Influence of Flexibilizers on the Thermal and Combustion Properties of Soundproof Enclosures in Ultrahigh Voltage Converter Transformer Equipment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiments
2.2.1. Thermogravimetry Measurements
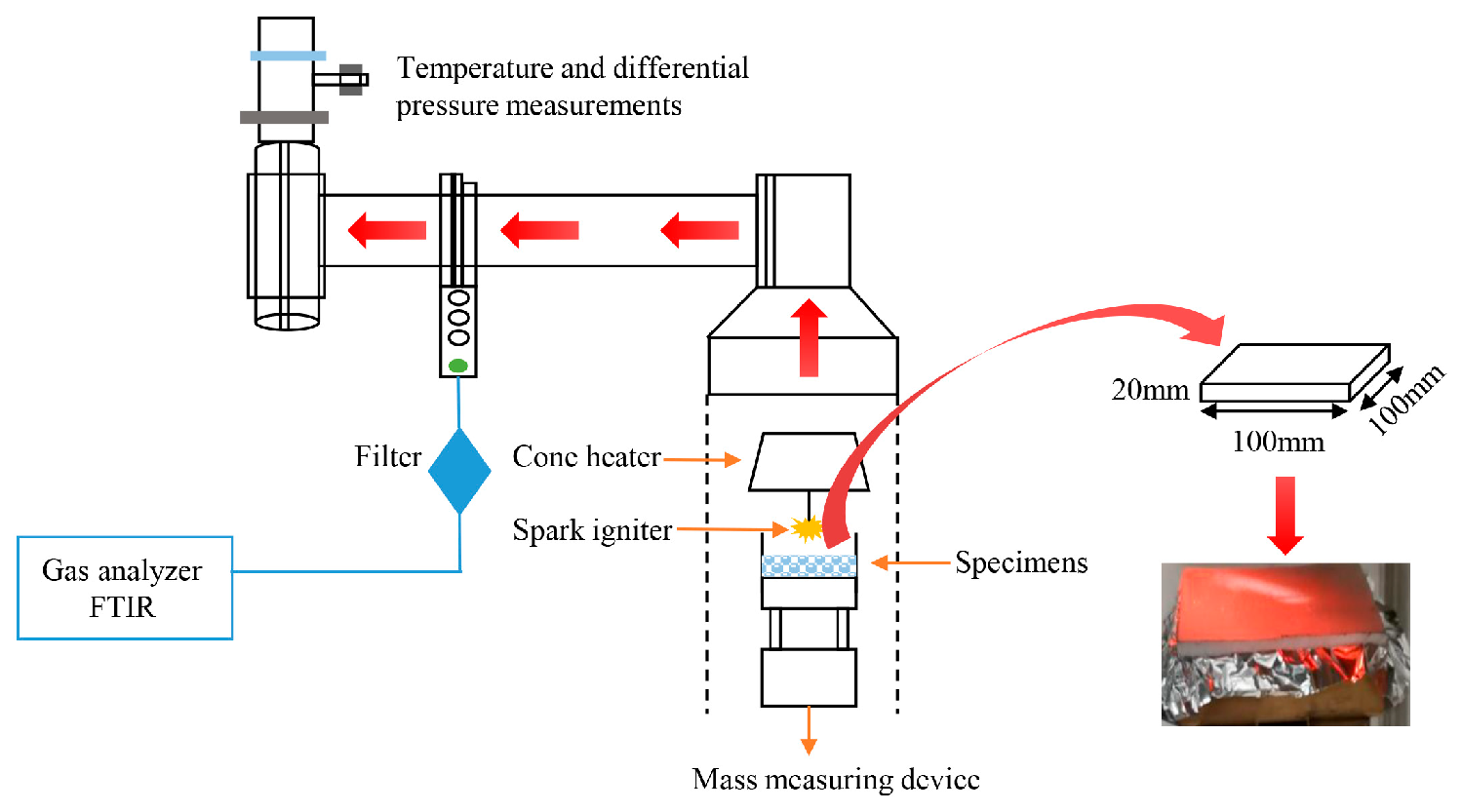
2.2.2. Cone Calorimetry Measurements
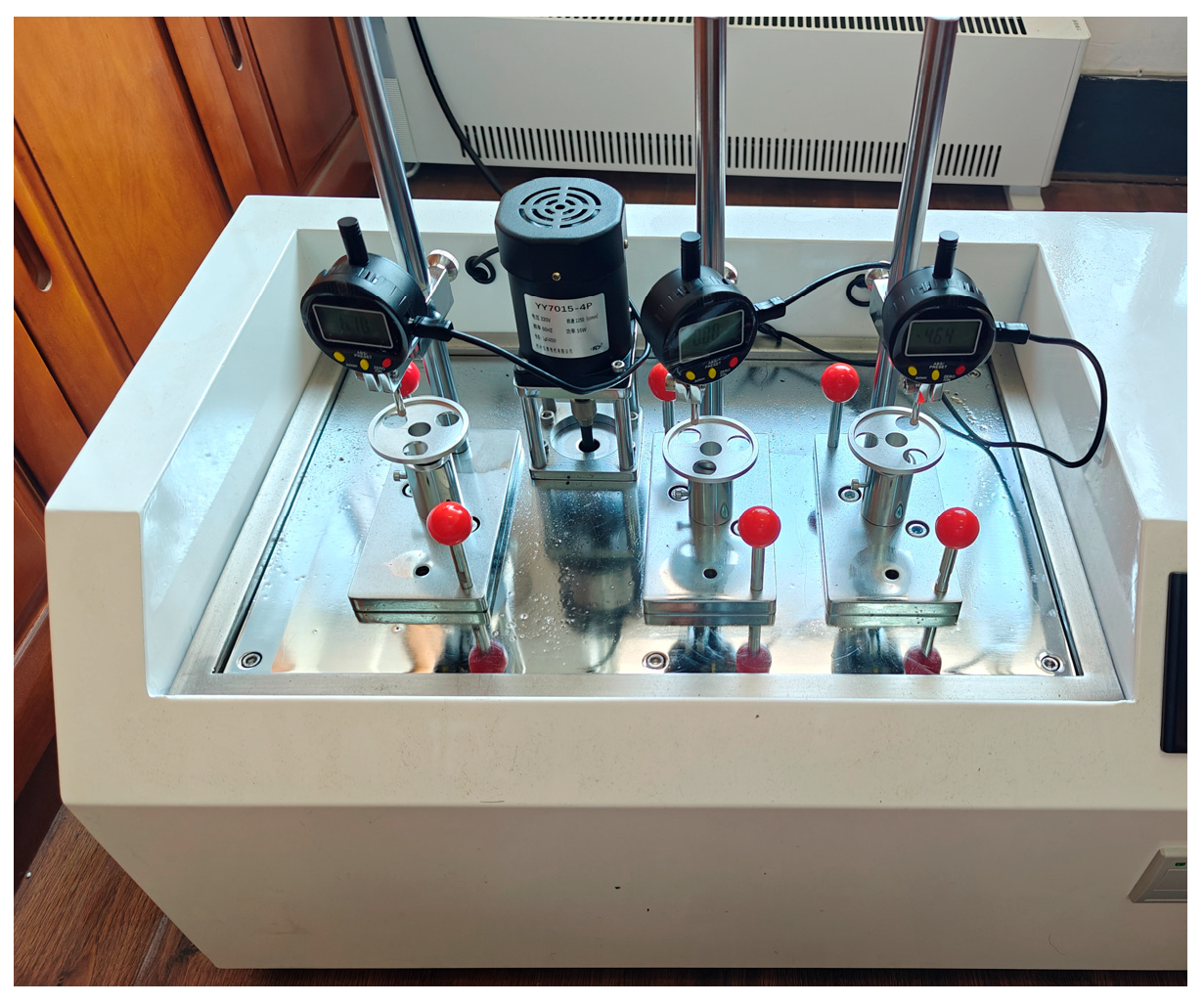
2.2.3. Thermal Deformation Experiments
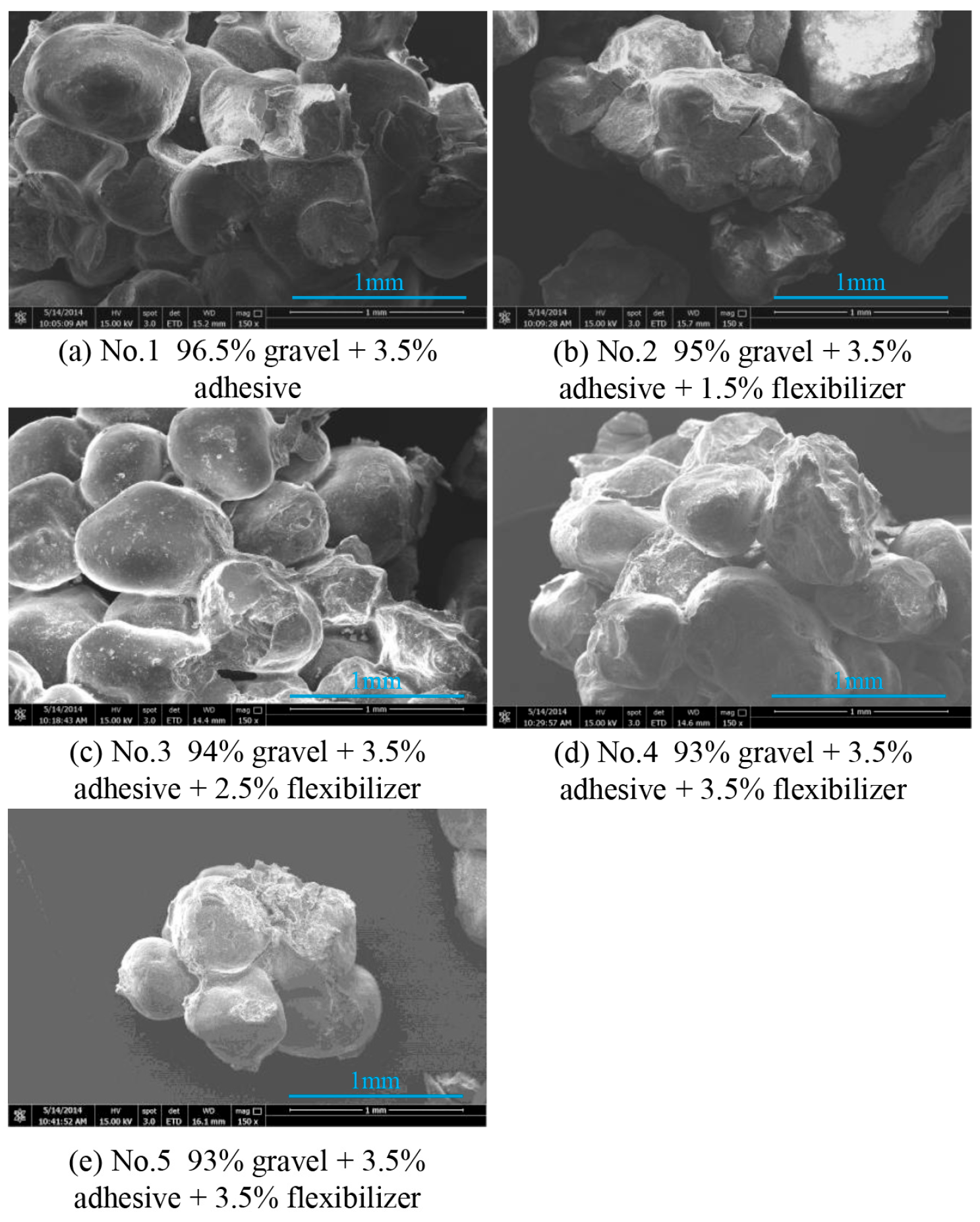
2.2.4. Scanning Electron Microscope Experiments
2.3. Theoretical Methods
2.3.1. Pyrolysis Kinetics
2.3.2. Distributed Activation Energy Model Method
2.3.3. Entropy Method
- (1)
- Data standardization: The raw data are normalized according to the nature of the indicator using distinct equations for forward and reverse indicators to ensure consistent comparability.
- (2)
- Non-negative transformation. To avoid zero values in subsequent calculations, an infinitesimal offset (0.00001) is introduced to each Yij value. This minor adjustment ensures computational stability without compromising data reliability.
- (3)
- Proportional calculation. The relative contribution (Pij) of each indicator value is calculated using Equation (8), which quantifies its relative contribution within the overall evaluation matrix. This step generates a standardized distribution across all assessment criteria.
- (4)
- Entropy quantification. The information entropy (eij) for each evaluation indicator is determined through Equation (9). A lower entropy value corresponds to a higher information content, indicating greater relative importance of the indicator within the analytical framework.
- (5)
- Variation coefficient determination. The variation coefficient (gj) is computed to quantify the relative variability of each indicator. As a critical metric in entropy-based weighting, it reflects the principle that indicators exhibiting greater dispersion provide higher discriminatory power and are therefore assigned increased weight in the evaluation system. The calculation procedure is as follows:
- (6)
- Weight determination. The relative importance weight (wj) for each evaluation indicator is calculated using Equation (11), where each weight is proportional to the indicator’s variation coefficient.
- (7)
- Composite index calculation. The composite index (sⱼ) is calculated based on the values of Pij and wj, as expressed by
3. Results and Discussion
3.1. Structure-Property Relationship
3.2. Microstructure Analysis
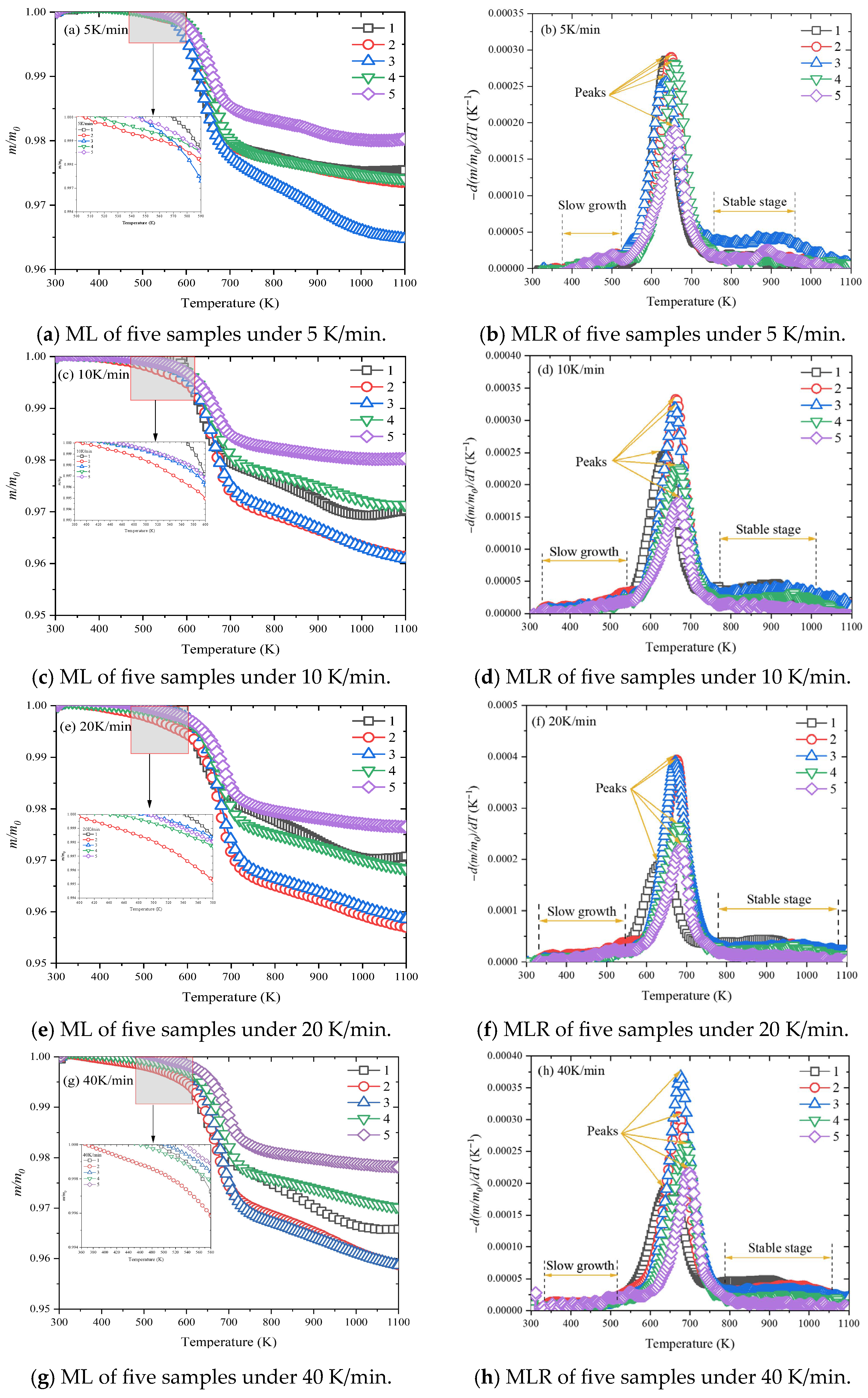
3.3. Thermogravimetric Analysis
3.3.1. Thermogravimetric Behaviors
3.3.2. Kinetic Analysis
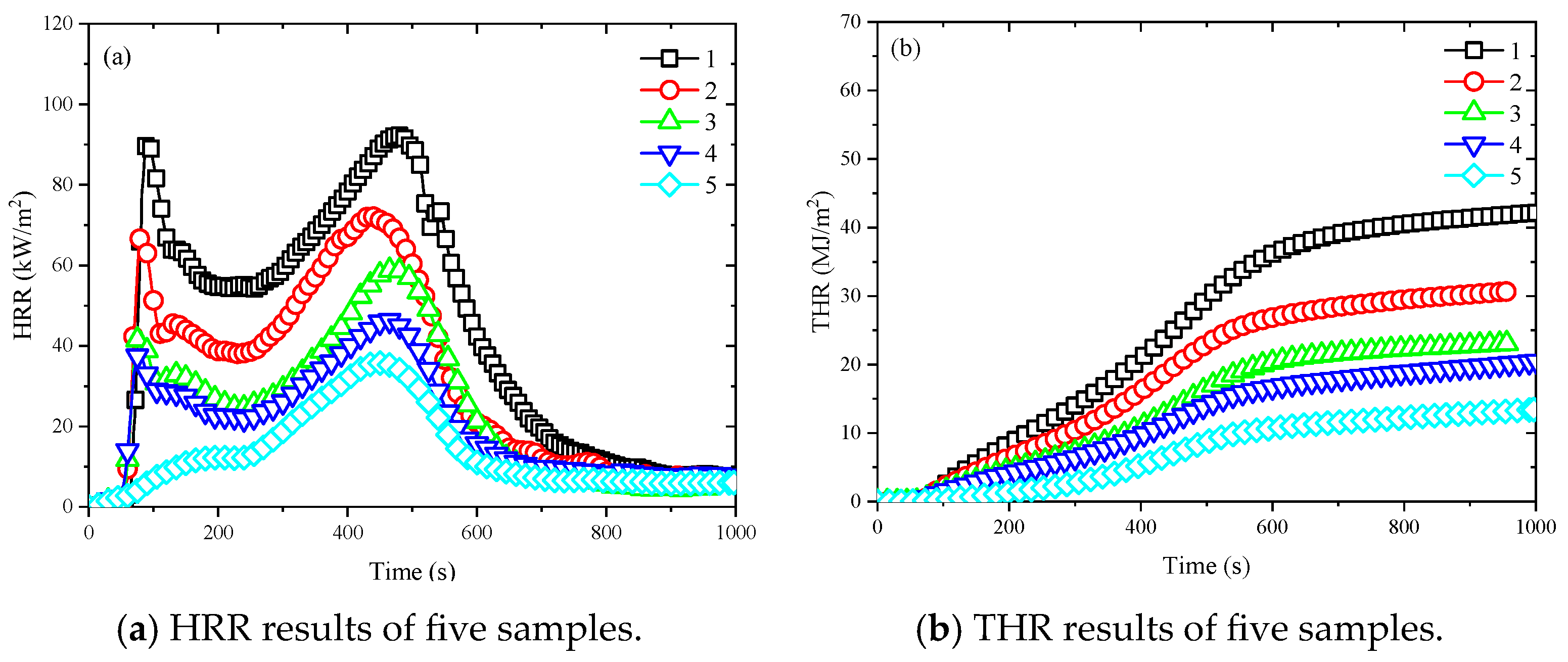
3.4. Heat Release Rate and Total Heat Release
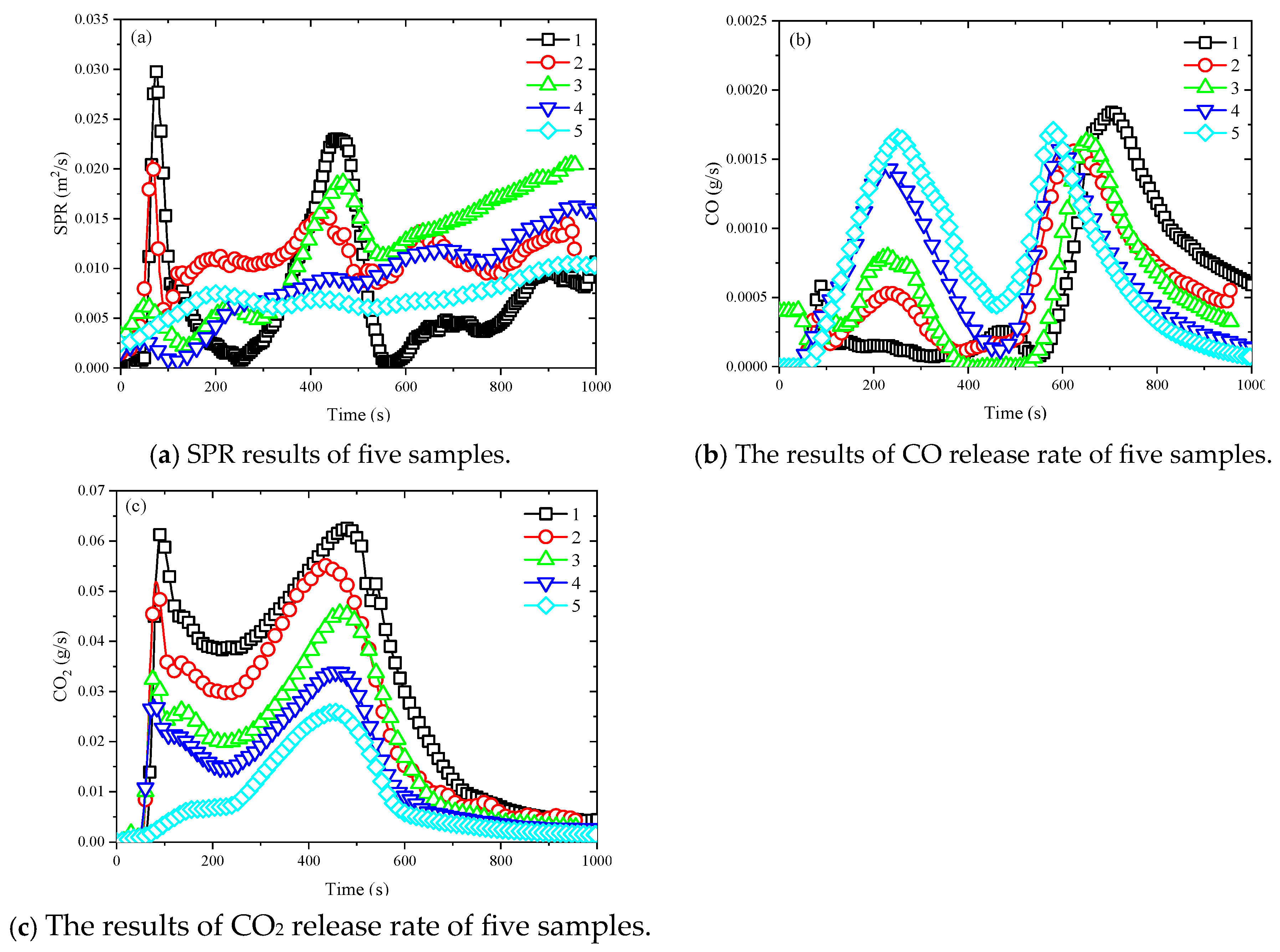
3.5. Combustion Products
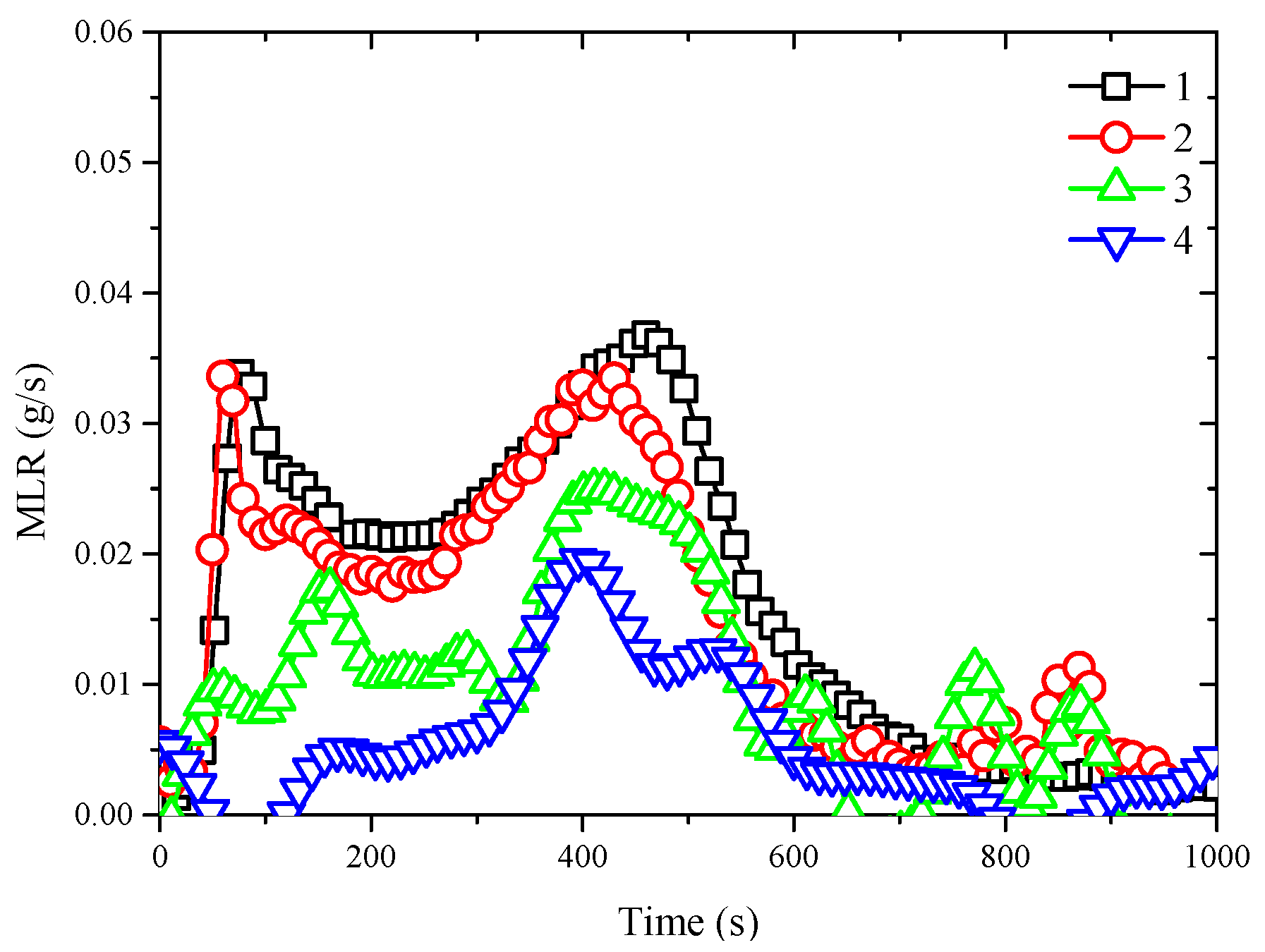
3.6. Mass Loss
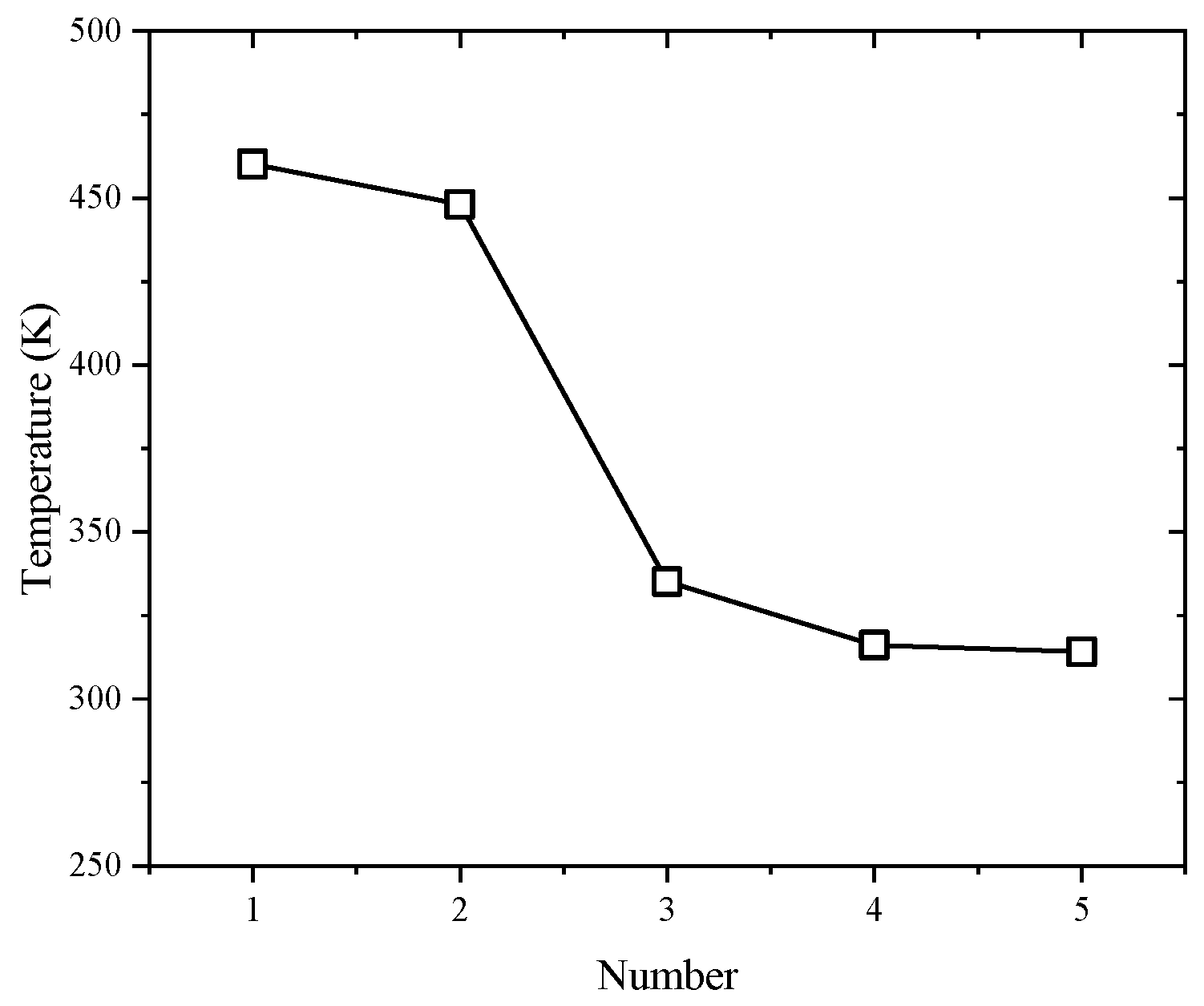
3.7. Thermal Deformation Analysis
3.8. Comprehensive Performance Analysis
4. Conclusions
- (1)
- The incorporation of a flexibilizer was found to reduce the thermal stability of the samples, as evidenced by three key observations. Firstly, the initial mass loss temperatures decreased from a range of 480–570 K to 370–545 K under five heating rates. Secondly, the activation energy exhibited a complex dependence on flexibilizer content, showing an initial decrease, followed by an increase, and then a subsequent decrease. The vast majority of samples displayed activation energy below 250 kJ/mol in the control sample. Finally, the thermal deformation temperature dramatically decreased from 460 K to 314 K.
- (2)
- The incorporation of the flexibilizer significantly enhanced fire safety performance by effectively suppressing heat release. The first HRR peak was reduced from 73.57 kW/m2 to 3.67 kW/m2, and the second peak decreased from 81.25 kW/m2 to 36.55 kW/m2. The THR values were reduced from 42.13 MJ/m2 to 13.43 MJ/m2. The average SPR increased from 7.73 m2/s to 11.53 m2/s and then decreased to 7.01 m2/s. The CO release rate for the first peak rose from 1.49 × 10−4 g/s to 1.66 × 10−3 g/s, while the second peak decreased from 1.84 × 10−3 g/s to 1.56 × 10−3 g/s. Similarly, the first peak of CO2 release rate dropped from 6.15 × 10−2 g/s to 6.15 × 10−3 g/s, and the second peak decreased from 6.25 × 10−2 g/s to 2.58 × 10−2 g/s.
- (3)
- The overall fire performance scores showed a significant reduction from 0.2801 for unmodified samples to a range of 0.1147–0.2522 for formulations containing the flexibilizer, indicating a reduction in fire hazards. Combustion characteristics and fire risk were influenced by the material composition, allowing flexibility in formulation design based on specific fire safety requirements. Among the tested samples, sample No. 5 (92% gravel + 3.5% binder + 4.5% flexibilizer) exhibited the lowest fire hazards. However, it should be noted that as these materials are intended for use in non-laboratory environments, their long-term performance may be affected by aging. Thus, future studies should consider the effects of aging on material properties.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shang, F.; Zhang, J.; Zhou, Y.; Li, G. Improvement design of UHV converter transformer noise reduction device. Fire Sci. Technol. 2021, 40, 1367–1372. (In Chinese) [Google Scholar]
- Zhang, J.; Li, G.; Wang, Y.; Li, C.; Shang, F.; Lu, S. Numerical simulation study on fire of typical UHV converter transformer. Fire Sci. Technol. 2020, 39, 1115–1120. (In Chinese) [Google Scholar]
- Wang, Y.; Li, C.; Zhang, J.; Shang, F.; Lu, S.; Fan, M.; Wang, L. Fire accident characteristics and fire extinguishing countermeasures of oil-immersed transformer. Saf. Environ. Eng. 2019, 6, 166–171. (In Chinese) [Google Scholar]
- Tang, X.; Xie, Q.; Qiu, R.; Yang, Y. Development of a relationship between kinetic triplets and heating rates to improve pyrolysis kinetic modeling of polymer. Polym. Degrad. Stab. 2018, 154, 10–26. [Google Scholar] [CrossRef]
- Onsree, T.; Tippayawong, N.; Zheng, A.; Li, H. Pyrolysis behavior and kinetics of corn residue pellets and eucalyptus wood chips in a macro thermogravimetric analyzer. Case Stud. Therm. Eng. 2018, 12, 546–556. [Google Scholar] [CrossRef]
- Ding, Y.; Fukumoto, K.; Ezekoye, O.A.; Lu, S.; Wang, C.; Li, C. Experimental and numerical simulation of multi-component combustion of typical charring material. Combust. Flame 2020, 211, 417–429. [Google Scholar] [CrossRef]
- Fan, M.; Naughton, A. Mechanisms of thermal decomposition of natural fibre composites. Compos. Part B Eng. 2016, 88, 1–10. [Google Scholar] [CrossRef]
- Zhang, W.; Pan, R.; Wang, J.; Lu, C.; Liu, M.; Ding, Y. Study on pyrolysis characteristics of typical thermal insulation materials under light aging. Energy 2025, 320, 135238. [Google Scholar] [CrossRef]
- Kuznetsov, G.; Volkov, R.; Sviridenko, A.; Strizhak, P.J.P.S.; Protection, E. Fast detection of compartment fires under different heating conditions of materials. Process Saf. Environ. Prot. 2022, 168, 257–274. [Google Scholar] [CrossRef]
- Liu, W.; Xu, X.; Zhang, J.; Zhong, Y.; Li, X.; Ding, Y. Thermal decomposition process of fireproof sealant measured with thermogravimetric and fourier transform infrared spectroscopy analysis and estimated using shuffled complex evolution. Fire 2024, 7, 25. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Q.; Pan, R.; Chao, J.; Yu, H.; Ding, Y. Study on fire risk of typical thermal insulation materials based on pyrolysis, combustion and flame spread experiments. Polym. Bull. 2025, 82, 4005–4029. [Google Scholar] [CrossRef]
- Jing, J.; Zhang, Y.; Fang, Z. Diphenolic acid based biphosphate on the properties of polylactic acid: Synthesis, fire behavior and flame retardant mechanism. Polymer 2017, 108, 29–37. [Google Scholar] [CrossRef]
- Lai, X.; Tang, S.; Li, H.; Zeng, X. Flame-retardant mechanism of a novel polymeric intumescent flame retardant containing caged bicyclic phosphate for polypropylene. Polym. Degrad. Stab. 2015, 113, 22–31. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, Y.; Zhang, C.; Liu, D.; Li, F.; Yaqing, L. A novel phosphoramidate and its application on cotton fabrics: Synthesis, flammability and thermal degradation. J. Anal. Appl. Pyrolysis 2017, 125, 109–116. [Google Scholar] [CrossRef]
- Feng, G.; Zhou, Y.; Hu, Y.; Yan, M.; Jia, P. Influence of a nitrogen-containing oil-based plasticizer on mechanical, thermal stability and fire performance of plasticized poly(vinyl chloride) and study of its mechanism of flame retardancy with Py-GC/MS. Ind. Crops Prod. 2015, 77, 883–894. [Google Scholar]
- Wang, L.; Wang, C.; Liu, P.; Jing, Z.; Ge, X.; Jiang, Y. The flame resistance properties of expandable polystyrene foams coated with a cheap and effective barrier layer. Constr. Build. Mater. 2018, 176, 403–414. [Google Scholar] [CrossRef]
- Jian, R.; Li, C.; Chen, S.; Long, J.; Wang, Y. A novel flame-retardant acrylonitrile-butadiene-styrene system based on aluminum isobutylphosphinate and red phosphorus: Flame retardance, thermal degradation and pyrolysis behavior. Polym. Degrad. Stab. 2014, 109, 184–193. [Google Scholar] [CrossRef]
- Zhang, W.; Pan, R.; Wang, J.; Shan, W.; Ding, Y. Effect of light aging on combustion characteristics and fire hazard of organic thermal insulation materials. J. Build. Eng. 2025, 104, 112346. [Google Scholar] [CrossRef]
- Ma, H.; Pan, R.; Zhang, W.; Chao, J.; Ding, Y. Research on fire risk of typical thermal insulation materials based on Entropy method and Fuzzy Cluster Analysis method. Polym. Bull. 2025, 6, 1–17. [Google Scholar] [CrossRef]
- GB/T 1634.1-2019; Plastics—Determination of Temperature of Deflection Under Load—Part 1: General Test Method. China Standard Press: Beijing, China, 2019.
- Zhang, W.; Pan, R.; Wang, J.; Pei, B.; Ding, Y. Accuracy of kinetic parameters in multiple methods for separating multi-step thermal degradation reactions of biomass into single-step reactions. Energy 2025, 314, 134183. [Google Scholar] [CrossRef]
- Zhang, W.; Jia, J.; Zhang, J.; Ding, Y.; Zhang, J.; Lu, K.; Mao, S. Pyrolysis and combustion characteristics of typical waste thermal insulation materials. Sci. Total. Environ. 2022, 834, 155484. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, S.; Weng, M.; Liu, Y. Fire risk assessment for large-scale commercial buildings based on structure entropy weight method. Saf. Sci. 2017, 94, 26–40. [Google Scholar] [CrossRef]
- Saeed, L.; Tohka, A.; Zevenhoven, R.; Haapala, M. Two-stage combustion of PVC-containing wastes with HCI recovery: An experimental assessment. Energy Sources 2006, 27, 669–686. [Google Scholar] [CrossRef]
- Shi, L.; Chew, M.Y.L. Fire behaviors of polymers under autoignition conditions in a cone calorimeter. Fire Saf. J. 2013, 61, 243–253. [Google Scholar] [CrossRef]
- Chen, R.; Xu, X.; Zhang, Y.; Lu, S.; Lo, S. Characterization of ignition and combustion characteristics of phenolic fiber-reinforced plastic with different thicknesses. J. Therm. Anal. Calorim. 2020, 140, 645–655. [Google Scholar] [CrossRef]
- Li, A.; Huang, B.; Wu, H.; Zhang, W.; Zhou, R.; Ding, Y. Effects of sample thickness on the combustion and smoke characteristics of chlorinated polyvinyl chloride. J. Appl. Polym. Sci. 2022, 139, 51541. [Google Scholar] [CrossRef]
- Baalisampang, T.; Saliba, E.; Salehi, F.; Garaniya, V.; Chen, L. Optimisation of smoke extraction system in fire scenarios using CFD modelling. Process Saf. Environ. Prot. 2021, 149, 508–517. [Google Scholar] [CrossRef]
- Luche, J.; Rogaume, T.; Richard, F.; Guillaume, E. Characterization of thermal properties and analysis of combustion behavior of PMMA in a cone calorimeter. Fire Saf. J. 2011, 46, 451–461. [Google Scholar] [CrossRef]


| Number | Components |
|---|---|
| 1 | 96.5% gravel + 3.5% binder |
| 2 | 95% gravel + 3.5% binder + 1.5% flexibilizer |
| 3 | 94% gravel + 3.5% binder + 2.5% flexibilizer |
| 4 | 93% gravel + 3.5% binder + 3.5% flexibilizer |
| 5 | 92% gravel + 3.5% binder + 4.5% flexibilizer |
| Parameters | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| FPI-1 | 1.247 | 1.152 | 0.828 | 0.650 | 0.062 |
| FPI-2 | 1.377 | 1.239 | 0.992 | 0.790 | 0.619 |
| FGI-1 | 0.808 | 0.829 | 0.619 | 0.492 | 0.051 |
| FGI-2 | 0.169 | 0.167 | 0.124 | 0.101 | 0.081 |
| Indicators | ej | gj | wj |
|---|---|---|---|
| Ti (K) | 0.7852 | 0.2148 | 0.0930 |
| TP (K) | 0.8159 | 0.1841 | 0.0797 |
| RP (×1000 K−1) | 0.8505 | 0.1495 | 0.0647 |
| Rv (×1000 K−1) | 0.6930 | 0.3070 | 0.1329 |
| HRR (kW/m2) | 0.7801 | 0.2199 | 0.0952 |
| THR (MJ/m2) | 0.7621 | 0.2379 | 0.1030 |
| SPR (g/s) | 0.7552 | 0.2448 | 0.1059 |
| CO release rate (g/s) | 0.7698 | 0.2302 | 0.0996 |
| CO2 release rate (g/s) | 0.7979 | 0.2021 | 0.0875 |
| HDT (K) | 0.6796 | 0.3204 | 0.1387 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Shang, F.; Guo, Y.; Zhang, W.; Ding, Y. Influence of Flexibilizers on the Thermal and Combustion Properties of Soundproof Enclosures in Ultrahigh Voltage Converter Transformer Equipment. Fire 2025, 8, 381. https://doi.org/10.3390/fire8100381
Zhang J, Shang F, Guo Y, Zhang W, Ding Y. Influence of Flexibilizers on the Thermal and Combustion Properties of Soundproof Enclosures in Ultrahigh Voltage Converter Transformer Equipment. Fire. 2025; 8(10):381. https://doi.org/10.3390/fire8100381
Chicago/Turabian StyleZhang, Jiaqing, Fengju Shang, Yi Guo, Wenlong Zhang, and Yanming Ding. 2025. "Influence of Flexibilizers on the Thermal and Combustion Properties of Soundproof Enclosures in Ultrahigh Voltage Converter Transformer Equipment" Fire 8, no. 10: 381. https://doi.org/10.3390/fire8100381
APA StyleZhang, J., Shang, F., Guo, Y., Zhang, W., & Ding, Y. (2025). Influence of Flexibilizers on the Thermal and Combustion Properties of Soundproof Enclosures in Ultrahigh Voltage Converter Transformer Equipment. Fire, 8(10), 381. https://doi.org/10.3390/fire8100381








