Abstract
Vasovagal syncope (VVS) affects 17% of children, significantly impairing quality of life. Machine learning (ML) models achieve high predictive accuracy of VVS in adults using blood pressure (BP) monitoring, but pediatric implementation remains challenging. The aim of the study was to evaluate whether ML models incorporating anthropometric data and heart rate variability (HRV) can predict VVS without BP monitoring in children with prior syncope or suspected VVS. We analyzed 87 participants (7–18 years) with VVS history. HRV indices (time-domain, frequency-domain, and nonlinear) were extracted from 5 min supine and standing ECG recordings using NeuroKit2. Multiple algorithms were tested with 10-fold cross-validation; SHAP analysis identified feature importance. AdaBoost achieved the performance of 71.0% accuracy, 76.3% sensitivity, and 63.3% specificity—78% of adult BP-dependent algorithm sensitivity. Weight, multifractal detrended fluctuation analysis during standing, and normalized low-frequency power were most influential. Alterations in symbolic dynamics and multiscale entropy indicated compromised autonomic complexity. ML models with anthropometric and HRV data show potential as an adjunctive screening tool to identify children at higher risk for syncope recurrence, requiring clinical confirmation.
1. Introduction
Vasovagal syncope (VVS) affects approximately 17% of children [1,2]. Whilst generally benign, recurrent episodes significantly impair quality of life [1]. In adults, machine learning (ML) approaches have achieved high predictive accuracy (95–98% sensitivity) for VVS using combined heart rate and blood pressure (BP) data during head-up tilt testing [3]. However, continuous BP monitoring limits practical implementation, particularly in pediatric populations [4]. Heart rate variability (HRV), which reflects autonomic modulation, offers predictive potential, though HRV-only algorithms in adults demonstrated reduced performance (sensitivity of 85–90% and specificity of 62–64%) [3,5]. Importantly, anthropometric factors such as age, sex, and body mass index (BMI) independently predict syncope occurrence in children [2]. Therefore, we aimed to evaluate whether ML models incorporating anthropometric data, clinical information, and short-term HRV parameters from supine and standing phases can achieve predictive performance for pediatric VVS comparable to that reported in adults.
2. Materials and Methods
The study was conducted on 96 participants (35 boys and 61 girls) aged 7–18 years with a history of VVS. Anthropomorphic characteristics (sex, age, height, weight, and BMI) and clinical data (lifetime number of syncopal episodes and sport participation) were collected using a questionnaire and used as features for ML modeling. RR intervals (RRis) time series were obtained from ECG examinations (Task Force Monitor System; CNSystems Medizintechnik GmbH, Graz, Austria) performed at supine (5 min) and upright rest (5 min), 10 min in total, sampled at 1 kHz, stationary-confirmed (Phillips–Perron test).
The study was approved by the University Bioethical Committee (KB/182/2016) and followed the rules and principles of the Helsinki Declaration; all parents or legal guardians and patients 16 years old and older gave their informed written consent.
Signals were preprocessed using the coRRection software [6]. Technical and physiological artifacts (ectopic beats and premature atrial and/or ventricular beats) present in the ECG signal were replaced by interpolated RRis from adjacent RRis. Corrected RR series were not filtered or detrended using the software tools. Time-domain, frequency-domain, and nonlinear HRV indices were calculated using the NeuroKit2 library in Python. All the patients were examined in the same room under controlled conditions.
Fourteen datasets were constructed, combining anthropometric data, clinical information, and HRV indices derived from 1 to 5 min windows during supine positioning, with or without standing-phase data. Each patient record was binary-labeled according to tilt test outcome (positive/negative).
ML classification was performed using HRV features as input variables and the tilt test result as the target. Stratified 10-fold cross-validation was applied, and class imbalance was addressed using the Synthetic Minority Oversampling Technique, applied only to the training set (upsampling to 100 samples per class) to preserve the original distribution in the test set. Multiple ML models (logistic regression, decision trees, random forests, support vector machines, K-nearest neighbors, AdaBoost, XGBoost, and multilayer perceptron) were tested with hyperparameter optimization. Performance metrics (accuracy, sensitivity, specificity, precision, and F1 score) were calculated for each cross-validation fold and then aggregated across folds as mean ± standard deviation (SD). To interpret the ML model’s predictions, SHAP (SHapley Additive exPlanations) values were calculated for each feature, quantifying their contribution to individual predictions. SHAP analysis provides model-agnostic interpretability by computing the marginal contribution of each feature across all possible feature combinations, with positive SHAP values indicating increased probability of syncope prediction and negative values indicating decreased probability. Feature importance was determined by the mean absolute SHAP value across all observations, and results were visualized using beeswarm plots to display both the magnitude and direction of feature effects on model output. All analyses were conducted using Python 3.11.9.
3. Results
Results from 9 participants were excluded due to poor signal quality; consequently, data from 87 participants (30 boys and 57 girls) were analyzed. Mean ± SD characteristics were age 14.5 ± 2.5 years, body mass 56.1 ± 13.4 kg, height 1.65 ± 0.12 m, and BMI 20.2 ± 3.2 kg/m2. Twenty participants (23%) reported engaging in sport 2–3 times per week. More than four lifetime syncopal episodes were reported by 35 patients (40%), and 51 (59%) had a positive tilt test outcome. The results of the ML analysis are summarized in Table 1. The highest performance in terms of accuracy, sensitivity, and F1 score was achieved by the AdaBoost model (for 980 weak learners utilized in the algorithm and for the learning rate coefficient of 0.07) using the dataset that combined anthropometric data, clinical information, and HRV parameters derived from 5 min of ECG recordings in both supine and standing positions.

Table 1.
Metrics obtained from the machine learning analysis, presented as mean ± standard deviation, based on datasets containing HRV parameters calculated from different ECG recording windows. “–” indicates that the dataset did not include HRV parameters from the standing position.
The SHAP plot (Figure 1) illustrates the contribution of individual features to the ML model’s predictions for VVS in children. Features are ranked by their overall importance with each dot representing a single patient. The horizontal position indicates the SHAP value (impact on model output), where positive values increase the probability of syncope prediction and negative values decrease it. The color gradient represents feature values, with blue indicating lower values and pink/red indicating higher values for each respective variable. Weight emerged as the most influential feature, showing the widest distribution of SHAP values. Higher weight values (pink/red dots) were predominantly associated with positive SHAP values. The second most important feature was multifractal detrended fluctuation analysis (MFDFA)_alpha2_Peak during standing, a nonlinear HRV measure reflecting long-range fractal scaling properties of heart rate dynamics during orthostatic stress. Among frequency-domain indices, LFn (normalized low-frequency power) during both standing and supine positions ranked highly, with lower LFn values during standing (blue dots) showing positive SHAP contributions. Several MFDFA parameters from standing position appeared prominently, including MFDFA_alpha1_Width_standing, suggesting that alterations in multifractal properties during orthostatic challenge provide important discriminative information. Nonlinear measures from symbolic dynamics (SymDyn) and multiscale entropy (MSEn) families such as SymDynMaxMin_2LV_supine and CompositeMSEn_supine also demonstrated moderate importance, with their respective value distributions showing variable directional effects on predictions. Anthropometric variables, specifically height, ranked lower in importance, indicating that while body size contributes to the model, HRV-derived features provide more discriminative power for syncope prediction.
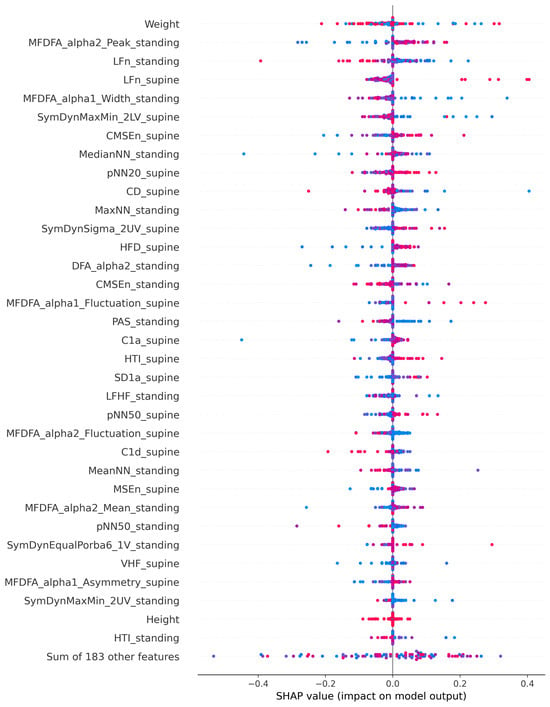
Figure 1.
SHAP plot showing feature importance for vasovagal syncope prediction model. Blue indicates lower values, pink/red indicates higher values of a feature.
4. Discussion
To our knowledge, this is the first study to develop ML models for pediatric VVS prediction using only HRV and anthropometric data. Our models achieved 71.0% accuracy, 76.3% sensitivity, and 63.3% specificity, which represents 78% of the sensitivity achieved by BP-dependent algorithms in adults (97.6%) [3] while eliminating the need for continuous monitoring, which is particularly challenging in pediatric populations [4]. Performance approximates adult HRV-only algorithms (85.7% sensitivity and 62.1% specificity) [5], suggesting comparable autonomic response patterns despite developmental differences.
The superior performance with 5 min dual-posture recordings demonstrates the importance of capturing autonomic transitions [3,5]. Specificity (63.3%) aligns with adult HRV-only methodologies [3,5]. Importantly, HRV-based approaches can be implemented in pediatric practice utilizing wearable devices with sufficient sampling rates for accurate RR interval detection in different conditions and reliable HRV analysis, such as Pneumonitor, which has already been validated in pediatric cardiac patients [7,8]. The identification of weight as a primary predictor of syncope risk aligns with existing evidence demonstrating that lower BMI is associated with increased syncope recurrence and poorer therapeutic response to standard interventions in pediatric VVS [1,9,10], potentially reflecting inadequate blood volume reserves and reduced lower limb muscle pump function critical for venous return during orthostatic stress [2]. Orthostatic challenge induces the sympathetic activation and parasympathetic withdrawal necessary for maintaining blood pressure and cerebral perfusion [11]. This autonomic shift disrupts the correlated, complex fractal structure of the heart rate toward uncorrelated or stochastic behavior, reflecting immediate sympathetic-mediated cardiovascular adjustments [12,13]. The reduced MFDFA α2 peak values during standing suggest decreased long-range fractal correlations in heart rate dynamics, which has been associated with loss of short-term fractal organization and reduced autonomic complexity during postural change maneuvers [14,15]. The lower normalized low-frequency power (LFn) during standing in children who developed syncope reflects inadequate sympathetic enhancement during orthostatic stress, contrasting with the expected sympathetic activation pattern observed in healthy controls during head-up tilt testing [16,17,18]. Alterations in SymDyn patterns and CMSEn during the supine position indicate compromised complexity in cardiac autonomic control, as these nonlinear indices quantify the prevalence of sympathovagal balance and the richness of interactive processes within the central autonomic network [19,20]. VVS and other pediatric orthostatic intolerance disorders, including postural orthostatic tachycardia syndrome (POTS), share overlapping pathophysiological mechanisms involving impaired autonomic cardiovascular regulation during postural stress [21]. Children and adolescents with POTS demonstrate attenuated vagal baroreflex function and sympathetic predominance during head-up tilt, quantifiable through HRV analysis [22]. Notably, baseline HRV parameters have proven predictive of the therapeutic response to beta-blockers in pediatric POTS, establishing the clinical utility of autonomic assessment in guiding management [23]. The comparable alterations in HRV metrics observed in our cohort (i.e., disrupted complexity across multiple domains including fractal scaling properties, frequency-domain indices, and nonlinear entropy measures) suggest shared pathophysiological mechanisms and support the potential for HRV-based risk stratification across different phenotypes of pediatric orthostatic intolerance.
From the analyzed ML methods, AdaBoost performed best for the specific dataset utilized for the study. The reason for this cannot be explained unequivocally. The answer might lie in the method itself. It is one of the “boosting” methods and compared to other approaches in the family, the AdaBoost method adapts the weights for misclassified data, which makes it well-suited for small, yet still high-dimensional datasets. The fact that this method was the best will in no way favor it in our future research on this topic.
Our model achieved moderate accuracy (71%); it should be emphasized that this tool is intended as an adjunctive risk assessment instrument rather than a standalone diagnostic tool. The sensitivity of 76.3% suggests reasonable capability for identifying children who may benefit from closer monitoring or preventive interventions. However, the specificity of 63.3% indicates that clinical judgment and comprehensive evaluation remain essential. The realistic clinical role of this model would be (i) continuous monitoring to identify periods of increased syncope risk, (ii) supporting clinical decision-making regarding lifestyle modifications, and (iii) providing objective data to complement subjective symptom reporting.
The study has several limitations. Recordings were obtained during single sessions, limiting our ability to assess model generalizability across temporally separated measurements and account for day-to-day variability that would be encountered during continuous monitoring. The moderate specificity (63.3%) indicates potential for false-positive predictions, emphasizing the need for integration with comprehensive clinical assessment. Future research should implement multi-session longitudinal data collection with temporally stratified validation and explore pediatric-specific hybrid models incorporating BP or BP surrogates to enhance predictive performance while maintaining feasibility for wearable technologies.
5. Conclusions
ML models utilizing anthropometric and HRV data demonstrated feasibility for adjunctive risk assessment of VVS recurrence in children, with performance (76.3% sensitivity and 63.3% specificity) achieving 78% of the sensitivity reported for blood pressure-dependent algorithms in adults while eliminating the need for continuous invasive monitoring. While these performance metrics are insufficient for standalone diagnosis, the model shows potential as a complementary tool to support clinical decision-making in identifying children at higher risk for syncope recurrence. The comparable performance to adult HRV-only methodologies suggests that autonomic response patterns can inform risk stratification despite developmental differences in pediatric populations. Future clinical implementation would require integration with comprehensive clinical assessment, with the model serving to enhance rather than replace clinical judgment. Further development may include incorporation of additional hemodynamic parameters, validation with wearable-grade signals in larger cohorts, and assessment of real-time monitoring capabilities.
Author Contributions
Conceptualization, P.W. and J.S.G.; methodology, P.W., J.S.G., M.R., and M.M.; formal analysis, P.W., J.S.G., M.R., and M.M.; investigation, P.W.; data curation, P.W., E.S.-W., and A.P.-M.; writing—original draft preparation, P.W., J.S.G., M.R., and M.M.; writing—review and editing, all authors; project administration, P.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Acknowledgments
During the preparation of this manuscript, the authors used Claude.ai to polish English text. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sun, R.; Kang, Y.; Zhang, M.; Wang, H.; Shi, L.; Li, X. Development of prognostic nomogram model to predict syncope recurrence in children with vasovagal syncope. Front. Cardiovasc. Med. 2023, 10, 1099115. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Peng, Y.; Zou, R.; Wang, Y.; Cai, H.; Li, F.; Luo, X.; Zhang, J.; He, Z.; Wang, C. The relationship between demographic factors and syncopal symptom in pediatric vasovagal syncope. Sci. Rep. 2023, 13, 22724. [Google Scholar] [CrossRef] [PubMed]
- Virag, N.; Erickson, M.; Taraborrelli, P.; Vetter, R.; Lim, P.B.; Sutton, R. Predicting vasovagal syncope from heart rate and blood pressure: A prospective study in 140 subjects. Heart Rhythm 2018, 15, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kim, J.T.; Kwak, S.S.; Hourlier-Fargette, A.; Avila, R.; Vogl, J.; Tzavelis, A.; Chung, H.U.; Lee, J.Y.; Kim, D.H.; et al. Wireless, Skin-Interfaced Devices for Pediatric Critical Care: Application to Continuous, Noninvasive Blood Pressure Monitoring. Adv. Healthc. Mater. 2021, 10, e2100383. [Google Scholar] [CrossRef]
- Bin Lee, H.B.; Park, G.; Jung, M.; Yong Shin, S.; Cho, S.; Hwan Cho, J. Machine Learning Model Using Heart Rate Variability for the Prediction of Vasovagal Syncope. IEEE Access 2024, 12, 151153–151160. [Google Scholar] [CrossRef]
- Mikielewicz, M.; Gąsior, J.S.; Młyńczak, M. CoRRection–an open source software tool for RR intervals processing. Pol. J. Med. Phys. Eng. 2025, 31, 10–19. [Google Scholar] [CrossRef]
- Gąsior, J.S.; Młyńczak, M.; Rosoł, M.; Wieniawski, P.; Walecka, I.; Cybulski, G.; Werner, B. Validity of the Pneumonitor for RR intervals acquisition for short-term heart rate variability analysis extended with respiratory data in pediatric cardiac patients. Kardiol. Pol. 2023, 81, 491–500. [Google Scholar] [CrossRef]
- Gąsior, J.S.; Młyńczak, M.; Rosoł, M.; Wieniawski, P.; Pietrzak, R.; Werner, B. Validity of the Pneumonitor for Analysis of Short-Term Heart Rate Asymmetry Extended with Respiratory Data in Pediatric Cardiac Patients. J. Clin. Med. 2024, 13, 4654. [Google Scholar] [CrossRef]
- Zhu, W.; Bian, X.; Lv, J. Advances in diagnosis, management, and long-term outcomes of pediatric vasovagal syncope: A comprehensive review. Front. Cardiovasc. Med. 2025, 12, 1481749. [Google Scholar] [CrossRef]
- Tao, C.Y.; Chen, S.; Li, X.Y.; Tang, C.S.; Du, J.B.; Jin, H.F. Body mass index is a promising predictor of response to oral rehydration saline in children with vasovagal syncope. Chin. Med. J. 2020, 134, 463–468. [Google Scholar] [CrossRef]
- de Souza, A.C.; Cisternas, J.R.; de Abreu, L.C.; Roque, A.L.; Monteiro, C.B.; Adami, F.; Vanderlei, L.C.; Sousa, F.H.; Ferreira, L.L.; Valenti, V.E. Fractal correlation property of heart rate variability in response to the postural change maneuver in healthy women. Int. Arch. Med. 2014, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.K.; Havlin, S.; Stanley, H.E.; Goldberger, A.L. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos 1995, 5, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, P.; Parati, G.; Lombardi, C.; Quintin, L.; Di Rienzo, M. Assessing the fractal structure of heart rate by the temporal spectrum of scale exponents: A new approach for detrended fluctuation analysis of heart rate variability. Biomed. Tech. 2011, 56, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Perseguini, N.M.; Takahashi, A.C.; Rebelatto, J.R.; Silva, E.; Borghi-Silva, A.; Porta, A.; Montano, N.; Catai, A.M. Spectral and symbolic analysis of the effect of gender and postural change on cardiac autonomic modulation in healthy elderly subjects. Braz. J. Med. Biol. Res. 2011, 44, 29–37. [Google Scholar] [CrossRef]
- Castiglioni, P.; Parati, G.; Di Rienzo, M.; Carabalona, R.; Cividjian, A.; Quintin, L. Scale exponents of blood pressure and heart rate during autonomic blockade as assessed by detrended fluctuation analysis. J. Physiol. 2011, 589, 355–369. [Google Scholar] [CrossRef]
- Shim, S.H.; Park, S.Y.; Moon, S.N.; Oh, J.H.; Lee, J.Y.; Kim, H.H.; Han, J.W.; Lee, S.J. Baseline heart rate variability in children and adolescents with vasovagal syncope. Korean J. Pediatr. 2014, 57, 193–198. [Google Scholar] [CrossRef]
- Kouakam, C.; Lacroix, D.; Zghal, N.; Logier, R.; Klug, D.; Le Franc, P.; Jarwe, M.; Kacet, S. Inadequate sympathovagal balance in response to orthostatism in patients with unexplained syncope and a positive head up tilt test. Heart 1999, 82, 312–318. [Google Scholar] [CrossRef]
- Piccirillo, G.; Elvira, S.; Bucca, C.; Viola, E.; Cacciafesta, M.; Marigliano, V. Abnormal passive head-up tilt test in subjects with symptoms of anxiety power spectral analysis study of heart rate and blood pressure. Int. J. Cardiol. 1997, 60, 121–131. [Google Scholar] [CrossRef]
- Guzzetti, S.; Borroni, E.; Garbelli, P.E.; Ceriani, E.; Della Bella, P.; Montano, N.; Cogliati, C.; Somers, V.K.; Malliani, A.; Porta, A. Symbolic dynamics of heart rate variability: A probe to investigate cardiac autonomic modulation. Circulation 2005, 112, 465–470. [Google Scholar] [CrossRef]
- Cysarz, D.; Van Leeuwen, P.; Edelhäuser, F.; Montano, N.; Porta, A. Binary symbolic dynamics classifies heart rate variability patterns linked to autonomic modulations. Comput. Biol. Med. 2012, 42, 313–318. [Google Scholar] [CrossRef]
- Stewart, J.M.; Boris, J.R.; Chelimsky, G.; Fischer, P.R.; Fortunato, J.E.; Grubb, B.P.; Heyer, G.L.; Jarjour, I.T.; Medow, M.S.; Numan, M.T.; et al. Pediatric Writing Group of the American Autonomic Society. Pediatric Disorders of Orthostatic Intolerance. Pediatrics 2018, 141, e20171673. [Google Scholar] [CrossRef]
- Orjatsalo, M.; Alakuijala, A.; Partinen, M. Heart Rate Variability in Head-Up Tilt Tests in Adolescent Postural Tachycardia Syndrome Patients. Front. Neurosci. 2020, 14, 725. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Chen, S.; Liu, P.; Wang, Y.; Tang, C.; Jin, H.; Du, J. Heart Rate Variability Predicts Therapeutic Response to Metoprolol in Children with Postural Tachycardia Syndrome. Front. Neurosci. 2019, 13, 1214. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).