Abstract
Metal oxides have been widely used in various applications such as biomedical, commercial and environmental, due to their unique physicochemical properties. As the use of metal oxides increase worldwide, their exposure to the living systems also increases. It is therefore necessary to understand their potential harmful effects on human and environment health. In this study, dermal fibroblasts were exposed to bulk zinc oxide (0.1, 1, 10, 50, and 100 μg/mL) for 6 and 48 h. After exposure, changes in cell viability, morphology, membrane damage and zinc oxide uptake were investigated. The response of dermal fibroblasts exposed to different concentrations of bulk zinc oxide was monitored in real-time using an impedance-based assay system. Results demonstrated that zinc oxide at 50 and 100 μg/mL showed significant toxic effects compared to the control cell cultures.
1. Introduction
Zinc oxide is an inorganic compound widely used in the food, cosmetic and pharmaceutical industries. It has been included into polymeric materials to incorporate the antimicrobial and antifungal properties for wound dressings, food packaging and dental filling materials. Also, zinc oxide has been used in various pharmaceutical and cosmetic products due to antibacterial and UV-blocking properties [1]. Some chemical substances can easily be uptaken by the cells and interact with the biological molecules inside the cells affecting a variety of cell responses, leading to the decrease in cell viability, proliferation, etc. [2]. Therefore, the potential toxic effects of such chemicals that directly affect human health need to be comprehensively evaluated.
In recent years, impedance-based high-throughput assay systems are increasingly becoming the preferred system for analyzing the effects of toxic chemical substances on cultured mammalian cells, hence providing information on cell death, proliferation and morphological changes in real time without the use of any labels [3]. This study provides in vitro toxicity data on the responses of dermal fibroblasts cultured with different concentrations of zinc oxide to determine its potential toxic effects using an impedance-based system.
2. Materials and Methods
2.1. Preparation of Zinc Oxide Dispersions
Zinc oxide powder (Sigma, St Louis, MO, USA) was exposed to UV irradiation for sterilization for 30 min before cell culture experiments. The stock solutions of zinc oxide were prepared in cell culture medium and sonicated for 30 min to form the dispersions. Thereafter, the working solutions (0.1, 1, 10, 50, and 100 μg/mL) were prepared using cell culture medium from the stock solution.
2.2. Culture of Dermal Fibroblasts
Mouse dermal fibroblasts were purchased from ATCC. Frozen cells were thawed in the waterbath at 37 °C until only a small ice crystal remains. The cells were cultured in DMEM-F12 medium (containing 10% FBS, 2 mM l-glutamine and 1% penicillin streptomycin) inside the incubator set to 5% CO2, 37 °C and 95% humidity. The cell medium was replaced with fresh medium twice a week.
2.3. In Vitro Cytotoxicity Assays
To determine cell viability and membrane damage, in vitro cytotoxicity tests, i.e., MTT (Sigma) and LDH (Sigma) assays, were performed. For that purpose, at ~70–80% confluence cells were detached from the culture flask (Corning, New York, NY, USA) with trypsin-EDTA solution and seeded onto 96-well plates at a density of 1 × 104 cells per well. After 24 h, the cells were treated with zinc oxide with different concentrations for 6 and 48 h. At specific time point, the cell viability and LDH release were determined using the MTT and LDH kits, respectively.
2.4. Impedance-Based Measurements
The responses of dermal fibroblasts cultured with different concentrations of bulk zinc oxide were monitored by the xCELLigence Real-Time Cell Analysis (RTCA) instrument DP (Roche, Basel, Switzerland). After background measurement, the cells were seeded in the wells of electrode plates, cultured inside the incubator and allowed to adhere for 15 h. Then, zinc oxide suspensions were added into each well of the E-plates. The changes in the cell index were continuously monitored for 80 h.
3. Results and Discussion
In this study, dermal fibroblast cells were exposed to different concentrations of bulk zinc oxide. The cellular responses to zinc oxide were determined using in vitro toxicity assays (MTT and LDH), as well as by the xCELLigence Real-Time Cell Analyzer system. The changes in the morphology of cells exposed to zinc oxide were monitored using inverted microscopy. The results showed that the cells exposed to 50 and 100 μg/mL zinc oxide gradually lost their characteristic phenotype, started to shrink and obtained irregular shapes (Figure 1).
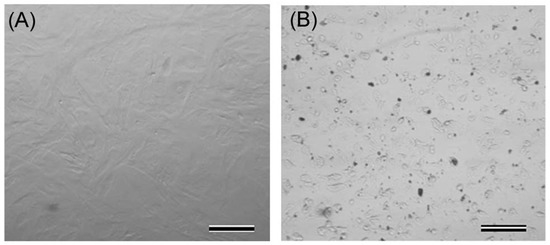
Figure 1.
Representative phase contrast microscopy images demonstrating control (A) and 100 μg/mL zinc oxide-treated cells (B). Scale bars: 100 µm.
In vitro toxicity assay showed that zinc oxide caused significant decrease in cell viability and significant increase in LDH release at 50–100 μg/mL for 48h (Figure 2). Similar results were obtained with different cell type in previous studies [4,5].
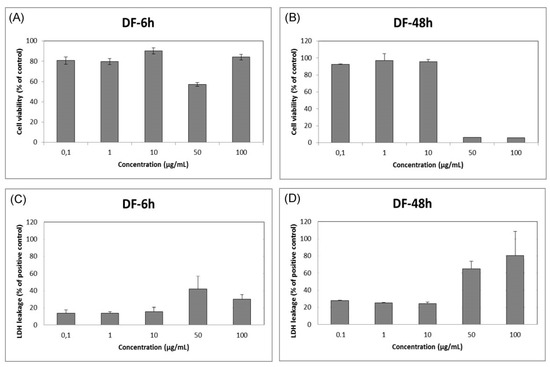
Figure 2.
In vitro cytotoxicity assays by (A,B) MTT assay; (C,D) LDH assay.
Real-time impedance measurements demonstrated that cell index values were almost zero in the presence of 50 and 100 μg/mL zinc oxide after 12 h (Figure 3).
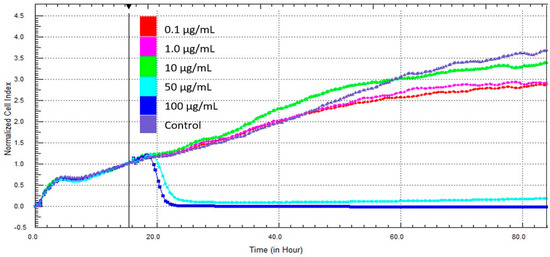
Figure 3.
Real-time monitoring of cell responses of mDF.
The results showed that 50–100 μg/mL zinc oxide had a higher risk of cellular toxicity compared to lower concentrations under studied experimental conditions.
This study provides the toxic dose levels of bulk zinc oxide to determine its relative safety when used with dermal fibroblasts. Impedance-based high-throughput system seems to be an efficient assay method for evaluating cellular toxicity.
Acknowledgments
I thank Y. Murat Elçin and A. Eser Elçin very warmly for their advice and support. The data of this work has not been published (or in consideration) elsewhere.
References
- Pasquet, J.; Chevalier, Y.; Couval, E.; Bouvier, D.; Noizet, G.; Morlière, C.; Bolzinger, M.A. Antimicrobial activity of zinc oxide particles on five micro-organisms of the Challenge Tests related to their physicochemical properties. Int. J. Pharm. 2014, 460, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Remya, N.S.; Syama, S.; Sabareeswaran, A.; Mohanan, P.V. Investigation of chronic toxicity of hydroxyapatite nanoparticles administered orally for one year in wistar rats. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Şeker, Ş.; Elçin, A.E.; Yumak, T.; Sınağ, A.; Elçin, Y.M. In vitro cytotoxicity of hydrothermally synthesized ZnO nanoparticles on human periodontal ligament fibroblast and mouse dermal fibroblast cells. Toxicol. In Vitro 2014, 28, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Sahu, D.; Kannan, G.M.; Vijayaraghavan, R.; Anand, T.; Khanum, F. Nanosized zinc oxide induces toxicity in human lung cells. ISRN Toxicol. 2013, 2013, 316075. [Google Scholar] [CrossRef] [PubMed]
- Jeng, H.A.; Swanson, J. Toxicity of metal oxide nanoparticles in mammalian cells. J. Environ. Sci. Health A 2006, 41, 2699–2711. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).