Abstract
A large part of the wastewater generated by the Chemical and Transformation Industries are discharged with the presence of organic pollutants, in many cases they contain refractory organic compounds such as formaldehyde in a very low concentration for their recovery to be profitable, but it is high enough for to constitute a source of important pollution, which causes a loss of biodiversity and retards sustainable development. In the present work, the elimination of formaldehyde by the catalytic wet oxidation reaction is evaluated as part of the tertiary treatment of aqueous effluents in a three-phase reaction system, using copper and cobalt mixed oxides catalysts supported in alumina (alpha phase), the results of the characterization of the catalyst used are also shown, by conventional techniques.
1. Introduction
A large part of the wastewater generated by the chemical industry and the transformation are discharged with the presence of organic pollutants, in many cases contain refractory organic compounds such as formaldehyde in a very low concentration for their recovery to be profitable, but it is high enough to be a source of significant contamination []. Given this scenario, it is necessary to search for technologies that effectively treat this type of contaminated effluents and contribute to reducing the impact caused by a loss of biodiversity and delaying sustainable development [].
The heterogeneous catalysis is widely used in industrial processes for its advantages, such as: high activity, selectivity and easy recovery, so the use of catalysts to improve the processes of the chemical industry and environmental protection is considered one of the pillars of green chemistry.
The application of systems based on CeO2 as catalysts for the elimination of pollutants is very broad and includes as metals supported mainly to noble metals, however, the high cost of these, promotes the investigation of other catalytically active phases that include metals, such as: Copper, Cobalt. The catalysts based on mixed oxides Ce-Co, have a good activity in the oxidation of CO, and show that the incorporation of Co improves the initial reaction speed, given that the Co influences directly, improving the catalytic activity with respect to the catalyst monometallic [].
The catalysts whose active phase is based on CeO2, are of great interest due to their applications in catalysis and since they can be the basis of different mixed oxides, the importance of cerium oxide is due to its ability to function as a buffer of storage of oxygen linked to the facility to transit between the oxidation states of Ce4+ to Ce3+, that is, of its redox properties [].
However, the use of CeO2 is limited due to its low specific area.
In order to increase its thermal stability to CeO2, several studies have been carried out incorporating transition metal cations (Al3+, Si4+, Ti4+, Zr4+, Li3+, etc.) into the crystalline structure, observing, in some cases, favorable changes in redox properties and/or in its thermal stability [].
So in this work formaldehyde mineralization is raised by a catalytic technique, which is the Catalytic Wet Oxidation (CWO) using as a catalyst a mixed oxide of Ce-Cu-Co supported in alumina and characterized by different techniques such as: XRD, SEM/EDS, TPR and FTIR.
2. Materials and Methods
For the synthesis of the mixed oxide catalyst, solutions of precursor salts of: Ce(NO3)3·6H2O with 99% purity, for copper oxide Cu(NO3)2·2H2O at 99% and for cobalt oxide were realized used the precursor salt of Co(NO3)2·6H2O with 98% purity, Sigma-Aldrich brand varying the molar concentrations of the solutions being the solution of precursor salt of cerium the highest concentration. The wet impregnation method was used using a porous commercial alumina material (alpha phase) as a support. The materials after impregnation were allowed to dry at room temperature for 24 h and subsequently calcined at 350 °C for 2 h.
To be characterized by the following techniques: X-Ray Diffraction (XRD) in a Philip model X’pert diffractometer with a scanning angle of 2θ, Fourier Transform Infrared Spectroscopy (FTIR), was performed on a Varian model spectrometer Excalibur 3600 working in the region of the infrared medium, the Elemental Chemical Analysis (SEM/EDS) in a supra 55VP microscope of the Carl Zeiss brand with a secondary electron detector, Thermo Programmed Reduction (TPR), in a brand equipment Bel Japan using a reducing atmosphere.
Oxidation of formaldehyde was carried out by means of the CWO reaction in a Parr-type slurry reactor, having as operating variables the temperature of 25 and 60 °C and the pressure of 1 and 2 atmospheres of air used as oxidizing agent, with a mass ratio of catalyst per litter solution of 1 g/L, with an initial concentration of 100 ppm of formaldehyde; in all the case studies, the reaction time was 4 h and monitored every 30 min by taking the pH reading and doing chromatographic analysis of each sample taken in liquid phase.
The CO2 product of the CWO was bubbled into a solution of Sr(OH)2 in order to capture the gas product of the mineralization in the form of carbonate, which was identified by FTIR, also the residual catalyst was analyzed by FTIR for identify possible products adsorbed on its surface.
3. Results and Discussion
In Figure 1, the XRD diffractogram of the mixed oxide catalyst Ce-Cu-Co/α-alumina is shown, where the characteristic pattern of the commercial alumina in its alpha phase and the cerium oxide is observed, as well as the characteristic signals of the cerium oxide, with greater intensity compared to those of copper, or those of cobalt, these signals correspond to the cubic structure centered on the faces, characteristic of fluorite, and according to what is reported in the literature, also characteristic of the mixed oxides based on cerium, in the planes (111), (200), (220), (311), (222) and (400) []. A greater intensity was observed in the characteristic peaks of cerianite compared to copper or cobalt peaks because the cerium precursor solution was prepared with higher concentration.
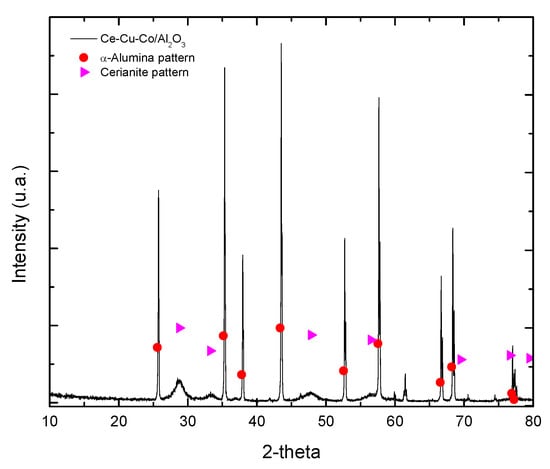
Figure 1.
XDR of the mixed oxide catalyst Ce-Cu-Co/α-alumina.
The SEM/EDS analysis shows the typical morphology of the alumina support in its alpha phase with a homogeneous distribution of incorporating particles corresponding to the metals of Ce, Co and Cu, with a higher percentage of weight in Ce. In the Figure 2a shows the micrograph, analyzed by backscattered electrons (AsB), of an alpha-alumina grain coated with small particles corresponding to metals (whiter and brighter areas). Figure 2b shows the micrograph of the analysis with secondary electrons (SE2) and at higher magnifications, where the characteristic crystals of commercial alumina in alpha phase, coated with metal oxides, are observed. Figure 2c presents the elemental analysis (SEM/EDS) where a higher weight percentage of Ce content is observed, compared to the Cu and Co content, since during the synthesis of the catalyst, a higher concentration was used for the precursor solution of cerium oxide.
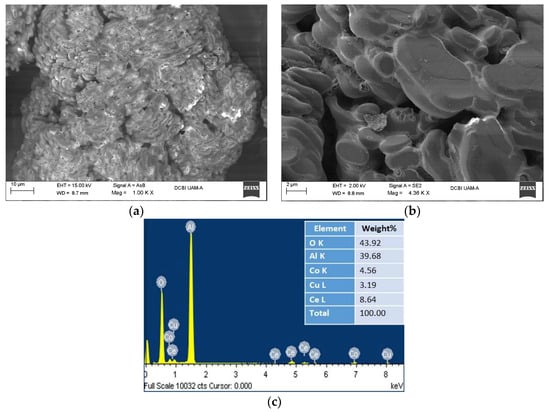
Figure 2.
SEM analysis for catalyst Ce-Cu-Co/α-alumina: (a) AsB detector (1000X), (b) SE2 detector (4360X), (c) EDS detector (elemental analysis).
Figure 3, presents the TPR of both the alumina commercial support and the Ce-Cu-Co/Al2O3 powder catalyst, the support has two signs of hydrogen consumption at high temperatures, the first starting at 450 °C, with a maximum at 550 °C and the second with a maximum at 630 °C, while the catalyst presents three peaks of hydrogen consumption, the first with a maximum at 170 °C, the second at 190 °C and the third at 223 °C, which suggests that there is a strong interaction between the three metal oxides induced by the establishment of an intimate contact between the three metals [].
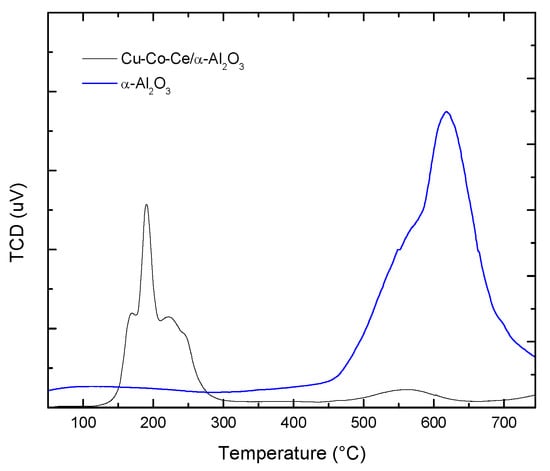
Figure 3.
TPR of α-Al2O3 support and catalyst Ce-Cu-Co/α-alumina.
The results of catalytic activity have shown that it is possible to eliminate formaldehyde through advanced oxidation processes such as CWO (Figure 4a). The effect of pressure and temperature are very marked since the highest percentage of elimination of the contaminant was obtained at the temperature of 60 °C and at the pressure of two atmospheres of oxidizing agent. The conversion rate of formaldehyde ranged from 30 to 65%. The pH reading showed a decrease in acid scale, obtaining values in a range of 6.60 to 4.53 that could be indicative of the presence of carboxylic acids of low molecular weight at low concentrations not detectable by gas chromatography. FTIR analyses [] made to the catalysts after reaction showed adsorbed secondary products (not shown here).
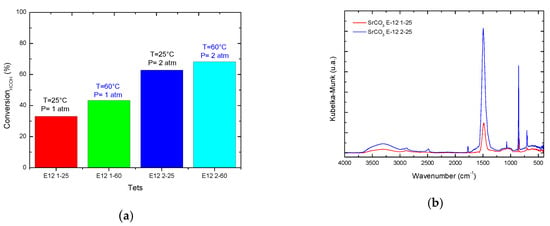
Figure 4.
(a) Results of conversion of CWO of HCOH at pressure 1 and 2 atm and temperature 25 and 60 °C, (b) FTIR of the precipitate of the CWO reaction.
On the other hand, the analysis by FTIR of the precipitate generated confirmed the formation of strontium carbonate [] which confirms the degradation of the contaminant by the CWO to the mineralization (Figure 4b).
4. Conclusions
The SEM/EDS and XRD of the samples, verify the presence of the metals that make up the mixed oxide on the alumina support. The TPR of the material shows a strong interaction between the three metals and the alumina support, so we can conclude that the preparation method was adequate. The results of CWO showed that the elimination of HCOH at the temperature of 60 °C and the pressure of two atmospheres of oxidizing agent is possible and the results of the FTIR analysis of the precipitate generated confirmed the formation of the carbonate product of the mineralization of the pollutant to CO2.
Author Contributions
M.G.-A. and M.T.-R. conceived of and designed the experiments and analyzed the data, E.R.-C. performed the experiments and V.M.-Á. wrote the paper.
Acknowledgments
Rodríguez thanks the CONACyT scholarship and the co-authors thank the Divisional Electronic Microscopy Laboratory of the UAM-A.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Silva, A.M.T.; Castelo-Blanco, I.M.; Quinta-Ferreira, R.M.; Levec, J. Catalytic studies in wet oxidation of effluents from formaldehyde industry. Chem. Eng. Sci. 2003, 58, 963–970. [Google Scholar] [CrossRef]
- UNESCO Etxea, 2010. Servicios de los ecosistemas y bienestar humano. Available online: www.unescoetxea.org/dokumentak/Ecossitemas_bienestar.pdf (accessed on 16 May 2018).
- Shao, J.; Zhang, P.; Tang, X.; Zhang, B.; Song, W.; Xu, Y. Effect of Preparation Method and Calcination Temperature on Low-Temperature CO Oxidation over Co3O4/CeO2 Catalysts. Catal. Chin. J. Catal. 2007, 28, 163–169. [Google Scholar] [CrossRef]
- Trovarelli, A.; De Leitenburg, C.; Dolcetti, G. Design Better Cerium-Based Oxidation Catalysts. CHEMTECH 1997, 27, 32–37. [Google Scholar]
- Trovarelli, A. Catalysis by Ceria and Related Materials; Catalytic Science Series 2; World Scientific Publishing Company: London, UK, 2002; pp. 253–254. [Google Scholar]
- Yu, S.W.; Huang, H.H.; Tang, C.W.; Wang, C.B. The effect of accessible oxygen over Co3O4–CeO2 catalysts on the steam reforming of ethanol. Int. J. Hydrogen Energy 2014, 39, 20700–20711. [Google Scholar] [CrossRef]
- Cuevas, S.A.; Gutiérrez, M.; Aguilar, J.; Mugica, V.; Noreña, L.E.; Torres, M. Remoción de formaldehído de efluentes acuosos mediante oxidación húmeda catalítica. ACI 2011, 2, 13–23. [Google Scholar]
- Ma, L.; Wanga, D.; Li, J.; Baib, B.; Fub, L.; Li, Y. Ag/CeO2 nanospheres: Efficient catalysts for formaldehyde oxidation. Appl. Catal. B Environ. 2014, 148–149, 36–43. [Google Scholar] [CrossRef]
- Alavi, M.A.; Morsali, A. Syntheses and characterization of Sr(OH)2 and SrCO3 nanostructures by ultrasonic method. Ultrason. Sonochem. 2010, 17, 132–138. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).