Abstract
Inkjet 3D printing is with success used for preparation microfluidics devices as a microfluidic chips for DNA separation. Utilized this technic help us to prepare a chip in shorter time and cheaper, but length of microchannel are limited by possibility of support material removing. One of the Solution for solve this limitations is divide a microfluidics chips for modules and printing them separately. I this paper we present a modular configurable system for gel electrophoresis. Results of carrying methylene-blue from first module to second module are showed. New methodology for electrophoresis is show.
1. Introduction
It is well known that on-chip gel electrophoresis is a powerful tool of genetic material analysis. The chips are made as disposables with fixed configuration and with integrated all necessary components—inlet/outlets, T-junction dozers, separation microchannel, detection area etc. They are made of glass, plastics and recently also with photocurable polymer by inkjet 3D printing (i3Dp) [1]. However one of the i3Dp feature is that diameter and length of the microchannel is limited by support material removing efficiency. It causes that our previous printed chips for gel electrophoresis had separation channel length limited to about 30 mm with diameter of 500 μm. Additionally, extraction of the selected fraction(s) of the sample for post-analyze procedures is impossible in all traditional separation chips—they are made as monolithic block of the chip’s material.
2. Materials and Methods
Here we propose a modular configuration that utilizes few main modules (Figure 1) to build required configuration. Diameter of the embedded separation microchannels is 500 μm with 30 mm length for a single module. Utilized inkjet 3D printing technique is able to produce microchannels with smallest size (i.e., 200 μm [2]), but is strongly correlated with possibly of support removing material from long printed microchannels. Developed modules were designed in Inventor Professional 2017 (Autodesk, USA). The modules were designed for provide on-chip gel electrophoresis of genetic material. Thus module for injection of the sample, module with different lengths of separation channel (10–30 mm), fluorimetric detection and double T cross section module were developed. The Projet 3510 (3D Systems, USA) inkjet 3D printer is used to print the modules. Visijet M3 Crystal building material and wax-like Visijet S300 support material (3D Systems, USA) are used. After printout the modules are cleaned according to the procedure described by us earlier [2]. Briefly, the support material is melted way at 60 °C, than the modules are cleaned in mineral oil bath with ultrasonic agitation at 60 °C. Neext the chips are cleaned in a detergent and raised in deionized water. The clean and dry modules are connected each to other according to desired configuration (Figure 2a). Finally the modules are filed with separation gel (POP-4, A&A Biotechnology, Poland).
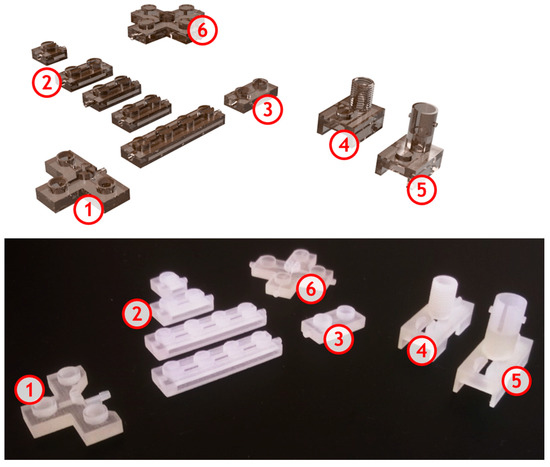
Figure 1.
3D printed microfluidic modules for on-chip gel electrophoresis – visualization and view of printed modules: 1—T-type injector; 2—separation microchannels with various lengths; 3—final module; 4,5—optical detection modules with SMA/ST optical fibre connections, 6—double-T cross-section.
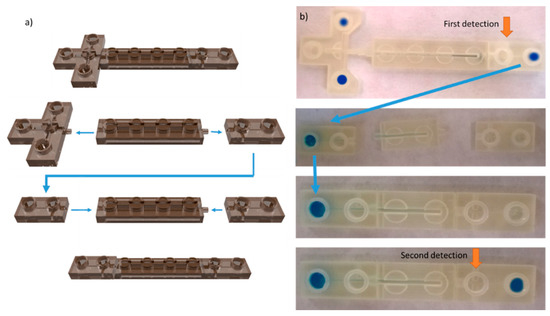
Figure 2.
Computer visualization (a) and view of the reconfigurable modular microfluidic chip for gel electrophoresis (b) during electrophoretic separation and relocation of the sample between two configurations of the chips. First configuration contains T-type injector, 30 mm—long separation channel and final module. After separation in the first configuration the final module (with immobilised analyte confirmed by fluorescence detection) is detached and relocated as a first module in the second configuration. Finally, the separation is continued in the second configuration with fluorescence detection at points as marked.
3. Results
In the preliminary experiments blue methylene was used to observe sample injection and movement in the microchannels (sample volume 0.7 μL, 200 V/cm electric field) and to test sample relocation between two configurations (Figure 2). The fluorescence detection was performed at the ends of separation channels (Figure 2b) with orthogonal configuration of red laser as excitation source and miniature non-cooled camera as detector [3].
The carried out experiment confirm that: (1) it is possible to build separation chip from microfluidic blocks; (2) electrophoretic transport and separation in the modular chip is properly controlled and observed—the same behavior as in standard chip is noted (Figure 3); (3) sample relocation between modular configurations is possible (Figure 3), (4) i3Dp enables printing of user-defined modules for on-chip gel electrophoresis with total separation channels length longer than previously reported.
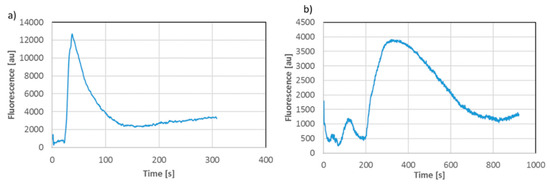
Figure 3.
Blue methylene electrophoretic transport in the first microfluidic configuration (a) and after relocation of the sample to the second configuration (b), florescence measurement points are marked with arrows on Figure 2b.
In the second experiment 20–200 bp DNA ladder labeled with Cy5 dye was properly separated in 60 mm (2 × 30 mm) long separation channel was (Figure 4). Thus usefulness of the developed configurable on-chip gel electrophoresis is confirmed.
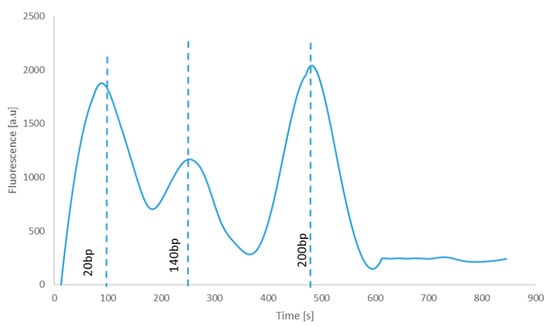
Figure 4.
DNA ladder (20–200 bp) separation in the modular chip with 60 mm long separation microchannel and fluorescence detection.
4. Conclusions
In this paper we present for the first time a concept of modular microfluidic chips for on-chip gel electrophoresis of genetic material. There are two main innovations that are proposed here—methodology of the analyse and fabrication of the microfluidic modules (patent pending). The microfluidic chip is a set of modular blocks that are easily connected each to other, filed with sieve matrix (gel) and configured according to analyse requirements. The analyse can be stopped at any moment and the microfluidic module with “frozen” analyte fraction(s) can be relocated as a new part of next set of the modular chips that form new separation chip. Thus, the same fraction(s) can be analysed many times without contamination and as long as it is necessary. The second innovation is application of inkjet 3D printing technique as a tool for precise fabrication of customized microfluidic modules for on-chip gel electrophoresis.
Acknowledgments
The works are financed by Polish National Science Centre (NCN) under frame of the project no 2013/10/E/ST8/0342.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Adamski, K.; Walczak, R.; Kubicki, W. 3D Printed Electrophoretic Lab-on-Chip for DNA Separation. Procedia Eng. 2016, 168, 1454–1457. [Google Scholar] [CrossRef]
- Walczak, R.; Adamski, K. Inkjet 3D printing of microfluidic structures—On the selection of the printer towards printing your own microfluidic chips. J. Micromech. Microeng. 2015, 25, 085013. [Google Scholar] [CrossRef]
- Walczak, R.; Adamski, K.; Lizanets, D. Inkjet 3D printed check microvalve. J. Micromech. Microeng. 2017, 27, 047002. [Google Scholar] [CrossRef]
- Walczak, R.; Kubicki, W.; Dziuban, J. Low cost fluorescence detection using a CCD array and image processing for on-chip gel electrophoresis. Sens. Actuators B Chem. 2017, 240, 46–54. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).