W.A.; Mayeux, R.; Myers, R.H.; Pericak-Vance, M.A.; Risch, N.; van Duijn, C.M. Effects of Age, Sex, and Ethnicity on the Association Between Apolipoprotein E Genotype and Alzheimer Disease: A Meta-analysis. JAMA 1997, 278, 1349–1356. [Google Scholar] [CrossRef]
- Fleisher, A.S.; Chen, K.; Liu, X.; Ayutyanont, N.; Roontiva, A.; Thiyyagura, P.; Protas, H.; Joshi, A.D.; Sabbagh, M.; Sadowsky, C.H.; et al. Apolipoprotein E epsilon4 and age effects on florbetapir positron emission tomography in healthy aging and Alzheimer disease. Neurobiol Aging 2013, 34, 1–12. [Google Scholar] [CrossRef]
- Groot, C.; Grothe, M.J.; Mukherjee, S.; Jelistratova, I.; Jansen, I.; van Loenhoud, A.C.; Risacher, S.L.; Saykin, A.J.; Mac Donald, C.L.; Mez, J.; et al. Differential patterns of gray matter volumes and associated gene expression profiles in cognitively-defined Alzheimer’s disease subgroups. NeuroImage Clin. 2021, 30, 102660. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, N.; Ossenkoppele, R.; Smith, R.; Strandberg, O.; Ohlsson, T.; Jogi, J.; Palmqvist, S.; Stomrud, E.; Hansson, O. Greater tau load and reduced cortical thickness in APOE epsilon4-negative Alzheimer’s disease: A cohort study. Alzheimer’s Res. Ther. 2018, 10, 77. [Google Scholar] [CrossRef]
- Reiman, E.M.; Arboleda-Velasquez, J.F.; Quiroz, Y.T.; Huentelman, M.J.; Beach, T.G.; Caselli, R.J.; Chen, Y.; Su, Y.; Myers, A.J.; Hardy, J.; et al. Exceptionally low likelihood of Alzheimer’s dementia in APOE2 homozygotes from a 5000-person neuropathological study. Nat. Commun. 2020, 11, 667. [Google Scholar] [CrossRef]
- Josephs, K.A.; Whitwell, J.L.; Ahmed, Z.; Shiung, M.M.; Weigand, S.D.; Knopman, D.S.; Boeve, B.F.; Parisi, J.E.; Petersen, R.C.; Dickson, D.W.; et al. Beta-amyloid burden is not associated with rates of brain atrophy. Ann. Neurol. 2008, 63, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Rhein, V.; Song, X.; Wiesner, A.; Ittner, L.M.; Baysang, G.; Meier, F.; Ozmen, L.; Bluethmann, H.; Drose, S.; Brandt, U.; et al. Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice. Proc. Natl. Acad. Sci. USA 2009, 106, 20057–20062. [Google Scholar] [CrossRef] [PubMed]
- Puzzo, D.; Argyrousi, E.K.; Staniszewski, A.; Zhang, H.; Calcagno, E.; Zuccarello, E.; Acquarone, E.; Fa, M.; Li Puma, D.D.; Grassi, C.; et al. Tau is not necessary for amyloid-beta-induced synaptic and memory impairments. J. Clin. Investig. 2020, 130, 4831–4844. [Google Scholar] [CrossRef]
- Costoya-Sánchez, A.; Moscoso, A.; Silva-Rodríguez, J.; Pontecorvo, M.J.; Devous, M.D.; Sr Aguiar, P.; Schöll, M.; Grothe, M.J.; Alzheimer’s Disease Neuroimaging Initiative and the Harvard Aging Brain Study. Increased Medial Temporal Tau Positron Emission Tomography Uptake in the Absence of Amyloid-β Positivity. JAMA Neurol. 2023, 80, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Ossenkoppele, R.; Binette, A.P.; Groot, C.; Smith, R.; Strandberg, O.; Palmqvist, S.; Stomrud, E.; Tideman, P.; Ohlsson, T.; Jögi, J.; et al. Amyloid and Tau PET positive cognitively unimpaired individuals are at high risk for future cognitive decline. Nat. Med. 2022, 28, 2381–2387. [Google Scholar] [CrossRef] [PubMed]
- Susanto, T.A.; Pua, E.P.; Zhou, J.; Alzheimer’s Disease Neuroimaging Initiative. Cognition, brain atrophy, and cerebrospinal fluid biomarkers changes from preclinical to dementia stage of Alzheimer’s disease and the influence of apolipoprotein e. J. Alzheimer’s Dis. 2015, 45, 253–268. [Google Scholar] [CrossRef]
- Schmitz, T.W.; Soreq, H.; Poirier, J.; Spreng, R.N. Longitudinal Basal Forebrain Degeneration Interacts with TREM2/C3 Biomarkers of Inflammation in Presymptomatic Alzheimer’s Disease. J. Neurosci. 2020, 40, 1931–1942. [Google Scholar] [CrossRef]
- Schmitz, T.W.; Spreng, R.N.; Alzheimer’s Disease Neuroimaging Initiative. Basal forebrain degeneration precedes and predicts the cortical spread of Alzheimer’s pathology. Nat. Commun. 2016, 7, 13249. [Google Scholar]
- Yu, M.C.; Chuang, Y.F.; Wu, S.C.; Ho, C.F.; Liu, Y.C.; Chou, C.J. White matter hyperintensities in cholinergic pathways are associated with dementia severity in e4 carriers but not in non-carriers. Front. Neurol. 2023, 14, 1100322. [Google Scholar] [CrossRef]
- Hu, L.; Wong, T.P.; Cote, S.L.; Bell, K.F.; Cuello, A.C. The impact of Abeta-plaques on cortical cholinergic and non-cholinergic presynaptic boutons in alzheimer’s disease-like transgenic mice. Neuroscience 2003, 121, 421–432. [Google Scholar] [CrossRef]
- Bell, K.F.; Cuello, A.C. Altered synaptic function in Alzheimer’s disease. Eur. J. Pharmacol. 2006, 545, 11–21. [Google Scholar] [CrossRef]
- Mesulam, M.; Shaw, P.; Mash, D.; Weintraub, S. Cholinergic nucleus basalis tauopathy emerges early in the aging-MCI-AD continuum. Eur. J. Pharmacol. 2004, 55, 815–828. [Google Scholar] [CrossRef]
- Ballinger, E.C.; Ananth, M.; Talmage, D.A.; Role, L.W. Basal Forebrain Cholinergic Circuits and Signaling in Cognition and Cognitive Decline. Neuron 2016, 91, 1199–1218. [Google Scholar] [CrossRef]
- Fontana, I.C.; Kumar, A.; Nordberg, A. The role of astrocytic alpha7 nicotinic acetylcholine receptors in Alzheimer disease. Nat. Rev. Neurol. 2023, 19, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Ferrari-Souza, J.P.; Lussier, F.Z.; Leffa, D.T.; Therriault, J.; Tissot, C.; Bellaver, B.; Ferreira, P.C.L.; Malpetti, M.; Wang, Y.T.; Povala, G.; et al. APOEepsilon4 associates with microglial activation independently of Abeta plaques and tau tangles. Sci. Adv. 2023, 9, eade1474. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.M.; He, Y. Emerging hypotheses regarding the influences of butyrylcholinesterase-K variant, APOE epsilon 4, and hyperhomocysteinemia in neurodegenerative dementias. Med. Hypotheses 2009, 73, 230–250. [Google Scholar] [CrossRef] [PubMed]
- Steward, A.; Biel, D.; Dewenter, A.; Roemer, S.; Wagner, F.; Dehsarvi, A.; Rathore, S.; Otero Svaldi, D.; Higgins, I.; Brendel, M.; et al. ApoE4 and Connectivity-Mediated Spreading of Tau Pathology at Lower Amyloid Levels. JAMA Neurol. 2023, 80, 1295–1306. [Google Scholar] [CrossRef]
- Chuang, Y.F.; Varma, V.; An, Y.; Tanaka, T.; Davatzikos, C.; Resnick, S.M.; Thambisetty, M. Interaction between Apolipoprotein E and Butyrylcholinesterase Genes on Risk of Alzheimer’s Disease in a Prospective Cohort Study. J. Alzheimer’s Dis. 2020, 75, 417–427. [Google Scholar] [CrossRef]
- Lane, R.; Feldman, H.H.; Meyer, J.; He, Y.; Ferris, S.H.; Nordberg, A.; Darreh-Shori, T.; Soininen, H.; Pirttila, T.; Farlow, M.R.; et al. Synergistic effect of apolipoprotein E epsilon4 and butyrylcholinesterase K-variant on progression from mild cognitive impairment to Alzheimer’s disease. Pharmacogenet Genom. 2008, 18, 289–298. [Google Scholar] [CrossRef]
- Bigler, E.D.; Lowry, C.M.; Anderson, C.V.; Johnson, S.C.; Terry, J.; Steed, M. Dementia, quantitative neuroimaging, and apolipoprotein E genotype. Am. J. Neuroradiol. 2000, 21, 1857–1868. [Google Scholar]
- Yasuda, M.; Mori, E.; Kitagaki, H.; Yamashita, H.; Hirono, N.; Shimada, K.; Maeda, K.; Tanaka, C. Apolipoprotein E ε4 allele and whole brain atrophy in late-onset Alzheimer’s disease. Am. J. Psychiatry 1998, 155, 779–784. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Petersen, R.C.; Xu, Y.C.; O’Brien, P.C.; Waring, S.C.; Tangalos, E.G.; Smith, G.E.; Ivnik, R.J.; Thibodeau, S.N.; Kokmen, E. Hippocampal atrophy and apolipoprotein E genotype are independently associated with Alzheimer’s disease. Ann. Neurol. 1998, 43, 303–310. [Google Scholar] [CrossRef]
- Tijms, B.M.; Vromen, E.M.; Mjaavatten, O.; Holstege, H.; Reus, L.M.; van der Lee, S.; Wesenhagen, K.E.J.; Lorenzini, L.; Vermunt, L.; Venkatraghavan, V.; et al. Cerebrospinal fluid proteomics in patients with Alzheimer’s disease reveals five molecular subtypes with distinct genetic risk profiles. Nat. Aging 2024, 4, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Sauty, B.; Durrleman, S. Impact of sex and APOE-epsilon4 genotype on patterns of regional brain atrophy in Alzheimer’s disease and healthy aging. Front. Neurol. 2023, 14, 1161527. [Google Scholar] [CrossRef] [PubMed]
- Laws, K.R.; Irvine, K.; Gale, T.M. Sex differences in cognitive impairment in Alzheimer’s disease. World J. Psychiatry 2016, 6, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, M.T.; Iulita, M.F.; Cavedo, E.; Chiesa, P.A.; Schumacher Dimech, A.; Santuccione Chadha, A.; Baracchi, F.; Girouard, H.; Misoch, S.; Giacobini, E.; et al. Sex differences in Alzheimer disease—The gateway to precision medicine. Nat. Rev. Neurol. 2018, 14, 457–469. [Google Scholar] [CrossRef]
- Dubal, D.B. Sex difference in Alzheimer’s disease: An updated, balanced and emerging perspective on differing vulnerabilities. Handb. Clin. Neurol. 2020, 175, 261–273. [Google Scholar]
- Beam, C.R.; Kaneshiro, C.; Jang, J.Y.; Reynolds, C.A.; Pedersen, N.L.; Gatz, M. Differences Between Women and Men in Incidence Rates of Dementia and Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 64, 1077–1083. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).