Abstract
We clarified the effect of mixing temperature on the synthesis of gold nanoparticles by a microfluidic device using tannic acid and citric acid as reducing reagents. In this study, gold nanoparticles were synthesized on the microfluidic device with a flow rate of 0.05 mL/min at the temperature of room temperature (23 °C) and 80 °C. The results confirmed that the mean diameter and coefficient of variation of gold nanoparticles synthesized at 80 °C were 13.7 nm and 0.28 and the coefficient of variation increased by 65% in comparison with that at room temperature.
1. Introduction
Gold nanoparticles (GNPs) exhibit an absorption peak at a wavelength of approximately 530 nm in a visible light region. This absorption is applied to catalysis and conductive materials caused by localized plasmon resonance (LSPR) [1]. The GNPs can be produced by liquid-phase reduction using gold ion, protective reagent, and reducing reagent. In the liquid-phase reduction using citric acid [2], the GNPs with a diameter of approximately 10 nm can be synthesized by heating the mixture solution and the size distribution of GNPs depends on the temperature of the solution [2]. Non-thermal synthesis in a microfluidic device using sodium citrate and tannic acid as reducing reagents was reported [3]. This synthesis can be controlled by the size distribution of GNPs by changing the flow rate of the solution and monodisperse GNPs with a mean diameter of 10 nm can be synthesized at a specific flow rate. But because the control of the size distribution by a wide range is required for each application, the synthesis temperature effect on the particle size distribution must be clarified. In this paper, we proposed thermal reduction synthesis of GNPs using a microfluidic device.
2. Materials and Methods
In this study, a PDMS/glass microfluidic device was used for synthesis to prevent glass breakage due to heating. A microfluidic device with a Y-shaped microchannel of 260 μm width and 70 μm depth was fabricated by plasma bonding of a PDMS sheet and glass substrate with a microchannel processed by micropowder blasting (Figure 1a).
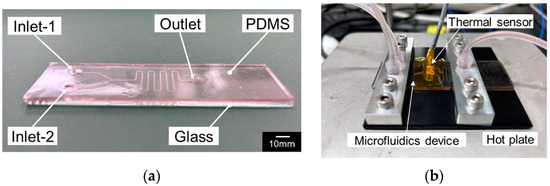
Figure 1.
(a) Photograph of (a) the fabricated microfluidic device and (b) experimental setup.
In the synthesis, 5mL of hydrogen tetrachloroaurate (III) tetrahydrate aqueous solution (solution-A, 0.368 mM) and 5 mL mixture of 4mL sodium citrate (1.69 mM), 0.1 mL tannic acid (5.82 mM), and 75.9 mL distilled water (solution-B) were simultaneously injected into Inlet-1 and Inlet-2 by a syringe pump at the flow rate of 0.05 mL/min and the synthesized GNPs were collected from the outlet in a bottle through the silicone tube (Figure 1b). In this study, the device was set on a hot plate and the temperature of the device was controlled at room temperature (23 °C) and 80 °C (Figure 1b). The synthesized GNP solution was allowed to stand for seven days.
Seven days after the synthesis, the absorbance spectrum of the synthesized GNP solution was recorded and the mean diameter, standard deviation, and coefficient of variation (standard deviation/mean diameter) were analyzed by TEM observation.
3. Results and Discussion
The synthesized GNPs showed a red color at the temperature of 23 °C and a red–purple color at the temperature of 80 °C (Figure 2a). The absorption spectrum of the synthesized GNPs confirmed that the full width at half maximum at the peak was increased by increasing the synthesis temperature (Figure 2b). This expected that the size distribution of the GNPs synthesized at 80 °C was large in comparison with that at 23 °C. From the analysis of TEM images (Figure 2c), it was confirmed that the mean diameter of the synthesized GNPs showed 12.1 nm at the temperature of 23 °C and 13.7 nm at the temperature of 23 °C. On the other hand, the coefficient of variation of the synthesized GNPs showed 0.17 at the temperature of 23 °C and 0.28 at the temperature of 80 °C.
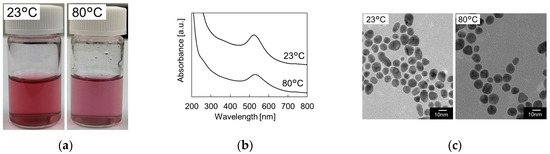
Figure 2.
(a) Photograph and (b) absorbance spectrum of GNPs. (c) TEM images of the synthesized GNPs at the flow rate of 0.05 mL/min.
These results indicated that the mean diameter of GNPs synthesized at the temperature of 80 °C increased by 13% and the coefficient of variation increased by 65%. Increasing synthesis temperature was attributed to the wide size distribution of GNPs.
Author Contributions
Conceptualization, S.S., M.H. and H.Y.; methodology, S.S. and M.H.; validation, S.S.; formal analysis, S.S.; investigation, S.S.; resources, H.Y.; data curation, S.S.; writing—original draft preparation, S.S.; writing—review and editing, S.S., M.H. and H.Y.; visualization, S.S.; supervision, H.Y.; project administration, H.Y.; funding acquisition, H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Alvarez, M.; Khoury, J.T.; Schaaff, T.G.; Shafigullin, M.N.; Vezmar, I.; Whetten, R.L. Optical absorption spectra of nanocrystal gold molecules. J. Phys. Chem. B 1997, 101, 3706–3712. [Google Scholar] [CrossRef]
- Turrevich, J.; Stevenson, P.C.; Hillier, J. A Study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Yagyu, H.; Hamamoto, M.; Wang, Y. Analyzing the critical mixing time for the liquid-phase reduction synthesis of monodisperse gold nanoparticles using glass microfluidics. Microfluid. Nanofluidics 2022, 26, 1–10. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).