Abstract
Lithium-ion batteries (LIBs) are widely used as energy storage units in electric vehicles, mobile phones, and other electric devices due to their high voltage, large capacity, and long cycle life. Lithium-ion batteries are prone to thermal runway (TR), resulting in fires and explosions, which can seriously hinder the commercial development of LIBs. A series of safety methods has been studied to prevent TR of LIBs. The safety methods for suppressing TR in LIBs were reviewed, including safety equipment method, material modification method, thermal management method, and cooling method. The mechanism, advantages and disadvantages, and future applications of the TR suppression method are discussed. The effectiveness of the proposed safety method was evaluated through technical analysis and experimental testing, and the inhibitory effects of different safety methods on battery TR were summarized. The future trend of suppressing TR is discussed by summarizing and generalizing existing technologies for suppressing thermal runaway. This study provides a reference for exploring more effective methods to mitigate TR in the future.
1. Introduction
Lithium-ion batteries (LIBs) are now dominating the power sources market for portable electronics such as smartphones, laptops, digital cameras, and electric vehicles (EVs) because of their high energy density [1,2], long lifespan [3,4], and environmental friendliness [5,6]. With the vigorous development of the energy storage and electric vehicle industries, the production of LIB products for these critical sectors needs to grow in tandem. According to forecasts by research firm Wood Mackenzie, global LIB production capacity will quadruple in 2030 compared to 2019, reaching 1.3 TWh [7].
However, LIBs are prone to thermal runaway (TR) during application, which can cause fires or even explosions [8,9]. In recent years, there has been a proliferation of safety accidents caused by TR, ranging from fires in large energy storage power plants to many other accidents and incidents in portable devices. For example, in 2018, fires and an explosion at the 25 MWh DC Optical Storage and Charging Integration Project in Beijing Jimei Dahongmen. Battery accidents also occur frequently in cell phones and electric vehicles, such as Samsung Galaxy Note 7 battery fires and a series of Tesla electric vehicles fires. The U.S. Postal Service stopped the international shipment of LIBs in 2012 because of overheating and explosion issues [10]. The further development of LIBs in the energy storage industry has been limited due to safety accidents caused by TR [11,12].
The TR of LIB is a complex electrochemical reaction process that typically exhibits a range of serious thermal, chemical, fire, and explosion hazards [13,14]. TR of LIBs can be initiated in a variety of ways, such as overheating, short-circuiting, mechanical abuse, and manufacturing flaws [15,16]. In addition, the defects in the manufacturing process can also lead to TR of LIBs.
A large number of safety methods have been adopted to alleviate TR of LIBs. Battery manufacturers employ some safety methods at the cell level [17] and packaging level [18] to mitigate or inhibit TR. Positive temperature coefficient (PTC) thermistors, current interrupt devices (CIDs), safety vents, and protection circuitry were adopted to prevent TR from occurring in devices [19]. Separators, electrolytes, and electrodes determine the electrochemical performance of batteries, and they even affect the safety performance of batteries. Shutdown separators, electrolyte additives, safe electrodes, and safe electrolytes were used to reduce the risk of TR of LIBs. A battery thermal management system (BTMS) was employed to remove heat from LIBs, thus regulating the operating temperature in order to avoid TR of LIBs caused by overheating [20,21]. As an active prevention method, spray can effectively alleviate the TR of LIBs and prevent the TR propagation at the module level.
This article summarizes the safety methods for mitigating TR of lithium batteries from the aspects of safety materials, safety equipment, active prevention, and passive mitigation, as shown in Figure 1. We elaborated on the working mechanisms of current methods for mitigating TR and analyzed the advantages and disadvantages of current methods. The effectiveness of mitigating TR was characterized by parameters such as heating rate, heat generation, and delayed TR time. The suppression effect of different mitigation methods on battery TR was studied through technical analysis and experimental testing. The current issues, key challenges, and future trends in suppressing TR were discussed, providing a reference for exploring effective methods to alleviate TR in the future.
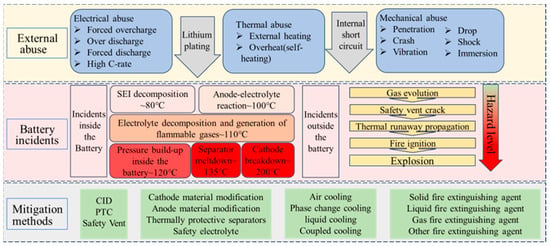
Figure 1.
Mitigation methods for each stage of battery TR under different triggering conditions.
2. Safety Device for Mitigating TR
2.1. Positive Temperature Coefficient (PTC)
Positive temperature coefficient (PTC) is a typical semiconductor resistor with temperature sensitivity. When it exceeds a certain temperature (Curie temperature), its resistance value increases step by step with the increase in temperature. The PTC material used for LIBs consists of a conducting polymer with a high resistance-change rate [22], which has sufficiently low-room-temperature resistance in the milliohm range of 1 mΩ–10 mΩ [23]. A conductive polymer, a mixture of carbon black and PE polymer layer, was placed between two metal circular discs into LIBs. The conducting polymer will convert to amorphous state above glass transition temperature during working conditions. The volumetric expansion of conducting polymer cause by phase change will increase the distance and conduction pathway between carbon black particles, which result in the nonlinearity and a sharp increase in PTC resistance. When the temperature drops below the glass transition temperature, the polymer returns to a crystalline state [24,25]. Therefore, when the battery’s temperature exceeds 100 °C due to a short circuit, PTC in a high resistance state protects individual batteries from the influence of overcurrent. This working mechanism, to a large extent, prevents TR from occurring in a single battery.
Figure 2a shows the cap structure of a typically cylindrical battery. The computer tomography (CT) images in Figure 2b show the corresponding components in the actual 18650 battery. There is also no PTC thermistor in the battery shown in Figure 2b, as this type of battery is designed for high-discharge-rate applications as high as 20 A, such as Samsung INR18650-25R [26].
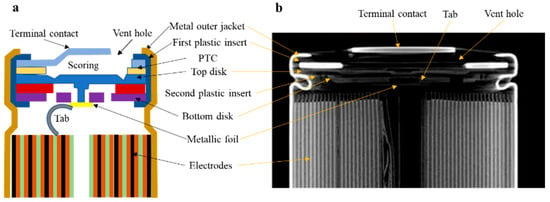
Figure 2.
A typical cylindrical LIB (a) schematic diagram of the cap structure and (b) a CT scan image of the cap structure [23].
Internal short circuits in batteries may promote TR [27]. It is necessary to cut down short circuits and overcurrent, which can mitigate TR. The overcurrent suppression performance of composite PTC thermistor with positive temperature coefficient significantly increases between 120 °C and 150 °C. The P3BT-523 composite electrode hardly discharged after exposure to 150 °C for 5 min, thus indicating that PTC could suppress internal short circuits. Kise et al. [28,29] prepared a new PTC cathode to trigger a protection mechanism at 140 °C. A new positive temperature coefficient (PTC) electrode was prepared simply by coating a thin layer of redox-active poly (3-butylthiophene) (P3BT) in between the electroactive Li[Ni0.5Co0.2Mn0.3]O−2 layer and the Al foil substrate to improve the safety of LIBs [30]. The effectiveness of this method is demonstrated by the fact that the P3BT-523 composite electrode hardly discharges after being exposed to 150 °C for 5 min, and the discharge voltage of the composite electrode drops sharply below the cutoff voltage at a current density of 3 °C. A novel cathode configuration was achieved by sandwiching a positive temperature coefficient (PTC) material layer between the Al foil and active cathode material. At high temperatures (>80 °C), the PTC effect was triggered in the protective layer by PVDF volume expansion, which effectively disrupted electron flow, leading to a dramatic increase in cathode resistance and, thus, preventing TR from occurring [31].
However, the distance between the PTC thermistor and internal hot spot of the battery results in a response delay of the PTC [32]. The coating method will increase the redundancy of the internal system of the battery, and the coating is prone to failure in the event of battery puncture. In addition, the temperature at which the TR is triggered is typically low, about 120 °C. Therefore, a PTC electrode containing PTC components was used to greatly increase the resistance of the battery when the battery would overheat [33]. Feng et al. [34] designed a novel PTC electrode that shows a complete current-limiting effect until the temperature reaches 100–130 °C, which can alleviate TR in the early stage. A PTC cathode with self-activation heating protection was developed to cut off the electric current at 110 °C [35]. Zhong et al. [32] prepared PTC cathodes by mixing LiFePO4, carbon black, and PE. Their results showed that a mixed electrode could limit the current until the temperature of the PTC is over 90 °C. Li et al. [36] investigated a Ni particle mixed-polymer layer with lower electrode polarization. The electrochemical reactions can be fully suppressed to prevent advancement of TR at 90 °C. A positive-temperature-coefficient graphite anode as a TR firewall of LiCoO2/graphite LIBs (LIBs) was prepared by introducing thermally sensitive polymer microspheres (TSPMs) into the anode to improve battery safety under abusive conditions [37]. Compared with the batteries without TSPMs, under abusive conditions, including overcharge at 1 C/10 V, thermal shock from 20 to 130 °C, and external short circuit with a resistance of approximate to 80 mΩ, the batteries with TSPMs effectively mitigate TR. Other methods have been adopted to enhance the thermal stability of LIBs, such as a conductive matrix of cathode [38], which makes the entire electrode have PTC functionality. In addition, the validity of these methods can be demonstrated in other ways, such as by Reference [39].
The PTC matches the current and voltage of the individual cells before embedding. The PTC thermistor reacts quickly to avoid electrochemical reactions in the event of an external short circuit or overcurrent. Therefore, the PTC thermistor is effective at the single-cell level. Nevertheless, in commerce, batteries are connected in series, parallel, series–parallel, and more complex ways in the quest for greater capacity and voltage [40]. For the battery pack, the PTC thermistor of each battery in this pack may not trip simultaneously in the event of an external short circuit and excessive temperature. The first PTC tripped in the battery pack may undergo the large short-circuit voltage drop that exceeds its withstanding voltage [41]. A failure of PTC may result in ignition and TR, as confirmed by evidence that a PTC in a 14-cell series package was ignited [42].
Obviously, when short circuits and overcurrent occur inside the battery, batteries with a PTC mechanism are beneficial for suppressing further development of the TR. Moreover, the effectiveness of these methods in suppressing TR is achieved by comparing the changes in electrode current, resistance, and temperature before and after use. A more novel approach is to confirm the effectiveness of protection by analyzing the battery components before and after a short circuit.
According to the previous analysis, the design of future PTC electrodes tends to introduce PTC function inside the electrodes, which can not only reduce system redundancy but also ensure battery electrical performance. Secondly, it is necessary to ensure fast response of the fit, and, finally, the problem of not being able to be used in module-level battery systems needs to be solved.
2.2. Current Interrupt Devices (CIDs)
Current interrupt devices are a protective device that cuts off the electrical connection of equipment when the internal pressure or current exceeds its predetermined level [43]. Thus, CIDs can be roughly classified into pressure-responsive CIDs and temperature-responsive CIDs.
2.2.1. Pressure-Responsive CIDs
A pressure-responsive CID structure in cylindrical cells was triggered during battery expansion, as shown in Figure 3 [44]. However, CID cylindrical LIBs can permanently damage a battery once it is triggered, and a CID can disrupt the electrical connection when the pressure exceeds the threshold to protect the battery from TR during overcharge. Li et al. [34]. tested two types of CID activation pressures (K2 18650E and LG M36) using simulation and experimental methods, and their research indicated that both types of CID could be activated at 100 °C, with activation pressures of 1.781 ± 0.355 and 1.799 ± 0.284 MPa, respectively.
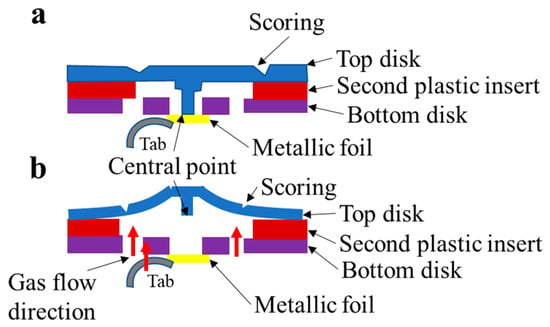
Figure 3.
An example of a pressure-responsive CID structure in cylindrical cells. (a) normal state (b) working state.
Unfortunately, high-voltage (>50 V) CID could generate arc under overcharge conditions, and contact surface might be erosion or ablation by high current density. Sparks and heat generated by the arc will ignite combustible gas and the battery electrolyte, which result in TR in the battery [45]. At present, there is relatively little research on the impact of CID on TR of LIBs. And the use of CID inevitably increases battery internal resistance and reduces energy density, so the top of most 18650 LIBs is both a CID and a safety vent. Future research on the impact of CID on TR should be more in-depth and comprehensive.
2.2.2. Temperature-Responsive CIDs
An obvious signal of TR is temperature. Internal short circuits may lead to TR, which attributes to the internal materials of LIBs being prone to decomposition under high temperatures. Thermal fuses and shape memory alloy will cut off the current to prevent internal short-circuiting.
The internal current was disconnected by thermal fuses under overheat to decrease the risk of TR. Generally, when the temperature of LIBs is over approximately 150 °C, an internal short circuit may arise. Thermal fuses made of low-melting-point materials can cut off the internal current when the temperature of LIBs ranges from 85 °C to 120 °C.
However, the insulation distance between relative lead terminals was cut down by thermal fuses at work, thus causing insufficient insulating capacity and permanent battery failure.
Shape-memory alloy, reversibly driven by temperature or magnetic field, can undergo deformation to protect the battery at high temperatures and restore deformation at low temperatures.
2.2.3. Summary of Current Interrupt Devices
Usually, the earliest heating stage of a battery is due to an increase in the internal resistance of the battery. The heat generation induced by side reaction increases when the temperature reaches the threshold. Therefore, cutting off the current in the early stage can better prevent the occurrence of TR. Unlike PTC, CID can cut off a current at a high voltage (>50 V). The use of CID can still lead to TR, which is completed by arcing tests by contact opening and an arc behavior analysis.
Additionally, different degrees of aging seriously affect the activation time of CID; for example, the time interval between current interrupt device activation and the onset temperature of TR for cells with higher SOC was reduced by 183 s, and the maximum surface temperature increased by 34 °C [46]. Under overcharge conditions, the single fresh cells experience slower activation of the current interrupt device (CID) compared to the aged cells, the cathode displayed severe degradation in spite of the CID activation, and the anode exhibited lithium plating on the edges of the electrode. At the module level, the fresh module experiences fire, while the aged module shows sequential CID activation with no TR [47]. A method based on high-temperature superconducting (HTS) current-limiting technology to improve the impact of overcurrent on batteries was designed [48]. In normal operation, the thin film is in the superconducting state, and its influence on the system can be ignored. An overcurrent will be limited to be evidently lower than the action threshold of the internal protection system by the quench resistance of the thin film. Through validation experiments conducted in our laboratory, the proposed protection method operates as expected to improve the stability and reliability of the battery cells.
2.3. Safety Vent
The safety vent (Figure 2) is a protective device in the battery that is made of gaskets with perforated film and spikes, which are located on the positive-end cover of 18650-type LIBs [49]. Gas accumulation during TR increases the internal pressure of the battery [50]. If the pressure cannot be discharged reasonably, it may cause fire and explode [51,52]. The LFP battery with oval safety valve has the lowest TR hazard, as shown by the TR hazard assessment model based on the gray/fuzzy/analytic hierarchy process [53]. However, the vents are not always effective; they have led to more serious TR hazards due to their failure, as seen with the Sony VCT5 18650 Li-ion battery and Ampking 20700 LIBs [54]. Computed tomography (CT) scanning was used to investigate the internal structure of the battery to assess the failure mechanisms of the failed vents. The results show that the failed vents caused the cylindrical casing (can) to rupture during TR.
Many studies have proven that the characteristics of the safety vent (location, quantity, size, production process, lifecycle, etc.) enormously impact the reliability and safety of battery [55]. The presence of a safety vent can result in the uneven distribution of ohmic resistance within the battery [56], and it can intensify the heating of the battery [57]. Meanwhile, Ouyang et al. [58] evaluated the impact of safety ventilation on the thermal characteristics of batteries with different states of charge (SOCs) and cathode chemical properties by measuring the rate of temperature rise and mass loss. The following results were obtained: (i) Batteries with safety vents reduce temperature-rise rates, and they delay ignition and explosion. (ii) The mass losses of the TR process were 10.2 g (21.3%) and 8.5 g (18.7%), corresponding to the battery in the presence/absence of a safety vent. (iii) The battery, in the absence of a safety vent, may experience TR at lower temperatures. (iv) The battery without a safety vent shows more serious hazard during TR. The TR behaviors of a battery without a safety vent evolve quickly, preventing side reactions and heat release.
Furthermore, the location of the safety vent plays a significant role in the LIBs, as it may delay the occurrence of TR and minimize the hazard of TR. Compared to bottom rupture, a side-wall rupture results in a temperature rise to the adjacent cell in a battery package [59]. An annular-shaped break groove with break-aiding was designed to promote the rapid rupture of the safety vent to avoid press accumulation [60]. However, uneven press may impact the emission of gas. A symmetrical safety vent design was employed to resolve uneven press.
A double safety vent in a battery can encourage ventilation efficiency to prevent explosion during TR. A novel 18650 cell design with a second vent at the base was evaluated by ultra-high-speed synchrotron X-ray imaging (>20,000 frames per second), which is shown to avoid the critical stages that lead to rupture [26].
In general, a safety vent can effectively prevent the rupture and explosion of a battery, and it can be found to impact the thermal features of a battery to a certain extent [61]. Adding to the number of safety vents can reduce the thermal impact of battery rupture, significantly improve the thermal safety of the battery, and effectively inhibit the occurrence of TR [62]. In conventional manufacturing, the cap plate and the safety vent are fabricated separately and subsequently welded to each other. In the future, a manufacturing process including a backward extrusion and coining process is suggested to produce an integral safety vent which also has the benefit of increasing production efficiency [63].
3. Safety Materials for Mitigating TR
The thermal energy generated by the battery TR comes from the joule heat of battery short circuit and the electrochemical reaction between the materials inside the battery. Simulations and experiments have been conducted to research the features of TR in LIBs. The main side reactions of TR include the SEI membrane decomposition, the reaction between anode and electrolyte, the cathode decomposition, the reaction of the binder, and the reaction of the electrolyte. The material of internal components in a battery, to some extent, determines the electrochemical performance and safety performance of lithium batteries. Gao et al. [64] compared the thermal safety and electrochemical performance of graphite anode with commercial conductive polymer poly (3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT: PSS) and standard polyvinylidene fluoride (PVDF) adhesive, and thermal analysis with differential scanning calorimetry (DSC) was used to elucidate its effectiveness in suppressing TR. Compared to PVDF, PEDOT: PSS adhesive reduces the heat generated by solid electrolyte interface (SEI) decomposition between 100 and 150 °C, especially when excluding carbon black (CB) additives. PVDF/CB, PEDOT: PPS, and PEDOT: PSS/CB composite adhesives produce 143, 37.5, and 102 J g−1, respectively. Therefore, they enhance the safety of the battery’s internal components through material design, alleviate the further development of TR, and avoid greater hazard of TR [65].
3.1. Improving Anode Materials
Graphite is widely used in the market as a positive electrode material for batteries due to its high capacity (372 mAh·g−1), large output voltage, and low flat working potential (0.1 V vs. Li+/Li). However, the slow dynamics of Li leads to the formation of dendrites, thus easily causing TR in the battery [66,67]. The decomposition of the solid electrolyte interface (SEI) formed on the surface of graphite occurs at 90–120 °C and may also occur at the relatively low temperature of 69 °C [68], leading to continuous exothermic reaction between lithified graphite and the electrolyte [69], thus promoting TR. In order to avoid the growth of dendrites, a nano carbon coating was used as the surface coating of the anode [70]. After cyclic testing of carbon-coated electrodes, the charge transfer resistance significantly decreased, establishing a more stable solid electrolyte (SEI) layer.
Many approaches were employed to enhance the thermal stability of anodes to avoid TR, for example, material modification, surface coating, and composite materials [71,72]. Another effective suppression method was adopted to prevent TR. Adding thermally responsive polymer microspheres to the anode material will reduce ion conduction when the polymer layer melts and overheats. The use of additives in electrode composites enhances the temperature stability of the SEI layer built on the surface of the anode. Surface modification of electrode materials can help improve the capacity and thermal safety of LIBs. For example, Mo et al. [73] coated the anode material with polyaniline, and the capacity of the composite material remained 83.2% after 80 cycles at 60 °C. Polyimide formed by polymerization of phthalic anhydride was used as an anode material for LIBs, and its thermal properties were compared with graphite anode materials. The effectiveness of differential scanning calorimetry (DSC) in suppressing TR was demonstrated. The results showed that the heat release of polyimide and graphite was 242 J g−1 and 658 J g−1, respectively. Polymer adhesives can affect the thermal stability and deformation of spherical graphite used in the anode of LIBs [74]. Compared with PVDF and CMC/SBR adhesives, PAA adhesive effectively suppresses heat release at low temperatures up to 200 °C (43% (PVDF) and 23% (CMC/SBR) with less heat).
Furthermore, transition metals (MxOy, M = Fe, Co, Ni, Mn, Mo, Cr, Nb, etc.) can provide about 2–3-times-higher reversible capacity than conventional graphite and are eco-friendly, corrosion resistant, and economical as future anode materials [75]. Liu et al. [76] investigated the safety associated with LIBs consisting of LiFePO4 cathodes, and SiO2 anodes, which exhibited a 93.6% retention of discharge capacity at the 1500th cycle. Meanwhile, the nail temperature slightly increased with the maximum change during the nail penetration of only 2 °C. Also, no fire or even smoke was observed, suggesting that the cell is sufficiently safe without any TR. This was attributed to the formation of a SiO2 matrix on the anode surface, which prevents any side reactions and cuts off the internal current path during pin pricking. A similar study indicated that the silicon nanowire anode can bear the mechanical load to prevent the battery from TR under the condition of mechanical extrusion. Metal oxide composites can radically inhibit the occurrence of TR.
The synthesis and research of new substitute elements can enhance the thermal safety of materials (such as titanium niobium oxide, germanium-based materials, etc.) [77]. These modification strategies are usually studied on the materials themselves, such as particle size reduction, element doping, oxygen vacancy introduction, composite conductive phases, and surface coatings [78]. These technologies optimize the safety and electrochemical performance of anode materials by adjusting their microstructure and surface properties. However, these strategies did not take into account the impact relationship between materials and other component materials, resulting in deficiencies in the thermal safety, battery capacity, and economy of anode materials.
Currently, more methods are being used to enhance anode materials. Compared with existing reviews, more and more methods are becoming more systematic in addressing the thermal safety issues of materials through innovative technological means. For example, high-entropy oxide (HEO) is a novel single-phase material composed of multiple equimolar or quasi-equimolar main elements. Due to design flexibility and the interaction between multiple functional components, HEO can exhibit improved overall performance as a LIB anode [79]. Chemical vapor deposition (CVD) is used to co-pyrolyze silane and gaseous hydrocarbons into porous carbon scaffolds. These anodes demonstrate promising performance and improved economic feasibility [80]. Due to the fact that metal organic framework (MOF)-derived materials can inherit the advantages of MOFs, metal phosphides derived from MOFs have been widely used as anodes for LIBs and exhibit excellent electrochemical performance. The derivation strategy of MOFs can be used to adjust and optimize the composition and structure of metal phosphide composite materials, such as constructing rich heterojunction structures or designing stable carbon skeleton structures [81]. These modifications effectively improve the electronic structure and structural stability of metal phosphide composite materials. This writing can effectively enhance the stability of the battery. However, this writing method is still in the laboratory stage, and there are no data indicating its effectiveness in suppressing TR.
In summary, experimental and thermodynamic tests have shown that the modification of anode materials can effectively prevent the occurrence of TR in batteries, mainly manifested in reducing electrode heat generation and deformation, preventing dendrite growth, and reducing reactions with the electrolyte. Specifically, silicon-based anodes can effectively delay TR and reduce TR damage. However, chemical crosstalk can still lead to TR in the battery. It is suggested that the future anode design should have a better match with the battery electrolyte, mainly reflected in reducing the electrochemical reaction between the two and increasing thermal stability.
3.2. Cathode Material Modification
The cathode material undergoes continuous reversible embedding/de-embedding of ions during fast charging/discharging, which indicates that the energy and power of the battery is largely determined by the cathode material (fast-charging cathode materials for Li-ion and sodium-ion batteries). The safety of cathode materials is crucial to the electrochemical performance and safety of batteries, attributed to the earlier occurrence of the reaction between the cathode and electrolyte compared to the decomposition reaction of the anode during TR. Thermal stability can be enhanced by cutting off the chain reaction between cathode and electrolyte through element substitution and protective coating to mitigate TR in the battery [82].
Element substitution is conducive to stabilizing the crystal structure to optimize the stability of layered oxides. Cationic metals such as Co, Mn, and Mg partially replace Ni or Mn in LiNiO2 or Li1.05Mn1.95O4, thereby improving thermal performance. For nickel-rich NMC and NCA, the formation of H3 phase occurs at high voltage. In addition, the H2-to-H3 phase transition in LiNiO2 is accompanied by anisotropic lattice changes along the c-axis, resulting in significant volume changes (9%). Conversely, fully delithiated FePO4 has the same skeleton as LiFePO4, providing stability during continuous lithiation/delithiation processes [83]. When the charging voltage is higher than 4.2 V vs. Li+/Li (>0.75 lithium insertion), microcracks will be induced in LiNiO2 particles. This phase transition is the main factor causing capacity degradation and safety issues in nickel-rich cathode materials. Therefore, cations such as Co3+, Mn4+, and Al3+ are used instead of Ni to extend the cycle life, reduce initial capacity loss, and improve thermal stability and storage performance. A higher upper cut-off potential and higher nickel content can lower the starting temperature of SHR (dT/dT > 20 °C min−1), meaning that the thermal stability of NMC is strongly influenced by the upper cut-off voltage and nickel content. This is attributed to the fact that an increase in Ni content can accelerate the release of oxygen and reduce the starting temperature of phase transition (from layered (R-3m) to disordered spinel (LiMn2O4 type, Fd-3m) structure) during heating. Liu et al. [84] doped Ni and Mn into LiNiO2 to increase the TR decomposition temperature from 220 °C to 310 °C, wherein Mn-rich composition maintains better cycle life and thermal safety. Zhou et al. [85] and Cho et al. [86] partially replaced Co in Li (Ni1/3Mn1/3Co1/3) O2 with Ni and Al, and the results showed that Al doping can enhance the thermal stability of the cathode. Adding flame-retardant additives can improve the thermal safety of the cathode. Chen et al. [87]. added nanoscale magnesium hydroxide to the LiNi0.5Co0.2Mn0.3O2 (NCM523) cathode, effectively interrupting the TR process of the battery. The result obtained by the safety assessment (includes nail penetration, impact, and overcharge tests) was that the TR temperature of the battery increased from 120 °C to 176 °C. It is worth noting that not all additives can increase the thermal stability of the cathode, for example, VC additives have a negative impact on the thermal performance and safety characteristics of the cathode in LIBs [88].
Protective coatings can protect the cathode surface from direct contact with the electrolyte, prevent side reactions and phase transitions, and reduce the disorder of cations in crystal sites [89]. Inorganic film (MgO, Mg2TiO4, ZnO, ZrO2, ZrFx, ZrP2O7, Li2ZrO3, SiO2, SnO2, TiO2, TiP2O7, NaAlO2, Al2O3, AlPO4, AlF3, FeF3, etc.), organic film, and protective film can enhance the inertness of the cathode, making the battery have good thermal stability [90,91]. The TiO2 coating was used to increase the stability of the charge-transfer resistance for a LiNi0.5Co0.2Mn0.3O2 cathode [92]. Compared to unused, the triggering temperature of TR in batteries with TiO2-coated cathode materials is 257 °C. (The triggering temperature for TR without TiO2 coating is 251 °C.) Fluoride coatings have excellent thermal stability due to their excellent inertness, wherein AlF3 coating delayed the initial TR temperature of NCM by 20 °C [93]. Solid oxides have a better trend in reducing heat generation. Cho et al. [94] coated a nickel-rich LiNi0.6Co0.2Mn0.2O2 cathode material with SiO2, resulting in a 35% reduction in heat generation of the cathode material and reduced degradation of the active core material. Chen et al. [95] coated on LiNi0.6Co0.2Mn0.2O2 powder with nano Al2O3 particles via ultrasonic coating. During cyclic testing, it was found that the Al2O3 coating significantly reduced the impedance of LiNi0.6Co0.2Mn0.2O2. The excellent thermal stability of phosphate conversion coating is due to the strong P-O bond in the crystal structure, which can reduce the release of oxygen during TR. Lee [96] applied Li3PO4 surface coating on nickel-rich LiNi0.6Co0.2Mn0.2O2 (NCM) material via the citric acid assisted sol–gel method. The results indicated that the Li3PO4 surface coating not only prevented interfacial reactions between the cathode and electrolyte, but also suppressed the phase transition of charged NCMs at high temperatures.
The existing review covers various types of organic materials, organic sulfur compounds, organic radical compounds, organic carbonyl compounds, conductive polymers, and imine compounds. And the advantages, challenges, and sustainable development of this field were studied, emphasizing the potential of organic cathode materials to achieve higher energy density, improve cycling stability, and contribute to environmental sustainability [97]. The comprehensive analysis of organic cathode materials provides insights into their electrochemical performance, electrode reaction mechanism, and design strategies, such as molecular structure modification, hybridization with inorganic components, porous structure, conductive additives, electrolyte optimization, binder selection, and electrode structure, to improve their efficiency and performance. In addition, future research in the field of organic cathode materials should focus on addressing current limitations, such as low energy density, cycle stability, poor discharge capacity, potential safety issues, and improving their performance. For example, high-nickel layered cathode materials such as LiNiO2 and LiNi1-x-yCoxMnyO2 (NCM) have higher specific capacity and lower cost than traditional materials such as LiCoO2 [98]. However, they face significant challenges, including cation mixing, oxygen evolution, transition metal ion dissolution, and structural instability during cycling processes [99]. This article discusses various modification strategies, including innovative synthesis methods, element doping, surface coating, single-crystal design, and concentration gradient structures, to improve the stability and performance of high-nickel cathodes. Therefore, it is necessary to improve structural stability, conductivity, cycle life, and capacity decay; explore new redox-active organic compounds; and pave the way for the next generation of high-performance energy storage devices.
Surface modification of cathode materials is one of the promising strategies to overcome these limitations. This method reduces the negative impact of cathode electrolyte interaction, enhances the transport of lithium ions, and improves the thermal stability of the material. This article discusses modern methods for surface modification of cathode materials, their impact on key battery performance parameters, and the prospects for further research.
Surface modification of cathode materials is one of the promising strategies to overcome these limitations. This method reduces the negative impact of cathode–electrolyte interaction, enhances the transport of lithium ions, and improves the thermal stability of the material. Furthermore, physical insulation is used to slow down the reaction between the cathode and electrolyte. The BaZrO3 thermal barrier with ultra-low thermal conductivity effectively hinders the rapid heat exchange between the electrode and electrolyte, reducing severe surface side reactions [100]. Although using physical methods can suppress surface side reactions, it still cannot completely interrupt the progress of side reactions, and under extreme conditions, it can still cause TR in the battery. The selection of cathode materials requires a balance between electrochemical performance and safety performance. In the future, cathode materials need to reduce heat generation and reaction with electrolytes to alleviate TR.
3.3. Safety Electrolytes
Traditional electrolytes are composed of lithium salts (LiPF6, LiBF4 [60], LiSO3CF3, etc.) and organic solvents (dimethyl carbonate (DMC), methyl ethyl carbonate (EMC) or diethyl carbonate (DEC), ethyl carbonate (EC), methyl carbonate (EMC), and propylene carbonate [101]). Electrolytes provide pathways for ion transport during battery operation. However, organic solvents exhibit poor thermal performance at low temperatures; for example, the flash points of DMC, EMC, and EMC are 15 °C, 22 °C, and 33 °C, respectively [102]. The high temperature of TR will cause the electrolyte to decompose into flammable gas and toxic gas (HF, POF5 and POF3). Electrolytes can also react with the cathode to produce oxygen to promote the development of TR, and can lead to fires and explosions. Moreover, electrolytes can also affect the SEI characteristics of the anode and cathode surfaces, and can easily lead to dendrites. Dendrites are one of the main culprits causing internal short circuits.
Generally, a flame-retardant (FR) additive was used to improve the thermal stability of electrolyte and electrochemical stability with electrodes, which can reduce the risk of TR [103]. Organophosphorus compounds or halogenated compounds are selected as FR additives. Halogens are prone to environmental pollution, so organophosphorus compounds are more promising for application. Phosphorus-containing radicals such as *PO and *PO2 can block the combustion-essential radicals *H and *OH radicals, which can decrease the generation of heat [104]. Organophosphorus chemistry was used to simultaneously combat the safety and related issues of LMB. Moreover, 15wt% DMMP achieves strong flame retardancy without affecting electrochemical performance (set value: ~1–2 s). In addition, it can also generate stable CEI [105]. Lei et al. [106]. introduced two flame retardants with high vapor pressure and low vapor pressure into carbonate electrolytes. And under strict testing conditions, only smoke was released, and the maximum temperature of TR decreased by 300 °C. The evaluation results of thermal abuse indicate that the start time of TR is delayed by 86 s. Wu et al. [107] tested the effectiveness of electrolytes containing flame retardant additives in alleviating TR using differential scanning calorimetry and thermogravimetric analysis. According to the results of differential scanning calorimetry, the total calorific value decreases sharply with the increase in TTFP concentration. The enthalpy of heat release decreased from 608.58 to 257.58 J g−1.The results showed that adding TTPP to the electrolyte effectively delayed the exothermic peak, reduced heat, improved the liquid flash point, and shortened the self-extinguishing time. Then, a series of thermodynamic models were used to evaluate the thermal stability of lithiated anodes containing various electrolytes [108]. The results showed that the activation energy of silicon-based lithiation anode increased from 68.46 kJ/mol to 91.32 kJ/mol.
Although phosphate ester additives have satisfactory flame retardancy, they seriously affect the electrochemical performance of batteries. Therefore, researchers use the following methods to balance electrical and thermal performance: (i) The FR is confined in the diaphragm, and the temperature-responsive triphenyl phosphate (TPP) can be released into the electrolyte at 160 °C (the melting point of TPP) to achieve the function of flame retardant [109]. A 5wt% TPP additive significantly reduces the flammability of the electrolyte, but it does not negatively affect the cycling performance of the battery. (ii) Electrolytes and electrodes were utilized to form a stable SEI layer and prevent the formation of dendrites. Xue et al. [110] used lithium bis(fluorosulfonyl)imide group (L.FSI) and ethyl fluorocarbonate (FEC) as additives, which can form dense and uniform LiF-rich stable SEI on lithium metal anode and inhibit the formation of lithium dendrites. (iii) The formation of composite electrolytes through the co-blending of solvents, salts, and additives, and the synergistic effect between two or more additives have the potential to enhance thermal safety. And the increase in salt concentration contributes to the formation of organic-rich solid electrolyte interfaces (SEIs) with better stability [111].
Ionic liquids are composed of anions and cations and have properties such as high melting point, low vapor pressure, non-volatility, and non-flammability. These properties can be used as a substitute for electrolytes [112]. The addition of 1-ethyl-3-methylimidazole and trifluoromethanesulfonimide to electrolytes exhibited excellent properties of flame retardant [113]. Ionic liquids have drawbacks, such as increasing the viscosity of electrolytes, reducing ionic conductivity, and high cost, making them difficult to widely apply in commercial applications in the future [114]. However, the positive effects of ionic liquids on reducing the flammability of electrolytes in contact with charged electrodes and improving the thermal stability of electrolytes have been demonstrated through self-extinguishing time testing and differential scanning calorimetry, respectively [115].
Polymer electrolytes (solid polymer electrolytes and gel polymer electrolytes) are used to replace conventional electrolytes due to their light weight, film formation, strong viscoelasticity, and high stability, and avoid the risk of electrolyte leakage [116]. In addition, solid electrolytes are excellent in inhibiting dendrite growth. For example, Zhang et al. [117] obtained multimeric electrolytes via in situ stochastic polymerization of electrolyte solution precursors containing VC and EAVE to block ion in case of overheating. Liu et al. [118] used lithium sulfonate (SSEBS-i) as a solid electrolyte, and constant current cycling demonstrated its excellent lithium retardation ability and inhibition of lithium dendrite growth. Dong et al. [119] prepared a novel intelligent polymer electrolyte by via situ polymerization of methyl methacrylate and anhydride monomers in a nitrile-based anionic deep eutectic solvent. Impressively, through accelerated calorimeter testing, this electrolyte did not experience TR even at temperatures as high as 250 °C. Despite their outstanding safety performance, the lower ionic conductivity (10−8~10−5 s/cm) at room temperature, poor cycling performance, and low voltage operation limit the application of solid electrolytes. Gel electrolytes are a compromise solution to enhance the conductivity of solid electrolytes while reducing the leakage of liquid electrolytes [57]. Finegan et al. [120] prepared a hybrid gel polymer electrolyte of poly (vinylidene fluoride) and ethylene oxide and obtained an excellent ionic conductivity of 4.8 × 10−5 s/cm at room temperature. Zhu et al. [121] developed an in situ crosslinking method using a four-armed crosslinker via cationic ring-opening polymerization (CROP) and prepared crosslinked gel polymer electrolytes (C-GPEs). The cells can provide the best cycle performance and high non-flammability (300 cycles, 80% retention) of (cationic open-loop polymerization) CROP-based cells to date. Man et al. [122] designed a smart adaptive gel polymer electrolyte (GPE) based on a novel copolymer with functional groups that can help lithium-ion migration through the synergistic effect of side groups. It not only meets the cycling performance of the battery at room temperature, but also achieves thermal shutdown function and flame retardation at high temperature. Wang et al. [123] prepared ultra-thin solid polymer electrolytes (SPEs) with reduced phonon scattering. The results indicate that the introduction of EMIM:DCA successfully breaks the random intermolecular attraction of the PU polymer chain and significantly decreases phonon scattering to enhance the internal thermal conductivity of the polymer. Thus, the thermal conductivity of the as-obtained SPEs increases by approximately six times, and the TR of the battery is effectively inhibited.
Modified electrolytes such as lithium salts, solvents, and additives have been widely studied. Even though these modification methods can enhance the safety of lithium batteries to some extent, there is still a lot of work to be performed, such as the development of all solid-state lithium-ion batteries (ASSLBs) based on halide solid-state electrolytes (SSEs), which show potential for application in fixed energy storage devices and may eventually become an important component of future smart grids [124]. However, it is necessary to explore the performance of halide SSEs with different halide anions in terms of ionic conductivity, activation energy, electronic conductivity, interface contact stability, and electrochemical window, and to develop corresponding optimization strategies for each of the abovementioned electrochemical indicators.
The new electrolyte design scheme will take into account the thermal runaway behavior in LIBs, including high temperature, jetting, combustion, explosion, and release of toxic gases, as well as the propagation of thermal faults in battery packs [125]. Water-based lithium-ion batteries (ALIBs) utilize the advantages of water as a solvent, providing inherent safety, high ion conductivity, cost-effectiveness, and environmental sustainability, making them promising candidates for large-scale energy storage applications [126]. However, three key challenges greatly hinder their practical application: the limited electrochemical stability window of water, the inevitable hydrogen evolution reaction (HER), and the significant corrosion of metal current collectors in aquatic environments.
In the future, the design of electrolytes will not only satisfy the cycling performance of the battery at room temperature; it will also achieve thermal shutdown function and flame retardation under TR. Conventional liquid electrolytes reduce the risk of TR via several methods: adding flame-retardant additives to limit the combustion of the electrolyte; changing the interaction between the electrolyte and electrode to reduce the generation of dendrites; and using a composite electrolyte to meet the safety and electrochemical performance of the battery. Solid and gel electrolytes are important future research areas in the suppression of TR. Therefore, executing the functionalized design for electrolytes to cut off these reactions has been recognized as a critical solution to mitigate TR [127]. However, it is still important to note that the cell volume expands during the cell cycle, and optimizing the interfacial contact between electrolyte and electrode remains challenging.
3.4. Thermally Protective Separators
The separator is a porous insulating membrane that allows only lithium-ions to pass through and protects the battery by preventing physical contact between the anode and the cathode [128]. PE; PP; and their combinations, PE-PP double-layer separator and PP-PE-PP three-layer separator, are the separator materials for most commercial LIBs. The diaphragm shrinks during overheating and physical puncture, leading to an internal short circuit between the two electrodes that triggers TR and battery explosion [13]. Celgard LLC sandwiched the PE layer between two PP layers to avoid internal short-circuiting (PP/PE/PP), which is attributed to the fact that, at 135 °C, the PE partially melts, while the PP remains mechanically intact [129]. However, commercial polyolefin separators have disadvantages, such as a low temperature threshold and poor thermal stability, which can easily cause internal short circuits in batteries [130,131]. Liao et al. [132] prepared a method using papermaking, which exhibited self-extinguishing properties after ignition and had a low heat release rate (HRR) (approximately 65 W g−1) and total heat release rate (THR) (approximately 3. 2 kJ g−1) value.
The growth of lithium dendrites tends to puncture the diaphragm, leading to short circuits within the cell and triggering TR [133]. The preparation of composite diaphragms by surface coating, surface grafting fingers, and co-blending to meet the demand for thermal stability, mechanical strength, and electrical properties of diaphragms is the trend of commercial development [134,135]. Inorganic nanoparticles were introduced to coat the surface of the coated polyolefin diaphragm to achieve better thermal stability [136]. For example, coating HDPE separators with ceramic nanoparticles (NPs) results in a good, thermally stable type [137]. The results of differential scanning calorimetry indicate that no decomposition occurs at 280 °C. Reactive Al2O3 nanostructured materials were coated onto polyethylene (PE) membranes. The test results show that the thermal shrinkage of the separator coated with reactive alumina is significantly reduced at 140 °C [138]. The polyimide Al2O3 membrane developed through electrospinning technology can serve as a direct substitute for polyolefin membranes. In situ multi-mode calorimetry analysis of the entire battery showed that the heat release of an MCMB/PI-Al2O3/LiFePO4 battery during TR events was only 25 J g−1 [139]. However, the coatings are prone to flaking and separation, and coatings with inhomogeneous coating can produce inhomogeneous ion fluxes, which can accelerate the formation of lithium dendrites [140]. Lee et al. [141] fabricated the boehmite-coupled PE nanocomposite diaphragm to avoid flaking and separation of the coating and inhibit dendrite growth (puncture strength of 0.74 N m and thermal shrinkage of 3.2% at 140 °C for 30 min). A better solution is to prepare membranes through ion track etching and scraper coating, which not only promotes interface compatibility and enhances lithium-ion conduction, but also maintains good mechanical strength at 500 degrees Celsius. Engineering roll-to-roll technology can become a promising candidate in suppressing TR and improving battery safety [142].
Separators have excellent mechanical strength and thermal stability in order to avoid TR in the battery. Preparing a separator that meets multiple requirements is still a challenge. Tang et al. [143] provided ideas for the preparation of multifunctional diaphragms. A porous composite membrane based on para-aramid nanofibers (ANFs) with the introduction of polyethylene oxide (PEO) was successfully prepared by electrostatic spinning, and the cell of ANFs/PEO diaphragm showed better electrochemical performance than Celgard 2400 diaphragm. Separation via heat treatment possesses a preferable mechanical strength (tensile strength reaches 41.52 MPa) and remarkable thermal stability (almost no thermal contraction at 200 °C for 1 h). In the future, high porosity, good flexibility, excellent thermal stability, and excellent electrolyte wettability are the basic characteristics of high-performance membranes. An effective preparation method is referred to as the polyimide air gel diaphragm prepared based on molecular structure design, which has a high TR temperature (204.9 °C vs. 140.4 °C) and a low maximum temperature rise (0.06 °C min−1 vs. 8 °C min−1) [144].
4. Thermal Management Method for Mitigating TR
For commercial applications, a large number of batteries need to be stacked in series/parallel/series–parallel in a confined space. For example, Tesla offers a battery pack consisting of 7104 cylindrical cells, although the built-in PTC and CID can effectively prevent the TR of a single cell. However, NASA studies have shown the failure of PTC and CID to mitigate TR in multi-cell configurations [41], which can cause the temperature of the surrounding battery to rise, triggering the propagation of TR or even cause widespread fires and explosions. This is catastrophic for commercial applications. Therefore, it is necessary to mitigate TR disasters from the module level, as well as to prevent TR propagation. Not only that, but the uneven temperature distribution among LIB cells can lead to inconsistency in the internal resistance of LIB cells, which can easily trigger heat build-up. The temperature control of the battery pack is within a reasonable range for commercial applications. The temperature of the battery pack needs to be controlled within a reasonable range for commercial applications. The LIB can operate between −20 °C and 60 °C [145], while the operating temperature to maintain optimal performance is between 20 °C and 40 °C [146]. In addition, the temperature difference in the battery pack is preferably kept at 5 °C [147]. This subsection provides an overview of the method to suppress TR from a thermal-management perspective, including air-cooled thermal management, liquid-cooled thermal management, heat-pipe thermal management, phase-change-material thermal management, and coupled thermal management. Although immersion cooling has a better cooling effect, its application in TR is not found, so this section does not discuss the immersion-cooling strategy; for thermal-management aspects, the reader can refer to the literature [148].
4.1. Air-Cooled Thermal Management
Air-cooled thermal management has many advantages such as simple structure, light weight, easy maintenance, low cost, and easy battery replacement, and is one of the more traditional methods of battery thermal management systems (BTMSs) [148,149]. Air cooling is shown in Figure 4, e.g., Honda’s Insight electric vehicle.
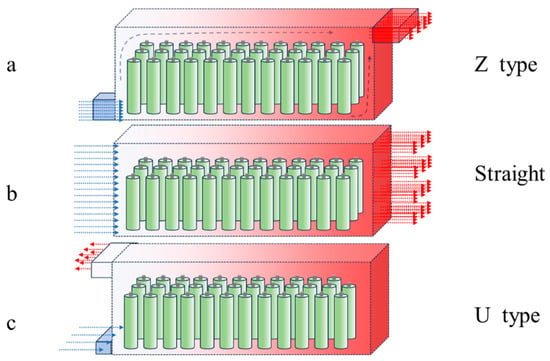
Figure 4.
The type of air cooling (a) Z-type air cooling,(b) straight type air cooling, (c) U-type air cooling.
Rami [150] used air cooling for thermal management of 18560-type batteries and found that when the battery is in a high-rate discharge situation, the temperature is difficult to control, and the increase in ambient temperature makes this problem more difficult to solve. This is attributed to the poor sealing and low heat-dissipation efficiency of natural convection cooling, which is easily affected by environmental factors. Naming Yang et al. [151] designed a forced cooling of air thermal management system for cylindrical battery cooling. It was found that the arrangement of the cells affects the airflow lines, resulting in a large temperature difference within the pack and reducing the cooling effect. Lopez et al. [152] used numerical simulations to study the cooling effect of the battery under the condition of forced-air cooling with gaps left between the cells as air ducts, and the results showed that the cooling effect of the battery is related to the ventilation method, the shape of the air duct, the fan spacing, and the air volume size. Increasing the inter-cell spacing in the module and proper lug configuration can have a significant impact on TR propagation [153]. Subsequently, Daniels et al. [154], for varying cell arrangements (aligned, staggered, and cross) and fault positions, validated a three-dimensional numerical model of the module and used it to quantify the temperature responses of the first TR-prone cell surface sectors and the influence of different flow conditions, heat generation, and ambient temperatures on the TR initiation in terms of the rate of temperature rise and the TR onset time. The results indicate that, under laminar flow conditions, the arrangement with edge fault positions delays the initiation of TR, while under turbulent conditions, the position of corner faults delays TR. Regardless of the fault location, staggered arrangement will cause multiple TRs to start. The arranged arrangement has the most TR starting sites on the side of the first TRP cell, while the staggered and crossed arrangement has the most at the front. Forced-air cooling can significantly reduce the temperature of the entire cell module, depending on many factors, such as cell module layout, ambient temperature, cooling air-temperature flow rate, flow area, and airflow path length.
The rational design of duct parameters facilitates battery heat dissipation. Therefore, Chen et al. [155] optimized the parallel ventilation method, and the maximum temperature difference of the battery pack can be reduced by 29% by increasing the spacing around the battery where the highest temperature is located, while decreasing the spacing around the battery where the lowest temperature is located for a constant heat production rate of the battery. Optimization of the duct shape can enhance cooling effectiveness [156]. However, forced-air cooling performs reasonably well in controlling the peak temperature and temperature uniformity of NiMH battery systems that generate less heat, but it is not ideal for dissipating heat from Li-ion batteries with higher heat densities. Estimates indicate that air cooling can handle normal EV driving conditions if the heat discharged from the battery pack is below approximately 4 kW [157]. Forced-air cooling is limited in suppressing the temperature rise of lithium batteries [158]. The air cooling approach has a lower heat capacity, and the air pump provides a higher volume flow rate to meet the cooling demand of the battery. This results in more parasitic power generated by the system. On the other hand, forced-air cooling is difficult to suppress the propagation of TR when the battery undergoes TR. Forced-air cooling may also deliver more oxygen in the event of an open fire, promoting flame combustion and exacerbating the propagation of TR.
4.2. PCM Thermal Management
Phase change materials (PCMs) absorb heat while undergoing a phase change without changing temperature. The appreciable latent heat of phase change can regulate the cell temperature and slow down the sharp rise in temperature during TR [159]. Therefore, it is used for battery thermal management to mitigate the temperature rise during TR in LIBs. Javani et al. [160] performed numerical simulations for electric vehicles with and without phase change materials and showed that the maximum battery temperature was reduced by 7.8 °C, and the battery temperature distribution was more uniform for the battery with phase change materials compared to the heat sink system without phase change materials. Stephen Wilke et al. [161] demonstrated the effectiveness of phase change composites in preventing propagation when TR occurs in LIBs. The results showed that when the lithium battery was subjected to pinch short-circuit experiments, the TR propagated completely in the battery pack without the phase change composite, while it failed to propagate in the battery pack with the phase change composite. Similar studies are as follows: by S. Ahmadi Atouei et al. [162]. The use of phase change materials not only provides good heat dissipation, but also controls the temperature rise without the need for an additional heat Sink. Kizille et al. [163] demonstrated the effectiveness of using PCM in 18650 battery modules (as in Figure 5a) by numerical simulations. The PCM-filled battery module exhibited a more uniform temperature distribution, while the PCM effectively suppressed the propagation of TR. Thus, battery fires and explosions were avoided (Figure 5d,e).
Different types of phase change materials have been developed, including organic (e.g., paraffins, fatty acids, and polyols), inorganic (salt hydrates, and metals and their alloys), and eutectic phase change materials [164]. For example, paraffins have a higher specific heat capacity (2140 to 2900 J/kg·K) than most submerged cooling and are therefore preferred as phase change materials. According to the form of phase change, they can be divided into solid–solid phase change material (SSPCM), solid–liquid phase change material (SLPCM), solid–gas phase change material (SGPCM), and liquid–gas phase change metal (LGPCM).
Phase change materials can mitigate TR onset and inhibit TR propagation by absorbing heat. Dai et al. [165] used paraffin wax as a phase change material to mitigate battery TR and showed that paraffin wax delayed the onset of TR by 277 s and used its heat absorption to reduce the battery temperature. In another work by Dai et al. [166], the inhibitory effect of non-combustible EPCM/CNT on TR propagation was studied. TR propagation tests showed that the triggering times of the three batteries were successfully delayed by 129,474 and 551 s, respectively, and the propagation interval was greatly extended. The low thermal conductivity and diffusivity are not conducive to heat exchange between the phase change material and the environment and may further worsen the TR propagation [167]. For example, when Ouyang et al. [168] used PCMs with low thermal conductivity and diffusivity, TR propagation occurred in the module. High-thermal-conductivity materials were added to improve the thermal properties of phase change materials, such as carbon fibers [168], nanomagnets [169], carbon nanotubes [169], metal foams [170,171], and expanded graphene [172,173]. The development of phase change materials for battery cooling was reviewed by Lucia Ianniciello et al. [174,175]. Heat transfer can be significantly enhanced by adding carbon fibers to the phase change material. Simulation results show that the composite phase change material can reduce the maximum battery-temperature rise by 45% compared to the pure phase change material. The addition of metal-based phase change materials reduces the battery system’s temperature difference by 70%. Babapoor and Karimi et al. [176,177] added graphite with a mass fraction of 16%~20% to paraffin to improve the thermal conductivity and reduce the temperature rise of the cell. Wilke et al. [166] used composite phase change materials to suppress the propagation of TR and reduced the maximum temperature by 60 °C. The thermal conductivity of the composite phase change material can be increased by adding carbon materials, and containing a higher percentage of carbon fiber phase change composites allows for a more uniform distribution of the cell. The heat build-up is avoided [178]. However, not all additions of nanomaterials are beneficial. Farid Bahiraei et al. [179,180,181] embedded three carbon-based nanomaterials in paraffin wax; although they improved the heat transfer of solid-phase paraffin wax, they also increased the dynamic viscosity of the composite and inhibited the heat transfer in the molten state. Lin et al. [182] proposed that gas–liquid phase change materials reduce the composite phase change material of the dynamic viscosity. Gas–liquid phase change can provide a lower and more uniform temperature distribution.
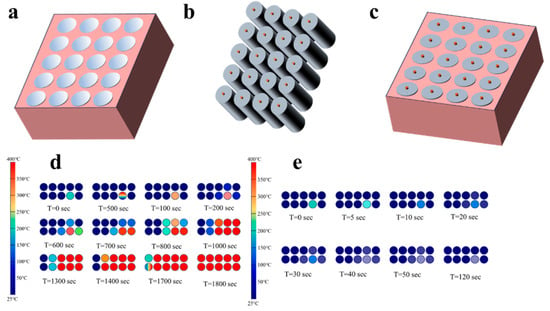
Figure 5.
(a) Schematic representation of 5S4P module configuration. (b) 5S4P battery module (c) 5S4P battery module with phase change material.Propagation of TR spreading in (d) air cooling and (e) PCM cooling [179].
Other forms of composite phase change materials also exhibit excellent cooling performance in regard to suppressing TR. Li et al. [74] prepared sodium acetate trihydrate urea/expanded graphite inorganic salt hydrate-compliant phase change materials, which underwent solid–liquid phase change at 50.3 °C, corresponding to a latent heat storage density of 181 kJ/kg, a thermal decomposition temperature of 114.0 °C, and a corresponding thermochemical storage density of 567.3 kJ/kg. The composite phase change material can meet the cooling requirement of the battery in minor accidents (e.g., external short circuit). Moreover, the two-stage thermal storage can suppress the propagation of TR caused by thermal or mechanical abuse. To prevent ignition of the cooling material by an open flame generated during TR, Weng et al. [183] combined aerogel with flame-retardant PCM to minimize thermal propagation of the TR (Figure 6). The PCM-EG prevented flame burning and reduced the peak temperature of the cell module, while the aerogel significantly delayed the cell TR.
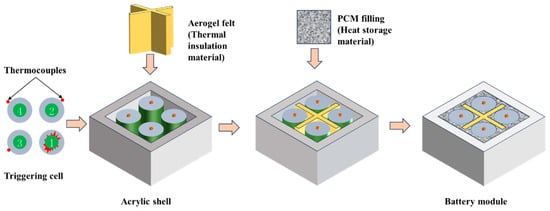
Figure 6.
Structure and assembly of designed flame retarded PA/aerogel felt battery module [176].
Phase change materials can effectively control the battery temperature rise and inhibit TR propagation via three main aspects: (i) acting as a thermal barrier to stop the conduction of heat in the battery module; (ii) absorbing the heat energy generated by TR; and (iii) effectively reducing the cell temperature difference during high-magnification operation, preventing heat accumulation, and avoiding the formation of TR conditions. The effectiveness of using phase change materials in alleviating TR and suppressing TR propagation has been proven by many methods, for example, one-dimensional heat transfer models [184], numerical studies [185], and TR propagation tests. The thermal performance of conventional phase change materials can be improved by material modification [167]. Although composite phase change-only materials are artificially considered to be future materials for slowing down thermal failure propagation, there are still some problems: for example, the combustibility of phase change materials has been studied by adding flame retardants to them and achieving good inhibitory effects [186,187]. Phase change composites (PCCs) are considered to be future materials for slowing down thermal failure propagation. Wang et al. [179] found that graphene-reinforced PCMs are not effective in stopping TR propagation, due to strong heat transfer between adjacent cells. When the latent heat of the phase change material is depleted, it leads to thermal failure. Therefore, the coupled cooling system was proposed and is presented in the following.
4.3. Coupled Cooling Systems
Pure PCM can effectively reduce the battery temperature to prevent TR and inhibit the propagation of TR in the battery. However, in extreme heat disasters, it may still lead to TR or the spread of TR when the latent heat of PCM is depleted. In order to optimize thermal management performance, reduce parasitic power consumption, and meet the heat-dissipation requirements under normal operation and TR conditions. And it can effectively alleviate TR and its spread in extreme heat disasters. Zhang et al. [188] conducted TR experiments with and without a heat source and found that, compared to active and passive sources, HTMS can use 76.7% and 100% of heat sinks and PCM latent heat capacity, respectively, while passive sources can only use 13% of PCM thermal storage capacity. Coupled cooling systems combined with different heat-dissipation methods are emerging ways to suppress TR in batteries, for example, PCM–air cooling [189,190], PCM–heat pipe [191,192], PCM–liquid cooling [193,194], heat pipe–liquid cooling [195,196], and PCM–heat pipe–air cooling systems.
Khateeb et al. [197] demonstrated for the first time the successful application of PBTM to Li-ion batteries in electric vehicles. Zhang et al. [198] studied the TR behavior of a hybrid PCM liquid cooling system. In a pure PCM system, the battery TR propagation is only delayed. And TR propagation can be successfully suppressed in the system with PCM coupled with liquid cooling. Zhang et al. [199] proposed an innovative battery thermal management system consisting of nonuniform thermally conductive phase change materials and auxiliary liquid cooling. The system can not only meet the heat-dissipation requirements under normal operating conditions, but also control the TR in the middle row and the cell temperature in the other rows below 200 °C. Wu et al. [200] conducted a similar study where the high thermal conductivity of graphite flakes was used to transfer the heat from a thermally runaway cell to a PCM when TR occurred in a single cell. The high thermal conductivity of PCM is beneficial for reducing battery temperature, but may lead to TR propagation. The TR propagation was prevented when the cell was spaced at 14 mm. Wang et al. [201] designed a PCM-coupled OHP cooling system where a single OHP system could extend the time to heat up the cell to 50 °C to 498 s, which was increased to 934 s for the PCM/OHP system.
Yin et al. [202] used PCM coupled with air cooling to demonstrate that the cooling performance of a cooling system with a composite phase change material is approximately 1.25–1.30 times that of a cooling system without a composite phase change material at constant power input of 4 to 30 W. Mehrabi Kermani et al. [203] compared air cooling, PCM/foam copper cooling, and PCM-coupled cooling systems and showed that these three methods reduced the cell surface temperature by 22.3%, 18.6%, and 40.3%, respectively, at heating powers of 5 W–40 W; in the TR test, the time required to limit the temperature to below 60 °C was extended by 38.8%, 28.3%, and 83.4%, respectively. However, PCM-coupled forced-air cooling may not meet the heat-dissipation requirements of the battery under extreme operating conditions (high discharge and overcharge) and TR. Bai et al. [204] used a PCM-coupled water-cooling plate for the cooling system of a lithium battery. The cooling-system schematic and numerical-simulation results are shown in Figure 6 and Figure 7. The system prevented TR during five charge/discharge cycles at a cooling-plate width of 5 CM.
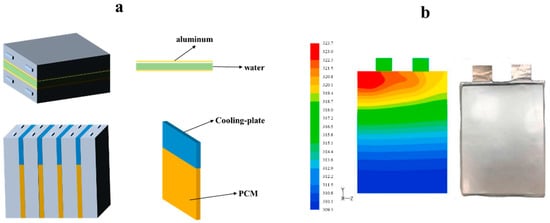
Figure 7.
(a) Schematic of lithium-ion battery module with PCM/water-cooling plate. (b) Simulated temperature cloud of pouch battery at the end of 2 C discharging [194].
A PCM-coupled cooling system can be used in the future lithium battery thermal management system to mitigate TR; it can reduce the temperature rise of the battery under normal operation, and it can also prevent the propagation of TR of the battery. PCM-coupled air cooling could not satisfy the heat-dissipation requirements under TR conditions. PCM-coupled liquid cooling or micro-channel form of cooling system can ensure the normal operation and extreme conditions of heat-dissipation requirements. In extreme conditions, heat dissipation will increase the energy consumption of the system. However, the maximum energy savings of the coupled cooling system under normal operating conditions is up to 81.80% [205].
5. Spray/Jet Method for Mitigating TR
The spray/spray method is a fire-extinguishing method that releases the extinguishing agent to the fire-extinguishing area under the action of power source drive [206]. As shown in Figure 8, this method can give full play to utilize the cooling effect of the fire-extinguishing agent and play a key role in alleviating TR. Typical fire-extinguishing agents are employed to alleviate TR of LIBs, such as Novec1230, CO2, LN, perfluorohexanone, heptafluoropropane, water, water mist, dry powder, etc. [207].
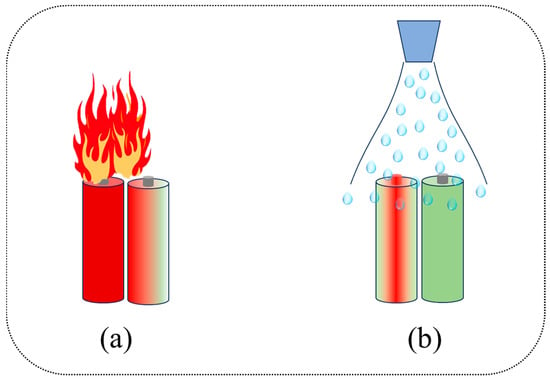
Figure 8.
(a) Without spray/jet method for mitigating TR. (b) Use of spray/spray method to alleviate TR of lithium battery.
Dry powder and halogenated alkane extinguishing agents (HFC-227ea and C6F12O) can quickly extinguish lithium battery fires, but they cannot prevent the exothermic reaction inside the battery. Halogenated alkane-based extinguishing agents are unable to inhibit TR propagation at low usage levels [208,209] and can only prevent TR propagation at low SOC (~50%) [209], attributing to the low heat capacity of the fire-extinguishing agent. Experiments by Sun et al. [210] showed that HFC-227ea, C6F12O, and water spray dissipated 24.8 kJ, 111 kJ, and 459.8 kJ, respectively, in fire-extinguishing tests. Despite the strong cooling performance of liquid nitrogen, the high price and danger restrict the development of liquid nitrogen [211]. In particular, CO2 cannot completely suppress the flame, nor can it inhibit TR propagation [212].
TR of LIBs is essentially a high-energy thermal disaster. Therefore, a significant cooling capacity can present better cooling effects. Water spraying can effectively suppress the TR of 21700 type LIBs [213]. The longer the water-spraying time is, the better the suppression of TR of LIBs [214,215]. The fine water spray has superior cooling effects compared to CO2 and HFC-227ea. Liu et al. [216] released water mist at a critical inflection point during TR development, and it successfully suppressed TR and dissipated more than 1000 kJ of heat during WM release. However, the water mist cannot stop the TR caused by continuous charging. The Leidenfrost effect reduces the contact efficiency between the water and the battery, which in turn leads to poorer cooling. The heat transfer of water was enhanced by adding water-mist additives to change the physical properties of water [217,218], such as sodium dodecyl sulfate (SDS), F-500, cetyl trimethyl ammonium bromide (CTAB), and polyoxyethylene (20) monolaurate sorbate (Tween 20). Xu et al. [219] showed that the heat dissipation of Tween 20 was 12.46 ± 0.20 kJ, an increase of 23.6% compared to the pure water test. A 3% F-500 solution has about three times the cooling capacity of WM [220].
Single water mist is susceptible to fire plume and has difficulty reaching the cell surface [221]. Wang et al. [222] designed a new additive package to promote flame extinguishment and enhance cooling performance. Zhang [213], and Liu and Duan [223] et al. used a gas extinguishing agent and water-mist synergistic extinguishing method with a better suppression effect than a single extinguishing agent.
Spray/jet cooling takes away the heat generated by the TR of the battery through forced heat exchange, which can effectively control the TR of LIBs. Rapid and effective control of TR requires a reasonable choice of extinguishing agent. The ideal extinguishing agent should have fast extinguishing speed, environmental friendliness, insulation, and high thermal conductivity. It may also need to pay attention to the use of extinguishing agents to reduce the production of toxic gases and particulate matter, reduce the adverse impact on the environment, and ensure the safety of rescue personnel. Compared to existing reviews, efficient, green, and environmentally friendly fire-prevention and -extinguishing materials are crucial for quickly extinguishing fires and minimizing the risk of reignition. Developing strategies for the protection period, temperature-rise stage, and occurrence of major and minor fires throughout the entire development process of LIB can achieve safety protection for the entire lifecycle of LIB [224]. At the same time, it is necessary to develop refined and intelligent fire-prevention technologies, combined with the characteristics of lithium-ion battery fires and the needs of different scenarios, to achieve fire protection in various scenarios, such as large, medium, small, and micro, ensuring the safe and stable operation of lithium-ion batteries in various application environments.
6. Conclusions and Future Outlook
In recent years, lithium-ion batteries (LIBs) have been extensively employed for sustainable development. However, frequent battery accidents related to thermal runaway could hinder their broader adoption. This review aims to summarize inherent methods for suppressing TR of LIBs and various mitigation methods were compared in Table 1. The results demonstrate that different approaches exhibit distinct impacts on battery thermal performance and varied inhibitory effectiveness, offering insights into the future directions of LIB safety research.

Table 1.
Summary of lithium-ion battery mitigation methods.
The material properties of crucial components of batteries directly influence battery safety, including the cathode, anode, separator, and electrolyte, and have a direct influence on safety. However, achieving a balance between electrochemical performance and safety remains a challenging task for the future. It should be noted that some material modifications may still have negative effects on electrochemical and thermal performance. Among cathode materials, NMC and LFP are still promising cathode materials. Silicon, due to its beneficial properties, has emerged as a significant anode material in current applications. At present, electrolyte additives are used to reduce the flammability of electrolytes. Solid electrolytes and gel electrolytes are the fundamental methods to improve the safety of electrolytes. Thus, future research on battery safety materials should prioritize reducing heat generation, minimizing flammability, and preventing electrochemical crosstalk, all while maintaining electrochemical performance.
Protective devices serve as a second line of defense against TR incidents by shielding battery packs from abnormal current flow. PTC thermistors, CIDs, safety vents, and protection circuitry are undergoing continuous development to minimize resistance, weight, spatial requirements, and cost, thereby enhancing ion conductivity and long-term cycling performance. Multiple vents may be more conducive to releasing internal pressure and preventing explosions in the future. For example, Sony and LG have added a bottom vent to some cylindrical cells for better venting.
The demand for enhanced battery management systems (BMSs) is expected to grow significantly with the expanding range of LIB applications. The BMS must not only satisfy the normal operating temperature of the battery but also cope with the heat-dissipation requirements under extreme conditions. This problem can be effectively solved by coupled thermal management system. However, battery safety components typically add weight and occupy additional space within the battery pack, compounded by a current lack of unified industry standards. Therefore, standardization, compactness, and effectiveness will be critical targets or the next phase of commercial BMSs.
As an emergency measure, the spray/jet method can mitigate or slow the propagation of TR when the preventive measures mentioned above have failed. The occurrence of TR can be suppressed when the spray/jet method is applied below the critical temperature. However, TR cannot be avoided when the spray/jet method is applied above the critical temperature. Even after TR initiation, spray cooling can reduce battery surface temperatures, preventing wider thermal disasters.
Although various measures have been taken to prevent TR of LIBs, it is still impossible to avoid the occurrence of TR events. Emergency measures are necessary to minimize associated risks and hazards.
Author Contributions
Conceptualization, J.D.; formal analysis, J.C.; investigation, Z.B.; writing—original draft preparation, Z.H.; writing—review and editing, Z.H. and J.C.; project administration, J.Z.; funding acquisition, J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to acknowledge financial support sponsored by the National Natural Science Foundation of China (No. 52304256), the China Postdoctoral Science Foundation (No. 2023M732823), and Key R&D program in Shaanxi Province (2023-GHZD-29).
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TR | Thermal runaway |
| LIBs | Lithium-ion batteries |
References
- Chen, L.; Fan, X.; Hu, E.; Ji, X.; Chen, J.; Hou, S.; Deng, T.; Li, J.; Su, D.; Yang, X.; et al. Achieving High Energy Density through Increasing the Output Voltage: A Highly Reversible 5.3 V Battery. Chem 2019, 5, 896–912. [Google Scholar] [CrossRef]
- Yang, Y.; McDowell, M.T.; Jackson, A.; Cha, J.J.; Hong, S.S.; Cui, Y. New nanostructured Li2S/silicon rechargeable battery with high specific energy. Nano Lett. 2010, 10, 1486–1491. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yan, C.; Chen, Y.; Si, W.; Deng, J. Three-Dimensionally “Curved” NiO Nanomembranes as Ultrahigh Rate Capability Anodes for Li-Ion Batteries with Long Cycle Lifetimes. Adv. Energy Mater. 2014, 4, 1300912. [Google Scholar] [CrossRef]
- Yang, C.-P.; Yin, Y.-X.; Zhang, S.-F.; Li, N.-W.; Guo, Y.-G. Accommodating lithium into 3D current collectors with a submicron skeleton towards long-life lithium metal anodes. Nat. Commun. 2015, 6, 8058. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of LIBs. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, Y.; Wu, Y. A comparative study on air transport safety of LIBs with different SOCs. Appl. Therm. Eng. 2020, 179, 115679. [Google Scholar] [CrossRef]
- Cao, J.; Ju, X.; Peng, Y.; Zhou, X.; Hu, Y.; Li, L.; Wang, D.; Cao, B.; Yang, L.; Peng, F. Experimental study on fire hazard of LiCoO2-based LIBs with gel electrolyte using a cone calorimeter. J. Energy Storage 2020, 32, 101884. [Google Scholar] [CrossRef]
- Agostini, M.; Brutti, S.; Navarra, M.A.; Panero, S.; Reale, P.; Matic, A.; Scrosati, B. A high-power and fast charging Li-ion battery with outstanding cycle-life. Sci. Rep. 2017, 7, 1104. [Google Scholar] [CrossRef]
- Hu, M.; Pang, X.; Zhou, Z. Recent progress in high-voltage lithium ion batteries. J. Power Sources 2013, 237, 229–242. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243. [Google Scholar] [CrossRef]
- Kim, G.Y. Challenges for Rechargeable Li Batteries†. Am. Chem. Soc. 2009, 22, 587–603. [Google Scholar]
- Hendricks, C.; Williard, N.; Mathew, S.; Pecht, M. A failure modes, mechanisms, and effects analysis (FMMEA) of LIBs. J. Power Sources 2015, 297, 113–120. [Google Scholar] [CrossRef]
- Wang, Q.; Mao, B.; Stoliarov, S.I.; Sun, J. A review of lithium-ion battery failure mechanisms and fire prevention method. Prog. Energy Combust. Sci. 2019, 73, 95–131. [Google Scholar] [CrossRef]
- Baird, A.R.; Archibald, E.J.; Marr, K.C.; Ezekoye, O.A. Explosion hazards from lithium-ion battery vent gas. J. Power Sources 2020, 446, 227257. [Google Scholar] [CrossRef]
- Quintiere, J.G. On methods to measure the energetics of a lithium ion battery in TR. Fire Saf. J. 2020, 111, 102911. [Google Scholar] [CrossRef]
- Said, A.O. Dynamics and Hazards of Cascading Failure in Lithium Ion Cell Arrays: Analysis, Passive Mitigation, and Active Suppression. Doctoral Dissertation, University of Maryland, College Park, MD, USA, 2020. [Google Scholar]
- Kasnatscheew, J.; Streipert, B.; Roeser, S.; Wagner, R.; Laskovic, I.C.; Winter, M. Determining oxidative stability of battery electrolytes: Validity of common electrochemical stability window (ESW) data and alternative method. Phys. Chem. Chem. Phys. 2017, 19, 16078–16086. [Google Scholar] [CrossRef]
- Lee, C.; Said, A.O.; Stoliarov, S.I. Passive Mitigation of TR Propagation in Dense 18650 Lithium Ion Cell Assemblies. J. Electrochem. Soc. 2020, 167, 090524. [Google Scholar] [CrossRef]
- Balakrishnan, P.G.; Ramesh, R.; Kumar, T.P. Safety mechanisms in LIBs. J. Power Sources 2006, 155, 401–414. [Google Scholar] [CrossRef]
- Fraser, R. Coupled Electrochemical-Thermal Simulations and Validation of Minichannel Cold-Plate Water-Cooled Prismatic 20 Ah LiFePO4 Battery. Electrochem 2021, 2, 643–663. [Google Scholar]
- Chitta, S.D.; Akkaldevi, C.; Jaidi, J.; Panchal, S.; Fowler, M.; Fraser, R. Comparison of lumped and 1D electrochemical models for prismatic 20Ah LiFePO4 battery sandwiched between minichannel cold-plates. Appl. Therm. Eng. 2021, 199, 117586. [Google Scholar] [CrossRef]
- Li, Q.; Park, O.K.; Lee, J.H. Positive temperature coefficient behavior of HDPE/EVA blends filled with carbon black. In Advanced Materials Research; Trans Tech Publications, Ltd.: Stafa-Zurich, Switzerland, 2008. [Google Scholar]
- Asare, E.; Evans, J.; Newton, M.; Peijs, T.; Bilotti, E. Effect of particle size and shape on positive temperature coefficient (PTC) of conductive polymer composites (CPC)—A model study. Mater. Des. 2016, 97, 459–463. [Google Scholar] [CrossRef]
- Huang, W.; Feng, X.; Han, X.; Zhang, W.; Jiang, F. Questions and Answers Relating to Lithium-Ion Battery Safety Issues. Cell Rep. Phys. Sci. 2021, 2, 100285. [Google Scholar] [CrossRef]
- Faivre, A. Glassy state and Glass transition. Matér. Tech. 2015, 103, 404. [Google Scholar] [CrossRef]
- Finegan, D.P.; Darcy, E.; Keyser, M.; Tjaden, B.; Heenan, T.M.M.; Jervis, R.; Bailey, J.J.; Vo, N.T.; Magdysyuk, O.V.; Drakopoulos, M.; et al. Identifying the Cause of Rupture of Li-Ion Batteries during TR. Adv. Sci. 2018, 5, 1700369. [Google Scholar] [CrossRef]
- Abaza, A.; Ferrari, S.; Wong, H.K.; Lyness, C.; Moore, A.; Weaving, J.; Blanco-Martin, M.; Dashwood, R.; Bhagat, R. Experimental study of internal and external short circuits of commercial automotive pouch lithium-ion cells. J. Energy Storage 2018, 16, 211–217. [Google Scholar] [CrossRef]
- Kise, M.; Yoshioka, S.; Hamano, K.; Kuriki, H.; Nishimura, T.; Urushibata, H. Alternating current impedance behavior and overcharge tolerance of LIBs using positive temperature coefficient cathodes. J. Electrochem. Soc. 2006, 153, A1004–A1011. [Google Scholar] [CrossRef]
- Kise, M.; Yoshioka, S.; Kuriki, H. Relation between composition of the positive electrode and cell performance and safety of lithium-ion PTC batteries. J. Power Sources 2007, 174, 861–866. [Google Scholar] [CrossRef]
- Zhang, H.; Pang, J.; Ai, X.; Cao, Y.; Yang, H.; Lu, S. Poly(3-butylthiophene)-based positive-temperature-coefficient electrodes for safer LIBs. Electrochim. Acta 2016, 187, 173–178. [Google Scholar] [CrossRef]
- Jin, H.-Z.; Han, X.-F.; Radjenovic, P.M.; Tian, J.-H.; Li, J.-F. Facile and Effective Positive Temperature Coefficient (PTC) Layer for Safer LIBs. J. Phys. Chem. C 2021, 125, 1761–1766. [Google Scholar] [CrossRef]
- Zhong, H.; Kong, C.; Zhan, H.; Zhan, C.; Zhou, Y. Safe positive temperature coefficient composite cathode for lithium-ion battery. J. Power Sources 2012, 216, 273–280. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. TR caused fire and explosion of lithium-ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Feng, X.M.; Ai, X.P.; Yang, H.X. A positive-temperature-coefficient electrode with thermal cut-off mechanism for use in rechargeable lithium batteries. Electrochem. Commun. 2004, 6, 1021–1024. [Google Scholar] [CrossRef]
- Xia, L.; Li, S.L.; Ai, X.P.; Yang, H.X.; Cao, Y.L. Temperature-sensitive cathode materials for safer LIBs. Energy Environ. Sci. 2011, 4, 2845–2848. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Lu, H.; Jia, M.; Zhang, Z. A Positive-Temperature-Coefficient Layer Based on Ni-Mixed Poly(Vinylidene Fluoride) Composites for LiFePO4 Electrode. Int. J. Electrochem. Sci. 2013, 8, 5223–5231. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, Z.; Ma, Z.; Nan, J. Positive-Temperature-Coefficient Graphite Anode as a TR Firewall to Improve the Safety of LiCoO2/Graphite Batteries under Abusive Conditions. Energy Technol. 2020, 8, 1901037. [Google Scholar] [CrossRef]
- Li, H.; Wang, F.; Zhang, C.; Ji, W.; Qian, J.; Cao, Y.; Yang, H.; Ai, X. A temperature-sensitive poly(3-octylpyrrole)/carbon composite as a conductive matrix of cathodes for building safer Li-ion batteries. Energy Storage Mater. 2019, 17, 275–283. [Google Scholar] [CrossRef]
- Beletskii, E.V.; Alekseeva, E.V.; Anishchenko, D.V.; Levin, O.V. Li-Ion Battery Short-Circuit Protection by Voltage-Driven Switchable Resistance Polymer Layer. Batteries 2022, 8, 171. [Google Scholar] [CrossRef]
- Lisbona, D.; Snee, T. A review of hazards associated with primary lithium and LIBs. Process Saf. Environ. Prot. 2011, 89, 434–442. [Google Scholar] [CrossRef]
- Manzo, M.A.; Brewer, J.C.; Bugga, R.V.; Darcy, E.C.; Jeevarajan, J.A.; Mckissock, B.I.; Schmitz, P.C. NASA Aerospace Flight Battery Program: Generic Safety, Handling and Qualification Guidelines for Lithium-Ion (Li-Ion) Batteries; Availability of Source Materials for Lithium-Ion (Li-Ion) Batteries; Maintaining Technical Communications Related to Aerospace Batteries (NASA Aerospace Battery Workshop); NASA: Greenbelt, MD, USA, 2010. [Google Scholar]
- Li, W.; Crompton, K.R.; Hacker, C.; Ostanek, J.K. Comparison of current interrupt device and vent design for 18650 format lithium-ion battery caps: New findings. J. Energy Storage 2022, 46, 103841. [Google Scholar] [CrossRef]
- Xu, B.; Lee, J.; Kwon, D.; Kong, L.; Pecht, M. Mitigation method for Li-ion battery TR: A review. Renew. Sustain. Energy Rev. 2021, 150, 111437. [Google Scholar] [CrossRef]
- Diao, W.; Xu, B.; Pecht, M. Charging induced electrode layer fracturing of 18650 LIBs. J. Power Sources 2021, 484, 229260. [Google Scholar] [CrossRef]
- Augeard, A.; Singo, T.; Desprez, P.; Abbaoui, M. Contribution to the Study of Electric Arcs in LIBs. IEEE Trans. Compon. Packag. Manuf. Technol. 2016, 6, 1068–1078. [Google Scholar] [CrossRef]
- Kwo-Hsiung, Y.; John, K.; Chubin, W.; Roman, D.; Volodymyr, Y. Cell Performance Comparison between C14- and C15-Predomiated AB2 Metal Hydride Alloys. Batteries 2017, 3, 29. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, Z.; Liu, J.; Gao, T.; Yan, W. Influence of state of charge inconsistency on electrical performance and TR characteristics in 2 serials 1 parallel lithium-ion battery pack. Fire Mater. 2024, 48, 439–455. [Google Scholar] [CrossRef]
- Juarez-Robles, D.; Azam, S.; Jeevarajan, J.A.; Mukherjee, P.P. Degradation-Safety Analytics in Lithium-Ion Cells and Modules Part II. Overcharge and External Short Circuit Scenarios. J. Electrochem. Soc. 2021, 168, 050535. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, X.; Sahraei, E.; Wierzbicki, T. Deformation and failure mechanisms of 18650 battery cells under axial compression. J. Power Sources 2016, 336, 332–340. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, R.; He, Y.; Ma, J.; Guo, H.; Yang, Y.; Zhang, L. A Lightweight Multiscale Attention Semantic Segmentation Algorithm for Detecting Laser Welding Defects on Safety Vent of Power Battery. IEEE Access 2021, 9, 39245–39254. [Google Scholar] [CrossRef]
- Ouyang, D.; Liu, Y.; Hamam, I.; Wang, J.; Dahn, J. A comparative study on the reactivity of charged Ni-rich and Ni-poor positive electrodes with electrolyte at elevated temperatures using accelerating rate calorimetry. J. Energy Chem. 2021, 60, 523–530. [Google Scholar] [CrossRef]
- Huang, Q.; Ma, L.; Liu, A.; Ma, X.; Li, J.; Wang, J.; Dahn, J.R. The reactivity of charged positive Li1−n[NixMnyCoz]O2 electrodes with electrolyte at elevated temperatures using accelerating rate calorimetry. J. Power Sources 2018, 390, 78–86. [Google Scholar] [CrossRef]
- Jia, Z.; Min, Y.; Qin, P.; Mei, W.; Meng, X.; Jin, K.; Sun, J.; Wang, Q. Effect of safety valve types on the gas venting behavior and TR hazard severity of large-format prismatic lithium iron phosphate batteries. J. Energy Chem. 2024, 89, 195–207. [Google Scholar] [CrossRef]
- Yao, X.-Y.; Kong, L.; Pecht, M.G. Reliability of Cylindrical Li-ion Battery Safety Vents. IEEE Access 2020, 8, 101859–101866. [Google Scholar] [CrossRef]
- Zhang, N.; Li, J.; Li, H.; Liu, A.; Huang, Q.; Ma, L.; Li, Y.; Dahn, J.R. Structural, Electrochemical, and Thermal Properties of Nickel-Rich LiNixMnyCozO2 Materials. Chem. Mater. 2018, 30, 8852–8860. [Google Scholar] [CrossRef]
- Li, H.; Chen, H.; Zhong, G.; Wang, Y.; Wang, Q. Experimental study on TR risk of 18650 lithium-ion battery under side-heating condition—ScienceDirect. J. Loss Prev. Process Ind. 2019, 61, 122–129. [Google Scholar] [CrossRef]
- Robinson, J.B.; Darr, J.A.; Eastwood, D.S.; Hinds, G.; Lee, P.D.; Shearing, P.R.; Taiwo, O.O.; Brett, D.J.L. Non-uniform temperature distribution in Li-ion batteries during discharge—A combined thermal imaging, X-ray micro-tomography and electrochemical impedance approach. J. Power Sources 2014, 252, 51–57. [Google Scholar] [CrossRef]
- Duh, Y.S.; Sun, Y.; Lin, X.; Zheng, J.; Wang, M.; Wang, Y.; Lin, X.; Jiang, X.; Zheng, Z.; Zheng, S. Characterization on TR of commercial 18650 LIBs used in electric vehicles: A review. J. Energy Storage 2021, 41, 102888. [Google Scholar] [CrossRef]
- Anderson, N.; Tran, M.; Darcy, E. 18650 Cell Bottom Vent: Preliminary Evaluation into Its Merits for Preventing Side Wall Rupture; NASA: Greenbelt, MD, USA, 2016. [Google Scholar]
- Kong, L.; Li, C.; Jiang, J.; Pecht, M.G. Li-Ion Battery Fire Hazards and Safety Method. Energies 2018, 11, 2191. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, K.-H.; Ko, D.-C.; Lee, S.-B.; Kim, B.-M. Design of integrated safety vent in prismatic lithium-ion battery. J. Mech. Sci. Technol. 2017, 31, 2505–2511. [Google Scholar] [CrossRef]
- Chombo, P.V.; Laoonual, Y. A review of safety method of a Li-ion battery. J. Power Sources 2020, 478, 228649. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, K.H.; Lim, Y.J.; Kim, B.M. Manufacturing Integral Safety Vents in Prismatic LIBs. Trans. Mater. Process. 2015, 24, 293–298. [Google Scholar] [CrossRef]
- Gao, R.; Wang, S.; Xu, Z.; Li, H.; Chen, S.; Hou, H.; Wang, J. Octahedral Fe3O4/FeS composite synthesized by one-pot hydrothermal method as a high-performance anode material for LIBs. J. Alloys Compd. 2021, 864, 158796. [Google Scholar] [CrossRef]
- Kim, H.S.; Kong, M.; Kim, K.; Kim, I.J.; Gu, H.B. Effect of carbon coating on LiNi1/3Mn1/3Co1/3O2 cathode material for lithium secondary batteries. J. Power Sources 2007, 171, 917–921. [Google Scholar] [CrossRef]
- Shen, C.; Hu, G.; Cheong, L.Z.; Huang, S.; Zhang, J.G.; Wang, D. Direct Observation of the Growth of Lithium Dendrites on Graphite Anodes by Operando EC-AFM. Small Methods 2018, 2, 1700298. [Google Scholar] [CrossRef]
- Carter, R.E.; Love, C.T. Modulation of Lithium Plating in Li-Ion Batteries with External Thermal Gradient. ACS Appl. Mater. Interfaces 2018, 10, 26328–26334. [Google Scholar] [CrossRef]
- Chen, J.; Xu, L.; Li, W.; Gou, X. α-Fe2O3 Nanotubes in Gas Sensor and Lithium-Ion Battery Applications. Adv. Mater. 2005, 17, 582–586. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Madhavi, S.; Lou, X.W. α-FeO-mediated growth and carbon nanocoating of ultrafine SnO nanorods as anode materials for Li-ion batteries. J. Mater. Chem. 2012, 22, 2526–2531. [Google Scholar] [CrossRef]
- Arie, A.A.; Lee, J.K. Nano-carbon coating layer prepared by the thermal evaporation of fullerene C60 for lithium metal anodes in rechargeable lithium batteries. J. Nanosci. Nanotechnol. 2011, 11, 6569–6574. [Google Scholar] [CrossRef] [PubMed]
- Kopuklu, B.B.; Tasdemir, A.; Gursel, S.A.; Yurum, A. High stability graphene oxide aerogel supported ultrafine Fe3O4 particles with superior performance as a Li-ion battery anode. Carbon 2021, 174, 158–172. [Google Scholar] [CrossRef]
- Hussain, M.I.S.Z.; Hussain, S.B.; Oh, W.C.; Ullah, K.; Ullah, K. A review on graphene based transition metal oxide composites and its application towards supercapacitor electrodes. SN Appl. Sci. 2020, 2, 764. [Google Scholar] [CrossRef]
- Mo, L.; Zheng, H. Solid coated Li4Ti5O12 (LTO) using polyaniline (PANI) as anode materials for improving thermal safety for lithium-ion battery. Energy Rep. 2020, 6, 2913–2918. [Google Scholar] [CrossRef]
- Park, Y.-S.; Oh, E.-S.; Lee, S.-M. Effect of polymeric binder type on the thermal stability and tolerance to roll-pressing of spherical natural graphite anodes for Li-ion batteries. J. Power Sources 2014, 248, 1191–1196. [Google Scholar] [CrossRef]
- Chen, W.; Zhan, X.; Luo, B.; Ou, Z.; Shih, P.C.; Yao, L.; Pidaparthy, S.; Patra, A.; An, H.; Braun, P.V. Effects of Particle Size on Mg2+ Ion Intercalation into lambda-MnO2 Cathode Materials. Nano Lett. 2019, 19, 4712–4720. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-H.; Okano, M.; Mukai, T.; Inoue, K.; Yanagida, M.; Sakai, T. Improvement of thermal stability and safety of lithium ion battery using SiO anode material. J. Power Sources 2016, 304, 9–14. [Google Scholar] [CrossRef]
- Gou, Q.; Liao, H.; Chen, F.; Zeng, R.; Liu, H.; Yang, N.; Hou, Y.; Xie, G. Construction and modification of germanium-based anode materials in lithium-ion batteries. J. Alloys Compd. 2025, 1013, 178520. [Google Scholar]
- Chen, C.; Shi, H.; Jiang, H.; Zhu, J. Recent Progress on Titanium Niobium Oxide as Anode Material for Lithium-Ion Batteries. J. Electron. Mater. 2025, 54, 3346–3369. [Google Scholar] [CrossRef]
- Zhai, F.Y.; Yang, P.Y.; Zhang, W.F.; Zhu, X.-Y.; Cao, G.-P.; Zhang, H.-M.; Xing, Y.-L.; Lei, Y.-P.; Xiang, Y.; Zhang, S.-C. Creative high-entropy strategy: A booster to the design of anode materials for high-energy lithium-ion batteries. Rare Met. 2025, 1–26. [Google Scholar] [CrossRef]
- Xiao, Z.; Wu, H.; Quan, L.; Zeng, F.; Guo, R.; Ma, Z.; Chen, X.; Zhan, J.; Xu, K.; Xing, L.; et al. Micro-sized CVD-derived Si–C anodes: Challenges, strategies, and prospects for next-generation high-energy lithium-ion batteries. Energy Environ. Sci. 2025, 18, 4037–4052. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; Xu, C.; Wen, B.; Si, L.; Shen, W.; Feng, Z.; Mai, W.; Lin, X.; Wu, Y.; et al. Recent Advancements in Metal-Organic Frameworks-Derived Metal Phosphides as Anode for Lithium-Ion Batteries. ChemNanoMat 2025, 11, e202400504. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. TR mechanism of lithium-ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Xia, Y.; Zheng, J.; Wang, C.; Gu, M. Designing principle for Ni-rich cathode materials with high energy density for practical applications. Nano Energy 2018, 49, 434–452. [Google Scholar] [CrossRef]
- Kai, L.; Yayuan, L.; Dingchang, L.; Allen, P.; Yi, C. Materials for lithium-ion battery safety. Sci. Adv. 2018, 4, eaas9820. [Google Scholar]
- Zhou, F.; Zhao, X.; Jiang, J.; Dahn, J.R. Advantages of Simultaneous Substitution of Co in Li[Ni1/3Mn1/3Co1/3]O2 by Ni and Al. Electrochem. Solid-State Lett. 2009, 12, A81. [Google Scholar] [CrossRef]
- Cho, W.; Kim, S.-M.; Song, J.H.; Yim, T.; Woo, S.-G.; Lee, K.-W.; Kim, J.-S.; Kim, Y.-J. Improved electrochemical and thermal properties of nickel rich LiNi0.6Co0.2Mn0.2O2 cathode materials by SiO2 coating. J. Power Sources 2015, 282, 45–50. [Google Scholar] [CrossRef]
- Chen, J.; Cui, Y.; Jiang, L.; Xue, J.; Xu, H.; Nan, J. Thermal Safety and Runaway Blocking Mechanism for LIBs through Introducing Nanoscale Magnesium Hydroxide into the LiNi0.5Co0.2Mn0.3O2 Cathode. ACS Appl. Energy Mater. 2021, 4, 12780–12788. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, S.-O.; Mun, J.; Park, M.-S.; Kim, K.J.; Lee, K.Y.; Choi, W. Influence of salt, solvents, and additives on the thermal stability of delithiated cathodes in LIBs. J. Electroanal. Chem. 2017, 807, 174–180. [Google Scholar] [CrossRef]
- Li, T.; Yuan, X.-Z.; Zhang, L.; Song, D.; Shi, K.; Bock, C. Degradation Mechanisms and Mitigation Method of Nickel-Rich NMC-Based LIBs. Electrochem. Energy Rev. 2020, 3, 43–80. [Google Scholar] [CrossRef]
- Lee, S.-W.; Kim, M.-S.; Jeong, J.H.; Kim, D.-H.; Chung, K.Y.; Roh, K.C.; Kim, K.-B. Li3PO4 surface coating on Ni-rich LiNi0.6Co0.2Mn0.2O2 by a citric acid assisted sol-gel method: Improved thermal stability and high-voltage performance. J. Power Sources 2017, 360, 206–214. [Google Scholar] [CrossRef]
- Yoo, K.S.; Kang, Y.H.; Im, K.R.; Kim, C.S. Surface Modification of Li(Ni0.6Co0.2Mn0.2)O2 Cathode Materials by Nano-Al2O3 to Improve Electrochemical Performance in LIBs. Materials 2017, 10, 1273. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Ren, D.; Hsu, H.; Xu, G.-L.; Hou, J.; Wang, L.; Feng, X.; Lu, L.; Xu, W.; et al. Toward a high-voltage fast-charging pouch cell with TiO2 cathode coating and enhanced battery safety. Nano Energy 2020, 71, 104643. [Google Scholar] [CrossRef]
- Doughty, D.H. Vehicle Battery Safety Roadmap Guidance; National Renewable Energy Laboratory: Golden, CO, USA, 2012. [Google Scholar]
- Chen, Y.; Zhang, Y.; Wang, F.; Wang, Z.; Zhang, Q. Improve the structure and electrochemical performance of LiNi0.6Co0.2Mn0.2O2 cathode material by nano-Al2O3 ultrasonic coating. J. Alloys Compd. 2014, 611, 135–141. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, Y.; Lu, X.; Zhao, H.; Bai, Y. Ultra-High Temperature Operated Ni-Rich Cathode Stabilized by Thermal Barrier for High-Energy LIBs. Adv. Mater. 2024, 36, e2313500. [Google Scholar] [CrossRef]
- Lee, D.J.; Scrosati, B.; Sun, Y.K. Ni3(PO4)2-coated Li[Ni0.8Co0.15Al0.05]O2 lithium battery electrode with improved cycling performance at 55 °C. J. Power Sources 2011, 196, 7742–7746. [Google Scholar] [CrossRef]
- Andezai, A.; Iroh, J.O. Overview of Organic Cathode Materials in Lithium-Ion Batteries and Supercapacitors. Energies 2025, 18, 582. [Google Scholar] [CrossRef]
- Cai, M.; Yang, W.; Wu, Z. Modification Strategies of High-Nickel Layered Oxides Cathode for Lithium-ion Batteries. Batter. Supercaps 2025. [Google Scholar] [CrossRef]
- Belous, A.G.; Lisovskyi, I.V.; Khomenko, V.G. Surface modification of cathode materials with functional coatings for enhanced lithium-ion battery durability. J. Appl. Electrochem. 2025, 1–34. [Google Scholar] [CrossRef]
- Wen, J.; Yu, Y.; Chen, C. A Review on LIBs Safety Issues: Existing Problems and Possible Solutions. Mater. Express 2012, 2, 197–212. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, H.; Zhang, J. LIBs: Advanced Materials and Technologies; Crc Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Li, Z.; Huang, J.; Liaw, B.Y.; Metzler, V.; Zhang, J. A review of lithium deposition in lithium-ion and lithium metal secondary batteries. J. Power Sources 2014, 254, 168–182. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, R.; Sun, R.; Chen, R. Improved thermal stability of lithium ion battery by using cresyl diphenyl phosphate as an electrolyte additive. J. Power Sources 2010, 195, 7457–7461. [Google Scholar] [CrossRef]
- Kim, T. A facile treatment to improve safety and interfacial stability by an organophosphorus composite for lithium metal batteries. Mater. Lett. 2023, 338, 134023. [Google Scholar] [CrossRef]
- Liu, K.; Liu, C.; Hsu, P.C.; Xu, J.; Kong, B.; Wu, T.; Zhang, R.; Zhou, G.; Huang, W.; Sun, J. Core–Shell Nanofibrous Materials with High Particulate Matter Removal Efficiencies and Thermally Triggered Flame Retardant Properties. ACS Cent. Sci. 2018, 4, 894–898. [Google Scholar] [CrossRef]
- Lei, S.; Zeng, Z.; Wu, Y.; Liu, M.; Cheng, S.; Xie, J. Non-coordinating flame retardants with varied vapor pressures enabling biphasic fire-extinguishing electrolyte for high safety LIBs. Chem. Eng. J. 2023, 463, 142181. [Google Scholar] [CrossRef]
- Wu, H.-L.; Chong, Y.-H.; Ong, H.-C.; Shu, C.-M. Thermal stability of modified lithium-ion battery electrolyte by flame retardant, tris (2,2,2-trifluoroethyl) phosphite. J. Therm. Anal. Calorim. 2022, 147, 4245–4252. [Google Scholar] [CrossRef]
- Zhang, C.-Z.; Xie, L.-J.; Tang, Y.; Li, Y.; Jiang, J.-C.; Huang, A.-C. Thermal Safety Evaluation of Silane Polymer Compounds as Electrolyte Additives for Silicon-Based Anode LIBs. Processes 2022, 10, 1581. [Google Scholar] [CrossRef]
- Thanh-Ha, T.; Harmand, S.; Sahut, B. Experimental investigation on heat pipe cooling for Hybrid Electric Vehicle and Electric Vehicle lithium-ion battery. J. Power Sources 2014, 265, 262–272. [Google Scholar] [CrossRef]
- Xue, S.; Zhou, Y.; Liu, X.; He, M. A new fluorine-containing sulfone-based electrolyte for advanced performance lithium metal batteries. J. Energy Storage 2023, 64, 107137. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.-Q.; Shi, P.; Zhang, Q. Fluorinated Solid-Electrolyte Interphase in High-Voltage Lithium Metal Batteries. Joule 2019, 3, 2647–2661. [Google Scholar] [CrossRef]
- Qi, H.; Ren, Y.; Guo, S.; Wang, Y.; Li, S.; Hu, Y.; Yan, F. High-Voltage Resistant Ionic Liquids for LIBs. ACS Appl. Mater. Interfaces 2020, 12, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Hayyan, M.; Mjalli, F.S.; Hashim, M.A.; Alnashef, I.M.; Mei, T.X. Investigating the Electrochemical Windows of Ionic Liquids. J. Ind. Eng. Chem. 2013, 19, 106–112. [Google Scholar] [CrossRef]
- Bocharova, V.; Sokolov, A.P. Perspectives for Polymer Electrolytes: A View from Fundamentals of Ionic Conductivity. Macromolecules 2020, 53, 4141–4157. [Google Scholar] [CrossRef]
- Chernyshov, D.V.; Shin, W.C. Quaternary Ammonium-Based Room Temperature Ionic Liquids as Components of Carbonate Electrolytes for Li-ion Batteries: Electrochemical Performance and Thermal Properties. J. Electrochem. Sci. Technol. 2014, 5, 95–104. [Google Scholar] [CrossRef]
- Zhu, M.; Wu, J.; Wang, Y.; Song, M.; Long, L.; Siyal, S.H.; Yang, X.; Sui, G. Recent advances in gel polymer electrolyte for high-performance lithium batteries. J. Energy Chem. 2019, 37, 126–142. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, L.; Xu, H.; Zhang, X.; Chen, Z.; Gao, C.; Lu, C.; Liu, Z.; Jiang, M.; Cui, G. A polymer electrolyte with a thermally induced interfacial ion-blocking function enables safety-enhanced lithium metal batteries. eScience 2022, 2, 201–208. [Google Scholar] [CrossRef]
- Liu, K.-L.; Chao, C.-H.; Lee, H.-C.; Liao, C.-H.; Fang, J.; Wu, N.-L.; Chao, C.-Y. A single-ion conducting quasi-solid polymer electrolyte made from synthetic rubber for lithium metal batteries. Electrochem. Commun. 2023, 149, 107467. [Google Scholar] [CrossRef]
- Dong, T.; Xu, G.; Xie, B.; Liu, T.; Gong, T.; Sun, C.; Wang, J.; Zhang, S.; Zhang, X.; Zhang, H.; et al. An Electrode-Crosstalk-Suppressing Smart Polymer Electrolyte for High Safety LIBs. Adv. Mater. 2024, 36, e2400737. [Google Scholar] [CrossRef] [PubMed]
- Finegan, D.P. X-Ray Imaging of Failure and Degradation Mechanisms of LIBs. Ph.D. Thesis, University College London, London, UK, 2016. [Google Scholar]
- Zhu, J.; Zhang, J.; Zhao, R.; Zhao, Y.; Liu, J.; Xu, N.; Wan, X.; Li, C.; Ma, Y.; Zhang, H.; et al. In situ 3D crosslinked gel polymer electrolyte for ultra-long cycling, high-voltage, and high-safety lithium metal batteries. Energy Storage Mater. 2023, 57, 92–101. [Google Scholar] [CrossRef]
- Long, M.-C.; Wu, G.; Wang, X.-L.; Wang, Y.-Z. Self-adaptable gel polymer electrolytes enable high-performance and all-round safety lithium ion batteries. Energy Storage Mater. 2022, 53, 62–71. [Google Scholar] [CrossRef]
- Shi, X.; Jia, Z.; Wang, D.; Jiang, B.; Liao, Y.; Zhang, G.; Wang, Q.; He, D.; Huang, Y. Phonon Engineering in Solid Polymer Electrolyte toward High Safety for Solid-State Lithium Batteries. Adv. Mater. 2024, 36, 2405097.1–2405097.9. [Google Scholar] [CrossRef]
- Li, C.; Du, Y. Building a Better All-Solid-State Lithium-Ion Battery with Halide Solid-State Electrolyte. ACS Nano 2025, 19, 4121–4155. [Google Scholar] [CrossRef]
- Hosamane, S.; Kottam, N.; Chalil Suresh, A. Strategies to Boost the Safety and Ionic Conductivity of Lithium-Ion Batteries Using Solid State Electrolytes: A Review. Wiley Interdiscip. Rev. Energy Environ. 2025, 14, e544. [Google Scholar] [CrossRef]
- Liu, B.; Suo, L. Advanced Electrolyte Solution for Aqueous Lithium-Ion Batteries: Extending Their Lifespan. Adv. Funct. Mater. 2025. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, Z.; Wu, Y.; Zhong, W.; Lei, S.; Cheng, S.; Wen, J.; Xie, J. Reviewing recent progress of liquid electrolyte chemistry for mitigating TR in LIBs. Energy Storage Mater. 2024, 65, 103133. [Google Scholar] [CrossRef]
- Lei, T.; Chen, W.; Hu, Y.; Lv, W.; Lv, X.; Yan, Y.; Huang, J.; Jiao, Y.; Chu, J.; Yan, C. A Nonflammable and Thermotolerant Separator Suppresses Polysulfide Dissolution for Safe and Long-Cycle Lithium-Sulfur Batteries; John Wiley & Sons, Ltd.: London, UK, 2018. [Google Scholar]
- Deimede, V.; Elmasides, C. Separators for LIBs: A Review on the Production Processes and Recent Developments. Energy Technol. 2015, 3, 453–468. [Google Scholar] [CrossRef]
- Tian, Y.; Zeng, G.; Rutt, A.; Shi, T.; Kim, H.; Wang, J.; Koettgen, J.; Sun, Y.; Ouyang, B.; Chen, T.; et al. Promises and Challenges of Next-Generation “Beyond Li-ion” Batteries for Electric Vehicles and Grid Decarbonization. Chem. Rev. 2021, 121, 1623–1669. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shen, L.; Deng, S.; Cui, P.; Yao, X. 10 μm-Thick High-Strength Solid Polymer Electrolytes with Excellent Interface Compatibility for Flexible All-Solid-State Lithium-Metal Batteries. Adv. Mater. 2021, 33, e2100353. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Mu, X.; Li, Z.; Han, L.; Zhu, Y.; Wang, H.J.; Song, L.; Kan, Y.; Hu, Y. A Flame-Retardant, High Ionic-Conductivity and Eco-Friendly Separator Prepared by Papermaking Method for High-Performance and Fire Safety LIBs; Social Science Electronic Publishing: Rochester, NY, USA, 2022. [Google Scholar]
- Luo, D.; Chen, M.; Xu, J.; Yin, X.; Wu, J.; Chen, S.; Wang, L.; Wang, H. Polyphenylene sulfide nonwoven-based composite separator with superior heat-resistance and flame retardancy for high power lithium-ion battery. Compos. Sci. Technol. 2018, 157, 119–125. [Google Scholar] [CrossRef]
- Zheng, W.; Zhu, Y.; Na, B.; Lv, R.; Liu, H.; Li, W.; Zhou, H. Hybrid silica membranes with a polymer nanofiber skeleton and their application as lithium-ion battery separators. Compos. Sci. Technol. 2017, 144, 178–184. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, D.; Wu, K.; Fu, Q. Improved dielectric and energy storage properties of polypropylene by adding hybrid fillers and high-speed extrusion. Polymer 2020, 214, 123348. [Google Scholar] [CrossRef]
- Zuo, X.; Wu, J.; Ma, X.; Deng, X.; Cai, J.; Chen, Q.; Liu, J.; Nan, J. A poly(vinylidene fluoride)/ethyl cellulose and amino-functionalized nano-SiO2 composite coated separator for 5 V high-voltage LIBs with enhanced performance. J. Power Sources 2018, 407, 44–52. [Google Scholar] [CrossRef]
- Lv, W.; Zhang, X. Recent advances in lithium-ion battery separators with enhanced safety. In 60 Years of the Loeb-Sourirajan Membrane; Elsevier: Amsterdam, The Netherlands, 2022; pp. 269–304. [Google Scholar]
- da Silva, G.M.; Lima, T.J.; da Silva, D.D.; Henriques, I.B. Assessment of TR propagation in lithium-ion battery modules with different separator materials. Int. J. Therm. Sci. 2024, 197, 108836. [Google Scholar] [CrossRef]
- Palanisamy, M.; Lin, K.-W.; Lo, C.-T.; Pol, V.G. In Situ Thermal Safety Aspect of the Electrospun Polyimide-Al2O3 Separator Reveals Less Exothermic Heat Energies than Polypropylene at the TR Event of LIBs. ACS Appl. Mater. Interfaces 2022, 14, 28310–28320. [Google Scholar] [CrossRef]
- Jeon, H.; Choi, J.; Ryou, M.-H.; Lee, Y.M. Comparative Study of the Adhesion Properties of Ceramic Composite Separators Using a Surface and Interfacial Cutting Analysis System for LIBs. ACS Omega 2017, 2, 2159–2164. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, J.H.; Lee, J.; Kim, S.; Cho, S.O. Safety and performance enhanced boehmite-coupled polyethylene nanocomposite separator for LIBs by synergetic effects of silane treatment and electron irradiation. J. Power Sources 2023, 560, 232718. [Google Scholar] [CrossRef]
- Liu, J.; Cao, D.; Yao, H.; Liu, D.; Zhang, X.; Zhang, Q.; Chen, L.; Wu, S.; Sun, Y.; He, D.; et al. Hexagonal Boron Nitride-Coated Polyimide Ion Track Etched Separator with Enhanced Thermal Conductivity and High-Temperature Stability for LIBs. ACS Appl. Energy Mater. 2022, 5, 8639–8649. [Google Scholar] [CrossRef]
- Tang, W.; Liu, Q.; Luo, N.; Chen, F.; Fu, Q. High safety and electrochemical performance electrospun para-aramid nanofiber composite separator for lithium-ion battery. Compos. Sci. Technol. 2022, 225, 109479. [Google Scholar] [CrossRef]
- Deng, Y.; Pan, Y.; Li, C.; Zhang, Z.; Gong, L.; Fu, Y.; Shi, L.; Zhang, H.; Cheng, X. Advanced polyimide separator via co-precursor method for LIBs with low TR risks. J. Energy Storage 2022, 56, 106100. [Google Scholar] [CrossRef]
- Yrynen, A.V.; Salminen, J. Lithium-ion battery production. J. Chem. Thermodyn. 2012, 46, 80–85. [Google Scholar] [CrossRef]
- Greco, A.; Jiang, X.; Cao, D. An investigation of lithium-ion battery thermal management using paraffin/porous-graphite-matrix composite. J. Power Sources 2015, 278, 50–68. [Google Scholar] [CrossRef]
- Pesaran, A.A. Battery Thermal Models for Hybrid Vehicle Simulations. J. Power Sources 2002, 110, 377–382. [Google Scholar] [CrossRef]
- Mohammadian, S.K.; Rassoulinejad-Mousavi, S.M.; Zhang, Y. Thermal management improvement of an air-cooled high-power lithium-ion battery by embedding metal foam. J. Power Sources 2015, 296, 305–313. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, X.; Wang, B.; Zhang, C.; Xuan, D. Multiobjective optimization of air-cooled battery thermal management system based on heat dissipation model. Ionics 2021, 27, 1307–1322. [Google Scholar] [CrossRef]
- Sabbah, R.; Kizilel, R.; Selman, J.R.; Al-Hallaj, S. Active (air-cooled) vs. passive (phase change material) thermal management of high power lithium-ion packs: Limitation of temperature rise and uniformity of temperature distribution. J. Power Sources 2015, 182, 630–638. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, X.; Li, G.; Hua, D. Assessment of the forced air-cooling performance for cylindrical lithium-ion battery packs: A comparative analysis between aligned and staggered cell arrangements. Appl. Therm. Eng. 2015, 80, 55–65. [Google Scholar] [CrossRef]
- Lopez, C.F.; Jeevarajan, J.A.; Mukherjee, P.P. Experimental Analysis of TR and Propagation in Lithium-Ion Battery Modules. J. Electrochem. Soc. 2015, 162, A1905–A1915. [Google Scholar] [CrossRef]
- Yang, T.; Yang, N.; Zhang, X.; Li, G. Investigation of the thermal performance of axial-flow air cooling for the lithium-ion battery pack. Int. J. Therm. Sci. 2016, 108, 132–144. [Google Scholar] [CrossRef]
- Daniels, R.K.; Prabhakar, A. Experimental and numerical investigation on the effect of cell arrangement on TR propagation in air cooled cylindrical Li-ion battery modules. J. Energy Storage 2023, 72, 108191. [Google Scholar] [CrossRef]
- Chen, K.; Song, M.; Wei, W.; Wang, S. Structure optimization of parallel air-cooled battery thermal management system with U-type flow for cooling efficiency improvement. Energy 2018, 145, 603–613. [Google Scholar] [CrossRef]
- Li, X.; Zhao, J.; Yuan, J.; Duan, J.; Liang, C. Simulation and analysis of air cooling configurations for a lithium-ion battery pack. J. Energy Storage 2021, 35, 102270. [Google Scholar] [CrossRef]
- Murphree, T. Advanced Climate Analysis and Long Range Forecasting; Department of Meteorology, Naval Postgraduate School (NPS): Monterey, CA, USA, 2014. [Google Scholar]
- Dong, F.; Cheng, Z.; Zhu, J.; Song, D.; Ni, J. Investigation and optimization on cooling performance of a novel double helix structure for cylindrical LIBs. Appl. Therm. Eng. 2021, 189, 116758. [Google Scholar] [CrossRef]
- Talele, V.; Patil, M.S.; Panchal, S.; Fraser, R.; Fowler, M. Battery TR propagation time delay strategy using phase change material integrated with pyro block lining: Dual functionality battery thermal design. J. Energy Storage 2023, 65, 107253. [Google Scholar] [CrossRef]
- Javani, N.; Dincer, I.; Naterer, G.F. Numerical Modeling of Submodule Heat Transfer With Phase Change Material for Thermal Management of Electric Vehicle Battery Packs. J. Therm. Sci. Eng. Appl. 2015, 7, 031005. [Google Scholar] [CrossRef]
- Wilke, S.; Schweitzer, B.; Khateeb, S.; Al-Hallaj, S. Preventing TR propagation in lithium ion battery packs using a phase change composite material: An experimental study. J. Power Sources 2017, 340, 51–59. [Google Scholar] [CrossRef]
- Atouei, S.A.; Rezania, A.; Ranjbar, A.A.; Rosendahl, L.A. Protection and thermal management of thermoelectric generator system using phase change materials: An experimental investigation. Energy 2018, 156, 311–318. [Google Scholar] [CrossRef]
- Kizilel, R.; Sabbah, R.; Selman, J.R.; Al-Hallaj, S. An alternative cooling system to enhance the safety of Li-ion battery packs. J. Power Sources 2009, 194, 1105–1112. [Google Scholar] [CrossRef]
- Anisur, M.R.; Mahfuz, M.H.; Kibria, M.A.; Saidur, R.; Metselaar, I.H.S.C.; Mahlia, T.M.I. Curbing global warming with phase change materials for energy storage. Renew. Sustain. Energy Rev. 2013, 18, 23–30. [Google Scholar] [CrossRef]
- Dai, X.; Kong, D.; Du, J.; Zhang, Y.; Ping, P. Investigation on effect of phase change material on the TR of lithium-ion battery and exploration of flame retardancy improvement. Process Saf. Environ. Prot. 2022, 159, 232–242. [Google Scholar] [CrossRef]
- Dai, X.; Ping, P.; Kong, D.; Gao, X.; Zhang, Y.; Wang, G.; Peng, R. Heat transfer enhanced inorganic phase change material compositing carbon nanotubes for battery thermal management and TR propagation mitigation. J. Energy Chem. 2024, 89, 226–238. [Google Scholar] [CrossRef]
- Ouyang, D.; Weng, J.; Hu, J.; Chen, M.; Huang, Q.; Wang, J. Experimental investigation of thermal failure propagation in typical lithium-ion battery modules. Thermochim. Acta 2019, 676, 205–213. [Google Scholar] [CrossRef]
- Jiang, Z.; Ouyang, T.; Yang, Y.; Chen, L.; Fan, X.; Chen, Y.; Li, W.; Fei, Y. Thermal conductivity enhancement of phase change materials with form-stable carbon bonded carbon fiber network. Mater. Des. 2018, 143, 177–184. [Google Scholar] [CrossRef]
- Sahan, N.; Fois, M.; Paksoy, H. Improving thermal conductivity phase change materials-A study of paraffin nanomagnetite composites. Sol. Energy Mater. Sol. Cells 2015, 137, 61–67. [Google Scholar] [CrossRef]
- Hussain, A.; Abidi, I.H.; Tso, C.Y.; Chan, K.C.; Luo, Z.; Chao, C.Y.H. Thermal management of lithium ion batteries using graphene coated nickel foam saturated with phase change materials. Int. J. Therm. Sci. 2018, 124, 23–35. [Google Scholar] [CrossRef]
- Zhao, Y.; Zou, B.; Li, C.; Ding, Y. Active cooling based battery thermal management using composite phase change materials. Energy Procedia 2019, 158, 4933–4940. [Google Scholar] [CrossRef]
- Wu, S.; Yan, T.; Kuai, Z.; Pan, W. Thermal Conductivity Enhancement on Phase Change Materials for Thermal Energy Storage: A Review; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Jiang, G.; Huang, J.; Fu, Y.; Cao, M.; Liu, M. Thermal optimization of composite phase change material/expanded graphite for Li-ion battery thermal management. Appl. Therm. Eng. 2016, 108, 1119–1125. [Google Scholar] [CrossRef]
- Li, Z.; Sun, W.G.; Wang, G.; Wu, Z.G. Experimental and numerical study on the effective thermal conductivity of paraffin/expanded graphite composite. Sol. Energy Mater. Sol. Cells 2014, 128, 447–455. [Google Scholar] [CrossRef]
- Ianniciello, L.; Biwole, P.H.; Achard, P. Electric vehicles batteries thermal management systems employing phase change materials. J. Power Sources 2018, 378, 383–403. [Google Scholar] [CrossRef]
- Babapoor, A.; Azizi, M.; Karimi, G. Thermal management of a Li-ion battery using carbon fiber-PCM composites. Appl. Therm. Eng. 2015, 82, 281–290. [Google Scholar] [CrossRef]
- Karimi, G.; Azizi, M.; Babapoor, A. Experimental study of a cylindrical lithium-ion battery thermal management using phase change material composites. J. Energy Storage 2016, 8, 168–174. [Google Scholar] [CrossRef]
- Samimi, F.; Babapoor, A.; Azizi, M.; Karimi, G. Thermal management analysis of a Li-ion battery cell using phase change material loaded with carbon fibers. Energy 2016, 96, 355–371. [Google Scholar] [CrossRef]
- Bahiraei, F.; Fartaj, A.; Nazri, G.-A. Experimental and numerical investigation on the performance of carbon-based nanoenhanced phase change materials for thermal management applications. Energy Convers. Manag. 2017, 153, 115–128. [Google Scholar] [CrossRef]
- Bahiraei, F.; Ghalkhani, M.; Fartaj, A.; Nazri, G.-A. A pseudo 3D electrochemical-thermal modeling and analysis of a lithium-ion battery for electric vehicle thermal management applications. Appl. Therm. Eng. 2017, 125, 904–918. [Google Scholar] [CrossRef]
- Bahiraei, F.; Fartaj, A.; Nazri, G.-A. Electrochemical-thermal Modeling to Evaluate Active Thermal Management of a Lithium-ion Battery Module. Electrochim. Acta 2017, 254, 59–71. [Google Scholar] [CrossRef]
- Lin, S.; Ling, Z.; Li, S.; Cai, C.; Zhang, Z.; Fang, X. Mitigation of lithium-ion battery TR and inhibition of TR propagation using inorganic salt hydrate with integrated latent heat and thermochemical storage. Energy 2023, 266, 126481. [Google Scholar] [CrossRef]
- Weng, J.; Ouyang, D.; Yang, X.; Chen, M.; Zhang, G.; Wang, J. Alleviation of TR propagation in thermal management modules using aerogel felt coupled with flame-retarded phase change material. Energy Convers. Manag. 2019, 200, 112071. [Google Scholar] [CrossRef]
- Luo, W.; Zhao, L.; Chen, M. The effect of PCM on mitigating TR propagation in lithium-ion battery modules. Appl. Therm. Eng. 2024, 236, 121608. [Google Scholar] [CrossRef]
- Ni, R.; Zhang, D.; Wang, R.; Xie, Z.; Wang, Y. Prevention and suppression effects of phase change material on TR in batteries. Case Stud. Therm. Eng. 2023, 48, 103160. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, M.; Zhang, S.; Ouyang, D.; Weng, J.; Wei, R.; Chen, Y.; Zhao, L.; Wang, J. Experimental investigation on mitigation of TR propagation of lithium-ion battery module with flame retardant phase change materials. Appl. Therm. Eng. 2023, 235, 121401. [Google Scholar] [CrossRef]
- Huang, Q.; Li, X.; Zhang, G.; Weng, J.; Wang, Y.; Deng, J. Innovative thermal management and TR suppression for battery module with flame retardant flexible composite phase change material. J. Clean. Prod. 2022, 330, 129718. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J. Investigation of external heating-induced failure propagation behaviors in large-size cell modules with different phase change materials. Energy 2020, 204, 117946. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, G.; Zhang, G.; Yang, X. A novel thermal management structure using serpentine phase change material coupled with forced air convection for cylindrical battery modules. J. Power Sources 2020, 468, 228398. [Google Scholar] [CrossRef]
- Situ, W.; Zhang, G.; Li, X.; Yang, X.; Wei, C.; Rao, M.; Wang, Z.; Wang, C.; Wu, W. A thermal management system for rectangular LiFePO4 battery module using novel double copper mesh-enhanced phase change material plates. Energy 2017, 141, 613–623. [Google Scholar] [CrossRef]
- Huang, Q.; Li, X.; Zhang, G.; Zhang, J.; He, F.; Li, Y. Experimental investigation of the thermal performance of heat pipe assisted phase change material for battery thermal management system. Appl. Therm. Eng. 2018, 141, 1092–1100. [Google Scholar] [CrossRef]
- Lei, S.; Shi, Y.; Chen, G. A lithium-ion battery-thermal-management design based on phase-change-material thermal storage and spray cooling. Appl. Therm. Eng. 2020, 168, 114792. [Google Scholar] [CrossRef]
- Song, L.; Zhang, H.; Yang, C. Thermal analysis of conjugated cooling configurations using phase change material and liquid cooling techniques for a battery module. Int. J. Heat Mass Transf. 2019, 133, 827–841. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Q.; Zou, B.; Zhang, T.; Jin, L.; Qiao, G.; Nie, B.; Huang, Y.; Ding, Y. Performance of a liquid cooling-based battery thermal management system with a composite phase change material. Int. J. Energy Res. 2020, 44, 4727–4742. [Google Scholar] [CrossRef]
- Roe, C.; Feng, X.; White, G.; Li, R.; Wang, H.; Rui, X.; Li, C.; Zhang, F.; Null, V.; Parkes, M.; et al. Immersion cooling for LIBs—A review. J. Power Sources 2022, 525, 231094. [Google Scholar] [CrossRef]
- Zhao, J.; Lv, P.; Rao, Z. Experimental study on the thermal management performance of phase change material coupled with heat pipe for cylindrical power battery pack. Exp. Therm. Fluid Sci. 2017, 82, 182–188. [Google Scholar] [CrossRef]
- Khateeb, S.A.; Farid, M.M.; Selman, J.R.; Al-Hallaj, S. Design and simulation of a lithium-ion battery with a phase change material thermal management system for an electric scooter. J. Power Sources 2004, 128, 292–307. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, Z.; Yin, X.; Ling, G. Avoiding TR propagation of lithium-ion battery modules by using hybrid phase change material and liquid cooling. Appl. Therm. Eng. 2021, 184, 116380. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, L.; Zhang, Z.; Li, X.; Ma, R.; Ren, Y.; Wu, W. Non-uniform phase change material strategy for directional mitigation of battery TR propagation. Renew. Energy 2022, 200, 1338–1351. [Google Scholar] [CrossRef]
- Wu, W.; Wu, W.; Wang, S. Thermal optimization of composite PCM based large-format lithium-ion battery modules under extreme operating conditions. Energy Convers. Manag. 2017, 153, 22–33. [Google Scholar] [CrossRef]
- Wang, Q.; Rao, Z.; Huo, Y.; Wang, S. Thermal performance of phase change material/oscillating heat pipe-based battery thermal management system. Int. J. Therm. Sci. 2016, 102, 9–16. [Google Scholar] [CrossRef]
- Yin, H.; Gao, X.; Ding, J.; Zhang, Z.; Fang, Y. Thermal management of electronic components with thermal adaptation composite material. Appl. Energy 2010, 87, 3784–3791. [Google Scholar] [CrossRef]
- Mehrabi-Kermani, M.; Houshfar, E.; Ashjaee, M. A novel hybrid thermal management for Li-ion batteries using phase change materials embedded in copper foams combined with forced-air convection. Int. J. Therm. Sci. 2019, 141, 47–61. [Google Scholar] [CrossRef]
- Bai, F.; Chen, M.; Song, W.; Feng, Z.; Li, Y.; Ding, Y. Thermal management performances of PCM/water cooling-plate using for lithium-ion battery module based on non-uniform internal heat source. Appl. Therm. Eng. 2017, 126, 17–27. [Google Scholar] [CrossRef]
- Salva, N.N.; Tarzia, D.A. Explicit solution for a Stefan problem with variable latent heat and constant heat flux boundary conditions. J. Math. Anal. Appl. 2011, 379, 240–244. [Google Scholar] [CrossRef]
- Liang, G. 1. Mudawar, Review of spray cooling-Part 1: Single-phase and nucle.ate boiling regimes, and critical heat flux. Int. J. Heat Mass Transf. 2017, 115, 1174–1205. [Google Scholar] [CrossRef]
- Gao, Q.; Liu, Y.; Wang, G.; Deng, F.; Zhu, J. An experimental investigation of refrigerant emergency spray on cooling and oxygen suppression for overheating power battery. J. Power Sources 2019, 415, 33–43. [Google Scholar] [CrossRef]
- Said, A.O.; Stoliarov, S.I. Analysis of effectiveness of suppression of lithium ion battery fires with a clean agent. Fire Saf. J. 2021, 121, 103296. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, Q.; Xu, J.; Chen, H.; Lu, W.; Wang, Q. Experimental study on the efficiency of dodecafluoro-2-methylpentan-3-one on suppressing lithium-ion battery fires. RSC Adv. 2018, 8, 42223–42232. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, L.; Duan, Q.; Wang, S.; Sun, S.; Sun, J.; Wang, Q. Experimental study on suppressing TR propagation of LIBs in confined space by various fire extinguishing agents. Process Saf. Environ. Prot. 2022, 167, 299–307. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, P.; Duan, Q.; Zhao, C.; Wang, Q. Experimental investigation on the cooling and suppression effects of liquid nitrogen on the TR of lithium ion battery. J. Power Sources 2021, 495, 229795. [Google Scholar] [CrossRef]
- Wan, Q.; Li, K.; Wang, Y.; Chen, H.; Duan, Q.; Sun, J. The Efficiency of Dodecafluoro-2-Methylpentan-3-One on Suppressing the Lithium Ion Battery Fire. J. Electrochem. Energy Convers. Storage 2018, 15, 041001. [Google Scholar] [CrossRef]
- Zhang, L.; Duan, Q.; Liu, Y.; Xu, J.; Sun, J.; Xiao, H.; Wang, Q. Experimental investigation of water spray on suppressing lithium-ion battery fires. Fire Saf. J. 2021, 120, 103117. [Google Scholar] [CrossRef]
- Ditch, B.; Vries, J.D. Flammability Characterization of Lithium-ion Batteries in Bulk Storage. In Proceedings of the31st International Battery Seminar and Exhibit 2014 : Primary & Secondary Batteries—Other Technologies: 31st International Battery Seminar and Exhibit 2014, Ft. Lauderdale, FL, USA, 10–13 March 2014. [Google Scholar]
- Liu, T.; Hu, J.; Tang, Q.; Zhu, X.; Wang, X. Mitigating overcharge induced TR of large format lithium ion battery with water mist. Appl. Therm. Eng. 2021, 197, 117402. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, J. Research progress of water mist fire extinguishing technology and its application in battery fires. Process Saf. Environ. Prot. 2021, 149, 559–574. [Google Scholar] [CrossRef]
- Deng, J.; Hu, Z.; Chen, J.; Deng, T.; Zhang, Y.; Bai, Z.; Huang, L.; He, F. The enhanced cooling effect and critical control capability of nanofluids on suppressing thermal runaway of lithium-ion batteries. J. Energy Storage 2025, 106, 114733. [Google Scholar] [CrossRef]
- Das, L.; Pati, A.R.; Panda, A.; Munshi, B.; Sahoo, D.K.; Barik, K.; Mohapatra, S.S.; Sahoo, A. The enhancement of spray cooling at very high initial temperature by using dextrose added water. Int. J. Heat Mass Transf. 2020, 150, 119311. [Google Scholar] [CrossRef]
- Xu, J.; Duan, Q.; Zhang, L.; Liu, Y.; Sun, J.; Wang, Q. The enhanced cooling effect of water mist with additives on inhibiting lithium-ion battery TR. J. Loss Prev. Process Ind. 2022, 77, 104784. [Google Scholar] [CrossRef]
- Russoa, P.; Di Barib, C.; Mazzaroc, M.; De Rosac, A.; Morriellod, I. Effective Fire Extinguishing Systems for Lithium-ion Battery. Chem. Eng. Trans. 2018, 67, 727–732. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Duan, Q.; Chen, M.; Xu, J.; Zhao, C.; Sun, J.; Wang, Q. Experimental study on the synergistic effect of gas extinguishing agents and water mist on suppressing lithium-ion battery fires. J. Energy Storage 2020, 32, 101801. [Google Scholar] [CrossRef]
- Wang, W.; He, S.; He, T.; You, T.; Parker, T.; Wang, Q. Suppression behavior of water mist containing compound additives on LIBs fire. Process Saf. Environ. Prot. 2022, 161, 476–487. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, Q.; Xu, J.; Li, H.; Sun, J.; Wang, Q. Experimental study on a novel safety strategy of lithium-ion battery integrating fire suppression and rapid cooling. J. Energy Storage 2020, 28, 101185. [Google Scholar] [CrossRef]
- Bai, Z.; Li, X.; Deng, J.; Shu, C.-M.; Zhang, Y.; Zhang, P.; Ramakrishna, S. Overview of anti-fire technology for suppressing thermal runaway of lithium battery: Material, performance, and applications. J. Power Sources 2025, 640, 236767. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).