Abstract
The aviation industry needs to develop sustainable, fire-safe cabin interior materials. Although wood is eco-friendly, its high flammability makes it challenging to meet flame retardant standards. Enhancing wood fire safety requires the creation of an environmentally friendly and flame retardant coating. In this study, a new type of intumescent flame retardant (IFR) coating was applied to the wood surface using the layer-by-layer (LBL) technique, with fully bio-based chitosan (CS), agar, and phytic acid (PA) as key components. The coated wood demonstrated improved durability, flame resistance, and thermal stability. Particularly, the Wood-2 sample achieved a vertical burning test (UL-94) V-0 rate and a limiting oxygen index (LOI) of 53.1%, which exceeded most previous reported flame retardant coatings. Cone calorimeter test and infrared thermography analysis confirmed that a thick layer of intumescent char formed when the coating was exposed to heat, effectively hindering heat transfer and oxygen supply. This flame retardant effect is attributed to a synergistic mechanism involving nitrogen/phosphorus (N/P) elements. This study offers an environmentally friendly solution for wood flame retardancy and lays an experimental and theoretical foundation for the development of green aviation interior materials.
1. Introduction
Aviation safety is the lifeline of the civil aviation industry, and the fire resistance of cabin interior materials is crucial for ensuring passenger safety []. Currently, extensively used aviation interior materials, such as thermosetting polymers and textiles, rely on nonrenewable petroleum resources and are prone to producing toxic and harmful substances when burned, posing safety hazards [,]. Therefore, there is an urgent requirement to create new interior materials that are environmentally friendly, sustainable, and fire resistant.
In this context, wood is gaining attention as a candidate material for non-structural components, such as cabin interior linings and lockers, due to its advantages of renewability and degradability. However, its inherent flammability does not meet aviation safety requirements []. Thus, fire-retardant treatment of wood is essential for enabling its aviation applications [,,,].
Surface treatment is one of the flame retardant treatments for wood with low cost and high efficiency. This method involves directly coating, spraying, or depositing flame retardants onto the wood surface to form a protective layer after burning, which isolates the substrate from heat without altering its intrinsic properties [,,]. Traditional flame retardants were previously widely adopted due to their highly effective flame retardant qualities. However, these chemicals released substantial amounts of hazardous gases during combustion, such as hydrogen halides, which posed serious threats to the environment and human health [,,]. Later, halogen-free and eco-friendly flame retardants emerged as a primary research focus. Consequently, it is significant to develop a novel flame retardant treatment for wood that is eco-friendly, highly efficient, and preserves the wood’s original characteristics. The intumescent flame retardant (IFR) might develop a swelling char layer on the material’s surface, exerting a flame retardant effect in the condensed phase, while releasing non-flammable gases to accomplish flame retardant properties in the gas phase [,,]. Thus, the IFR system was considered an effective and eco-friendly flame retardant.
Recently, bio-based flame retardants have garnered considerable attention in flame retardant fields [,,]. Bio-based compounds have been used to construct the IFR system. These materials were primarily derived from renewable biomass, including plant and animal-based origins, and offer advantages such as wide availability, renewability, biocompatibility, and non-toxicity [,,]. Chitosan (CS), a natural polysaccharide containing abundant amino and hydroxyl groups in its molecular structure, serves as a carbon source and foaming agent in the IFR system [,,]. Phytic acid (PA), noted for its high phosphorus content, acts as an acid source in the IFR system [,,]. Zhang et al. [] developed a composite flame retardant coating for plywood by polymerizing zinc phytate chelated with Si-C-P, synthesized from CS and 9,10-Dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO), which significantly improved thermal stability and flame retardancy. Song et al. [] synthesized phytic ammonium using PA and further developed wood coatings with outstanding thermal stability and flame retardant characteristics. The optimized coating achieved a LOI value of 39.1% and a UL-94 V-0 rate. Yu et al. [] developed a multifunctional flame retardant coating by CS, polyphosphoric acid, melamine, and ammonium molybdenum phosphorus. The incorporation of CS promoted a highly crosslinked three-dimensional network structure in the coating, increasing flame retardancy and compatibility.
However, no previous study had reported the enhancement of wood flame retardancy through fully bio-based CS/agar/PA coating. In this study, a novel bio-based CS/agar/PA IFR coating was successfully deposited on the wood surface via the LBL technique. In this designed IFR system, agar functions as a complementary polysaccharide to CS, acting as a synergistic char-forming agent []. By alternately depositing different functional substances, the composition and structure of the coating can be precisely controlled, thereby enabling the regulation of coating properties. The flame retardant performance was evaluated using the cone calorimetry test (CCT) and infrared thermography analyzer, while thermal stability was investigated with a thermal gravimetric analyzer (TGA). Flame retardant characteristics were further assessed through limiting oxygen index (LOI) and UL-94 vertical burning test. The morphology and structure of the residual char were examined by scanning electron microscopy (SEM) and Raman spectroscopy, providing insight into the flame retardant mechanism. This work establishes an experimental foundation for developing fully bio-based IFR coating with improved environmental compatibility, flame retardancy, and durability, broadening the application range of bio-based materials in the aviation field and providing a new solution for improving the fire safety performance of aircraft interiors.
2. Materials and Methods
2.1. Materials
The wood was obtained from Chuzhou Xinyu Wood Co., Ltd., Chuzhou, China. PA (50 wt.% aqueous solution, CAS: 83-86-3), agar (reagent grade, CAS: 9002-18-0), and H3PO3 (reagent grade, CAS: 13598-36-2) were purchased from Aladdin Reagent Co., Ltd., Shanghai, China. CS (deacetylation degree ≥ 95%, CAS: 9012-76-4) was purchased from Shanghai Macklin Biochemical Co., Ltd., Shanghai, China.
2.2. Preparation of CS–Agar Solution
20 g CS was dispersed in 980 g of deionized water under stirring. Subsequently, 200 mL of the 1 mol/L H3PO3 solution was added to the CS dispersion at 50 °C under stirring until the CS was completely dissolved. H3PO3 was introduced to provide an acidic environment for the dissolution of CS. Then, 10 g of agar was added at 60 °C and stirred thoroughly to obtain CS–agar solution.
2.3. Preparation of CS/Agar/PA Coating by LBL Assembly
To ensure consistent coating formation, the CS–agar solution was uniformly applied to wood samples using a nylon brush. The coated samples were dried at 80 °C in an oven for a fixed duration of 5 h to avoid variations from inconsistent drying. This standardized brushing/drying cycle was strictly repeated twice for all Wood-1 samples. For Wood-2 and Wood-3, the brushing and drying processes were identical. Then the samples were immersed in PA solution with mass fractions of 3% and 5% at a controlled temperature of 25 °C for 10 min (with a consistent solution volume of 700 mL per batch to ensure uniform immersion), followed by drying at 80 °C for 5 h. Each sample went through this cycle twice. The detailed coating formulation for wood samples was summarized in Table 1, and the flowchart of sample preparation was illustrated in Figure 1.

Table 1.
Coating formulation for wood samples.
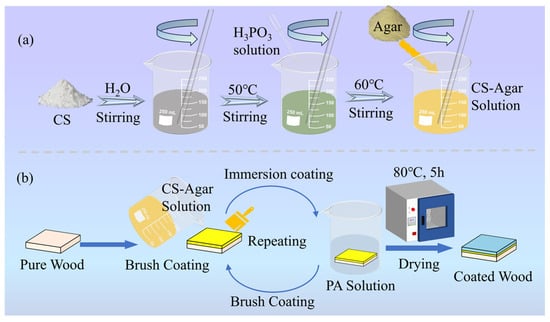
Figure 1.
Flowchart of sample preparation (a) preparation of the CS–agar solution (b) preparation of CS/agar/PA coating.
2.4. Measurements
The microstructure of the samples was analyzed using Fourier transform infrared, (FTIR, IRPrestige-21, Shimadzu Inc., Kyoto, Japan), X-ray photoelectron spectroscope (XPS, Thermo Scientific K-Alpha, Thermofisher Scientific Inc., Waltham, MA, USA), scanning electron microscopy (SEM, Prisma, FEI Inc., Hillsboro, OR, USA), and energy dispersive spectrometer (EDS, Phenom XL G2, Thermofisher Scientific Inc., Waltham, MA, USA).
Thermal stability of samples was analyzed using a thermal gravimetric analyzer (TGA 4000, PerkinElmer Inc., Shanghai, China) under N2 atmosphere. The samples were heated from 30 °C to 800 °C at a heating rate of 10 °C/min.
The flame retardant characteristics of wood samples were evaluated through limiting oxygen index (LOI), UL-94 vertical burning test and cone calorimetry test (CCT), with five replicates conducted for each test to reduce experimental noise. The LOI was measured with a JF-3 tester (Jiangning Analytical Instrument Inc., Nanjing, China) according to the GB/T 5454-1997 standard. For the UL-94 vertical burning test, a CZF-3 tester (Jiangning Analytical Instrument Inc., Nanjing, China) was employed according to the GB/T 2408-2008. The combustion behavior of wood samples was measured by CCT (Kunshan Combustion Technology Instrument Inc., Kunshan, China) according to the ISO 5660 with a heat flux of 50 kW/m2.
Mechanical characteristics of samples were tested with a WDW-100 electro-mechanical universal testing machine (Zhuo Di Instrument & Equipment Inc., Shanghai, China), with five replicates per sample type.
Thermal insulation capability was evaluated with a TA-60 infrared thermography analyzer (Shenzhen Dianyang Technology Inc., Shenzhen, China), with three replicates of the heating test (5 min and 8 min) performed for each sample.
The QFH-A paint film adhesion tester (Dongguan Huaguo Precision Instrument Inc., Dongguan, China) tested the adhesion between the coating and wood according to the GB/T17657-2013 standard.
The char residue of wood samples after CCT was examined using scanning electron microscopy (SEM, Prisma, FEI Inc., Hillsboro, OR, USA), X-ray photoelectron spectroscopy (XPS, Thermo Scientific K-Alpha, Thermofisher Scientific Inc., Waltham, MA, USA), and Raman (LabRam, Horiba, Solie Inc., Kyoto, Japan).
Thermogravimetric–Fourier-transform infrared (TG-FTIR) was performed using a Thermo Scientific Nicolet iS50 spectrometer (Thermofisher Scientific Inc., Waltham, MA, USA) under N2 atmosphere, with the temperature increasing from 30 °C to 800 °C at a heating rate of 10 °C/min.
3. Results
3.1. Characterization of CS/Agar/PA Coating
The FTIR spectra of agar, CS, H3PO3 and Wood-2 are shown in Figure 2a. The characteristic absorption peaks of -OH (3300–3400 cm−1) and C-O-C (1159 cm−1) were observed in the FTIR spectrum of the agar. The FTIR spectrum of CS showed peaks corresponding to -OH (3300–3400 cm−1), C-O (1090 cm−1), C=O (1658 cm−1), C-N (1588 cm−1), and -CH3 (1380 cm−1) []. The FTIR spectra of H3PO3 showed the characteristic absorption peaks of P-H (2279 cm−1) and P-O (1110 cm−1). Wood-2’s FTIR spectra retained the characteristic peaks of -OH (3300- 3400 cm−1) and C-O-C (1159 cm−1), while the peaks of C=O (1658 cm−1) and -CH3 (1380 cm−1) were broadened. A new characteristic peak at P-O-H (1064 cm−1) appeared, which could be the result of cross-linking or hydrogen bonding.
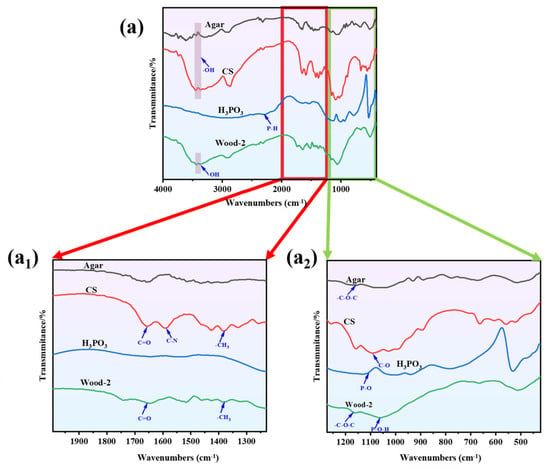
Figure 2.
(a) FTIR spectra of agar, CS, H3PO3, and Wood-2; (a1,a2) partial amplification of FTIR spectra.
XPS spectra of the Wood-2 sample were illustrated in Figure 3a–e. The survey XPS spectra revealed new bands of P (P 2s and P 2p). Furthermore, the C 1s spectra revealed three bands at 284.8 eV, 286.6 eV, and 288.2 eV, corresponding to the C-H, C-N, and C=O bonds. The N 1s spectra showed two bands at 400.3 eV and 402.1 eV, which were attributed to N-H and N-C bonds. The O 1s spectra showed three bands at 532.1 eV, 532.6 eV, and 533.8 eV, which represented O-P, O=C, and O-C bonds. The bands at 134.1 eV and 133.1 eV in the P 2p spectra correspond to P-O and P-C bonds []. XPS analysis of Wood-2 confirmed the successful introduction of phosphorus, demonstrating that CS, agar and PA form a composite structure through chemical bonds.
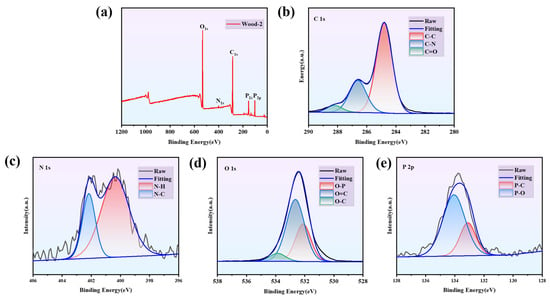
Figure 3.
XPS spectra of (a) survey scan, (b) C 1s, (c) N 1s, (d) O 1s, (e) P 2p of Wood-2.
3.2. Surface Morphology and Elemental Constituents
To investigate the morphological changes on the wood surface, SEM was utilized to characterize the surfaces of both coated and pure wood. As shown in Figure 4a, the natural structural characteristics of the pure wood surface were completely preserved, including clear surface textures, uniformly distributed duct cell openings, and regularly aligned wood rays, with no foreign material deposition observed. In contrast, as illustrated in Figure 4b–d, the coated wood samples (Wood-1, Wood-2, and Wood-3) showed significant morphological changes. The coatings formed a continuous layer that effectively covered the underlying wood fiber structure. The surface exhibited a characteristic texture with micro-scale features associated with the formation of a cross-linked biopolymer network. Despite this complex microstructure, the EDS analysis of the Wood-2 surface showed a uniform distribution of C, N, O, and P elements, confirming that the coating formed a continuous protective layer and successfully incorporated P elements.
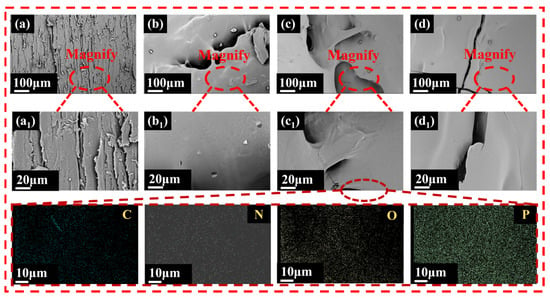
Figure 4.
SEM images of (a,a1) pure wood, (b,b1) Wood-1, (c,c1) Wood-2, and (d,d1) Wood-3.
3.3. Thermal Stability
TGA was used to investigate the thermal degradation behavior of pure wood and coated wood, and the results are shown in Figure 5 and Table 2. The TGA results showed that pure wood experienced a mass loss stage between 50 and 200 °C, which is primarily attributed to the evaporation of moisture []. The temperature corresponding to the maximum mass loss rate (Tmax) was 378 °C. In comparison, the coated wood samples showed significant changes in residual char and Tₘₐₓ, indicating that the coatings considerably influenced the thermal decomposition process of wood. The temperature of mass loss for 10 wt.% (T10%) of pure wood was 196.25 °C, which was substantially lower than that of the coated samples (Wood-1: 229.61 °C; Wood-2: 241.55 °C; and Wood-3: 248.57 °C). This could indicate that the CS/agar/PA synergistic barrier effectively delayed material mass loss. However, the temperature of mass loss for 50 wt.% (T50%) of pure wood was 368.82 °C, whereas the T50% of Wood-1 coated with CS–agar solution was reduced to 364.97 °C. The T50% of coated wood was further reduced with the addition of PA, due to the early degradation of CS and PA, which accelerated the decomposition of the wood. Although the coating improved the early thermal stability, the catalytic impact of PA at high high-temperature stage accelerated the overall thermal degradation process. More specifically, the coated wood had lower Tmax values than the pure wood (pure wood: 378 °C, Wood-1: 326 °C, Wood-2: 315 °C, Wood-3: 303 °C). This could be attributable to the low thermal stability of PA, which was consistent with prior research findings []. The phosphoric acid or polyphosphoric acid derived from PA decomposition promoted char formation during the thermal degradation process.
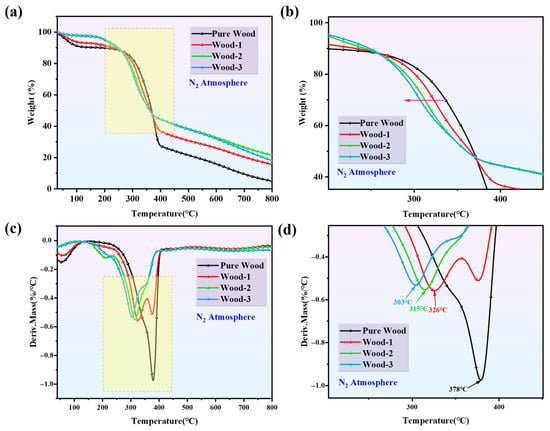
Figure 5.
(a) TG curves, (b) partially amplified TG curves, (c) DTG curves and (d) partially amplified DTG curves of wood samples in N2.

Table 2.
Thermal decomposition data of wood samples.
Notably, all coated wood samples exhibited a lower mass loss rate at Tmax. The residue char of Wood-1and Wood-3 samples at 800 °C were 15.44% and 18.02%. The residue char of the Wood-2 sample at 800 °C was 21.48%, which potentially prevented the wood from burning further, confirming the synergistic effect of the CS/agar/PA system in promoting char formation. Thus, the coating can modify the pyrolysis process and improve thermal stability. Furthermore, it also promoted char formation on the wood surface, which worked as an intumescent barrier and provided effective flame retardant qualities.
3.4. Flame Retardancy of Wood Samples
The flame retardancy of pure wood and coated wood was evaluated using the LOI and UL-94 test, with the results presented in Table 3 and Figure 6. Table 3 showed that pure wood was highly flammable, with a LOI value of only 26%. Coating with CS–agar solution significantly improved the flame retardancy. Wood-1 reached a LOI value of 48.9%, which is an increase of 22.9 percentage points compared to the value of 26% for pure wood. Thus, CS–agar solution demonstrated flame retardant qualities, as proven by the UL-94 vertical burning test. Surprisingly, Wood-2 samples with 3% PA had an LOI value of 53.1%. The results verified that the Wood-2 samples exhibited superior flame retardant qualities than the Wood-3 samples, consistent with their heat release behaviors.

Table 3.
LOI and UL-94 results for wood samples.
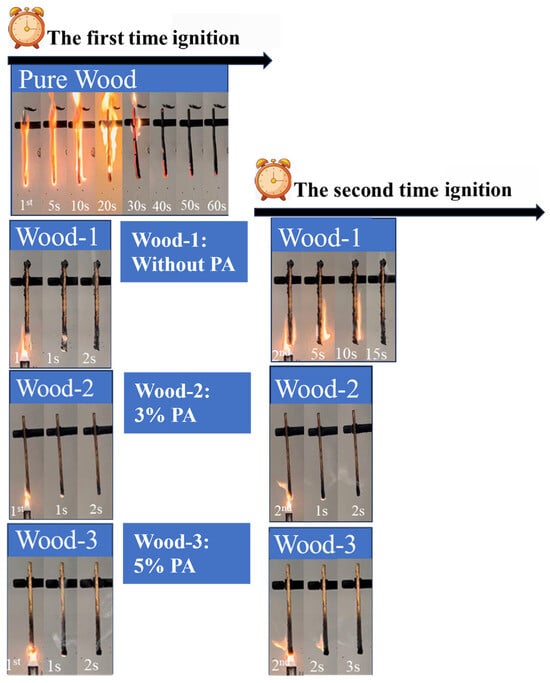
Figure 6.
Digital images of wood samples in UL-94 vertical burning test.
As shown in Figure 6, the UL-94 vertical burning test revealed that pure wood exhibited a burning duration exceeding 60 s after initial ignition. This result indicated that pure wood was unclassified according to the UL-94 standard. Therefore, a second ignition was not conducted. Application of the CS–agar solution partially mitigated the burning behavior, the burning duration of Wood-1 decreased to 17 s and passed the UL-94 V-1 rate. Notably, with the introduction of PA, both Wood-2 and Wood-3 samples achieved self-extinguishment within 5 s and passed the UL-94 V-0 rate. This indicated that the CS/agar/PA coating can effectively enhance the flame retardancy of wood, especially at 3% PA, exhibiting the optimal comprehensive flame retardancy performance.
Flame retardant coating can significantly improve the fire resistance and durability of wood substrates. CCT was conducted to evaluate the combustion behavior. HRR and THR curves are shown in Figure 7a,b. The critical parameters listed in Table 4 include the time to ignition (TTI), peak heat release rate (PHRR), total heat release (THR), and fire performance index (FPI). FPI calculated from PHRR and TTI [], a higher FPI value indicated the superior flame retardant performance [,]. The HRR curves for all samples, including the uncoated wood, exhibited two peaks, which is characteristic of the burning behavior of the material under the specific test condition. The first peak (P1HRR) was attributed to the combustion of gases released from coating pyrolysis, while the second peak (P2HRR) resulted from the cracking of the char layer. Combustible gases volatilized from the cracks, which generated a heat release rate peak []. Regarding TTI, pure wood showed a TTI of 32 s, while Wood-1 exhibited a slightly shortened TTI of 28 s, and Wood-2 and Wood-3 maintained a TTI of 29 s and 32 s, respectively. The slight TTI reduction in Wood-1 was attributed to the early thermal degradation of the CS–agar coating. However, Wood-2 and Wood-3 returned TTI to near or equal to the pure wood level.
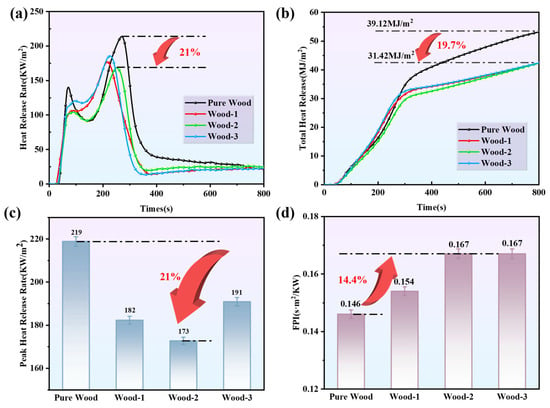
Figure 7.
(a) HRR and (b) THR curves; (c) PHRR values and (d) FPI values of wood samples.

Table 4.
Critical parameters for CCT of wood samples.
Compared with pure wood, the coated wood exhibited significantly enhanced flame retardancy. The THR and PHRR of all coated wood were substantially reduced. The PHRR and THR of Wood-2 were 173 kW/m2 and 31.42 MJ/m2, lower than the corresponding values for pure wood, which were 219 kW/m2 and 39.12 MJ/m2, with reductions of 21% and 19.7%, respectively. This was attributed to the protective char layer formed by the CS/agar/PA coating during combustion, which would isolate flame and heat contact with wood, thus delaying substrate damage. Upon heating, CS and agar decompose to produce a carbon layer, while releasing non-flammable gases like N2, NH3, and H2O, which dilute flammable gases []. In addition, the decomposition of PA produced acidic catalyst substances, promoting the formation of the carbon layer by CS and agar. As shown in Figure 7d, the FPI value of pure wood was 0.146, while the FPI value of Wood-1 increased to 0.154. The FPI value of Wood-2 and Wood-3 reached 0.167, indicating excellent flame retardant performance.
To test the durability of the coating, the samples were immersed in water, and the LOI values are shown in Figure 8. After 15 h immersion, the LOI values of the samples decreased to varying extents, which can be attributed to the partial hydration and leaching of hydrophilic components, including CS and PA, from the coating network upon prolonged water exposure. In general, the CS/agar/PA coating maintained adhesion to the wood substrate. Notably, Wood-2 retained a LOI value of 50.9% even after soaking, demonstrating its sustained flame retardant performance.
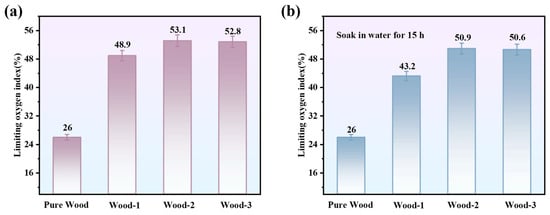
Figure 8.
(a) LOI values, (b) LOI values after 15 h of immersion in water.
3.5. Thermal Insulation Analysis
An infrared thermography analyzer was employed to evaluate the thermal insulation properties of the samples exposed directly to an ethanol flame. As shown in Figure 9, after 5 min of heating, the backside temperature of pure wood exceeded 200 °C, while those of coated wood were recorded at 172.2 °C for Wood-1, 164.6 °C for Wood-2, and 168.4 °C for Wood-3, respectively. After 8 min, the backside temperature of pure wood approached 300 °C, while all coated wood maintained a temperature below 200 °C. Notably, the Wood-2 sample exhibited the best performance, consistent with CCT and UL-94 tests. In addition, all coated wood surfaces formed a stable and dense char layer that provided effective thermal insulation and inhibited heat transfer. These results demonstrate that the coating could greatly enhance the wood’s flame retardant and thermal insulation qualities.
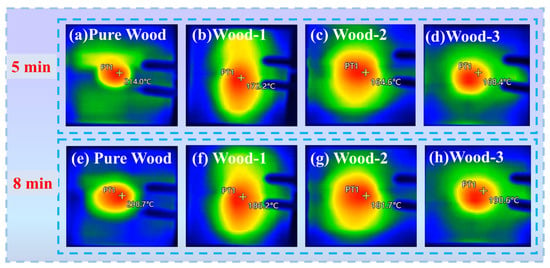
Figure 9.
Thermal infrared images of (a) pure wood, (b) Wood-1, (c) Wood-2, and (d) Wood-3 after 5 min of heating; (e) pure wood, (f) Wood-1, (g) Wood-2, and (h) Wood-3 after 8 min of heating.
3.6. Analysis of Residual Char
To analyze the condensed phase flame retardant mechanism, analysis of residual char morphology and composition was conducted [,,]. As shown in Figure 10, Wood-2 exhibited the thickest char layer, demonstrating that optimal PA incorporation facilitated char formation through phosphorus-rich crosslinking networks [,,]. Synergistic P-N interactions further strengthened the char layer, while the resulting crosslinked network enhanced thermal stability and insulation properties, explaining Wood-2’s outstanding performance in UL-94 and LOI tests. In contrast, Wood-3’s thinner char layer likely resulted from excessive PA content, which exhibited reduced density and continuity, thereby diminishing its protective effectiveness.

Figure 10.
Digital images of residual char. Main view of (a1) pure wood, (b1) Wood-1, (c1) Wood-2, and (d1) Wood-3; top view of (a2) pure wood, (b2) Wood-1, (c2) Wood-2, and (d2) Wood-3.
Furthermore, SEM characterized the microstructural features of residual char after CCT. As illustrated in Figure 11a–d, the pure wood’s residual char appeared with a loose, fragmented morphology characteristic. In contrast, the coated wood formed a continuous and compact char layer, attributable to the synergistic char-forming effects of CS and agar. Notably, bubble structures with occasional ruptures were observed on the char surface, likely resulting from NH3 release from CS throughout the combustion process []. Among these samples, Wood-2 exhibited optimal char integrity, forming an effective protective barrier that inhibited flame and heat transfer. This could be due to the catalytic effect of PA on char formation, indicating that PA could promote lignin and cellulose dehydration charring [].
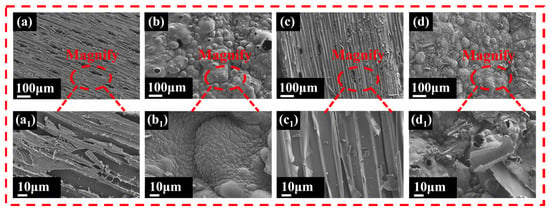
Figure 11.
SEM images of residual char. (a,a1) Pure wood, (b,b1) Wood-1, (c,c1) Wood-2, and (d,d1) Wood-3.
Raman spectra were employed to quantitatively assess the microstructural order and verify the quality of residual char. As shown in Figure 12a–d, the D band at 1349 cm−1 and the G band at 1584 cm−1 corresponded to the disordered graphite carbon structure and the ordered graphite structure. The intensity ratio of the D band to the G band (ID/IG) represented the graphitization level in residual char [,,]. Notably, Wood-2 displayed the lowest ID/IG ratio (1.51), contrasting with pure wood’s highest ID/IG value (2.77). This enhanced graphitization facilitated the formation of a carbon layer, which hindered heat penetration, decreased the thermal decomposition rate and ultimately enhanced fire resistance.
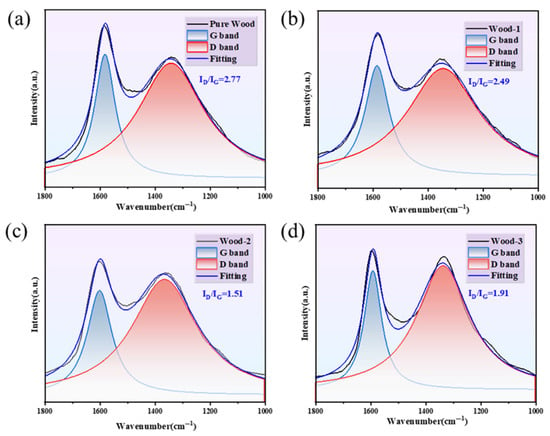
Figure 12.
Raman spectra of residual char, (a) pure wood; (b) Wood-1; (c) Wood-2; and (d) Wood-3.
Figure 13a,b showed the XPS spectra of Wood-2’s residual char. In the C 1s spectra, the band at 284.8 eV matched to the C-C bond in aromatic structure, while those at 286.4 eV and 288.2 eV corresponded to C-O-P and C=O bond, respectively. Notably, the presence of the 286.4 eV band verified that the C-O-P bond had formed through chemical reaction during combustion. For the O 1s spectra, the bands at 532.2 eV and 533.4 eV were ascribed to C-O-P and C-O-C bonds, the band at 531.2 eV was ascribed to C=O.
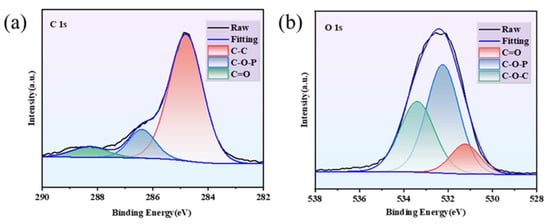
Figure 13.
XPS spectra of Wood-2′s residual char: (a) C 1s and (b) O 1s.
3.7. TG-FTIR Analysis
TG-IR was used to evaluate the pyrolysis products of pure wood and Wood-2 during thermal degradation, with the results presented in Figure 14. Under an N2 atmosphere, Wood-2 demonstrated a significant reduction in volatile pyrolysis products compared to pure wood. As shown in Figure 14a,b, these results indicated that Wood-2 had excellent barrier properties against oxygen and inhibited substrate degradation. In Figure 14c,d, the characteristic absorption peaks and release intensity of pyrolysis products were extracted from infrared spectra. Minimal differences were observed between pure wood and Wood-2, with both displaying peaks for H2O (-OH) at around 3700 cm−1, aromatic compounds at around 670 cm−1, and CO2 at around 2307 cm−1 and 2357 cm−1. Notably, Wood-2 exhibited new peaks above 400 °C, including NH3 at around 1528 cm−1. This indicated that the Wood-2 sample had a gas phase flame retardant effect by releasing non-flammable gases, including CO2 and NH3, during combustion, reducing the concentration of oxygen and combustible gases in the gas phase.
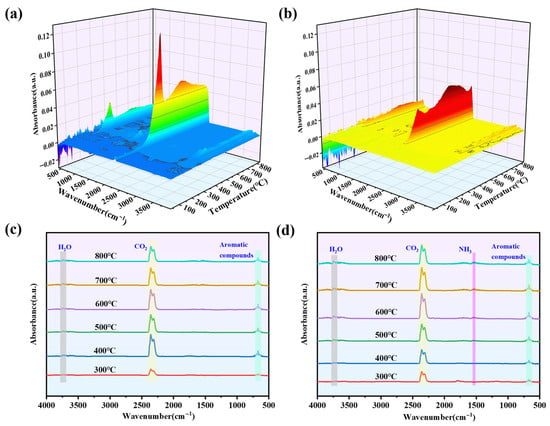
Figure 14.
(a) 3D TG-IR spectra of (a) pure wood and (b) Wood-2; FTIR spectra of the gas products at distinct temperatures: (c) pure wood and (d) Wood-2.
3.8. Flame Retardant Mechanism Analysis
According to the results above, the significant enhancement in flame retardancy of coated wood can be due to a synergistic impact between condensed phase and gas phase, as illustrated in Figure 15. During thermal degradation, PA decomposes into polyphosphoric acid and phosphates that catalyze the charring reaction of wood, CS, and agar into the carbon layer, which effectively suppresses volatile release and combustion intensity in the condensed phase. In the gas phase, PA accelerates the thermal degradation of CS and agar, releasing nitrogen-based inert gases (N2 and NH3) that dilute oxygen in the combustion zone. Additionally, P-containing (PO·) radicals derived from PA decomposition captured active H and OH radicals, thereby inhibiting the chain reaction and further reducing the combustion intensity [,,].
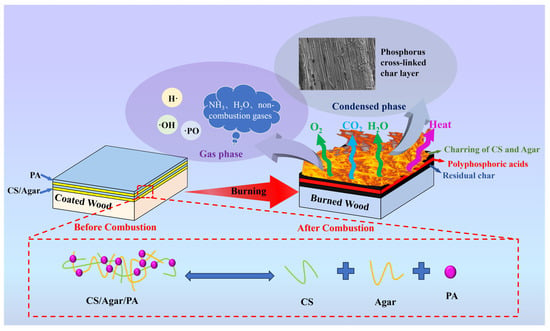
Figure 15.
The flame retardant mechanism for CS/agar/PA coating.
3.9. Mechanical Properties and Adhesion Test
To determine the impact of flame retardant coating on the mechanical characteristics of wood, a tensile strength test was performed on wood samples. As illustrated in Figure 16a, the tensile strength of pure wood, Wood-1, Wood-2 and Wood-3 were 21.03 MPa, 22.68 MPa, 20.11 MPa and 24.26 MPa, respectively. The results indicated only minor variations between the coated wood and the pure wood, suggesting that the coating has a negligible impact on its mechanical properties. Additionally, coating–substrate adhesion is a critical factor affecting the coating’s durability and flame retardant performance; an adhesion test was performed on the samples to evaluate this characteristic. As shown in Figure 16b–d, a single-edged tool was used to apply a constant force to make a cutting line on the surface of coated wood, and all the cuts scratched through the bottom of the coating. It was observed that the edge of the cut was entirely smooth, and the coatings remained intact, with no large fragments falling off, confirming satisfactory adhesion between the coating and wood substrate.
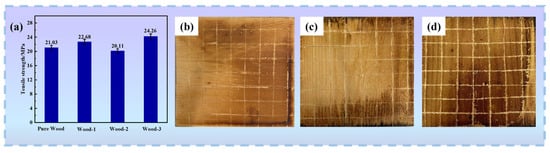
Figure 16.
Mechanical properties and adhesion test of wood samples: (a) tensile strength of wood samples; surface view of coated wood: (b) Wood-1, (c) Wood-2, and (d) Wood-3 after adhesion test.
4. Conclusions
To enhance the fire resistance of civil aviation cabin materials, this study successfully prepared a fully bio-based IFR system utilizing CS, agar and PA, and successfully fabricated a functional coating on a wood surface. The results showed that Wood-2 reached a high limiting oxygen index (53.1%), a UL-94 V-0 rating, and a notable reduction in peak heat release rate (19.7%) and total heat release (21%) compared to pure wood. TGA confirmed improved thermal stability, with a residual char yield of 21.48% at 800 °C. Raman spectra verified a notable increase in graphitization degree in the residual char. Moreover, mechanical characterization showed a negligible impact on tensile strength while maintaining high adhesion levels due to hydrogen bonding interactions between the coating and wood.
In conclusion, the IFR system functions through a flame retardant mechanism in both the gas phase and condensed phase. Upon heating, CS and agar decompose to release non-combustible gases, reducing the concentration of flammable gases and oxygen. When compounded with PA, the IFR system further catalyzes char formation on the wood surface, creating a protective barrier that insulates the underlying substrate from thermal degradation. This study establishes a sustainable and environmentally friendly strategy for enhancing the flame retardancy and durability of wood. The presented coating strategy offers significant potential for application to other flammable materials in civil aviation cabins, such as textiles and polymer composites, and provides a promising approach for the next generation of sustainable and fire-retardant aircraft interiors.
Author Contributions
Conceptualization, Q.L. and L.S.; methodology, L.S.; validation, Q.L. and L.S.; formal analysis, L.S.; investigation, L.S.; resources, Q.L. and P.Z.; data curation, Q.L. and P.Z.; writing—original draft preparation, L.S.; writing—review and editing, L.S.; supervision, Q.L. and P.Z.; project administration, Q.L. and P.Z.; funding acquisition, Q.L. and P.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (2024YFC3014400), the Fundamental Research Funds for the Central Universities (25CAFUC01007), the National Natural Science Foundation of China (52202416) and the Natural Science Foundation of Sichuan Province (2024YFHZ0027).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, D.; Yao, A.; Feng, K.; Zhou, H.; Wang, R.; Cheng, M.; Li, H.; Wang, D.; Ding, S. Next Frontiers of Aviation Safety: System-of-Systems Safety. Engineering 2025, 52, 262–277. [Google Scholar] [CrossRef]
- Di Giorgio, G. Safety and Accidents Involving Aircraft Manufactured from Polymer Composite Materials: A Review. Aerotec. Missili Spaz. 2023, 102, 337–353. [Google Scholar] [CrossRef]
- Loewenthal, O.; Doley, P.; Wang, C.; Yeoh, G.H.; Kabir, I.I. Investigating Intumescent Flame-Retardant Additives in Polyurethane Foam to Improve the Flame Resistance and Sustainability of Aircraft Cabin Materials. Fire 2024, 7, 351. [Google Scholar] [CrossRef]
- Perry, T.D. Aircraft Plywood and Adhesives. J. Aeronaut. Sci. 2012, 8, 204–216. [Google Scholar] [CrossRef]
- Song, K.; Ganguly, I.; Eastin, I.; Dichiara, A. High Temperature and Fire Behavior of Hydrothermally Modified Wood Impregnated with Carbon Nanomaterials. J. Hazard. Mater. 2020, 384, 121283. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ran, Y.; Shao, Y.; Zhu, J.; Du, C.; Yang, F.; Bao, Q.; Shan, Y.; Zhang, W. A Novel Intumescent MCA-Modified Sodium Silicate/Acrylic Flame-Retardant Coating to Improve the Flame Retardancy of Wood. Molecules 2024, 29, 3021. [Google Scholar] [CrossRef]
- Jian, H.; Liang, Y.; Deng, C.; Xu, J.; Liu, Y.; Shi, J.; Wen, M.; Park, H.-J. Research Progress on the Improvement of Flame Retardancy, Hydrophobicity, and Antibacterial Properties of Wood Surfaces. Polymers 2023, 15, 951. [Google Scholar] [CrossRef]
- Liang, Y.; Jian, H.; Deng, C.; Xu, J.; Liu, Y.; Park, H.; Wen, M.; Sun, Y. Research and Application of Biomass-Based Wood Flame Retardants: A Review. Polymers 2023, 15, 950. [Google Scholar] [CrossRef]
- Sethurajaperumal, A.; Manohar, A.; Banerjee, A.; Varrla, E.; Wang, H.; Ostrikov, K. Thermally-Insulating Vermiculite Nanosheets-Epoxy Nanocomposite Paint as Fire-Resistant Wood Coating. Nanoscale Adv. 2021, 3, 4235–4243. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Xu, M.; Liu, L.; Wang, W.; Gao, S.; Li, B. Effect of a Biomass Based Waterborne Fire Retardant Coating on the Flame Retardancy for Wood. Polym. Adv. Technol. 2021, 32, 4805–4814. [Google Scholar] [CrossRef]
- Shabir Mahr, M.; Hübert, T.; Sabel, M.; Schartel, B.; Bahr, H.; Militz, H. Fire Retardancy of Sol-Gel Derived Titania Wood-Inorganic Composites. J. Mater. Sci. 2012, 47, 6849–6861. [Google Scholar] [CrossRef]
- Malucelli, G. Biomacromolecules and Bio-Sourced Products for the Design of Flame Retarded Fabrics: Current State of the Art and Future Perspectives. Molecules 2019, 24, 3774. [Google Scholar] [CrossRef]
- Li, Y. Application of Halogen-Free EPDM Flame Retardants. Appl. Comput. Eng. 2023, 23, 280–286. [Google Scholar] [CrossRef]
- Holder, K.M.; Smith, R.J.; Grunlan, J.C. A Review of Flame Retardant Nanocoatings Prepared Using Layer-by-Layer Assembly of Polyelectrolytes. J. Mater. Sci. 2017, 52, 12923–12959. [Google Scholar] [CrossRef]
- Qiu, X.; Li, Z.; Li, X.; Zhang, Z. Flame Retardant Coatings Prepared Using Layer by Layer Assembly: A Review. Chem. Eng. J. 2018, 334, 108–122. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Ma, L.; Gao, J.; Ge, H.; Zhu, Z. Layered Charring Agent with Super Strong Carbonization for IFR System: Achieving Highly Efficient Flame Retardancy of PLA by Ultra-Low Addition IFR. Sustain. Mater.Technol. 2024, 40, e00924. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.; Wang, X. Synergistic Effects of Organically Modified Montmorillonite on the Flame-Retardant and Smoke Suppression Properties of Transparent Intumescent Fire-Retardant Coatings. Prog. Org. Coat. 2018, 122, 107–118. [Google Scholar] [CrossRef]
- Xia, L.; Dai, J.; Wang, X.; Xue, M.; Xu, Y.; Yuan, C.; Dai, L. Facile Fabrication of Multifunctional Cotton Fabric by AgNC@boronate Polymer/Crosslinked Chitosan. Carbohydr. Polym. 2022, 288, 119384. [Google Scholar] [CrossRef]
- Makhlouf, G.; Abdelkhalik, A.; Ameen, H. Preparation of Highly Efficient Chitosan-Based Flame Retardant Coatings with Good Antibacterial Properties for Cotton Fabrics. Prog. Org. Coat. 2022, 163, 106627. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Zhang, L.-P.; Cao, X.; Tian, X.-Y.; Ni, Y.-P. Facile Fabrication of Highly Efficient Chitosan-Based Multifunctional Coating for Cotton Fabrics with Excellent Flame-Retardant and Antibacterial Properties. Polymers 2024, 16, 1409. [Google Scholar] [CrossRef]
- Feng, J.; Sun, Y.; Song, P.; Lei, W.; Wu, Q.; Liu, L.; Yu, Y.; Wang, H. Fire-Resistant, Strong, and Green Polymer Nanocomposites Based on Poly(Lactic Acid) and Core–Shell Nanofibrous Flame Retardants. ACS Sustain. Chem. Eng. 2017, 5, 7894–7904. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, Y.; Du, X.; Song, P.; Fang, Z. Green and Scalable Fabrication of Core–Shell Biobased Flame Retardants for Reducing Flammability of Polylactic Acid. ACS Sustain. Chem. Eng. 2019, 7, 8954–8963. [Google Scholar] [CrossRef]
- Song, W.-M.; Zhang, L.-Y.; Li, P.; Ni, Y.-P.; Liu, Y. The Fabrication of Flame-Retardant Viscose Fabrics with Phytic Acid-Based Flame Retardants: Balancing Efficient Flame Retardancy and Tensile Strength. Int. J. Biol. Macromol. 2024, 260, 129596. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-M.; Yin, G.-Z.; Hobson, J.; Zhai, Z.; Zhao, J.; Shentu, B. Schiff Base Approach to Introduce Chitosan-Phytic Acid Complex for Flame-Retardant Cotton Fabrics. Eur. Polym. J. 2024, 221, 113525. [Google Scholar] [CrossRef]
- Huang, Z.; Li, S.; Tsai, L.-C.; Jiang, T.; Ma, N.; Tsai, F.-C. Flame Retardant Polypropylene with a Single Molecule Intumescent Flame Retardant Based on Chitosan. Mater. Today Commun. 2022, 33, 104689. [Google Scholar] [CrossRef]
- Chen, W.; Liu, H.; Yan, Q.; Chen, Q.; Hong, M.; Zhou, Z.-X.; Fu, H. Straightforward Synthesis of Novel Chitosan Bio-Based Flame Retardants and Their Application to Epoxy Resin Flame Retardancy. Compos. Commun. 2024, 48, 101949. [Google Scholar] [CrossRef]
- Yan, C.; Fang, Y.; Yang, R.; Yan, M.; Wang, W.; Song, Y.; Wang, Q. Preparation of Amino Wood Coatings with Superior Flame Retardancy, Smoke Suppression, and Transparency Using Fully Bio-Based Flame Retardants. Polym. Degrad. Stab. 2025, 234, 111213. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, A.; Cheng, Y.; Li, M.; Cui, Y.; Li, Z. Recent Advances in Biomass Phytic Acid Flame Retardants. Polym. Test. 2023, 124, 108100. [Google Scholar] [CrossRef]
- Tuble, K.A.Q.; Omisol, C.J.M.; Abilay, G.Y.; Tomon, T.R.B.; Aguinid, B.J.M.; Dumancas, G.G.; Malaluan, R.M.; Lubguban, A.A. Synergistic Effect of Phytic Acid and Eggshell Bio-Fillers on the Dual-Phase Fire-Retardancy of Intumescent Coatings Applied on Cellulosic Substrates. Chemosphere 2024, 358, 142226. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Li, F.; Zhao, J. In-Situ Polymerized Zinc Phytate Chelated Si-C-P Geopolymer Hybrid Coating Constructed by Incorporating Chitosan Oligosaccharide and DOPO for Flame-Retardant Plywood. Constr. Build. Mater. 2023, 397, 132416. [Google Scholar] [CrossRef]
- Song, F.; Liu, T.; Fan, Q.; Li, D.; Ou, R.; Liu, Z.; Wang, Q. Sustainable, High-Performance, Flame-Retardant Waterborne Wood Coatings via Phytic Acid Based Green Curing Agent for Melamine-Urea-Formaldehyde Resin. Prog. Org. Coat. 2022, 162, 106597. [Google Scholar] [CrossRef]
- Yu, K.; Wang, Y.; Qu, C.; Xue, X.; Zhao, J. Multifunctional Si-C-P Flame-Retardant Coatings Blended by Chitosan Hydrochloride/Melamine Polyphosphate/Ammonium Phosphomolybdate in Spodumene Tailings Geopolymer. Constr. Build. Mater. 2023, 405, 133340. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Chang, Z.; Meng, T.; Liu, F.; Zhou, H.; Zhang, D. Natural Polymer Agar for Enhancing Fire Resistance and Smoke Suppression of Intumescent Fire-Retardant Coating Used in Steel Structures. Polym. Degrad. Stab. 2024, 227, 110844. [Google Scholar] [CrossRef]
- Mamand, D.M.; Hadi, J.M.; Omer, R.A.; Aziz, S.B. FTIR, UV-VIS, and DFT Approach to Study the Structural, Optical and Thermal Properties of Chitosan Biopolymer. Dokl. Phys. Chem. 2024, 518, 137–154. [Google Scholar] [CrossRef]
- Tang, W.; Liang, G.; Wang, L.; Yuan, Y.; Dessie, W.; Liu, F.; Qin, Z.; Wang, Y.; Xiao, A.; Jin, X. Multi-Functional Flame Retardant Coatings Comprising Chitosan/ Gelatin and Sodium Phytate for Rigid Polyurethane Foams. J. Clean. Prod. 2023, 394, 136371. [Google Scholar] [CrossRef]
- Li, M.; Prabhakar, M.N.; Park, J.; Song, J. Flame-Retardant Innovations in Bio-Based Treatments for Lignocellulosic Natural Fibers: A Review. Int. J. Biol. Macromol. 2025, 311, 143728. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Sun, W.; Li, J.; Liu, H.; Liu, X. Eco-Friendly Flame Retardant and Dripping-Resistant of Polyester/Cotton Blend Fabrics through Layer-by-Layer Assembly Fully Bio-Based Chitosan/Phytic Acid Coating. Int. J. Biol. Macromol. 2021, 175, 140–146. [Google Scholar] [CrossRef]
- Movahedifar, E.; Vahabi, H.; Saeb, M.R.; Thomas, S. Flame Retardant Epoxy Composites on the Road of Innovation: An Analysis with Flame Retardancy Index for Future Development. Molecules 2019, 24, 3964. [Google Scholar] [CrossRef] [PubMed]
- Vahabi, H.; Laoutid, F.; Movahedifar, E.; Khalili, R.; Rahmati, N.; Vagner, C.; Cochez, M.; Brison, L.; Ducos, F.; Ganjali, M.R.; et al. Description of Complementary Actions of Mineral and Organic Additives in Thermoplastic Polymer Composites by Flame Retardancy Index. Polym. Adv. Technol. 2019, 30, 2056–2066. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Rahmati, N.; Movahedifar, E.; Hadavand, B.S.; Karami, Z.; Ghaffari, M.; Taheri, P.; Bakhshandeh, E.; Vahabi, H.; Ganjali, M.R.; et al. Properties of Nano-Fe3O4 Incorporated Epoxy Coatings from Cure Index Perspective. Prog. Org. Coat. 2019, 133, 220–228. [Google Scholar] [CrossRef]
- Cheng, X.; Shi, L.; Fan, Z.; Yu, Y.; Liu, R. Bio-Based Coating of Phytic Acid, Chitosan, and Biochar for Flame-Retardant Cotton Fabrics. Polym. Degrad. Stab. 2022, 199, 109898. [Google Scholar] [CrossRef]
- Chen, C.; Bai, X.; Xiao, G.; Wang, B.; Chen, C.; Mou, C.; Zhong, F.; Yang, Z.; Wang, M. Phosphorus-Nitrogen Co-Modification of Floral Hydrotalcite Synergized with Zinc Hydroxystannate for Enhancing Fire Resistance of Waterborne Epoxy Coatings. Colloids Surf., A 2024, 701, 134840. [Google Scholar] [CrossRef]
- Zhao, X.; Tian, M.; Wei, R.; Jiang, S. Facile Fabrication of a Novel Self-Healing and Flame-Retardant Hydrogel/MXene Coating for Wood. Sci. Rep. 2023, 13, 1826. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xiao, G.; Chen, C.; Chen, C.; Yang, Z.; Zhong, F.; Liu, Y.; Zou, R. Highly Thermally Stable Zirconium Oxide Deposited Layered Double Hydroxide for Enhancing Flame Retardancy of Waterborne Epoxy Coatings. Colloids Surf. A. 2021, 628, 127368. [Google Scholar] [CrossRef]
- Yi, L.; Yang, Q.; Yan, L.; Wang, N. A Facile Strategy to Construct ZnO Nanoparticles Reinforced Transparent Fire-Retardant Coatings for Achieving Antibacterial Activity and Long-Term Fire Protection of Wood Substrates. J. Build. Eng. 2023, 72, 106630. [Google Scholar] [CrossRef]
- Wei, A.; Wang, S.; Zou, Y.; Xiang, C.; Xu, F.; Sun, L. Preparation of a Flame-Retardant Curing Agent Based on Phytic Acid–Melamine Ion Crosslinking and Its Application in Wood Coatings. Polymers 2024, 16, 1557. [Google Scholar] [CrossRef]
- Lu, X.; Wei, A.; Wang, S.; Zou, Y.; Lu, Y.; Sun, L.; Xiang, C. Preparation and Application of a Urea–Formaldehyde-Blended Guanidinium Azole–Phytic Acid–Copper Flame-Retardant Resin Coating. Polymers 2024, 16, 3366. [Google Scholar] [CrossRef]
- Li, B.; Sun, H.; Zhang, L.; Zhao, X.; Bakar, A.A.; Mohamad, Z. Flame-Retarded Poly (Lactic Acid) Containing Phosphating Chitosan. Fire Mater. 2025, 49, 173–183. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Sadiku, E.R.; Ray, S.S.; Mochane, M.J.; Matabola, K.P.; Motloung, M. Flame Retardancy Efficacy of Phytic Acid: An Overview. J. Appl. Polym. Sci. 2022, 139, e52495. [Google Scholar] [CrossRef]
- He, T.; Chen, F.; Zhu, W.; Yan, N. Functionalized Lignin Nanoparticles for Producing Mechanically Strong and Tough Flame-Retardant Polyurethane Elastomers. Int. J. Biol. Macromol. 2022, 209, 1339–1351. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Wu, K.; Jiao, E.; Chen, W.; Hu, Z.; Xu, C.; Shi, J.; Wang, S.; Tan, Z. Surface Functionalization of Few-Layer Black Phosphorene and Its Flame Retardancy in Epoxy Resin. Chem. Eng. J. 2020, 382, 122991. [Google Scholar] [CrossRef]
- Yang, S.; Huo, S.; Wang, J.; Zhang, B.; Wang, J.; Ran, S.; Fang, Z.; Song, P.; Wang, H. A Highly Fire-Safe and Smoke-Suppressive Single-Component Epoxy Resin with Switchable Curing Temperature and Rapid Curing Rate. Compos. B Eng. 2021, 207, 108601. [Google Scholar] [CrossRef]
- Wang, W.; Wang, F.; Li, H.; Liu, Y. Synthesis of Phosphorus-Nitrogen Hybrid Flame Retardant and Investigation of Its Efficient Flame-Retardant Behavior in PA6/PA66. J. Appl. Polym. Sci. 2023, 140, e53536. [Google Scholar] [CrossRef]
- Xu, J.; Ao, X.; De La Vega, J.; Guo, F.; Xie, Z.; Liang, F.; Wang, D.-Y.; Wu, J. Poly(vinyl alcohol) Composite Aerogel toward Lightweight, Remarkable Flame Retardancy, and Thermal Insulation Properties by Incorporating Carbon Nanohorns and Phytic Acid. ACS Appl. Polym. Mater. 2024, 6, 8027–8039. [Google Scholar] [CrossRef]
- Shan, H.; Yan, L.; Xu, B.; Wang, D.; Wu, M. Polyphosphamide Containing Triazine and Melamine Cyanurate for Flame-Retardant PA6. ACS Appl. Polym. Mater. 2023, 5, 5322–5333. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).