The Effect of Smoke-Water on Seed Germination of 18 Grassland Plant Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Seed Collection
2.3. Smoke Solution Procedure
2.4. Seed Germination Trial
2.5. Data Analysis
3. Results
4. Discussion
4.1. Increased Germination
4.2. Decreased Germination
4.3. No Change in Germination
4.4. Other Factors
5. Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, N.; van Staden, J. Smoke as a germination cue: A review. Plant Growth Regul. 1997, 22, 115–124. [Google Scholar] [CrossRef]
- Franzese, J.; Ghermandi, L. Seed longevity and fire: Germination responses of an exotic perennial herb in NW Patagonian grasslands (Argentina). Plant Biol. 2011, 13, 865–871. [Google Scholar] [CrossRef]
- Kępczyński, J. Induction of agricultural weed seed germination by smoke and smoke-derived karrikin (KAR1), with a particular reference to Avena fatua L. Acta Physiol. Plant. 2018, 40, 87. [Google Scholar] [CrossRef]
- Zirondi, H.L.; de Pinho José, H.; Daibes, L.F.; Fidelis, A. Heat and smoke affect the germination of flammable resprouters: Vellozia species in the Cerrado. Folia Geobot. 2019, 54, 65–72. [Google Scholar] [CrossRef]
- Alahakoon, A.; Perera, G.; Merritt, D.; Turner, S.; Gama-Arachchige, N. Species-specific smoke effects on seed germination of plants from different habitats from Sri Lanka. Flora 2020, 263, 151530. [Google Scholar] [CrossRef]
- Hodges, J.A.; Price, J.N.; Nicotra, A.B.; Neeman, T.; Guja, L.K. Smoke and heat accelerate and increase germination in fire-prone temperate grassy ecosystems. Ecosphere 2021, 12, e03851. [Google Scholar] [CrossRef]
- Light, M. Special collection of articles on ‘smoke ecology and applications of plant-derived smoke’. S. Afr. J. Bot. 2018, 115, 217–218. [Google Scholar] [CrossRef]
- van Staden, J.; Jäger, A.; Light, M.; Burger, B.; Brown, N.; Thomas, T. Isolation of the major germination cue from plant-derived smoke. S. Afr. J. Bot. 2004, 70, 654–659. [Google Scholar] [CrossRef]
- de Lange, J.; Boucher, C. Autecological studies on Audouinia capitata (Bruniaceae). I. Plant-derived smoke as a seed germination cue. S. Afr. J. Bot. 1990, 56, 700–703. [Google Scholar] [CrossRef]
- Keeley, J.E.; Fotheringham, C.J. Smoke-induced seed germination in California chaparral. Ecology 1998, 79, 2320–2336. [Google Scholar] [CrossRef]
- Read, T.R.; Bellairs, S.M.; Mulligan, D.R.; Lamb, D. Smoke and heat effects on soil seed bank germination for the re-establishment of a native forest community in New South Wales. Austral Ecol. 2000, 25, 48–57. [Google Scholar] [CrossRef]
- Cox, R.D.; Chou, Y.; Wester, D.B. Smoke water and heat influence emergence of shortgrass prairie species. Fire Ecol. 2017, 13, 138–148. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Sparg, S.G.; Light, M.E.; Van Staden, J. Stimulation of rice (Oryza sativa L.) seedling vigour by smoke-water and butenolide. J. Agron. Crop Sci. 2006, 192, 395–398. [Google Scholar] [CrossRef]
- Merritt, D.J.; Flematti, G.R.; Ghisalberti, E.L.; Dixon, K.W. Karrikinolide—A phytoreactive compound derived from smoke with applications in horticulture, ecological restoration and agriculture. In Proceedings of the VI International Symposium on New Floricultural Crops, Madeira, Portugal, 11–15 June 2007; Volume 813, pp. 155–170. [Google Scholar]
- Jefferson, L.; Pennacchio, M.; Havens-Young, K. Ecology of Plant-Derived Smoke: Its Use in Seed Germination; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Govindaraj, M.; Masilamani, P.; Albert, V.A.; Bhaskaran, M. Plant derived smoke stimulation for seed germination and enhancement of crop growth: A review. Agric. Rev. 2016, 37, 87–100. [Google Scholar] [CrossRef][Green Version]
- Aslam, M.M.; Jamil, M.; Khatoon, A.; Hendawy, S.E.; Al-Suhaibani, N.A.; Malook, I.; Rehman, S.U. Physiological and biochemical responses of maize (Zea mays L.) to plant-derived smoke solution. Pak. J. Bot. 2017, 49, 435–443. [Google Scholar][Green Version]
- Mackenzie, D.D.; Naeth, M.A. Effect of plant-derived smoke water and potassium nitrate on germination of understory boreal forest plants. Can. J. For. Res. 2019, 49, 1540–1547. [Google Scholar] [CrossRef]
- Khatoon, A.; Rehman, S.U.; Aslam, M.M.; Jamil, M.; Komatsu, S. Plant-Derived Smoke Affects Biochemical Mechanism on Plant Growth and Seed Germination. Int. J. Mol. Sci. 2020, 21, 7760. [Google Scholar] [CrossRef]
- Osuna-Mascaró, C.; Agneray, A.C.; Galland, L.M.; Leger, E.A.; Parchman, T.L. Fine-scale spatial genetic structure in a locally abundant native bunchgrass (Achnatherum thurberianum) including distinct lineages revealed within seed transfer zones. Evol. Appl. 2023, 16, 979–996. [Google Scholar] [CrossRef]
- Ryan, K.C.; E Knapp, E.; Varner, J.M. Prescribed fire in North American forests and woodlands: History, current practice, and challenges. Front. Ecol. Environ. 2013, 11, e15–e24. [Google Scholar] [CrossRef]
- Gayton, D. Documenting fire history in a British Columbia Ecological Reserve. J. Ecosyst. Manag. 2013, 14, 1–10. [Google Scholar] [CrossRef]
- Fenner, M. The phenology of growth and reproduction in plants. Perspect. Plant Ecol. Evol. Syst. 1998, 1, 78–91. [Google Scholar] [CrossRef]
- Traveset, A.; Heleno, R.; Nogales, M. Ecology of seed dispersal. In Seeds: The Ecology of Regeneration in Plant Communities; Gallagher, R., Ed.; CABI: Wallingford, UK, 2014; pp. 62–93. [Google Scholar]
- Wikeem, B.; Wikeem, S. The Grasslands of British Columbia; Grasslands Conservation Council of BC: Kamloops, BC, Canada, 2004; 479p. [Google Scholar]
- Pitt, M.D.; Wikeem, B.M. Phenological patterns and adaptations in an Artemisia/Agropyron plant community. J. Range Manag. 1990, 43, 350–358. [Google Scholar] [CrossRef][Green Version]
- Turner, N.J. Thompson Ethnobotany: Knowledge and Usage of Plants by the Thompson Indians of British Columbia; Royal British Columbia Museum: Victoria, BC, Canada, 1990. [Google Scholar][Green Version]
- Pojar, J.; Klinka, K.; Meidinger, D. Biogeoclimatic ecosystem classification in British Columbia. For. Ecol. Manag. 1987, 22, 119–154. [Google Scholar] [CrossRef]
- Bureau of Land Management. Technical Protocol for the Collection, Study, and Conservation of Seeds from Native Plant Species for Seeds of Success; Bureau of Land Management: Washington, DC, USA, 2012.
- Coons, J.; Coutant, N.; Lawrence, B.; Finn, D.; Finn, S. An effective system to produce smoke solutions from dried plant tissue for seed germination studies. Appl. Plant Sci. 2014, 2, 1300097. [Google Scholar] [CrossRef]
- Zhou, J.; Kulkarni, M.G.; Huang, L.Q.; Guo, L.P.; Van Staden, J. Effects of temperature, light, nutrients and smoke-water on seed germination and seedling growth of Astragalus membranaceus, Panax notoginseng and Magnolia officinalis—Highly traded Chinese medicinal plants. S. Afr. J. Bot. 2012, 79, 62–70. [Google Scholar]
- Elsadek, M.A.; Yousef, E.A.A. Smoke-water enhances germination and seedling growth of four horticultural crops. Plants 2019, 8, 104. [Google Scholar] [CrossRef]
- Sabongari, S.; Aliero, B.L. Effects of soaking duration on germination and seedling growth of tomato (Lycopersicum esculentum Mill). Afr. J. Biotechnol. 2004, 3, 47–51. [Google Scholar] [CrossRef]
- Landis, T.D.; Dumroese, R.K. Propagation protocols on the Native Plant Network. Nativ. Plants J. 2000, 1, 112–114. [Google Scholar] [CrossRef][Green Version]
- Bonner, F.; Karrfalt, R. The Woody Plant Seed Manual; Agriculture Handbook No. 727; US Department of Agriculture, Forest Service: Washington, DC, USA, 2008; pp. 727–1223.[Green Version]
- Yusoff, N.; Rahman, M.F.A.; Yaakob, N.; Khandaker, M.; Mahmud, K. Environmental effects on germination and seedling emergence of weedy rice. J. Agrobiotechnology 2019, 10, 12–22. [Google Scholar][Green Version]
- Tobe, K.; Zhang, L.; Omasa, K. Seed germination and seedling emergence of three annuals growing on desert sand dunes in China. Ann. Bot. 2005, 95, 649–659. [Google Scholar] [CrossRef]
- Wolny, E.; Betekhtin, A.; Rojek, M.; Braszewska-Zalewska, A.; Lusinska, J.; Hasterok, R. Germination and the early stages of seedling development in Brachypodium distachyon. Int. J. Mol. Sci. 2018, 19, 2916. [Google Scholar] [CrossRef]
- Kucera, B.; Cohn, M.A.; Leubner-Metzger, G. Plant hormone interactions during seed dormancy release and germination. Seed Sci. Res. 2005, 15, 281–307. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. Seed germination ecophysiology of the woodland spring geophyte Erythronium albidum. Bot. Gaz. 1985, 146, 130–136. [Google Scholar] [CrossRef]
- Park, H.K.; Ko, C.; Lee, S.; Kim, S.; Yang, J.; Lee, K. Ecophysiology of seed dormancy and germination in four Lonicera (Caprifoliaceae) species native to Korea. J. Ecol. Environ. 2019, 43, 25. [Google Scholar] [CrossRef]
- Loewen, D.C.; Allen, G.A.; Antos, J.A. Autecology of Erythronium grandiflorum in western Canada. Can. J. Bot. 2001, 79, 500–509. [Google Scholar] [PubMed]
- Miller, M.T.; Allen, G.A.; Antos, J.A. Dormancy and flowering in two mariposa lilies (Calochortus) with contrasting distribution patterns. Can. J. Bot. 2004, 82, 1790–1799. [Google Scholar] [CrossRef]
- Tardiff, S.E.; Stanford, J.A. Grizzly bear digging: Effects on subalpine meadow plants in relation to mineral nitrogen availability. Ecology 1998, 79, 2219–2228. [Google Scholar] [CrossRef][Green Version]
- Lambert, A.M.; Miller-Rushing, A.J.; Inouye, D.W. Changes in snowmelt date and summer precipitation affect the flowering phenology of Erythronium grandiflorum (glacier lily; Liliaceae). Am. J. Bot. 2010, 97, 1431–1437. [Google Scholar] [CrossRef]
- Gupta, S.; Hrdlička, J.; Ngoroyemoto, N.; Nemahunguni, N.K.; Gucký, T.; Novák, O.; Kulkarni, M.G.; Doležal, K.; Van Staden, J. Preparation and Standardisation of Smoke-Water for Seed Germination and Plant Growth Stimulation. J. Plant Growth Regul. 2019, 39, 338–345. [Google Scholar] [CrossRef]
- Fischer, W.C.; Bradley, A.F. Fire Ecology of Western Montana Forest Habitat Types; US Department of Agriculture, Forest Service, Intermountain Research Station: Washington, DC, USA, 1987; Volume 223.
- Meng, Q.; Wang, Q.; Liu, H.; Jiang, L. A bio-inspired flexible fiber array with an open radial geometry for highly efficient liquid transfer. NPG Asia Mater. 2014, 6, e125. [Google Scholar] [CrossRef]
- Sugier, P.; Rysiak, A.; Sugier, D.; Winiarczyk, K.; Wołkowycki, D.; Kołos, A. Differentiation and Propagation Potential of Arnica montana L. Achenes as a Consequence of the Morphological Diversity of Flowers and the Position of Flower Heads on the Plant. Plants 2022, 11, 3424. [Google Scholar] [CrossRef] [PubMed]
- Pausas, J.G.; Lamont, B.B.; Paula, S.; Appezzato-Da-Glória, B.; Fidelis, A. Unearthing belowground bud banks in fire-prone ecosystems. New Phytol. 2018, 217, 1435–1448. [Google Scholar] [CrossRef] [PubMed]
- Scholten, M. Environmental Regulation of Dormancy Loss in Seeds of Lomatium dissectum. Master’s Thesis, Boise State University, Boise, ID, USA, 2011. [Google Scholar]
- Turner, N. Ancient Pathways, Ancestral Knowledge: Ethnobotany and Ecological Wisdom of Indigenous Peoples of Northwestern North America; McGill-Queen’s Native and Northern Series; McGill-Queen’s University Press: Kingston, ON, Canada, 2014; Volume 74. [Google Scholar]
- Hamer, D. Buffaloberry [Shepherdia canadensis (L.) nutt.] fruit production in fire-successional bear feeding sites. Rangel. Ecol. Manag. J. Range Manag. Arch. 1996, 49, 520–529. [Google Scholar] [CrossRef]
- Love, S.L.; Akins, C.J. Third summary of the native seed germination studies of Norman C Deno: Species with names beginning with letters F through K. Nativ. Plants J. 2019, 20, 123–144. [Google Scholar] [CrossRef]
- Macfarlane, R. Plate 395. Fritillaria affinis. Curtis’s Bot. Mag. 2000, 17, 168–172. [Google Scholar] [CrossRef]
- Barkley, Y. After the Burn: Assessing and Managing your Forestland After a Wildfire; Idaho Forest, Wildlife, and Range Experiment Station, University of Idaho: Moscow, ID, USA, 2002. [Google Scholar]
- Mensah, S.I.; Ekeke, C. Effects of different pretreatments and seed coat on dormancy and germination of seeds of Senna obtusifolia (L.) H.S. Irwin & Barneby (Fabaceae). Int. J. Biol. 2016, 8, 77–84. [Google Scholar]
- Stevens, R.; Monsen, S. Forbs for seeding range and wildlife habitats. In Restoring Western Ranges and Wildlands; Gen. Tech. Rep. RMRS-GTR-136-vol-2; US Department of Agriculture, Forest Service: Fort Collins, CO, USA, 2004; Volume 2, pp. 425–466+136. [Google Scholar]
- Turner, N.J. “Time to Burn:” Traditional use of Fire to Enhance Resource Production by Aboriginal Peoples in British Columbia. In Indians, Fire and the Land in the Pacific Northwest; Oregon State University Press: Corvallis, OR, USA, 1999; pp. 185–218. [Google Scholar]
- Bowen, P.C. Propagation protocol for production of Container (plug) Balsamorhiza sagittata (Pursh) Nutt. plants Rancho Santa Ana Botanic Garden Davis, California. In Native Plant Network; US Department of Agriculture, Forest Service, National Center for Reforestation, Nurseries and Genetic Resources: Fort Collins, CO, USA, 2006. Available online: http://npn.rngr.net/propagation (accessed on 23 September 2025).
- Egli, I.; Davidsson, L.; Juillerat, M.A.; Barclay, D.; Hurrell, R.F. The influence of soaking and germination on the phytase activity and phytic acid content of grains and seeds potentially useful for complementary feeding. J. Food Sci. 2002, 67, 3484–3488. [Google Scholar] [CrossRef]
- John, L.S.; Tilley, D. Plant Guide: Hooker’s balsamroot: Balsamorhiza hookeri (Hook.) Nutt; US Department of Agriculture, NRCS, Aberdeen Plant Materials Center: Aberdeen, ID, USA, 2012; p. 3.
- Love, S.L.; Akins, C.J. Fourth summary of the native seed germination studies of Norman C Deno: Species with names beginning with letters L through O. Nativ. Plants J. 2019, 20, 279–304. [Google Scholar] [CrossRef]
- García-Rodríguez, A.; Albrecht, J.; Szczutkowska, S.; Valido, A.; Farwig, N.; Selva, N. The role of the brown bear Ursus arctos as a legitimate megafaunal seed disperser. Sci. Rep. 2021, 11, 1282. [Google Scholar] [CrossRef]
- Mah, S. Relationship Between Vital Attributes of Ktunaxa Plants and Natural Disturbance Regimes in Southeastern British Columbia. Master’s Thesis, University of British Columbia, Vancouver, BC, Canada, 2000. [Google Scholar]
- Fuchs, M.A. Towards a Recovery Strategy for Garry Oak and Associated Ecosystems in Canada: Ecological Assessment and Literature Review; Environment Canada, Pacific and Yukon Region: Victoria, BC, Canada, 2001; p. 106. [Google Scholar]
- Dermott, C.A.; Sims, H.P.; Ziemkiewicz, P.F. Proceedings: Workshop on Native Shrubs in Reclamation; University of Alberta: Edmonton, AB, Canada, 1979. [Google Scholar]
- Cosyns, E.; Delporte, A.; Lens, L.; Hoffmann, M. Germination success of temperate grassland species after passage through ungulate and rabbit guts. J. Ecol. 2005, 93, 353–361. [Google Scholar] [CrossRef]
- Schupp, E.W. Quantity, quality and the effectiveness of seed dispersal by animals. Vegetation 1993, 107, 15–29. [Google Scholar] [CrossRef]
- Stoian-Dod, R.L.; Dan, C.; Morar, I.M.; Sestras, A.F.; Truta, A.M.; Roman, G.; Sestras, R.E. Seed germination within genus Rosa: The complexity of the process and influencing factors. Horticulturae 2023, 9, 914. [Google Scholar] [CrossRef]
- Plant Propagation Protocol ESRM 412 Native Plant Production. Available online: https://courses.washington.edu/esrm412/protocols/2015/LERE7.pdf (accessed on 24 September 2025).
- Meyer, G.A.; Witmer, M.C. Influence of seed processing by frugivorous birds on germination success of three North American shrubs. Am. Midl. Nat. 1998, 140, 129–139. [Google Scholar] [CrossRef]
- Parciak, W. Seed size, number, and habitat of a fleshy-fruited plant: Consequences for seedling establishment. Ecology 2002, 83, 794–808. [Google Scholar] [CrossRef]
- Bond, W.J.; Honig, M.; Maze, K.E. Seed size and seedling emergence: An allometric relationship and some ecological implications. Oecologia 1999, 120, 132–136. [Google Scholar] [CrossRef]
- Aragón, C.F.; Méndez, M.; Escudero, A. Survival costs of reproduction in a short-lived perennial plant: Live hard, die young. Am. J. Bot. 2009, 96, 904–911. [Google Scholar] [CrossRef]
- Li, F.L.; Liu, X.; Bao, W.K. Leaf lifespan is positively correlated with periods of leaf production and reproduction in 49 herb and shrub species. Ecol. Evol. 2016, 6, 3822–3831. [Google Scholar] [CrossRef]
- Grime, J.P. Plant Strategies, Vegetation Processes, and Ecosystem Properties, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2001; 417p. [Google Scholar]
- Liu, Z.; Liu, K.; Shi, X.; Lock, T.R.; Kallenbach, R.L.; Yuan, Z. Changes in grassland phenology and growth rate, rather than diversity, drive biomass production after fire. Agric. For. Meteorol. 2022, 322, 109028. [Google Scholar] [CrossRef]
- Noste, N.V.; Bushey, C.L. Fire Response of Shrubs of Dry Forest Habitat Types in Montana and Idaho; US Department of Agriculture, Forest Service, Intermountain Research Station: Washington, DC, USA, 1987; Volume 239.
- Gonzalez, S.; Salazar, C.V.; Ghermandi, L. Burning the seed bank: Seed response of eight species to different fire intensities in Patagonian steppe. Appl. Veg. Sci. 2022, 25, e12684. [Google Scholar] [CrossRef]
- López-Mársico, L.; Farías-Moreira, L.; Lezama, F.; Altesor, A.; Rodríguez, C. Light intensity triggers different germination responses to fire-related cues in temperate grassland species. Folia Geobot. 2019, 54, 53–63. [Google Scholar] [CrossRef]
- Ma, H.; E Erickson, T.; Merritt, D.J. Seed dormancy regulates germination response to smoke and temperature in a rhizomatous evergreen perennial. AoB Plants 2018, 10, ply042. [Google Scholar] [CrossRef]
- Ren, L.; Bai, Y. Smoke originating from different plants has various effects on germination and seedling growth of species in Fescue Prairie. Botany 2016, 94, 1141–1150. [Google Scholar] [CrossRef]
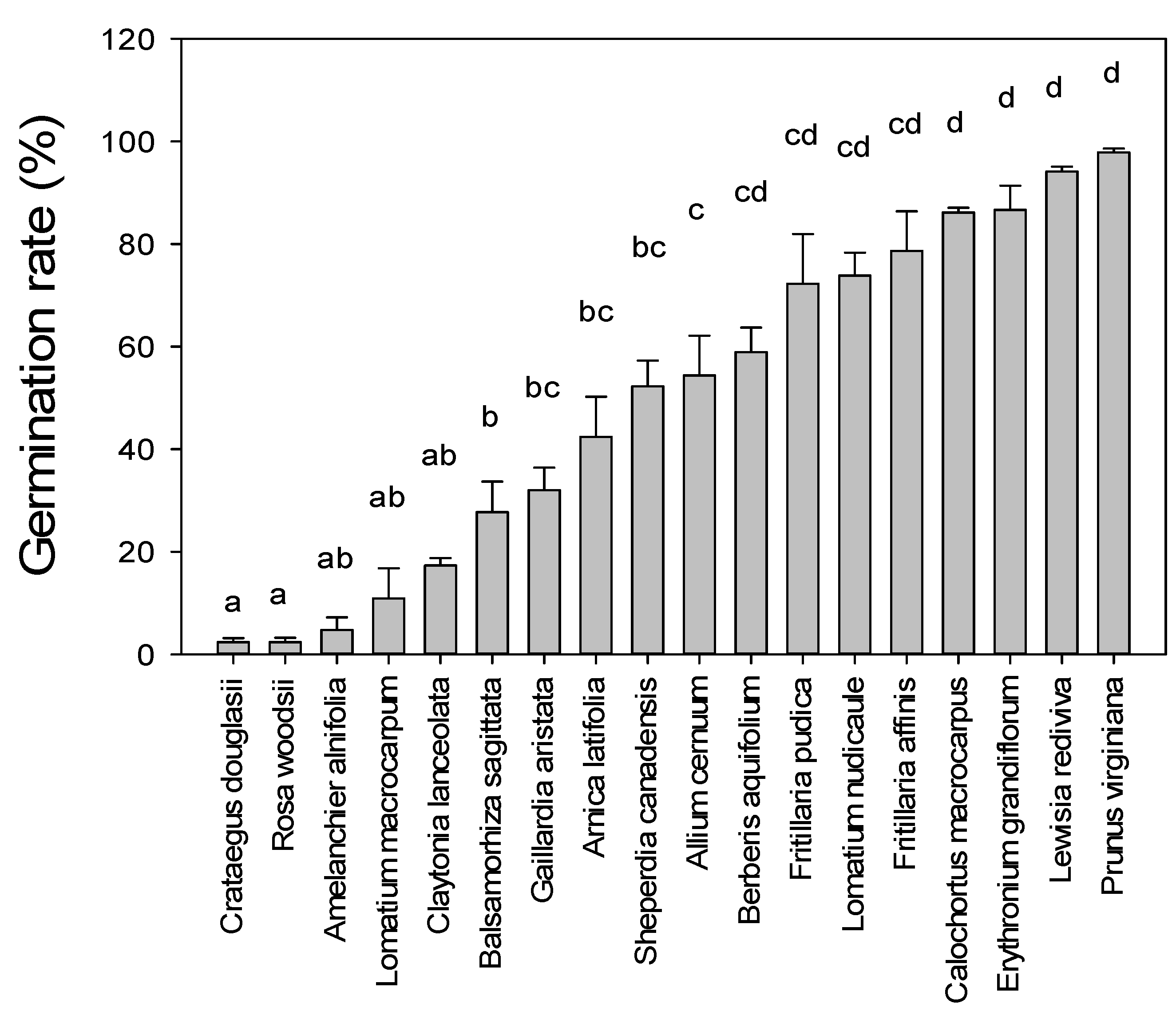
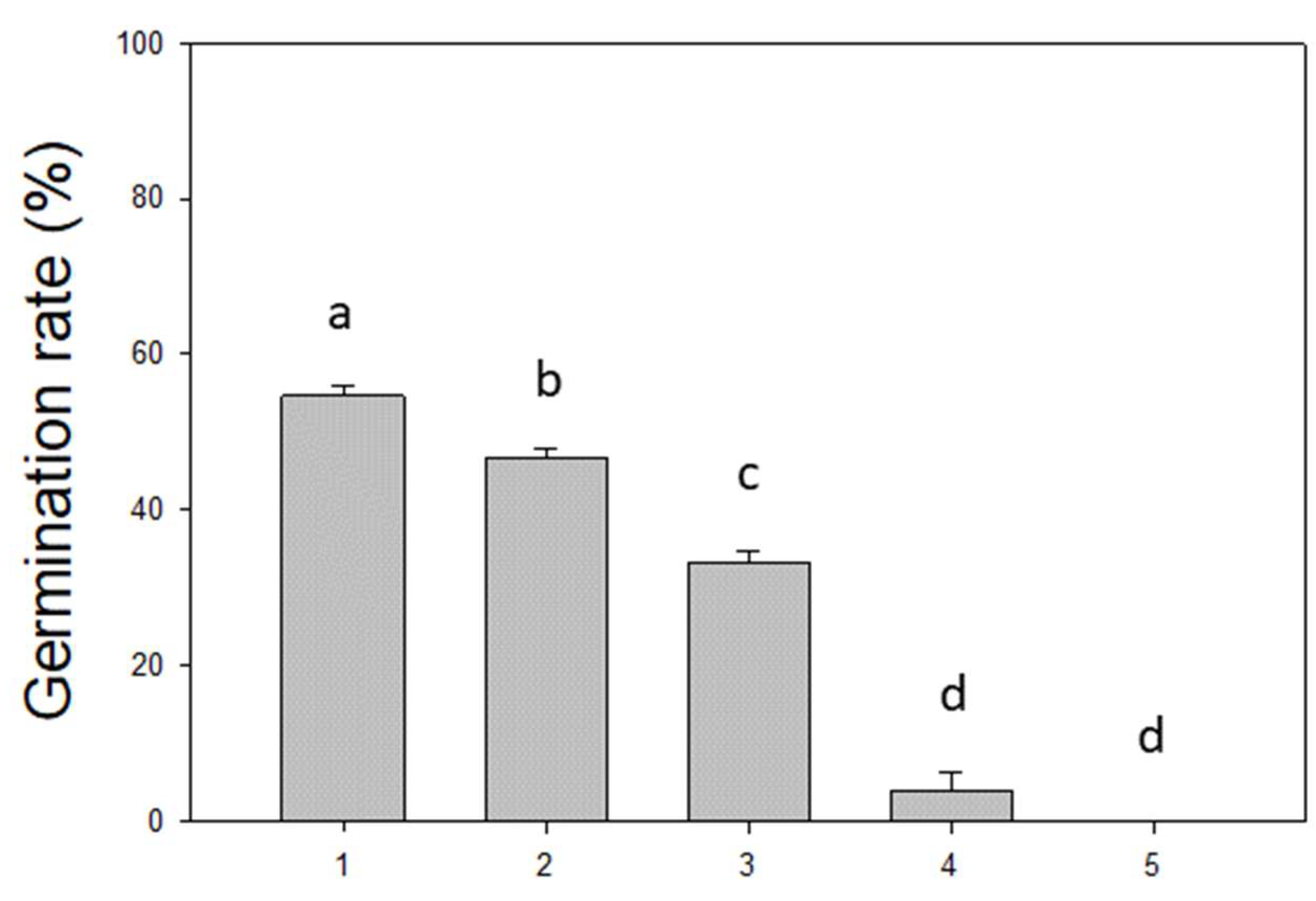
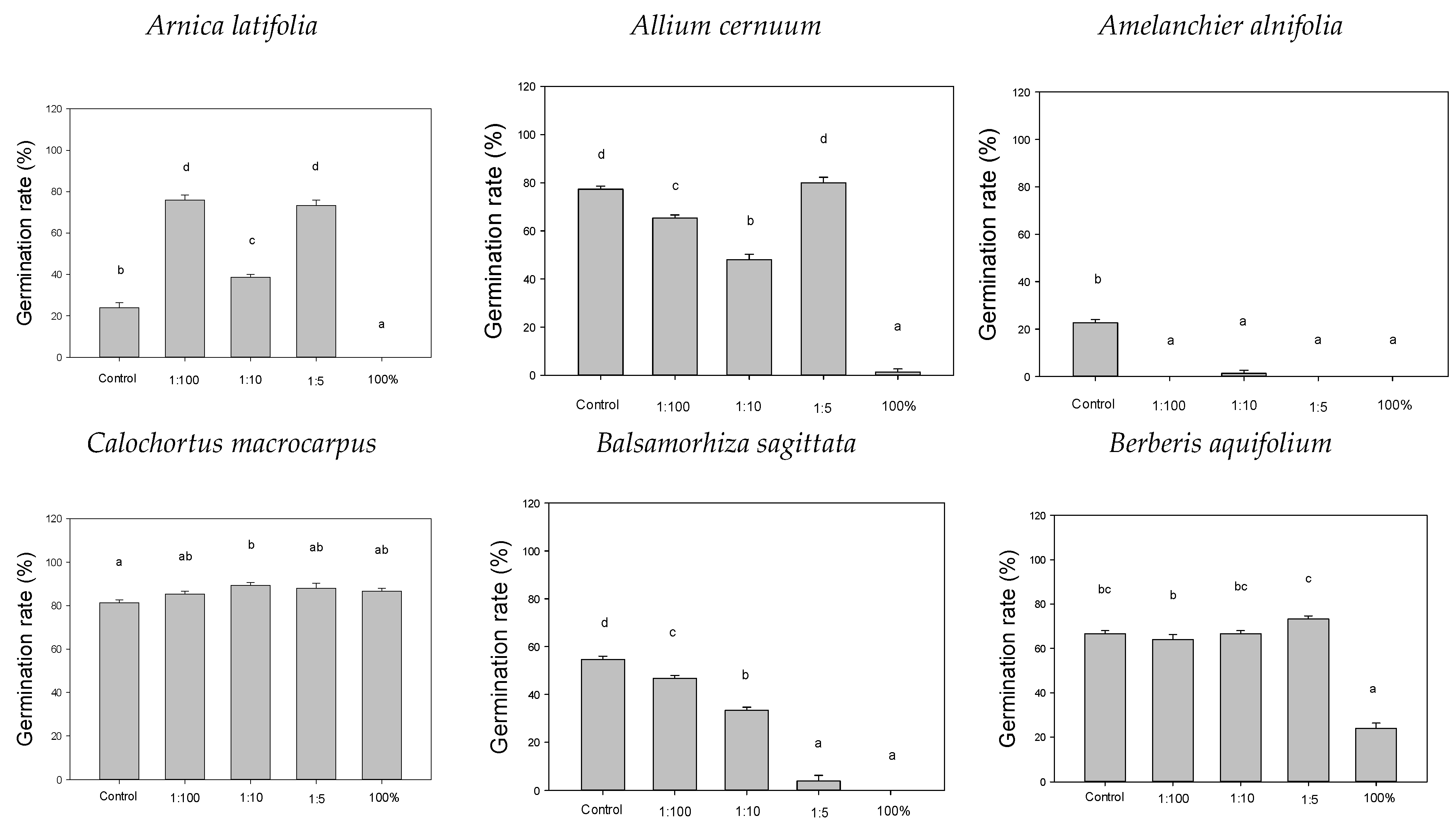
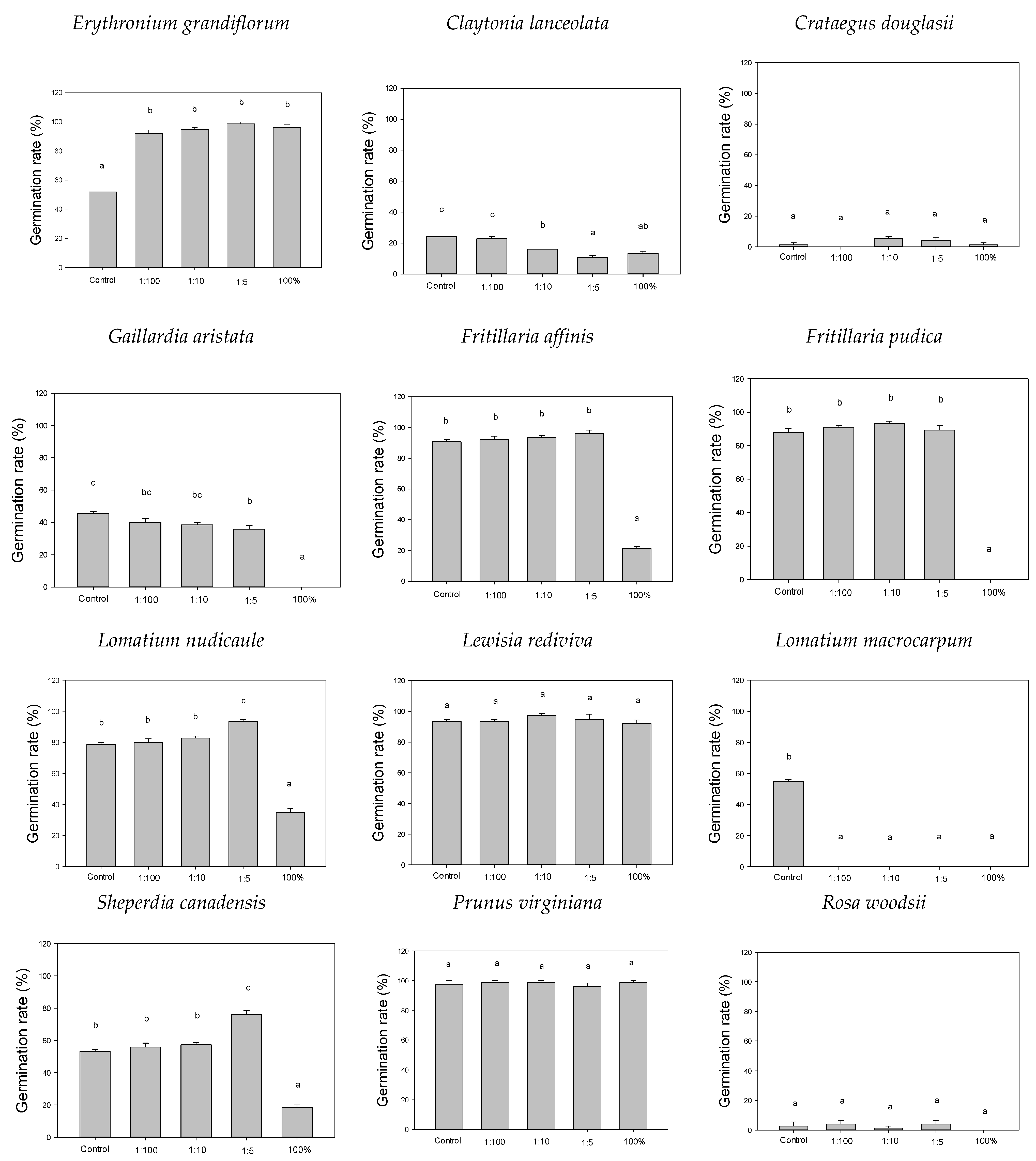
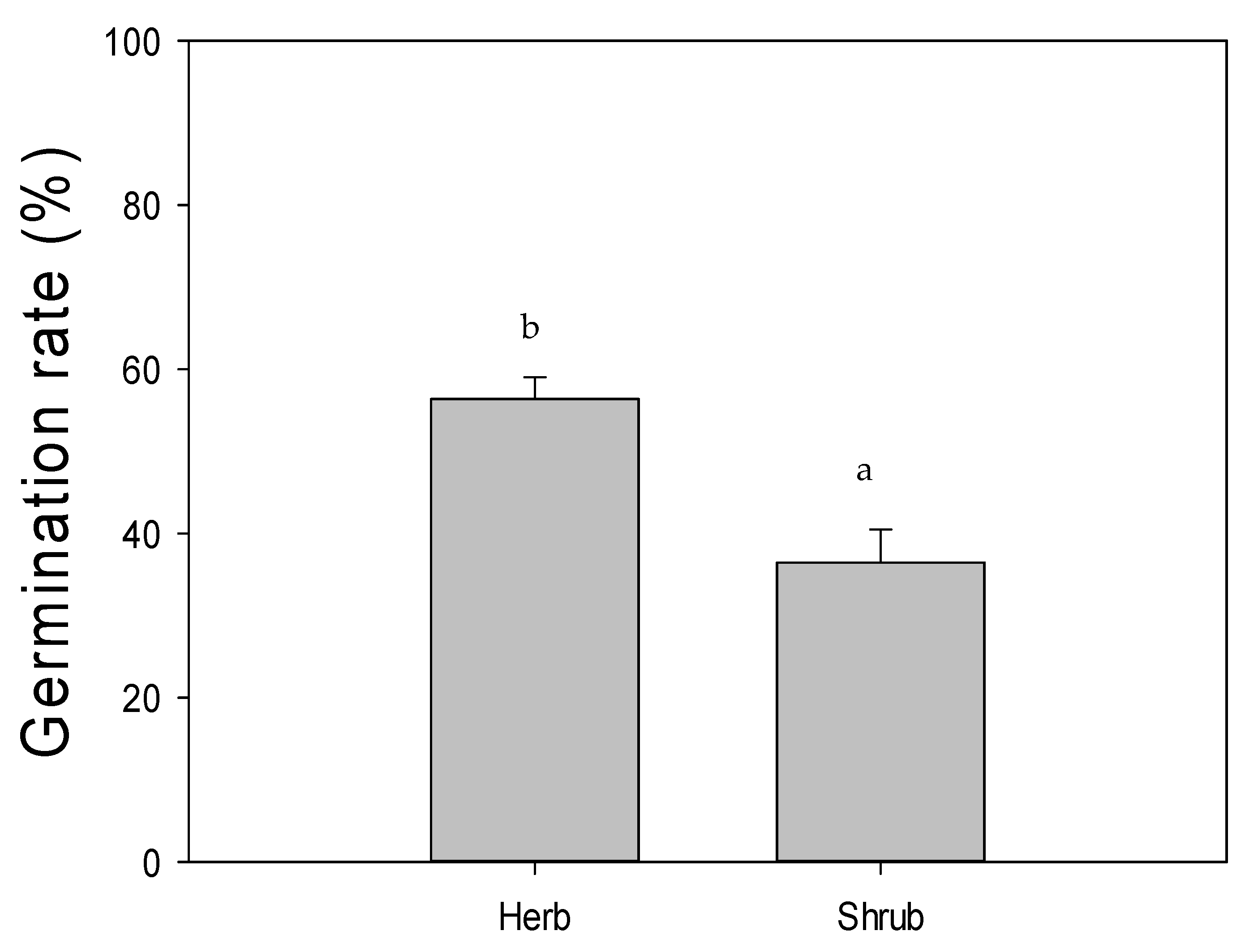
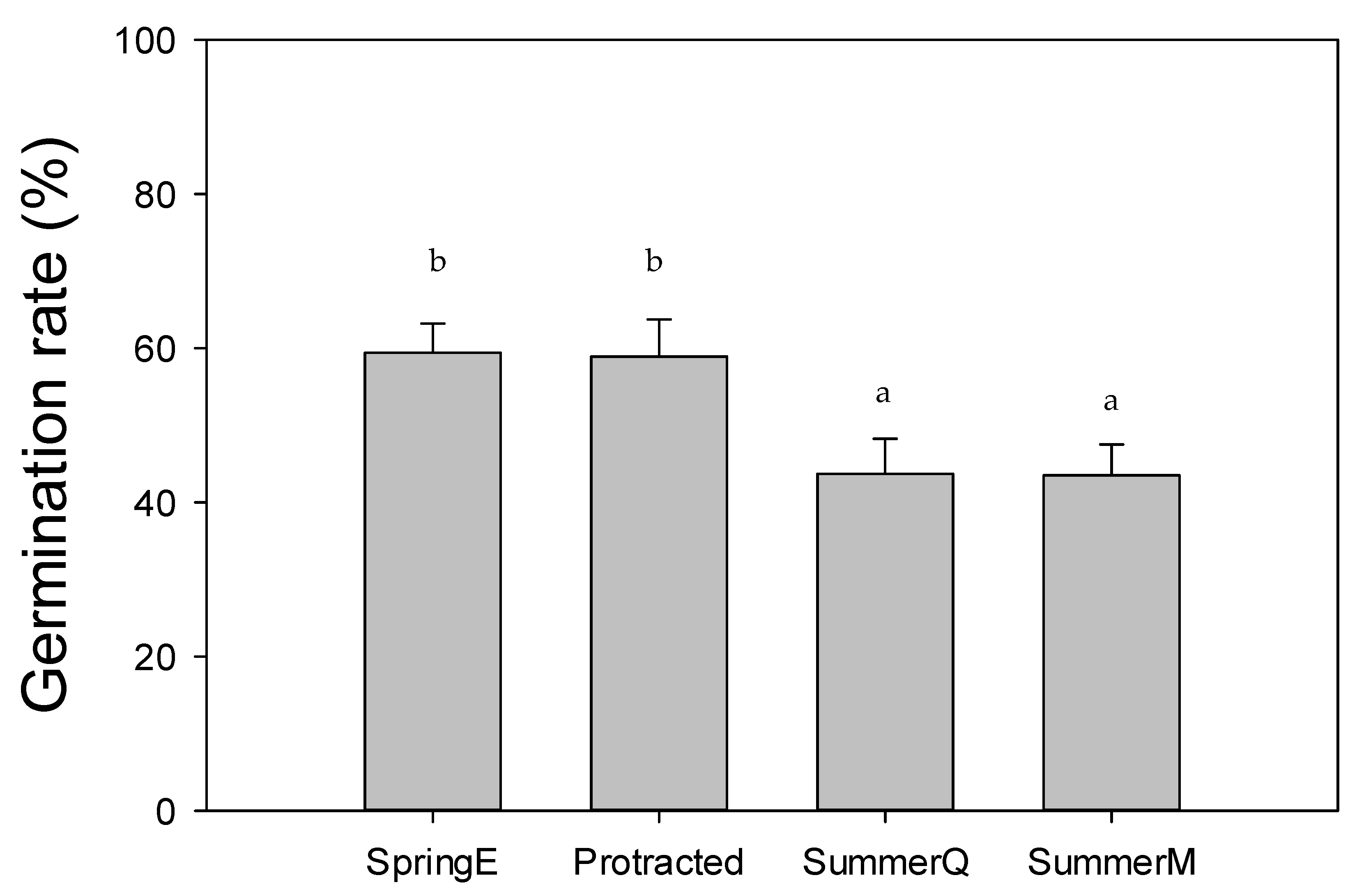
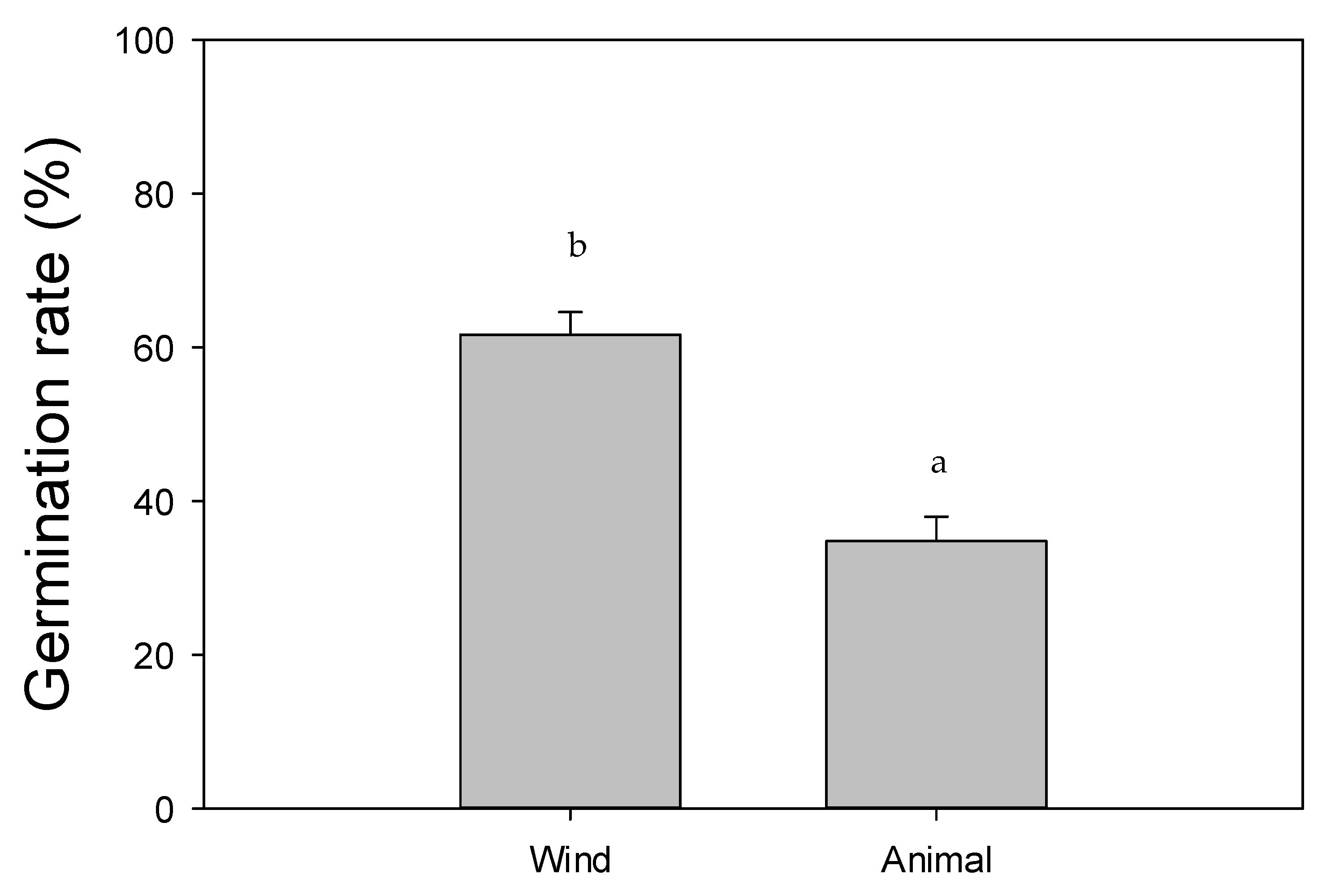
| Scientific Name | Common Name | Plant Form | Phenology | Seed Dispersal |
|---|---|---|---|---|
| Allium cernuum Roth | Nodding Onion | Forb | Summer Quiescent | Wind |
| Amelanchier alnifolia (Nutt.) Nutt. ex M. Roem. | Saskatoon | Shrub | Summer Mature | Animal |
| Arnica latifolia Bong. | Mountain Arnica | Forb | Summer Mature | Wind |
| Balsamorhiza sagittata (Pursh) Nutt. | Arrow Leaved Balsamroot | Forb | Spring Ephemeral | Animal |
| Berberis aquifolium Pursh | Oregon Grape | Shrub | Protracted Growth | Animal |
| Calochortus macrocarpus Douglas | Mariposa Lily | Forb | Summer Quiescent | Wind |
| Claytonia lanceolata Pall. ex Pursh | Western Spring Beauty | Forb | Spring Ephemeral | Wind |
| Crataegus douglasii Lindl. | Hawthorne | Shrub | Summer Mature | Animal |
| Erythronium grandiflorum Pursh | Glacier Lily | Forb | Spring Ephemeral | Wind |
| Fritillaria affinis (Schult. & Schult. f.) Sealy | Chocolate Lily | Forb | Spring Ephemeral | Wind |
| Fritillaria pudica (Pursh) Spreng. | Yellow Bell | Forb | Spring Ephemeral | Wind |
| Gaillardia aristata Pursh | Brown Eyed Susan | Forb | Summer Quiescent | Animal |
| Lewisia rediviva Pursh | Bitterroot | Forb | Summer Mature | Wind |
| Lomatium macrocarpum Coult. & Rose | Large Fruited Desert Parsley | Forb | Summer Mature | Wind |
| Lomatium nudicaule (Pursh) J.M. Coult. & Rose | Barestem Desert Parsley | Forb | Spring Ephemeral | Wind |
| Prunus virginiana L. | Choke Cherry | Shrub | Summer Mature | Animal |
| Rosa woodsii Lindl. | Prairie Rose | Shrub | Summer Quiescent | Animal |
| Shepherdia canadensis Nutt. | Soopolallie | Shrub | Summer Mature | Animal |
| Analyte | MDL (ng/mL) | MRL (ng/mL) | Free VPs (ng/mL) |
|---|---|---|---|
| 4-Methylguaiacol | 0.142 | 0.500 | 15,300 |
| Guaiacol | 0.095 | 0.500 | 26,000 |
| o-Cresol | 0.116 | 1.00 | 4740 |
| p-Cresol | 0.156 | 0.500 | 9260 |
| Species | Mean Squares | F-Ratio | p-Value |
|---|---|---|---|
| Allium cernuum | 3118.4 | 324.83 | <0.001 |
| Amelanchier alnifolia | 300.3 | 140.75 | <0.001 |
| Arnica latifolia | 3177.1 | 270.77 | <0.001 |
| Balsamorhiza sagittata | 1835.7 | 286.83 | <0.001 |
| Berberis aquifolium | 1179.7 | 122.89 | <0.001 |
| Calochortus macrocarpus | 28.3 | 3.79 | 0.04 |
| Claytonia lanceolata | 101.3 | 31.67 | <0.001 |
| Crataegus douglasii | 14.4 | 2.25 | 0.136 |
| Erythronium grandiflorum | 1144.0 | 134.06 | <0.001 |
| Fritillaria affinis | 3093.3 | 322.22 | <0.001 |
| Fritillaria pudica | 4907.7 | 511.22 | <0.001 |
| Gaillardia aristata | 994.7 | 116.56 | <0.001 |
| Lewisia rediviva | 12.3 | 0.89 | 0.507 |
| Lomatium macrocarpum | 1793.1 | 1681.00 | <0.001 |
| Lomatium nudicaule | 1540.3 | 144.40 | <0.001 |
| Prunus virginiana | 4.3 | 0.40 | 0.804 |
| Rosa woodsii | 9.1 | 0.77 | 0.567 |
| Sheperdia canadensis | 1299.7 | 135.39 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peterson, N.; Gardner, W.; Fraser, L.H. The Effect of Smoke-Water on Seed Germination of 18 Grassland Plant Species. Fire 2025, 8, 382. https://doi.org/10.3390/fire8100382
Peterson N, Gardner W, Fraser LH. The Effect of Smoke-Water on Seed Germination of 18 Grassland Plant Species. Fire. 2025; 8(10):382. https://doi.org/10.3390/fire8100382
Chicago/Turabian StylePeterson, Nicholas, Wendy Gardner, and Lauchlan H. Fraser. 2025. "The Effect of Smoke-Water on Seed Germination of 18 Grassland Plant Species" Fire 8, no. 10: 382. https://doi.org/10.3390/fire8100382
APA StylePeterson, N., Gardner, W., & Fraser, L. H. (2025). The Effect of Smoke-Water on Seed Germination of 18 Grassland Plant Species. Fire, 8(10), 382. https://doi.org/10.3390/fire8100382






