Abstract
We present an electronic nose that detects ovarian cancer based on gas emissions from blood plasma. There is currently no test available for screening or diagnostic testing of this disease, which is therefore often detected at aa late stage, resulting in a poor prognosis. Our approach correctly detected 85 out of 87 ovarian cancers, ranging from borderline to stage IV.
1. Introduction
Due to diffuse symptoms and a lack of screening tests, ovarian cancer is normally diagnosed in stages III-IV, with a five-year survival rate of only 4% upon diagnosis in stage IV. Stage I detection results in a much better prognosis, with a 5-year survival rate of 90%. Today, there is no reliable screening method or diagnostic test for ovarian cancer. There is an ELISA test based on a protein biomarker, CA-125, but the high false positive rate and poor sensitivity and specificity render it useless to clinicians [1]. S. Bratulic et al. recently demonstrated a multi-cancer early detection (MCED) approach able to detect 14 cancer types (among which is ovarian cancer) based on urine and plasma free glycosaminoglycan profiles as tumor biomarkers, with an up to 62% sensitivity to stage I disease at a 95% specificity [2]. While promising, the false-negative rate needs improvement for widespread adoption.
Metabolic activity produces endogenous volatile organic compounds (VOCs), which are emitted from the blood. The types of VOCs and their relative concentrations are characteristic of specific disease processes and can be exploited for diagnostic purposes. We present an electronic nose diagnostic tool that detects ovarian cancer in ten minutes through analysis of blood plasma gas emissions. Our approach could significantly improve the outlook for patients receiving the diagnosis.
2. Materials and Methods
The method is based on an electronic nose. Commercial gas sensors (Figaro TGS2X series: TGS2602, TGS2603, TGS2620, TGS2611E, TGS2600, TGS2611C, TGS2444, TGS2610) are configured in 4 banks with 8 sensors each, where the four banks operate at different temperatures. A 1 mL volume of blood plasma is placed into a sample holder, which is inserted into the e-nose sensor tunnel at the start of a measurement. After 20 s, a fan downstream of the sensor tunnel is started to transport the VOCs emitted from the blood plasma to the sensors. Data are collected at a sampling rate of 10 Hz for 600 s. The raw data are preprocessed through mean value normalization, filtering, and scaling to the standard deviation [3]. Data analyses were performed using feature extraction (384 features per measurement), after which principal component analysis is used for dimensionality and feature reduction, and a support vector machine (SVM) model is trained towards binary classification; positive or negative output.
3. Discussion
Figure 1a shows the signal of the same sensor for four cancer and four control samples after preprocessing. Due to the differing emissions, it is possible to identify features from the 32 sensor signals that can be used to distinguish between healthy and cancerous samples. We studied 87 ovarian cancer samples from patients with diagnoses ranging from borderline to stage IV in roughly equal distribution, alongside 26 negative control samples. The final features–observations dataset is a 385 × 113 matrix, which is processed by PCA before the training of the SVM model. Nine features are kept for the classification. Figure 1b shows the classification results. Using 5-fold cross validation, we have obtained a sensitivity of 97.7% (95% confidence interval (CI) 91.9–99.7%), a specificity of 84.6% (95% CI 65.1–95.6%), and an overall accuracy of 95%.
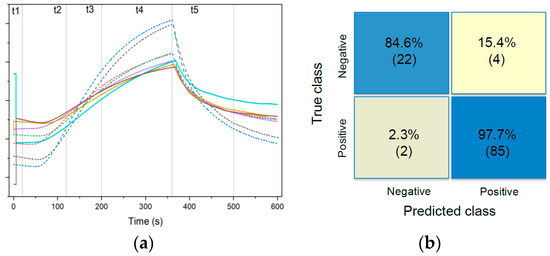
Figure 1.
(a) Sensor signal of one out of 32 sensors after preprocessing for four cancer samples (dashed) and four negative controls (solid); (b) confusion matrix showing true class vs. predicted class for 113 samples (87 cancers and 26 negative controls).
We are in the process of conducting a larger study, including other types of cancer. Data from gas chromatography/mass spectrometry show clear differences in the characteristic peaks and their relative intensities between different cancer types, indicating that there is reason to believe this method can be further developed for multi-cancer early detection.
Author Contributions
Conceptualization, J.E. and C.B.; methodology, J.E.; software, J.E.; validation, J.E, C.B. and D.P.; formal analysis, J.E.; investigation, J.E. and C.B.; resources, J.E., D.P. and C.B.; data curation, J.E. and C.B.; writing—original draft preparation, J.E.; writing—review and editing, J.E., D.P. and C.B.; visualization, J.E.; supervision, C.B.; project administration, D.P.; funding acquisition, J.E., D.P. and C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Sweden’s Innovation Agency, VINNOVA, grant number 2022-0346.
Institutional Review Board Statement
Ethical approvals for the study were consented by the Ethical Review Board at the Faculty of Medicine at Karolinska University Hospital Dnr: 2019-02140 and amendment Lund department 2 medicine Dnr 2022-03015-02.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data will be rendered available upon reasonable requests.
Conflicts of Interest
The authors declare no conflict of interest. VOC Diagnostics AB has no potential commercial conflict of interest.
References
- Moss, E.L.; Hollingworth, J.; Reynolds, T.M. The role of CA125 in clinical practice. J. Clin. Pathol. 2005, 58, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Bratulic, S.; Limeta, A.; Dabestani, S.; Birgisson, H.; Enblad, G.; Stålberg, K.; Hesselager, G.; Häggman, M.; Höglund, M.; Simonson, O.E.; et al. Noninvasive detection of any-stage cancer using free glycosaminoglycans. Proc. Natl. Acad. Sci. USA 2022, 119, e2115328119. [Google Scholar] [CrossRef] [PubMed]
- Bastuck, M. Improving the Performance of Gas Sensor Systems with Advanced Data Evaluation, Operation, and Calibration Methods; Dissertation No. 2009; Linköping Studies in Science and Technology: Linköping, Sweden, 2019; ISBN 978-91-7685-003-9. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).