Abstract
To investigate the fire prevention and suppression characteristics of coal gangue slurry grouting in goafs and the enhanced regulatory mechanisms of additives, the slurry-forming performance of coal gangue slurry was tested. The effects of heating temperature, grouting thickness, and heating duration on the surface temperature distribution characteristics were analyzed. Temperature-programmed experiments were conducted to examine the influence of various additives on the spontaneous combustion propensity of coal gangue, with a comparative analysis of the inhibitory effects between ammonium polyphosphate (APP) and other additives. The results demonstrate that the prepared coal gangue slurry exhibited no segregation or sedimentation, with a plasticity index consistent with standard grouting material requirements, confirming its superior stability. The central, maximum, and minimum surface temperatures of the slurry showed polynomial functional relationships with heating temperature. Surface temperature initially increased and then decreased with grouting thickness, with 10 cm identified as the critical thickness for temperature transition. Overall, the central, maximum, and minimum surface temperatures increased progressively with rising heating temperatures. In addition, under all tested conditions, the average surface temperature remained below 80 °C for slurries with >5 cm grouting thickness, meeting fire prevention requirements. However, the CO and CO2 concentrations increased significantly as heating temperatures rose from 100 °C to 300 °C. At grouting thicknesses of 9–12 cm, CO and CO2 emissions occurred only at 300 °C and decreased with increasing thickness. The coal gangue slurry modified with ammonium polyphosphate (APP) additives exhibited optimal antioxidant performance, significantly suppressing CO and CO2 emissions, which further diminished with higher additive dosages. The findings of this study provide critical insights into the fire prevention performance of coal gangue slurry grouting and the application of additives in this field.
1. Introduction
Coal remains China’s primary energy source, accounting for 91.2% of the country’s major energy mineral reserves [1,2]. During coal mining and washing processes, substantial coal gangue is generated and typically disposed of via surface stockpiling. Currently, China’s cumulative coal gangue stockpile has exceeded 6 billion tons, with annual emissions increasing by approximately 500–800 million tons/year. In 2022, the annual production of coal gangue surpassed 800 million tons for the first time, while the utilization rate remains below 40% [3,4]. Massive stockpiles occupy land resources, pollute the environment, and risk spontaneous combustion. In recent years, driven by China’s national low-carbon transition goals, coal gangue has been prioritized as a key bulk solid waste for comprehensive utilization. Supported by governmental initiatives regarding solid waste management and green industrial development, its applications in ecological restoration, backfill mining, construction, and energy production have gained significant momentum [5]. Therefore, safe, efficient, and environmentally benign coal gangue disposal is critical for sustainable coal resource development.
In recent years, goaf grouting backfill technology using coal gangue has gained prominence. This technique injects coal gangue slurry into underground spaces via surface boreholes, converting surface stockpiles into resources while stabilizing subterranean voids through particle packing, pore filling, and hydraulic hardening, thereby preventing subsidence. Researchers worldwide have extensively studied coal gangue grouting. Gu et al. [6] combined theoretical analysis, model testing, and field studies to explain overburden movement patterns and establish a porosity distribution model for slurry-filled spaces. Li et al. [7] developed a dimensionless formula for grout diffusion distance and analyzed diffusion stages through visualized simulations. Through grouting model tests, Wei et al. [8] demonstrated that reducing coal gangue particle size and increasing slurry concentration effectively mitigate gravitational effects on diffusion anisotropy. Meanwhile, elevated injection pressures nonlinearly enhance the grout diffusion range. Ma et al. [9] found that adding superplasticizers (lignosulfonates, polycarboxylates, or naphthalene-based) improves slurry fluidity and backfill efficiency.
However, the combustible materials contained in the coal gangue will continue to accumulate heat and eventually reach the ignition point for spontaneous combustion [10,11,12]. Surface stockpiles experiencing combustion risk structural destabilization and geological hazards [10,13,14]. Similarly, underground backfilled gangue retains flammability, threatening mine safety. Consequently, developing fire-resistant coal gangue slurry is essential for ensuring operational continuity and safety.
To enhance fire prevention and oxidation resistance, flame retardants and additives have been tested. Ma et al. [15] blended fly-ash-based slurry with diatomite and polyacrylamide to create a composite gel, reducing CO and CO2 emissions. Li et al. [16] identified that xanthan gum–Al3+ composite gels suppress oxygen consumption rates and delay C2H4 generation. Jiao et al. [17] utilized tea polyphenol microcapsules to inhibit spontaneous combustion. Sun et al. [18] developed a temperature-sensitive intelligent gel demonstrating fire suppression at 300–900 °C. Wang et al. [19] synthesized geopolymers from coal gangue as fillers for fireproof coatings. The current research on coal mine fire prevention predominantly focuses on novel oxygen-inhibiting gels or repurposing coal gangue extracts for non-mining fireproof materials, leaving critical gaps in understanding the interaction mechanisms between flame retardants and coal, as well as the fire-resistant and antioxidant performance enhancement of admixture-reinforced grouting materials. Addressing these gaps carries threefold urgency: retardant–gangue composite slurries enable in situ dynamic fire suppression in complex mining environments such as high-humidity and fissured coal seams, breaking through the applicability limitations of traditional static gels; converting vast stockpiled coal gangue into fire-control materials could simultaneously resolve solid waste pollution and fire prevention costs while advancing the circular economy; and existing mechanistic studies lack micro-to-macro cross-scale analysis of how retardants interfere with coal pyrolysis pathways or inhibit free radical chain reactions. This study focuses on the synergistic fire-suppression effects and free radical chain inhibition mechanisms of retardant–gangue composite slurries. By adapting to multi-physical field coupling environments in coal mines, including dynamic thermo-mechanical coupling and fracture seepage, the in situ fire control efficacy under complex working conditions is significantly enhanced. Furthermore, by elucidating the cross-scale relationship between the slurry’s microscopic interfacial reactions and macroscopic fire resistance, a resource utilization pathway for coal gangue that integrates engineering applicability with economic feasibility is pioneered.
After the mining of Ulan Mulun coal mine, the area usually contains a lot of combustible coal and coal gangue, which poses a serious risk of fire. Grouting or backfilling coal gangue slurry into goaf spaces provides a potential solution to mitigate coal gangue self-ignition. Based on the slurry-forming performance of coal gangue slurry, this study employs a grouting backfill fire prevention test platform to investigate changes in oxidation characteristics in coal gangue post-grouting, analyze the influence patterns of grouting thickness, heating duration, and heating temperature on fire prevention performance, and evaluate the regulatory effects of additive-modified slurry on oxidation suppression. Three additives were tested to determine the optimal type and dosage. The findings offer critical references for analyzing fire prevention performance in similar mining backfill applications.
2. Experiment
2.1. Materials
The coal gangue used in this study was sourced from Wulanmulun Coal Mine in Ordos. Its chemical composition includes SiO2 (59.38%), Fe2O3 (5.67%), Al2O3 (23.90%), CaO (1.18%), and MgO (2.02%). The raw material was crushed and reconstituted into coal gangue aggregates suitable for underground grouting backfill, with specific particle size distributions. The three admixtures selected in this paper, zinc borate (ZB), ammonium polyphosphate (APP) and aluminum hypophosphite (AHP), were all from Jinan Taizhong Kexing Company (Jinan, China).
Zinc borate (ZB) is an environmentally friendly non-halogenated flame retardant characterized by non-toxicity, low water solubility, high thermal stability, small particle size, low specific gravity, and excellent dispersibility. It is widely used in plastics, rubber, and coatings as an efficient flame suppression agent.
Ammonium polyphosphate (APP) exists in two solid forms: crystalline and amorphous. Crystalline APP appears as a white powder with stable properties at ambient temperature and no odor. Six crystalline polymorphs of APP have been identified, with Type I and Type II being the most utilized. Type I exhibits a low degree of polymerization, small molecular weight, and high solubility and is commonly employed in fireproof coatings and fertilizers. Type II features a high degree of polymerization, low water solubility, structural stability, and an elevated decomposition temperature, making it a focus in advanced flame retardation research [20,21].
Magnesium hydroxide (MH) is a white or colorless crystalline powder that is poorly soluble in water but soluble in acids, decomposing at 340 °C. It demonstrates high purity, strong reactivity, effective flame retardancy, and non-toxic, odorless, and non-corrosive properties.
2.2. Preparation of Coal Gangue Slurry
The coal gangue grouting backfill slurry was formulated with 20% coarse particles (1–3 mm) and a mass concentration of 68%. Coarse and fine coal gangue particles were thoroughly mixed, followed by the addition of an appropriate amount of water. The mixture was homogenized in a mixer for 10 min to obtain a uniform coal gangue grouting backfill slurry.
2.2.1. Analysis of the Slurry-Forming Performance of Coal Gangue Slurry
To evaluate the slurry-forming performance of coal gangue slurry as a fire suppression material, tests including settling velocity, critical stabilization time, plasticity index, and viscosity coefficient were conducted in accordance with the requirements for grouting material performance specified in the Technical Specification for Grouting Fire Prevention in Coal Mines (MT/T 702-2020). The test procedure is shown in Figure 1.
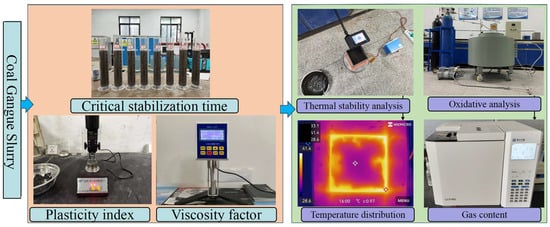
Figure 1.
Experimental flow.
2.2.2. Settling Velocity and Critical Stabilization Time Tests
A 1000 mL graduated cylinder was used to test the settling velocity and critical stabilization time of the backfill slurry. First, the prepared backfill slurry was poured into the cylinder; then, a glass rod was used to stir the slurry again to ensure uniform particle distribution inside; finally, the changes in the height of the solid–liquid interface of the backfill slurry were recorded at continuous intervals of 0.5, 1, 2, 5, 8, 10, 20, and 60 min.
2.2.3. Plasticity Index Test
The plasticity index of the coal gangue slurry was tested using a digital liquid–plastic limit tester. The prepared coal gangue slurry was poured into the mold and placed under the cone tip. The cone height was adjusted until it contacted the upper surface of the slurry, and the dial indicator was zeroed. The cone was then allowed to freely penetrate the slurry under automatic control, and the penetration depth was recorded to determine the plasticity index.
2.2.4. Viscosity Coefficient
The viscosity coefficient of the backfill slurry was measured using an NDJ-5S rheometer (Shandong Ouchang Trading Co., Ltd., Qingdao, China). The backfill slurry was poured into a beaker, the rheometer rotor was slowly inserted, and the slurry was stirred at 60 r/min for 120 s to obtain the viscosity coefficient.
2.3. Thermal Stability Test of Coal Gangue Slurry
The thermal stability analysis of coal gangue slurry aims to investigate the chemical stability of the slurry under the self-heating temperature of coal gangue. The self-heating temperature refers to the initial stage of oxidation reaction between coal gangue and air (80–90 °C), during which the temperature of coal gangue gradually rises and begins to release heat. The self-heating temperature of coal gangue typically remains below 200 °C. Based on this, thermal conductivity tests were conducted on coal gangue slurry under different heating temperatures, durations, and grouting thicknesses. If the surface temperature of the material reaches equilibrium without exceeding the self-heating temperature of coal gangue, the thermal insulation performance of the slurry at that thickness meets the requirements for fire prevention in grouting backfill.
The experimental setup for thermal stability testing included a heating plate, heating chamber, temperature control box, and infrared thermal imager. Tests were performed with six heating temperatures (100 °C, 120 °C, 140 °C, 160 °C, 180 °C, and 200 °C), four grouting thicknesses (5 cm, 10 cm, 15 cm, and 20 cm), and seven heating time intervals (0, 10 min, 20 min, 30 min, 40 min, 50 min, and 60 min). The central, maximum, and minimum surface temperatures of the slurry were recorded at each interval.
The experimental procedure was as follows: First, the coal gangue slurry was slowly poured into the heating chamber to the designated thickness. The chamber containing the slurry was then placed on the heating plate, and the target temperature was set via the temperature control box. Upon reaching the specified temperature, an infrared thermal imager was used to capture and record the temporal variations in surface temperature, thereby evaluating the thermal conductivity of the slurry under different temperatures and thicknesses. The main experimental parameters are summarized in Table 1.

Table 1.
Experimental program.
2.4. Study on the Oxidation Characteristics of Coal Gangue After Grouting Backfill
The principle of studying the oxidation characteristics of coal gangue after grouting backfill involves determining the rate and extent of oxidation reactions by measuring the gas products (primarily CO and CO2) generated during the reaction between coal gangue and oxygen at different temperatures. The oxidation reaction releases substantial heat, which promotes further reactions with oxygen and triggers chain reactions that accelerate the oxidation process. When the temperature reaches the spontaneous ignition point (approximately 300 °C), self-ignition occurs.
The experimental procedure for the oxidation testing of coal gangue after grouting backfill was as follows:
- (1)
- Six grouting thicknesses (0 cm, 5 cm, 7 cm, 9 cm, 11 cm, and 13 cm) and three heating temperatures (100 °C, 200 °C, and 300 °C) were used.
- (2)
- The reactor liner was stacked with different thicknesses of gangue blocks and then we injected gangue blocks of gangue slurry so they were just submerged. The mixture was placed at room temperature for 24 h to dry for the next operation.
- (3)
- The dried specimens were placed in the reaction vessel and evacuated using a vacuum pump.
- (4)
- Dry air was introduced, and the programmed temperature controller was set to heat the vessel to the target temperature.
- (5)
- After stabilizing at the target temperature, gas sampling bags were used to collect reaction products.
- (6)
- The gases were collected and immediately introduced into the gas chromatograph. Thereafter, the O2, N2, CO, and CO2 compositions of the gases were examined in three batches and averaged.
- (7)
- The detected data were compared with standard gas samples to determine the proportional content of each gas. To investigate the effects of additives on the oxidation performance of backfilled coal gangue, additional tests were conducted using a coal-based solid waste backfill fire prevention platform. These tests focused on coal gangue with additives and a grouting thickness of 9 cm.
3. Results Analysis
3.1. Slurry-Forming Performance of Coal Gangue Slurry
The slurry-forming performance parameters of coal gangue slurry are presented in Table 2. As shown in Table 2, when the coal gangue slurry contains 20% coarse particles (1–3 mm) with a mass concentration of 68%, both the settling velocity and critical stabilization time at the solid–liquid interface are 0 min, indicating excellent stability without coarse particle segregation. Additionally, the plasticity index aligns with standard grouting material requirements, while the viscosity coefficient exceeds the specified range of 1–2 MPa·s for conventional grouting materials. These results confirm that the coal gangue slurry exhibits stable slurry-forming properties and is suitable for grouting backfill applications.

Table 2.
Test results of coal gangue slurry pulping performance.
3.2. Influence of Heating Duration on the Thermal Stability of Coal Gangue Slurry
The relationship between the thermal stability of coal gangue slurry and heating duration is shown in Figure 2. At a heating temperature of 140 °C, the central, maximum, and minimum surface temperatures of the slurry exhibit polynomial functional growth (R2 > 0.99) with increasing heating time. For instance, at 0 min of heating, these temperatures measure 27.6 °C, 34.4 °C, and 26.9 °C, respectively. After 60 min of heating, they rise to 48.3 °C, 58.0 °C, and 46.8 °C, corresponding to increases of 75.0%, 68.6%, and 73.9%. This indicates continuous heat absorption by the slurry during prolonged heating.
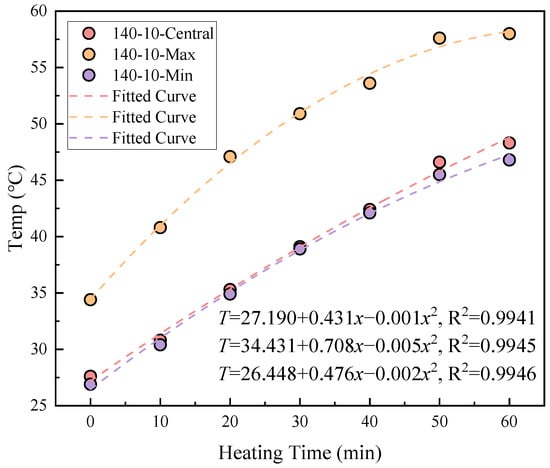
Figure 2.
Relationship between heating time and temperature.
Simultaneously, trace active components within the coal gangue slurry undergo hydration exothermic reactions upon contact with water, explaining why the initial central (27.6 °C) and minimum (26.9 °C) temperatures slightly exceed the ambient temperature (25 °C), while the maximum temperature reaches 34.4 °C at 0 min.
Furthermore, Figure 3 reveals that the central and minimum temperature zones remain adjacent throughout heating, whereas the maximum temperature consistently occurs at the slurry periphery. This demonstrates non-uniform heat distribution within the slurry, with temperatures gradually increasing from the center toward the edges after heating.
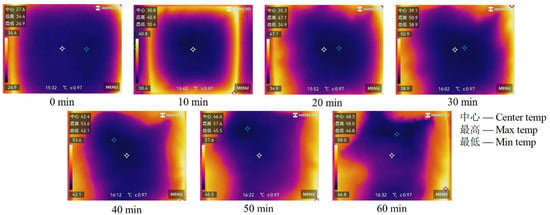
Figure 3.
Surface temperature distribution of coal gangue slurry with different heating times.
3.3. Influence of Grouting Thickness on the Thermal Stability of Coal Gangue Slurry
The relationship between the thermal stability of coal gangue slurry and grouting thickness is shown in Figure 4. At a heating temperature of 140 °C and a heating duration of 40 min, the surface temperature of the slurry initially increases and then decreases with increasing grouting thickness, peaking at 10 cm. Specifically, the central, maximum, and minimum surface temperatures rise from 30.4 °C, 39.7 °C, and 29.9 °C at 5 cm thickness to 42.4 °C, 53.6 °C, and 42.1 °C at 10 cm thickness, corresponding to increases of 39.4%, 35.0%, and 40.8%, respectively. Subsequently, these temperatures decrease to 27.5 °C, 29.4 °C, and 26.4 °C at 20 cm thickness, representing reductions of 54.2%, 82.3%, and 59.4% compared to the 10 cm thickness.
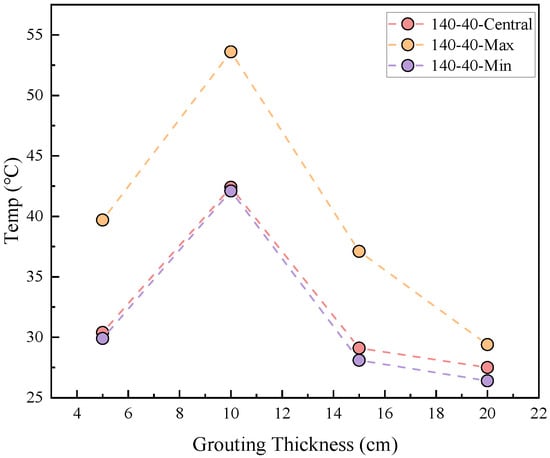
Figure 4.
Relationship between injection thickness and coal gangue slurry surface temperature.
This phenomenon indicates that grouting thickness significantly governs the temperature field evolution of coal gangue. The increased active components in thicker slurries enhance hydration exothermic reactions during prolonged heating, leading to higher surface temperatures at 10 cm compared to 5 cm. However, further increasing the thickness beyond 10 cm slows heat propagation due to the slurry’s low thermal conductivity, resulting in temperature reductions at 15–20 cm.
Figure 5 reveals the distinct temperature distribution patterns: at 5–10 cm thicknesses, the surface temperatures remain lower at the center and higher at the periphery, while at 15–20 cm thicknesses, the distribution becomes irregular with blurred boundaries (particularly at 20 cm). This is attributed to the stronger thermal inertia and lower inherent temperatures of thicker slurries, which suppress the temperature rise. These findings demonstrate that increasing grouting thickness effectively improves the thermal stability of coal gangue slurry.
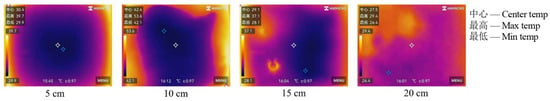
Figure 5.
Surface temperature distribution of coal gangue slurry under different thickness of grouting.
3.4. Influence of Heating Temperature on the Thermal Stability of Coal Gangue Slurry
The relationship between the thermal stability of coal gangue slurry and heating temperature is shown in Figure 6. Under conditions of 40 min heating duration and using a 10 cm grouting thickness, the central, maximum, and minimum surface temperatures of the slurry exhibit an overall increasing trend with rising heating temperatures. When heated to 100–120 °C, the central, maximum, and minimum surface temperatures range between 38.4 and 38.5 °C, 45.9 and 46.2 °C, and 33.8 and 32.6 °C, respectively. Subsequently, the central and minimum temperatures steadily increase to 57.3 °C and 49.6 °C at 200 °C, while the maximum temperature rises rapidly between 120 and 160 °C, peaks at 97.3 °C at 180 °C, and then decreases to 88.6 °C at 200 °C. Higher heating temperatures enhance heat transfer from the heating device to the slurry, resulting in increased heat absorption and elevated slurry temperatures under identical conditions, thereby driving the observed increases in surface temperatures. Figure 7 demonstrates the distinct temperature distribution characteristics: at 100–120 °C, minimal temperature variation is observed across the slurry surface; as temperatures rise to 140 °C, a center-to-periphery temperature gradient develops; however, localized high-temperature zones emerge at 160–200 °C, accompanied by uneven internal and surface temperature increases. This non-uniform thermal behavior may correlate with the irregular distribution of aggregate particles within the slurry, which disrupts heat conduction pathways.
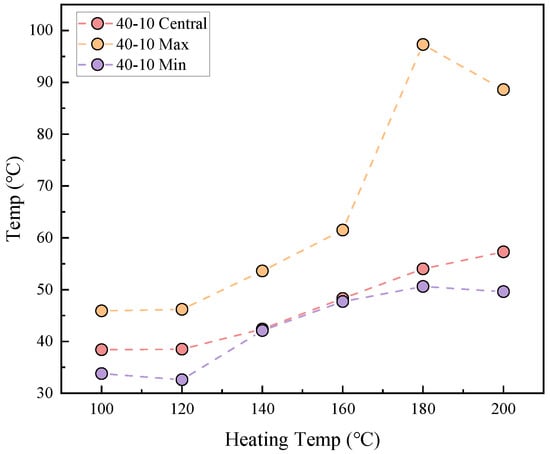
Figure 6.
Relationship between heating temperature and coal gangue slurry surface temperature.
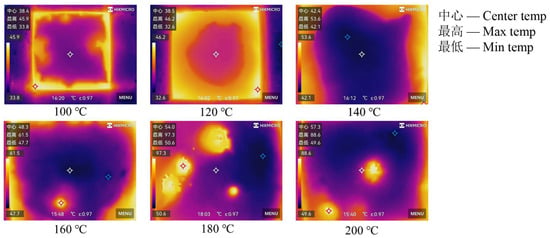
Figure 7.
Surface temperature distribution of coal gangue slurry under different heating temperatures.
3.5. Comprehensive Evaluation of the Thermal Stability of Coal Gangue Slurry Under Different Conditions
To comprehensively assess the thermal stability characteristics of coal gangue slurry under varying conditions, the average surface temperatures under different parameters were calculated (Figure 8). The temperature trends with heating duration, grouting thickness, and heating temperature align with Section 2.2, Section 2.3 and Section 2.4. The average surface temperature increases from 30.65 °C to 52.4 °C as the heating duration extends from 0 to 60 min; fluctuates between 27.9 °C and 34.8 °C with grouting thickness increasing from 5 cm to 20 cm; and rises from 39.8 °C to 73.9 °C with heating temperatures from 100 °C to 180 °C, then slightly decreases to 69.1 °C at 200 °C.
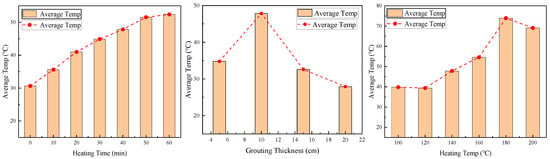
Figure 8.
Average surface temperature of coal gangue slurry under different conditions.
Jia et al. [22] assert that the spontaneous combustion of coal gangue parallels coal self-ignition, progressing through three stages: slow heating, auto-accelerated oxidation, and stable combustion. Spontaneous ignition occurs when temperatures exceed the critical range of 80–90 °C. Crucially, the average surface temperature remains below 80 °C for slurries with grouting thicknesses exceeding 5 cm, confirming their suitability for fire prevention in goaf grouting backfill applications.
3.6. Evaluation of the Oxidation Performance of Coal Gangue
The principle of studying the oxidation characteristics of coal gangue after grouting backfill involves determining the extent of oxidation reactions by measuring the gas products (primarily CO and CO2) generated during the reaction between coal gangue and oxygen at different temperatures. The oxidation reaction releases substantial heat, which promotes further reactions with oxygen and triggers chain reactions, accelerating the oxidation process. When the temperature reaches the spontaneous ignition temperature (approximately 300 °C), self-ignition occurs.
The oxidation performance of coal gangue is influenced by both its chemical composition (e. g., organic matter, pyrite, kaolinite, and illite) and external environmental factors such as the ambient temperature and oxygen concentration. Elevated temperatures accelerate oxidation reactions, while the oxygen concentration modulates the reaction rate. To investigate these effects, oxidation performance tests were conducted using a coal-based solid waste backfill fire prevention test platform (Figure 9) under varying grouting thicknesses.

Figure 9.
Coal-based solid waste filling fire prevention test platform.
3.6.1. Gas Content After the Oxidation of Coal Gangue
Figure 10 presents the gas content after coal gangue oxidation under varying grouting thicknesses and heating temperatures. The primary gases include N2, O2, CO, and CO2. The N2 and O2 concentrations remain stable despite increasing heating temperatures and grouting thicknesses. However, the CO and CO2 concentrations progressively rise with heating temperatures from 100 °C to 300 °C, indicating enhanced oxidation activity. This is attributed to the increased reactivity of combustible components within coal gangue at elevated temperatures, which accelerate oxidation rates and gas release.
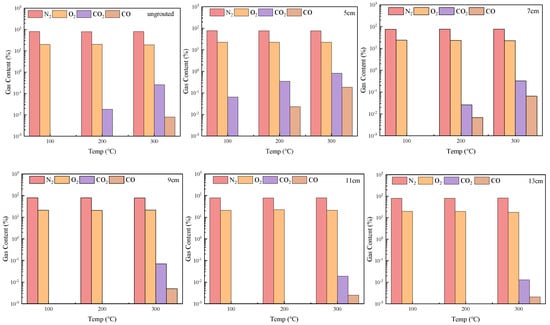
Figure 10.
Changes in gas content after coal gangue conservation reaction.
At grouting thicknesses of 9–12 cm, CO and CO2 emissions occur only at 300 °C and decrease with increasing thickness, demonstrating that thicker grouting effectively suppresses oxidation. As characteristic carbon oxides, CO and CO2 originate from the reaction between oxygen and carbon-containing free radicals on coal gangue surfaces [23,24,25].
Using 300 °C as a representative case (Figure 11), the concentrations of CO and CO2 initially increase and then decrease with grouting thickness, peaking at 5 cm. Unfilled coal gangue (0 cm) releases higher levels of CO/CO2 due to limited particle quantity and unrestricted oxidation. Thin grouting layers (e.g., 5 cm) enhance oxidation by enlarging particle fissures through water immersion, increasing oxygen contact areas. Conversely, thicker grouting (>5 cm) fills internal pores, isolating combustible materials from oxygen and reducing gas emissions.
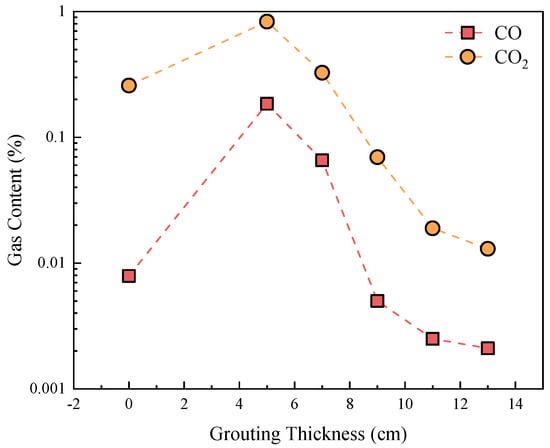
Figure 11.
CO and CO2 contents of coal gangue after oxidation reaction.
These findings confirm that grouting thickness and temperature critically govern oxidation performance. To prevent energy accumulation and spontaneous ignition, a recommended grouting thickness of 9–11 cm effectively inhibits oxidation.
3.6.2. Influence of Additive Type on Oxidation Reaction of Coal Gangue Slurry
The effects of different additives on the oxidation reaction of coal gangue slurry are shown in Figure 12. The N2 and O2 concentrations remain stable across all additive-modified slurries. However, significant variations occur in CO and CO2 emissions. At 100 °C and 200 °C, no CO or CO2 is generated in any additive-modified slurry. Figure 13 quantifies gas emissions at 300 °C, revealing that ammonium polyphosphate (APP)-modified slurry produces substantially lower CO and CO2 levels compared to the control group, while zinc borate- and magnesium hydroxide-modified slurries exhibit higher emissions (except for CO in magnesium hydroxide-modified slurry). This demonstrates distinct fire suppression efficiencies among additives, with APP showing superior oxidation inhibition and fire prevention capabilities.
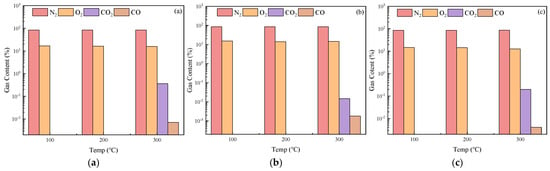
Figure 12.
Changes in gas content after oxidation reaction of additive-doped coal gangue ((a) ZB; (b) APP; (c) MH).
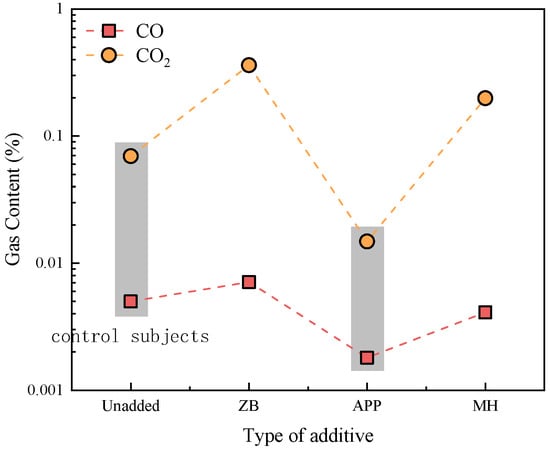
Figure 13.
CO and CO2 gas contents after oxidation reaction of additive-doped coal gangue.
3.6.3. Influence of Additive Dosage
The gas content released during the oxidation of coal gangue slurry modified with varying ammonium polyphosphate (APP) dosages is shown in Figure 14 and Figure 15. As previously stated, CO and CO2 emissions occur only at a heating temperature of 300 °C, and their concentrations gradually decrease with increasing APP content. At a grouting thickness of 9 cm and a heating temperature of 300 °C, the control group emits 0.0050% CO and 0.0695% CO2. These values decrease sharply to 0.0021% CO and 0.0179% CO2 at a 3% APP dosage (reductions of 58.0% and 74.2%, respectively), then gradually decline to 0.0015% CO and 0.0117% CO2 at a 9% APP dosage, representing maximum reductions of 70.0% and 83.2% compared to the control group. This suppression effect arises from enhanced oxygen isolation at coal gangue surfaces with higher APP contents, which impedes oxidation reactions and reduces gas emissions. Considering the flame-retardant efficiency of APP, a dosage of 3% is recommended.
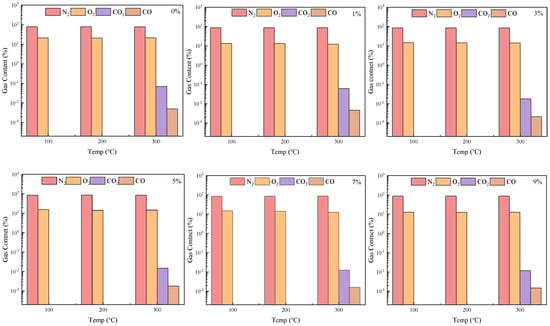
Figure 14.
Test results for oxidation performance of ammonium polyphosphate (APP) on slurry-filled coal gangue with different additive amounts.
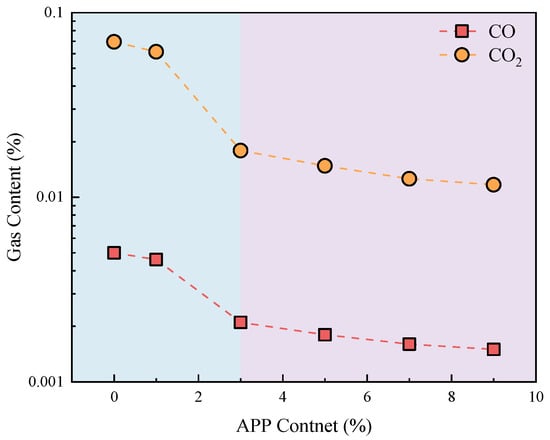
Figure 15.
Variation in CO and CO2 gas contents after coal gangue reaction with different addition amounts of ammonium polyphosphate (APP).
4. Fire Suppression Mechanisms
The macroscopic manifestations of coal oxidation result from synergistic interactions of microstructural evolution. During the grouting immersion of coal gangue, inevitable physicochemical alterations occur that subsequently influence the spontaneous combustion characteristics. By analyzing pore architecture, microcrystalline structures, and micromorphology before and after slurry immersion, combined with the established condensed-phase and gas-phase flame-retardation mechanisms of ammonium polyphosphate (APP), a microstructural explanation aligning with the macroscopic phenomenon of CO/CO2 emissions initially increasing and then decreasing with grouting thickness was derived. This phenomenon arises from APP’s dual-phase inhibition: condensed-phase carbon layer formation blocks oxygen diffusion, while gas-phase noncombustible emissions dilute reactive oxygen concentrations, collectively suppressing oxidation chain reactions.
Figure 16 illustrates the changes in the surface pore structures of coal gangue before and after grouting. Pre-grouting, coal gangue exhibits distinct pores and cracks, which are reduced in quantity, length, and size compared with those post-grouting. Water exerts dual effects on coal gangue self-ignition: initial promotion followed by inhibition as grouting thickness increases. During initial slurry immersion, hydraulic scouring removes pore impurities, enlarging pore structures and increasing the specific surface area. Concurrently, water-derived -OH groups replace weakly bonded hydrogen atoms on coal surfaces, elevating -OH density. A higher -OH content enhances oxidation reactivity with atmospheric oxygen, accelerating combustion [26]. During heating, residual pore water evaporation expands oxygen contact areas, while surface -OH groups intensify oxidation, leading to elevated CO/CO2 emissions in thinly grouted samples compared to untreated coal gangue. As the thickness of the grout increases, the oxygen in the slurry gradually decreases and the amount of -OH produced by the excitation of water molecules tends to be saturated. As demonstrated in Figure 17, the number of cracks gradually stabilizes and does not continue to expand, and the amount of CO and CO2 produced by oxidation is significantly reduced compared with the previous one.
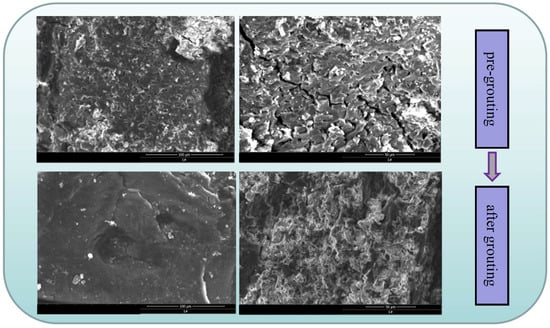
Figure 16.
Pore change of coal gangue surface before and after grouting.
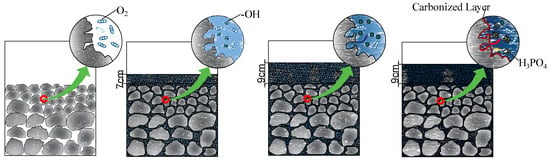
Figure 17.
Sectional view under different grouting thicknesses.
APP is thermally decomposed to generate polyphosphoric acid (H6P4O13) and metaphosphoric acid (HPO3), releasing phosphoric acid derivatives. The acidic environment promotes the dehydration reaction of hydrocarbons in the coal gangue and accelerates the cross-linking carbonization process. Phosphoric acid esterifies with hydroxyl groups in coal to form a stable phosphate cross-linking network that inhibits the release of volatile combustibles. The PO-, HPO-, and PO2- radicals generated by the decomposition of APP inhibit the oxidation chain reaction of coal through the following mechanisms: the PO- radical reacts with the -H generated by the oxidation of coal and blocks the chain-induced phase, the hydroxyl radical quenches, and HPO- reacts with -OH and terminates the chain-growth reaction. The P2O5 generated by APP decomposition reacts with the ash in the coal gangue to generate glassy phosphate, which enhances the densification and thermal stability of the carbon layer. The decomposition of APP releases the non-flammable gases, such as NH3 and NO, to reduce the oxygen concentration and inhibit the flame propagation, and the gas expands to form a closed pore structure within the viscous flow state carbon layer, which significantly reduces the thermal conductivity. Combustion is inhibited through a dual mechanism [27,28,29], as shown in Figure 18.
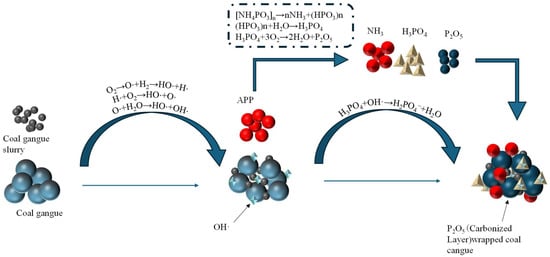
Figure 18.
Fire extinguishing mechanism of ammonium polyphosphate (APP).
5. Conclusions
This study, based on the engineering conditions at Wulanmulun Coal Mine, systematically evaluated the stability, surface temperature field distribution, and gas emission characteristics of coal gangue slurry through slurry-forming performance tests, thermal stability assessments, and oxidation behavior analysis. The key conclusions are as follows:
- The coal gangue slurry of this type exhibited no solid–liquid interface separation, with a plasticity index consistent with the requirements of standard grouting materials. The stable slurry-forming performance indicates its suitability as a grouting backfill material.
- With increasing heating duration, the central, maximum, and minimum surface temperatures of the coal gangue slurry followed polynomial growth functions (R2 > 0.99). As the grouting thickness increased, the surface temperature initially rose and then declined, peaking at a thickness of 10 cm. Additionally, the central, maximum, and minimum surface temperatures displayed an overall ascending trend as the heating temperature increased.
- When the heating temperature increased from 100 °C to 300 °C, the CO and CO2 concentrations progressively rose. At grouting thicknesses of 9–12 cm, CO and CO2 gases were only detected at 300 °C during coal gangue oxidation, with their concentrations decreasing as grouting thickness increased. The oxidation reaction rate, reflected by CO and CO2 concentrations, demonstrated that CO levels initially increased and then decreased as grouting thickness rose from 0 cm to 13 cm, reaching a maximum at 5 cm. Comparative analysis of additives with identical mass fractions revealed that the coal gangue slurry modified with ammonium polyphosphate (APP) released significantly lower amounts of CO and CO2 than the control group or slurries modified with zinc borate or magnesium hydroxide, confirming APP’s superior inhibitory effect.
- Increasing APP dosage progressively reduced CO and CO2 emissions from the coal gangue slurry, with maximum reductions of 70.0% and 83.2%, respectively, compared to the control group, markedly enhancing fireproofing performance. Mechanistic analysis indicates that APP decomposes upon heating, generating non-volatile phosphorus oxides and polyphosphoric acid that coat the coal gangue surface to isolate oxygen. Under high temperatures, APP dehydrates to form polyphosphoric or metaphosphoric acid, which scavenges free radicals such as -OH and inhibits combustion, as well as being a strong dehydrating agent. This promotes carbonization with char-forming substances, generating an expanded carbon layer reinforced by non-combustible gases, thereby effectively isolating air and blocking fire propagation. The fire prevention and suppression system based on the synergistic interaction between coal gangue slurry and APP not only meets the grouting requirements for coal mine goafs but also extends to other coal-bearing solid waste accumulation scenarios such as open-pit mines and abandoned mine backfilling, offering a universal solution for ecological restoration and fire hazard control in mining areas.
Author Contributions
Writing—original draft, R.W., X.L., S.W., X.S., H.Y. and Z.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 52364011), the Natural Science Foundation of Jiangxi Province (No. 20232BAB204034), and the Jiangxi University of Science and Technology Qingjiang Young Excellence Support Program Grant (No. JXUSTQJYX2019007).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to Data are contained within the articleprivacy.
Conflicts of Interest
Author Haigen Yu was employed by the company Changsha Institute of Mining Research Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ministry of Natural Resources. National Mineral Reserves Statistical Table. J. Nat. Resour. 2023. Available online: https://www.mnr.gov.cn/sj/sjfw/kc_19263/kczycltjb/202408/t20240802_2853983.html (accessed on 19 February 2025).
- Wang, S.; Liu, L.; Zhu, M.; Shen, Y.; Shi, Q.; Sun, Q.; Fang, Z.; Ruan, S.; He, W.; Yang, P.; et al. New Way for Green and Low-Carbon Development of Coal Under the Target of “Double Carbon”. J. China Coal Soc. 2024, 49, 152–171. [Google Scholar] [CrossRef]
- Zhou, N.; Yao, Y.; Song, W.; He, Z.; Meng, G.; Liu, Y. Present Situation and Prospect of Coal Gangue Treatment Technology. J. Min. Saf. Eng. 2020, 37, 136–146. [Google Scholar] [CrossRef]
- Pan, S.; Hu, Y.; Wang, H.; Yu, J.; Liu, Q. Current Status and Standard System of Comprehensive Utilization Technology for Coal Gangue. China Min. 2024, 33, 89–98. [Google Scholar] [CrossRef]
- Lan, C. Progress of Research on Comprehensive Utilization of Coal Gangue Resources. J. Inner Mong. Coal Econ. 2024, 17, 32–34. [Google Scholar] [CrossRef]
- Gu, W.; Yang, B.; Zhu, L.; Zhao, M. Study on Spatial Characteristics of Gangue Slurry Filling Mining and Engineering Practice. J. Min. Sci. Technol. 2023, 8, 409–418. [Google Scholar] [CrossRef]
- Li, J.; Zheng, K.; Xuan, D.; Xu, J.; Dong, Z. Experimental Study on Slurry Diffusion Law During the Non-Pressure Stage in Overburden Isolated Grouting. J. China Coal Soc. 2020, 45, 78–86. [Google Scholar] [CrossRef]
- Wei, Z. A Study on the Compaction Characteristics of Broken Rock in the Collapse Zone of Goaf and the Diffusion Mechanism of Grouting and Filling Slurry. Anhui Univ. Sci. Technol. 2023. [Google Scholar] [CrossRef]
- Ma, C.; Xuan, D.; Xu, J.; Li, J.; Zhang, Z.; Ning, X. Experimental Study on Water Reduction and Modification of Filling Slurry by Isolated Grouting in Overlying Strata. J. Min. Saf. Eng. 2023, 40, 1122–1128. [Google Scholar] [CrossRef]
- Wang, Q.; Ao, L.; Zhang, K.; Yang, K.; Tang, Y.; Tan, X. Research Progress on the Key Influencing Factors of Spontaneous Combustion of Coal Gangue and Control Methods. Clean Coal Technol. 2024, 30, 228–238. [Google Scholar] [CrossRef]
- Onifade, M. Countermeasures Against Coal Spontaneous Combustion: A Review. Int. J. Coal Prep. Util. 2021, 42, 2953–2975. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Shi, X.; Liu, S.; Shu, P.; Xia, S. Investigation of Thermal Behavior and Hazards Quantification in Spontaneous Combustion Fires of Coal and Coal Gangue. Sci. Total Environ. 2022, 843, 157072. [Google Scholar] [CrossRef]
- Fan, G.; Zhang, D.; Wang, X. Reduction and Utilization of Coal Mine Waste Rock in China: A Case Study in Tiefa Coalfield. Resour. Conserv. Recycl. 2014, 83, 24–33. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, R.; Wang, Z.; Zhang, X. Research on the Fire Extinguishing Performance of New Gel Foam for Preventing and Controlling the Spontaneous Combustion of Coal Gangue. Environ. Sci. Pollut. Res. 2023, 30, 88548–88562. [Google Scholar] [CrossRef]
- Ma, L. Preparation of Ash-Diatomite-PAM Composite Colloid Based on Activation Technology and Research on Fire-Extinguishing Mechanism. Taiyuan Univ. Technol. 2022. [Google Scholar] [CrossRef]
- Li, X. Preparation of Al-XG Composite Gel and Experimental Study on Inhibiting Coal Spontaneous Combustion. Henan Polytech. Univ. 2022. [Google Scholar] [CrossRef]
- Jiao, G. Study on Anti-Oxidation Type Microencapsulated Inhibitor for Preventing Coal Spontaneous Combustion. China Univ. Min. Technol. 2020. [Google Scholar] [CrossRef]
- Sun, L.; Li, W.; Duan, S.; Lv, X.; Shi, Q.; Qi, G.; Huang, Q. Temperature-Sensitive Intelligent Gel to Prevent Coal Spontaneous Combustion: Large-Scale Simulation Fire Extinguishing Experimental Study. Fuel 2025, 384, 133812. [Google Scholar] [CrossRef]
- Wang, P.; Tan, X.; Hao, H.; Wang, P.; Li, H.; Li, B.; Ma, J.; Guo, C. Gangue Geopolymer to Strength Fire Resistance of Intumescent Waterborne Coating. J. Coat. Technol. Res. 2025, 22, 985–998. [Google Scholar] [CrossRef]
- Huang, X.; Gao, H.; Wu, J.; Liu, X.; Zhang, P.; Huang, Y.; Yan, J.; Gao, Y.; Wang, L.; Rao, Z. Study on the Preparation of High Degree of Polymerization APP Flame Retardant. Non-Met. Mines 2021, 44, 33–36. [Google Scholar]
- Chen, Y.; Liu, X.; Wen, S.; Chen, J.; Gu, F.; Wu, J.; Meng, Y. Research Progress of Ammonium Polyphosphate Flame Retardant Modification and Application. China Plast. Ind. 2024, 52, 26–35. [Google Scholar] [CrossRef]
- Jia, B. Study on Mathematical Model of Spontaneous Combustion and Its Prevention and Harness Technology of Coal Gangue Dump. Master’s Thesis, Liaoning Technical University, Fuxin, China, 2002. [Google Scholar]
- Zhou, C.; Li, X.; Zhang, J.; Ren, X.; Yao, J.; Li, Z.; Li, T. Study on Oxidation Characteristics of Coal Gangue with Different Moisture Content Under Water Immersion Drying. Coal Sci. Technol. 2024, 52, 107–115. [Google Scholar] [CrossRef]
- Zhou, X.; Song, D.; Nie, R.; Bai, G.; Li, A.; Sun, B. Study on Formation Law of Carbon Oxides of Lignite in Combustion Phase. China Saf. Sci. J. 2016, 26, 59–63. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Xu, C. Research on Free Radical Variation Law in Spontaneous Combustion and Oxidation Process of Coal Gangue. Ind. Mine Autom. 2020, 46, 34–37. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Han, D.; Ren, X. Study on Correlation Between Exothermic Risk of Coal Gangue and Microscopic Groups. Saf. Coal Mines 2024, 55, 107–115. [Google Scholar] [CrossRef]
- Shao, Z.; Deng, C.; Tan, Y.; Chen, M.; Chen, L.; Wang, Y. An Efficient Mono-Component Polymeric Intumescent Flame Retardant for Polypropylene: Preparation and Application. ACS Appl. Mater. Interfaces 2014, 6, 7363–7370. [Google Scholar] [CrossRef]
- Wang, B.; Sheng, H.; Shi, Y.; Hu, W.; Hong, N.; Zeng, W.; Ge, H.; Yu, X.; Song, L.; Hu, Y. Recent Advances for Microencapsulation of Flame Retardant. Polym. Degrad. Stab. 2015, 113, 96–109. [Google Scholar] [CrossRef]
- Wang, Z. Preparation of Functionalized Ammonium Polyphosphate and Study on Structure and Properties of Its Flame Retardant Polymer Composites. Shenyang Univ. Chem. Technol. 2022. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).