Abstract
Organic electrochemical transistors (OECTs) are now well-known, robust and efficient as amplification devices for redox reactions, typically biologically ones. In contrast, electrolyte-gated organic field-effect transistors (EGOFETs) have never been described for that kind of application because field-effect transistors are known as capacitive coupled devices, i.e., driven by changes in capacitance at the electrolyte/gate or electrolyte/semiconductor interface. For such a kind of transistors, any current flowing at the gate electrode is seen as a drawback. However, we demonstrate in this paper that not only the gate potential can trigger the source-drain current of EGOFETs, which is the generally accepted mode of operation, but that the current flowing at the gate can also be used. Because EGOFETs can work directly in water, and as an example of application, we demonstrate the possibility to monitor microalgae photosynthesis through the direct measurement of photosynthetic O2 production within the transistor’s electrolyte, thanks to its electroreduction on the EGOFET’s gate. This paves the way for the use of EGOFETs for environmental monitoring.
1. Introduction
Electrolyte-gated organic field-effect transistors (EGOFETs) are original organic transistors for which the dielectric material in-between the gate and the semiconductor, mandatorily present in ISOFETs (ion-sensitive OFETs), is absent, thus the electrolyte is in direct contact with the semiconductor. In ISOFETs, the thickness of the dielectric governs the operating potential range of the device. Here, its absence allows particularly low operating potentials, of a few hundreds of millivolts. Another characteristic of EGOFETs compared to ISOFETs is that the gate is not necessarily a pseudo-reference electrode such as Ag/AgCl; it could be a simple metal wire such as Ti, Pt or Au, or even a carbon electrode. The electrolyte may be a solid one, as the first EGOFETs were designed, but could also be a liquid electrolyte such as deionized (DI) water or a current biological buffer such as PBS (phosphate buffer saline) [1] and are then operated at mild potentials, avoiding solvent oxidoreduction.
As for conventional FETs, EGOFETs are capacitance-driven devices. In practice, the current flowing through the semiconductor of these devices, called the drain current and noted ID, is directly proportional to the overall capacitance CG_OSC between the gate and the semiconductor. This capacitance CG_OSC comes from the series capacitances of both the gate/electrolyte (G_ELEC) and electrolyte/semiconductor (ELEC_OSC) interfaces, i.e., 1/CG_OSC = 1/CG_ELEC + 1/CELEC_OSC. Because the gate electrode and the OSC are dipping in an electrolyte, they are both subject to electrochemical reactions. One is the formation of an electrical double layer (EDL) corresponding to charge reorganization at the interfaces, directly related to the interfacial capacitances CG_ELEC and CELEC_OSC and associated with interfacial potential drops. In short, a small capacitance is associated with a large potential drop while a large capacitance is associated with a small potential drop. It is known that organic semiconductors present EDL capacitances one order of magnitude lower than that of metals. As a consequence, for a similar gate and OSC area, the potential drop is higher at the electrolyte/OSC interface and 1/CG_OSC ≈ 1/CELEC_OSC, i.e., CG_OSC ≈ CELEC_OSC. Under this condition, the drain current ID is independent on CG_ELEC, i.e., is independent on the gate/electrolyte interface. On the contrary, for small gate areas, the gate/electrolyte interface can be used for biosensing, as demonstrated elsewhere [2,3,4].
The other reactions expected to occur at the interface are redox reactions, i.e., electron transfers, which then produce a gate current. This explains why EGOFETs are subject to high gate currents (IG) compared to conventional OFETs. IG comes from oxidation or reduction of the solvent or of dissolved species such as molecular oxygen. We show here that a gate current leads to a change in a voltage drop at the interface so that a gate current IG can be used to drive the drain current ID of an EGOFET. In other words, an EGOFET can amplify a small gate current into a large drain current. As an example, we show that it is possible to amplify the reduction current of O2 at a Pt gate into a larger drain current. An application could be the continuous monitoring of photosynthetic organisms present into the electrolyte.
2. Materials and Methods
Materials and fabrication procedures were described elsewhere [3,5]. The cyanobacteria Anabaena flos-aquae (Af, strain ALCP B24) were provided by the National Museum of Natural History in Paris. For cyanobacteria growth, the Bold’s basal medium (B3N) was used, into which 2.97 µM of vitamin B1, 1.02 nM of vitamin H and 0.11 nM of vitamin B12 were added. A Pt microelectrode (diameter 100 µm) was used as a gate. Electrical characteristics were acquired with a Keithley 4200 SCS source meter.
3. Results and Discussion
As a preliminary experiment (not shown), oxygen or argon were alternately bubbled in the transistor’s electrolyte while drain and gate currents were monitored for a gate-source voltage VGS = −0.8 V and a drain-source voltage VDS = −0.6 V. Upon argon bubbling, the gate current decreased, while it increased upon oxygen bubbling. The amplitude of the gate current change was ca. 12 nA. We measured a parallel change of the drain current, with an amplitude of ca. 1000 nA. This experiment demonstrated that the EGOFET is able to amplify the current of a redox reaction occurring at the gate into a more intense drain current. In another experiment, 106 per mL Anabaena flos-aquae cyanobacteria were then added into the electrolyte compartment of the transistor (total of 2 × 105 A. flos-aquae in 200 μL of B3N). As all such photosynthetic organisms, A. flos-aquae consumes carbohydrates and oxygen for its respiration at any time but produces carbohydrates and O2 from CO2 and H2O during the light phase. O2 is not captured inside the cyanobacteria but released into the external medium, where it dissolves, which increases its local concentration. Therefore, the continuous monitoring of oxygen concentration in the medium allows following the life cycle of the cyanobacteria over time. Under the same conditions as above, the drain and gate currents were recorded under alternated dark and illuminated periods (Figure 1, left). This result shows that oxygen produced under illumination by the cyanobacteria is reduced at the gate electrode, leading to a gate current then to a drain current increase (in absolute value). Under optimized experimental conditions, the amplitude of the drain current change upon illumination is ca. 70–80 nA while that of the gate is ca. 0.1 nA, corresponding to an amplification of two to three orders of magnitude. A control experiment without cyanobacteria in the electrolyte showed no gate current but a small drain current change upon illumination, of ca. 10 nA, which is attributed to the photocurrent produced under semiconductor’s illumination (Figure 1, right).
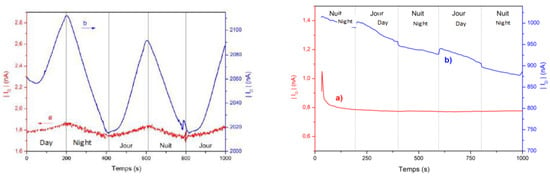
Figure 1.
(Left) gate current IG (curve a) and drain current ID (curve b) of an electrolyte-gated organic field-effect transistor (EGOFET) containing 106 cyanobacteria per mL of electrolyte, under illumination cycles of 200 s each. Gate diameter = 110 μm. VGS = −0.8 V; VDS = −0.6 V. (Right) gate and drain current under the same conditions, without cyanobacteria. Only the photocurrent appears on ID.
4. Conclusions
In this work, we demonstrated that the gate current of an electrolyte-gated organic field-effect transistor is not necessarily a drawback resulting from undesirable electrochemical side-reactions which must be systematically minimized: it can be advantageously used to control the drain current. In other words, an EGOFET is able to amplify up to several orders of magnitude a gate current into a drain current. This is made possible if the gate/electrolyte capacitance CG_ELEC is significantly lower than that of the electrolyte/semiconductor interface, CELEC_OSC, i.e., when the overall capacitance of the EGOFET is driven by CG_ELEC rather than CELEC_OSC. For a given gate voltage, it corresponds to the situation where the potential drop between the gate and the semiconductor is concentrated at the electrolyte/semiconductor interface, maximizing the field effect. We applied this property to the monitoring of the photosynthetic activity of cyanobacteria (Anabaena flos-aquae) directly added into the electrolyte compartment of the EGOFET. Compared to a regular amperometric O2 sensor, the device is able to amplify the reduction current of several orders of magnitude, typically from a few nA at a Ø100 μm gate to a few μA at the drain. As an example, we applied the device for monitoring the production of O2 from cyanobacteria. A perspective could be to use such a platform for monitoring the conditions of living organisms in surface waters, i.e., close to industrial areas.
Author Contributions
Conceptualization, B.P.; methodology, B.P., J.l.G. and R.B.; investigation, J.l.G.; data curation, B.P., V.N. and G.M.; writing, B.P. and J.l.G. All authors have read and agreed to the published version of the manuscript.
Funding
ANR (Agence Nationale de la Recherche) and CGI (Commissariat à l’Investissement d’Avenir) are acknowledged for their financial support of this work through Labex SEAM (Science and Engineering for Advanced Materials and devices) ANR-10-LABX-0096, ANR 11 IDEX 05 02.
Acknowledgments
J.l.G. thanks the Doctorate School ED388 “Chimie Physique et Chimie Analytique de Paris Centre” and Université de Paris for a PhD grant. We also warmly thank C. Yéprémian and A. Couté from the Museum National d’Histoire Naturelle de Paris for the cyanobacteria.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Kergoat, L.; Piro, B.; Berggren, M.; Horowitz, G.; Pham, M.C. Advances in organic transistor-based biosensors: From organic electrochemical transistors to electrolyte-gated organic field-effect transistors. Anal. Bioanal. Chem. 2012, 5, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Kergoat, L.; Herlogsson, L.; Piro, B.; Pham, M.C.; Horowitz, G.; Crispin, X.; Berggren, M. Tuning the threshold voltage in electrolyte-gated organic field-effect transistors. Proc. Nat. Acad. Sci. USA 2012, 109, 8394–8399. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.K.; Nguyen, T.N.; Anquetin, G.; Reisberg, S.; Noël, V.; Mattana, G.; Touzeau, J.; Barbault, F.; Pham, M.C.; Piro, B. Triggering the Electrolyte-Gated Organic Field-Effect Transistor output characteristics through gate functionalization using diazonium chemistry: Application to biodetection of 2,4-dichlorophenoxyacetic acid. Biosens. Bioelectron. 2018, 113, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.K.; Tran, H.V.; Vu, T.T.; Reisberg, S.; Noël, V.; Mattana, G.; Pham, M.C.; Piro, B. Peptide-modified electrolyte-gated organic field effect transistor. Application to Cu2+ detection. Biosens. Bioelectron. 2019, 127, 118–125. [Google Scholar] [CrossRef]
- Tibaldi, A.; Fillaud, L.; Anquetin, G.; Woytasik, M.; Zrig, S.; Piro, B.; Mattana, G.; Noël, V. Electrolyte-gated organic field-effect transistors (EGOFETs) as complementary tools to electrochemistry for the study of surface processes. Electrochem. Commun. 2019, 98, 43–46. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).