Abstract
The work detailed in this study utilized 20 kHz ultrasonic irradiation as a mechanism of hydrogen peroxide production. The effects of various operating parameters were investigated, including ultrasonic intensity; solution pH; source of water; initial dibutyl phthalate concentration and the presence of hydrogen peroxide. During the irradiation, the H2O2 concentration was monitored. The results indicate that H2O2 is produced by cavitation during ultrasonic irradiation. An increase in ultrasonic intensity increases the amount of hydrogen peroxide produced. The initial pH of the solution does not affect the efficiency of processes substantially. H2O2 is regarded as one of the most effective additives enhancing the sonochemical production of hydroxyl radicals and hydrogen peroxide. Above a 0.1 mM dose of H2O2, the amount of H2O2 formed decreased as the concentration of H2O2 increased. Thus, the concentration of hydrogen peroxide plays a crucial role in the extent to which the effectiveness of the combined process is enhanced. The negative effect on reactions of the presence of additional components in the reaction solution was also confirmed. It was therefore concluded that the experimental evaluation of optimum parameters of hybrid processes is a matter of importance.
1. Introduction
The range of ultrasound frequencies used commonly in sonochemistry is 20 kHz to 1 MHz [1,2]. Ultrasonic irradiation of liquids causes acoustic cavitations, i.e., the formation, growth and implosive collapse of bubbles. Such cavitation generates local sites of high temperature and pressure for short periods of time, with these being responsible for unusual sonochemical effects [3,4]. The cavitation process is influenced by several factors such as frequency, gas properties (solubility, heat capacity ratio, thermal conductivity) and solvent properties (vapor pressure, density, viscosity, surface tension). Sonochemistry usually deals with reactions in the liquid component. The mechanism of the sonochemical degradation of organic pollutants is usually based on the formation of short-lived radicals generated in violent cavitation events [5]. The sonochemical destruction of pollutants in the aqueous phase generally involves several reaction pathways, such as pyrolysis inside the bubble and hydroxyl radical-mediated reactions at the bubble–liquid interface and/or in the liquid bulk (Figure 1). The extreme temperature conditions generated by a collapsing bubble can also lead to the formation of radical chemical species. The radicals formed in this reaction are highly reactive and interact rapidly with other radical or chemical species in solution [6].
when ultrasound is applied, it will induce the sonolysis of water molecules and thermal dissociation of oxygen molecules, if present, to produce different kinds of reactive species such as OH, H, O and OOH. Reactive-species production ensues by way of the following reactions, with ultrasound “)))” denoting the ultrasonic irradiation (Equations (1)–(13)). Sonolysis of water also produces H2O2 and H2 gas via OH and H. Though oxygen enhances sonochemical activity, its presence is not essential for water sonolysis, as sonochemical oxidation and reduction processes can proceed in the presence of any gas. However, the presence of oxygen also allows the H. forming OOH to be scavenged, with this acting as an oxidizing agent [4,7,8]. The hydroxyl radicals generated during cavitation can be used in the oxidative degradation of organic pollutants in an aqueous system [9,10,11].
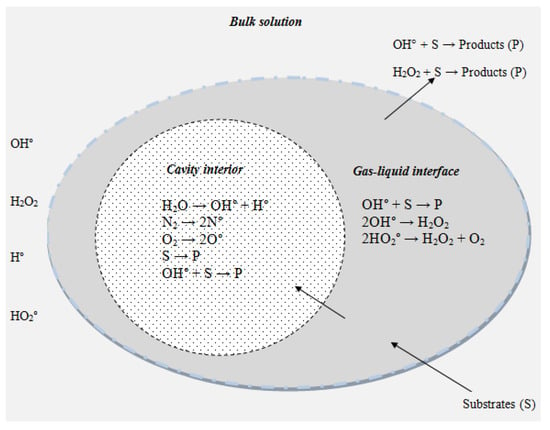
Figure 1.
The reaction zone in the cavitation process (adapted from Chowdhury et al., 2009).
During the past several years, ultrasound has been applied effectively as an emerging advanced oxidation process (AOP) for a wide variety of pollutants in wastewater treatment [12,13,14]. A growing number of studies have demonstrated that ultrasound irradiation results in a rapid and effective decomposition of phthalates [15], pesticides [16,17] phenols [18], chlorinated compounds [19], and pharmaceuticals [20] in aqueous solution. The main advantage is that the ultrasound process does not require added chemicals, oxidants or catalysts, and does not generate additional waste streams as compared with other processes (ozonation and adsorption). However, the ultrasonic degradation rate is found to be rather slow [21,22,23]. Several factors, including ultrasonic frequency, solution pH and the addition of hydrogen peroxide, may influence the sonochemical degradation of organic contaminants. Hydrogen peroxide is one of the most effective additives used to enhance sonochemical degradation of organic pollutants. During ultrasound irradiation, H2O2 can dissociate into hydroxyl radicals, though these have a very short lifetime and tend to combine and form H2O2. As hydrogen peroxide present at high concentration can act as a radical scavenger, especially for OH, it is important to evaluate the optimum concentration of H2O2. Unfortunately, this is difficult to do for each compound, because the formation of H2O2 depends on pollutant types and process parameters [5,21,24].
The objective of the work described here was to study the production of hydrogen peroxide in aqueous solution subject to sonication. The influence of sonochemical processes and of the initial concentration of hydrogen peroxide on the level of generation of hydrogen peroxide was also studied, with different process parameters considered. Certain previous studies have investigated the effects of H2O2 concentration on sonochemical reactions [5,22,23], though only one experiment [21] has monitored H2O2 under various input concentrations of H2O2 and in the presence or absence of target materials. However, in the investigation described here, the concentration of H2O2 was also monitored under different intensities of ultrasonic irradiation and pH values. In contrast, no experiments have hitherto investigated the formation of H2O2 in tap water. The H2O2 concentration in the sample containing dibutyl phthalate (DBP) contamination and H2O2 addition was also not checked.
2. Materials and Methods
2.1. Chemicals
Sodium hydroxide, potassium biphthalate, and potassium iodide (Chempur, Piekary Śląskie, Poland), as well as ammonium molybdate from POCH (Gliwice, Poland) of reagent grade were used to determine the H2O2 concentration. DBP of analytical standard was obtained from Sigma-Aldrich (Saint Louis, MO, USA). All reagents were prepared with deionized water, which was made by Purix CNX-100. HCl solution was purchased from POCH. HCl and NaOH were used for pH adjustment. H2O2 solution (30%) was obtained from Chempur.
2.2. Apparatus and Experimental Conditions
Ultrasonic irradiation experiments were conducted on the bench-scale in a reactor comprising an ultrasonic processer, a reactor cell and an ice-water bath. The source of ultrasound was a SONOPULS HD 3200 from Bandelin (Berlin, Germany), which is an ultrasonic processor equipped with a 1.3 cm-diameter titanium probe tip. The homogenizer operated at 20 kHz. Irradiation with ultrasonic waves at ultrasonic intensity of 3.6, 4.3 and 5.9 W/cm2 was applied.
All the experiments were conducted in a 250-mL glass beaker immersed in the ice bath. The reactor was filled with 100 mL of appropriate solutions, i.e., deionized water, deionized water with hydrogen peroxide, tap water, or aqueous DBP solution of the required concentration. The ultrasonic power was controlled by the panel setting, the sonication probe being dipped 1 cm below the water surface. The H2O2 concentration in the reaction solution was monitored over the course of the irradiation process. All the experiments were duplicated with an observed deviation of less than 5%. For this reason, average values are shown in the graphs. All tests were also conducted at room temperature and pressure.
2.3. Analytical Methods
The concentration of H2O2 generated during sonication was determined using the iodometric method. The iodide ion (I−) reacts with hydrogen peroxide (H2O2) to form a triiodide (I3−) ion, which absorbs at 352 nm. Sample aliquots (volume: 2.0 mL) from each experiment were mixed in a quartz cuvette containing 0.75 mL of 0.10 M potassium biphthalate and 0.75 mL of a solution containing 0.4 M potassium iodide, 0.06 M sodium hydroxide, and 10−4 M ammonium molybdate. The mixed solution (total volume: 3.5 mL) was allowed to stand for 2 min before absorbance was measured using a DR-5000 UV spectrophotometer.
3. Results and Discussion
When an aqueous solution is irradiated ultrasonically, OH radicals and H radicals are produced by cavitation. The hydroxyl radical exhibits a high oxidation potential and can oxidize organic substrates directly, causing their degradation or mineralization [1,4,5]. However, hydroxyl radicals have a very short lifetime, and tend to combine with one another to form H2O2 [2,3,10]. The production of hydrogen peroxide in the circumstances of different intensities of ultrasonic irradiation is as shown in Figure 2a. In the initial phase of the process, no difference in concentration of hydrogen peroxide is to be noted, irrespective of the intensity. However, after 15 min, it is clear that hydrogen peroxide production is at a higher level where the ultrasonic intensity is greater. The greatest production of hydrogen peroxide was found to be associated with higher–intensity irradiation. An increase in ultrasonic intensity may increase the number of active cavitation bubbles and the production of hydroxyl radicals. Similar results have been reported in the literature [4,21,24,25,26]. Under ultrasonic irradiation, there is a linear relationship between the H2O2 concentration generated and irradiation time (Figure 2a). All experiments showed R2 value greater than 0.9. Previous studies have confirmed that, during sonication at constant intensity, the rate of generation of hydroxyl radicals can be assumed constant, with a prominent product of sonication being hydrogen peroxide (reactions 1.6, 1.9 and 1.10), with this acting as an OH scavenger and accumulating linearly in solution during the process of ultrasonic irradiation [2,23]. In addition, conducted statistical analysis have shown that the highest correlation coefficient was observed for 90 min (R2 = 0.9998). It was also noticed that the decrease in the time resulted in a lower correlation coefficient (Figure 2b).
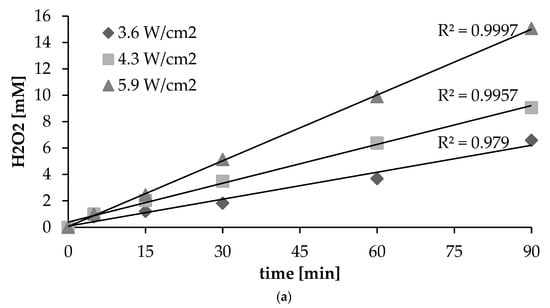
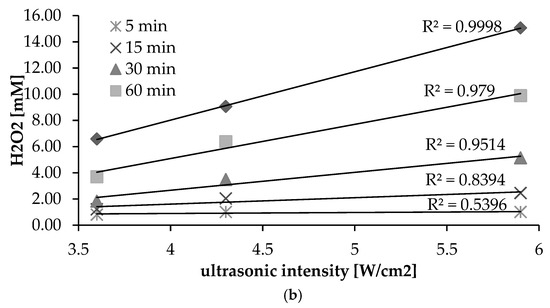
Figure 2.
(a) Influence of duration and intensity of ultrasonic irradiation (pH = 7) on the formation of H2O2; (b) relationships between ultrasonic intensity and formation of H2O2.
Solution pH is generally an important factor influencing the efficiency of processes. The influence of initial pH on the production of hydrogen peroxide in aqueous solution under sonication was studied, with results as presented in Figure 3. Initial pH cannot be seen to affect the efficiency of processes substantially. Reference to H2O2 measurements revealed no change in the trend for H2O2 production in line with changes in solution pH (3,7,11). Across the pH range studied, the rate of formation of hydrogen peroxide was independent of the initial value. Earlier research likewise shows no upward or downward trend for H2O2 production with the solution pH. However, a strong basic (pH > 11) environment does seem unfavorable for the accumulation of ultrasound-induced H2O2 [5]. On the other hand, the pH values of solutions are shown to influence the sonochemical degradation of organic pollutants markedly. For example, the sonochemical degradation rate of 4-nitrophenol has been reported to decrease with increasing pH, while the destruction of aniline is found to be favored by an alkaline solution [27]. For dimethyl phthalate, the degradation rate decreased somewhat with increasing pH across the 5–9 range [24]. Villaroel et al. [20] reported that ultrasonic degradation of acetaminophen in more pronounced in an acidic medium than in basic aqueous solution. The influence of the pH of solutions is probably therefore due to the chemical structure and properties of the substance involved. Hydrophobicity of the pollutant has certainly been revealed as one of the most important factors [20,28].
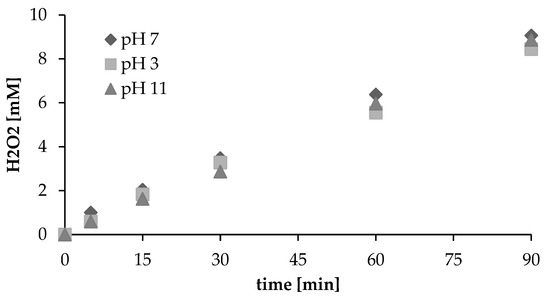
Figure 3.
Influence of pH values and duration of ultrasonic irradiation (at a constant intensity of 4.3 W/cm2) on the formation of H2O2.
In this study, the impact of H2O2 on the final amounts of hydrogen peroxide in solution was considered by reference to different H2O2 concentrations applied at the start of a sonication reaction. The results of adding hydrogen peroxide in the presence of ultrasonic irradiation are shown in Figure 4a. The presence of H2O2 can have both a positive and negative effect. In line with the results shown in Figure 4a, under a low (0.1 mM) concentration of hydrogen peroxide, the role of the latter is mainly to serve as a radical source, rather than a radical scavenger. However, formation of H2O2 is lower with higher input concentrations of H2O2, a result which confirms that the decay rates of H2O2 are slightly faster than the rates of formation where concentrations of H2O2 are high [21,23]. These studies also indicate that high concentrations of hydrogen peroxide will not prove effective in oxidizing organic pollutants. On the other hand, it is clearly of importance for the optimum concentration of H2O2 to be determined experimentally. Similar results were obtained earlier, in the course of research into the sonochemical removal of phenol and bisphenol A. The formation of hydrogen peroxide was found to decrease where input concentrations of H2O2 were successively greater [21].
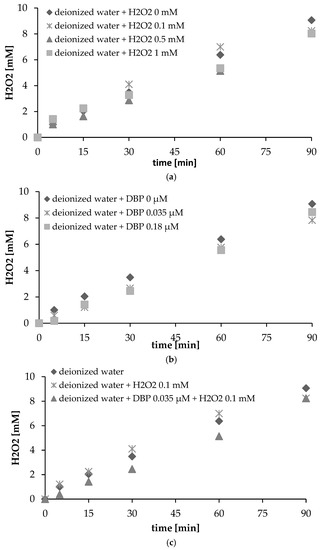
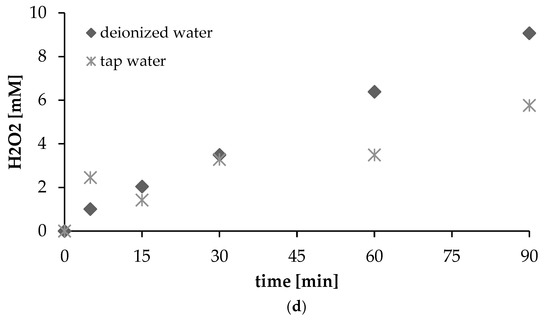
Figure 4.
(a) Formation of H2O2 with the effect of added H2O2 (pH = 7, intensity of ultrasonic irradiation = 4.3 W/cm2); (b) formation of H2O2 with the effect of added dibutyl phthalate (DBP) (pH = 7, intensity of ultrasonic irradiation = 4.3 W/cm2); (c) formation of H2O2 with the effect of added DBP + H2O2; (d) the formation of H2O2 in tap water and deionized water (pH = 7, intensity of ultrasonic irradiation =4.3 W/cm2).
The formation of H2O2 was also shown to be affected by the concentration of DBP (Figure 4b,c). In this investigation, the solutions of DBP were at 0.035 μM and 0.18 μM concentrations. Solution pH was not adjusted. These results demonstrate that the amount of H2O2 decreased with the input of both 0.035 and 0.18 μM DBP. Following sonication (4.3 W/cm2), the concentration of H2O2 was slightly lower than those of H2O2 in deionized water. The slight difference in the formation rate between the DBP solution and blank water was mainly the result of competition in the consumption of radicals through reactions with DBP molecules and intermediates, as opposed to self-decomposition. The hydrogen peroxide is used in the decomposition and digestion of the substance present (DBP). In an aquatic solution, the formation of H2O2 and hydroxyl radicals caused by cavitation serves to destroy organic materials [20]. In uncontaminated water, hydrogen peroxide is not consumed. The demand for hydrogen peroxide is greater with higher concentrations of DBP. The decomposition of hydrogen peroxide was also observed in the presence of DBP with an addition of H2O2 in aqueous solution (Figure 4c). In line with Le Chatelier’s principle, the presence of hydrogen peroxide may hamper the combination of hydroxyl free radicals, and increase the numbers of free radicals available for the decomposition of organic compounds. However, excessive amounts of hydrogen peroxide present in solution can reduce the rate at which sonolysis can decompose micropollutants [29]. A previous study [24] showed that the degree to which a compound was degraded by ultrasound was enhanced significantly by the addition of H2O2 to the solution, albeit with that enhancement significantly dependent on the compound concentration. In some research, the impact of H2O2 on dimethyl phthalate degradation has been considered in relation to different applied H2O2 concentrations. As observed in this study, the promoting effect of H2O2 seems to depend on concentration of DMP, with the presence of moderate (<5 mM) concentrations of H2O2 enhancing the compound degradation rate significantly where the DMP solution was at 0.02 mM, while giving little enhancement when in a 0.05 mM solution. Ku et al. [29] reported similar experimental results when it came to the decomposition by sonolysis of organic compounds in aqueous solution, with hydrogen peroxide added. Rates of sonolytic decomposition of 3-chlorophenol were found to be enhanced by the presence of the latter in aqueous solution. For instance, a sonication rate kept constant had an impact on 15 mg/L of 3-chlorophenol that was enhanced by more than 50% where 200 mg/L of hydrogen peroxide was added. Equally, excessive amounts of hydrogen peroxide present in aqueous solution were found to reduce the rate of decomposition by sonolysis of 3-chlorophenol. The sonication rate constant was, in turn, reduced markedly in experiments conducted with 700 mg/L of H2O2 added—indeed, to a level even lower than obtained where experiments were conducted without H2O2 present. The optimum concentration of H2O2 was found to vary in line with the initial concentration of 3-chlorophenol. For an experiment conducted with 20 mg/L of 3-chlorophenol, the reaction rate constant was kept high where 700 mg/L of hydrogen peroxide was present. However, the reaction rate constant was decreased slightly in the case of an experiment conducted with 1000 mg/L of H2O2 present.
Also compared was the influence on the effectiveness of the ultrasonic irradiation process that was due to the water applied in preparing a solution. The results with solutions based on deionized water and tap water are as shown in Figure 4d. In the case of deionized water, there is a proportional increase over time in the amount of H2O2. In contrast, the matrix in the tap water is seen to exert a negative effect on hydrogen peroxide production. A reduction in the rate of production of H2O2 is thus visible. Substances present naturally in tap water are potentially competitive substrates for the hydroxyl radical and hydrogen peroxide. It is well known that removal of organic pollutants depends on amounts of reactive species, with this, in turn, being dependent on the presence of additional substances in the reaction solution that can act as radical scavengers. Inorganic ions and organic substances are, in fact, well-known OH scavengers [30,31]. In a previous study [23], the ultrasonic degradation of bisphenol-A was also shown to be impacted upon negatively by humic acid. Thus, in real water samples and wastewaters, the presence of background substances reduces the effectiveness of ultrasonic oxidation. Indeed, other parameters of natural water samples including turbidity can also have a significant impact on the oxidation mechanism [32].
It should be mentioned that the operating conditions of the process radically affect the mechanism of the ultrasonic process. Usually, sonication conditions have to be controlled to ensure better efficiency of the experiments. For example, experiments are carried out under argon atmosphere to enhance the cavitation effect. This also causes protection from potential contamination from air. Additionally, 20 kHz is known to generate Ti particles as a result of erosion from the sonotrode. The presence of these particles can lead to the catalytic decomposition of H2O2. These studies were conducted without the control of these parameters, despite satisfactory results being achieved. It can be concluded that the use of all the optimum parameters would result in higher efficiency for producing hydrogen peroxide. This requires the use of a suitable reactor. However, the cost of the process can be much higher. Future studies can be directed towards the development of an economic ultrasonic reactor and optimal ultrasonic parameters.
4. Conclusions
- Hydrogen peroxide is a very effective oxidant for liquid-phase reactions. Ultrasonic irradiation plays an essential role in the formation of reactive species (H2O2, OH, H, O and OOH). H2O2 can be generated by the recombination of hydroxyl radicals as cavitation in aqueous solution takes place. Higher-intensity ultrasound is able to enhance H2O2 formation.
- Initial pH did not affect the efficiency of H2O2 formation substantially, but the pH values of solutions have a significant influence on the sonochemical degradation of organic pollutants. The main mechanism in the degradation of organic pollutants using ultrasound entails the generation of, and attack by, hydroxyl radicals.
- The use of hydrogen peroxide in conjunction with ultrasound is only beneficial to the point where optimum loading is achieved. At high concentrations, H2O2 can act as scavenger of radicals, especially hydroxyl radicals. The optimum value for the presence of H2O2 will depend on the nature of the pollutants and the operating conditions.
- The decomposition of hydrogen peroxide was also observed in the presence of DBP with and without an addition of H2O2 in aqueous solution. In contaminated water, hydrogen peroxide is consumed as an oxidant of organic compounds.
- Tap water contains mineral and organic chemicals that are considered to be inhibitors for most AOPs, because these chemicals are known as scavengers of hydroxyl radicals.
References
- Leong, T.; Ashokkumar, M.; Kentish, S. The fundamentals of power ultrasound—A review. Acoust. Aust. 2011, 39, 54–63. [Google Scholar]
- González-García, J.; Sáez, V.; Tudela, I.; Díez-Garcia, M.I.; Deseada Esclapez, M.; Louisnard, O. Sonochemical treatment of water polluted by chlorinated organocompounds: A review. Water 2010, 2, 28–74. [Google Scholar] [CrossRef]
- Chowdhury, P.; Viraraghavan, T. Sonochemical degradation of chlorinated organic compounds, phenolic compounds and organic dyes—A review. Sci. Total Environ. 2009, 407, 2474–2492. [Google Scholar] [CrossRef]
- Pétrier, C. 31: The use of power ultrasound for water treatment. In Power Ultrasonics; Woodhead Publishing: Oxford, UK, 2015; pp. 939–972. [Google Scholar]
- Wu, T.N.; Shi, M.C. pH-affecting sonochemical formation of hydroxyl radicals under 20 kHz ultrasonic irradiation. J. Environ. Eng. Manag. 2010, 20, 245–250. [Google Scholar]
- Dewulf, J.; Van Langenhove, H.; De Visscher, A.; Sabbe, S. Ultrasonic degradation of trichloroethylene and chlorobenzene at micromolar concentrations: Kinetics and modeling. Ultrason. Sonochem. 2001, 8, 143–150. [Google Scholar] [CrossRef]
- Thompson, L.H.; Doraiswamy, L.K. Sonochemistry: Science and engineering. Ind. Eng. Chem. Res. 1999, 38, 1215–1249. [Google Scholar] [CrossRef]
- Suslick, K.S.; Didenko, Y.; Fang, M.M.; Hyeon, T.; Kolbeck, K.J.; McNamara, W.B.; Wong, M. Acoustic cavitation and its chemical consequences. Philos. Trans. R. Soc. Lond. A 1999, 357, 335–353. [Google Scholar] [CrossRef]
- Adewuyi, Y.G. Sonochemistry: Environmental science and engineering applications. Ind. Eng. Chem. Res. 2001, 40, 4681–4715. [Google Scholar] [CrossRef]
- Hayashi, N.; Liang, J.; Choshi, H.; Kasai, E. Hexachlorobenzene removal from a model sediment by using ultrasonic irradiation. Water Sci. Technol. 2009, 59, 737–744. [Google Scholar] [CrossRef]
- Campbell, T.; Hoffmann, M.R. Sonochemical degradation of perfluorinated surfactants: Power and multiple frequency effects. Sep. Purif. Technol. 2015, 156, 1019–1027. [Google Scholar] [CrossRef]
- Włodarczyk-Makuła, M. Physical and Chemical Fates of Organic Micropollutants; Scholars’ Press: Saarbrucken, Germany, 2015. [Google Scholar]
- Pochwat, K.; Słyś, D.; Kordana, S. The temporal variability of a rainfall synthetic hyetograph for the dimensioning of stormwater retention tanks in small urban catchments. J. Hydrol. 2017, 549, 501–511. [Google Scholar] [CrossRef]
- Naumczyk, J.; Prokurat, I.; Marcinowski, P. Landfill Leachates Treatment by H2O2/UV, O3/H2O2, Modified Fenton, and Modified Photo-Fenton Methods. Int. J. Photoenergy 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Kida, M.; Książek, S.; Koszelnik, P. Preliminary studies on removal of dibutyl phthalate from aqueous solutions using ultrasound. Ecol. Eng. 2016, 48, 233–238. [Google Scholar] [CrossRef]
- Kida, M.; Ziembowicz, S.; Koszelnik, P. Removal of organochlorine pesticides (OCPs) from aqueous solutions using hydrogen peroxide, ultrasonic waves, and a hybrid process. Sep. Purif. Technol. 2018, 192, 457–464. [Google Scholar] [CrossRef]
- Gondrexon, N.; Renaudin, V.; Petrier, C.; Boldo, P.; Bernis, A.; Gonthier, Y. Degradation of pentachlorophenol aqueous solutions using a continuous flow ultrasonic reactor: Experimental performance and modeling. Ultrason. Sonochem. 1999, 54, 125–131. [Google Scholar] [CrossRef]
- Entezari, M.H.; Pétrier, C.; Devidal, P. Sonochemical degradation of phenol in water: A comparison of classical equipment with a new cylindrical reactor. Ultrason. Sonochem. 2003, 10, 103–108. [Google Scholar] [CrossRef]
- Naffrechoux, E.; Combet, E.; Fanget, B.; Petrier, C. Reduction of chloroform formation potential of humic acid by sonolysis and ultraviolet irradiation. Water Res. 2003, 37, 1948–1952. [Google Scholar] [CrossRef]
- Villaroel, E.; Silva-Agredo, J.; Petrier, C.; Taborda, G.; Torres-Palma, R.A. Ultrasonic degradation of acetaminophen in water: Effect of sonochemical parameters and water matrix. Ultrason. Sonochem. 2014, 21, 1763–1769. [Google Scholar] [CrossRef]
- Lim, M.; Son, Y.; Khim, J. The effects of hydrogen peroxide on the sonochemical degradation of phenol and bisphenol A. Ultrason. Sonochem. 2014, 21, 1976–1981. [Google Scholar] [CrossRef]
- Xu, L.J.; Chu, W.; Graham, N. Sonophotolytic degradation of phthalate acid esters in water and wastewater: Influence of compound properties and degradation mechanisms. J. Hazard. Mater. 2015, 288, 43–50. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, N.; Deng, Y.; Lin, T.F.; Ma, Y.; Li, L.; Sui, M. Degradation of bisphenol—A using ultrasonic irradiation assisted by low-concentration hydrogen peroxide. J. Environ. Sci. 2011, 23, 31–36. [Google Scholar] [CrossRef]
- Xu, L.J.; Chu, W.; Graham, N. A systematic study of the degradation of dimethyl phthalate using a high-frequency ultrasonic process. Ultrason. Sonochem. 2013, 20, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Kusiak, M.; Stępniak, L. Sonochemical methods of removing the organic impurities from water. Proc. ECOpole 2010, 4, 439–445. [Google Scholar]
- Kwarciak-Kozłowska, A.; Krzywicka, A. Effect of ultrasonic field to increase the biodegradability of coke processing wastewater. Arch. Waste Manag. Environ. Prot. 2015, 17, 133–142. [Google Scholar]
- Jiang, Y.; Pétrier, C.; Waite, T.D. Effect of pH on the ultrasonic degradation of ionic aromatic compounds in aqueous solution. Ultrason. Sonochem. 2002, 9, 163–168. [Google Scholar] [CrossRef]
- Isariebel, Q.P.; Carine, J.L.; Ulises-Javier, J.H.; Anne-Marie, W.; Henri, D. Sonolysis of levodopa and paracetamol in aqueous solutions. Ultrason. Sonochem. 2009, 16, 610–616. [Google Scholar] [CrossRef]
- Ku, Y.; Tu, Y.H.; Ma, C.M. Effect of hydrogen peroxide on the decomposition of monochlorophenols by sonolysis in aqueous solution. Water Res. 2005, 39, 1093–1098. [Google Scholar] [CrossRef]
- Pétrier, C.; Torres-Palma, R.; Combet, E.; Sarantakos, G.; Baup, S.; Pulgarin, C. Enhanced sonochemical degradation of bisphenol—A by bicarbonate ions. Ultrason. Sonochem. 2010, 17, 111–115. [Google Scholar] [CrossRef]
- Minero, C.; Pellizzari, P.; Maurino, V.; Pelizzetti, E.; Vione, D. Enhancement of dye sonochemical degradation by some inorganic anions present in natural waters. Appl. Catal. B 2008, 77, 308–316. [Google Scholar] [CrossRef]
- Nikfar, E.; Dehghani, M.H.; Mahvi, A.H.; Rastkari, N.; Asif, M.; Tyagi, I.; Gupta, V.K. Removal of Bisphenol A from aqueous solutions using ultrasonic waves and hydrogen peroxide. J. Mol. Liq. 2016, 213, 332–338. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).