Abstract
Excess generation of reactive oxygen species (ROS) could trigger a number of human diseases. We focused that antioxidants in beverages suppress the effect of ROS. Electrochemiluminescence (ECL) was determined by newly designed bi-potential screen printed electrodes and electron multiplying charge coupled device (EM-CCD) camera. Luminol based ECL was quenched due to the consumption of ROS by the antioxidants. Rapid and sensitive detection of antioxidants such as ascorbic acid and chlorogenic acid were demonstrated in beverage samples.
1. Introduction
When pathogens such as viruses enter the human body, the immune system generates reactive oxygen species (ROS). However, if ROS are generated in excess, they can attack the body and cause many diseases, including diabetes, high blood pressure and Parkinson’s disease [1]. It is known that certain food products such as green tea and citrus fruits have antioxidative properties. A large amount of evidence that antioxidants reduce the incidence of the above mentioned diseases has been reported [2]. Therefore, studying antioxidative properties of food is imperative for human healthcare. However, conventional methods often require long measurement time and large devices or complicated procedures, which render them cumbersome and impractical [3]. Thus, the objective of our research is to develop a fast and simple detection system for antioxidants. To this end, we focused on electrochemiluminescence (ECL) using luminol. ECL has emerged as a powerful analytical tool in a various field such as food analysis and physiological research. ECL has many advantages such as high sensitivity, and reliability. In addition, we adopted disposable screen-printed electrodes (SPEs) because they are easy to mass-produce and cost less compared to conventional electrodes. Utilizing these tools, we aimed to devise an in situ ROS-generating system based on the quenching of luminol ECL by antioxidants on the surface of comb-shaped screen printed bi-potential electrodes [4].
2. Materials and Methods
2.1. Reagents and Chemicals
Luminol was purchased from Sigma-Aldrich (MO, USA). 200 mM Tris buffer was adjusted to pH8.0 with HCl. 10 mM luminol stock solution was prepared by dissolving in 0.1 M NaOH solution under stirring until dissolved completely and stored at −20 °C until use. Trolox and chlorogenic acid were purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan). Beverages (green tea, apple juice) were purchased at a local convenience store. SPEs were purchased from Bio Device Technology, Ltd. (Japan).
2.2. Apparatus
ECL imaging was conducted using an image intensifier (model xx2050AH, Photonis, France) and electron multiplying charge coupled device (EM-CCD) camera (ImageEM C9100-13, Hamamatsu photonics K.K., Shizuoka, Japan) mounted on top of an upright microscope (model MVX10, Olympus, Tokyo, Japan). Photons entering the objective lens pass through the image intensifier, which amplifies the resulting light signal. The EM-CCD camera then captures the amplified signal. The ECL intensity was calculated using image analysis software (AquaCosmos Ver2.60, Hamamatsu Photonics K.K., Shizuoka, Japan). Comb-shaped screen-printed electrodes with two working electrodes were used to demonstrate the production of ROS and the oxidation of luminol. The electrodes comprised two working and counter electrodes made of carbon, and an Ag/AgCl reference electrode (Figure 1).
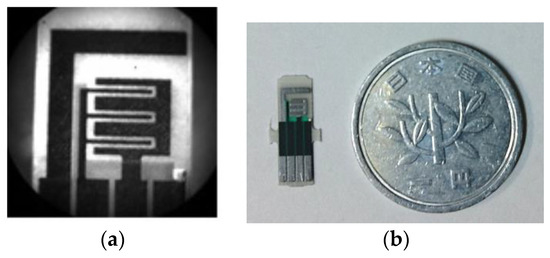
Figure 1.
(a) Bright field image of 3 comb-shaped screen printed electrode; (b) Size comparison of screen printed electrode with 1 yen coin.
2.3. ECL Measurement Procedure
Luminol solution was diluted to 50 μM in tris buffer, and 2 μL of this solution was dropped on the electrode surface. Then, the droplet was spread out across the electrode by a round glass slide (φ = 12 mm). To conduct the luminescent reaction, a negative voltage was first applied to one of the working electrodes to produce ROS. Next, a positive voltage was applied to the other electrode to oxidize luminol. Then, the oxidized luminol is excited by reacting with ROS, and luminescence is generated by the relaxation of the excited luminol to the ground state (Figure 2). First of all, for the application of positive voltage chronoamperometry (CA, 500 mV) and linear sweep voltammetry (LSV, scan rate of 50 mV/s, 0–700 mV) were compared. In addition, the negative voltage for ROS generation was optimized.
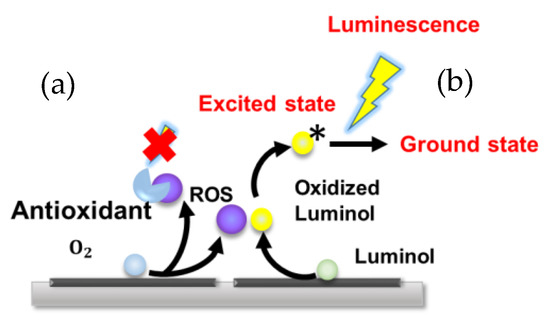
Figure 2.
The scheme of the principle of the luminol luminescence and quenching by antioxidant (a) Quenching scheme in presence of antioxidant; (b) Scheme of Luminol luminescence with self ROS generating system.
2.4. Antioxidant Detection with Using In Situ ROS Generating Bipotential System
Antioxidants were measured with using the previously described system. A constant voltage of −1000 mV was applied to one of the working electrodes to generate ROS, and a voltage sweep from 0 to 700 mV was applied to the second electrode for luminol oxidation. In the absence of antioxidants, a strong ECL signal is observed. However, if antioxidants are present in the sample, they deactivate the generated ROS, which decreases the ECL signal. Samples were mixed with equal volumes of 100 μM luminol. A range of concentrations (0.5–2 mM) of trolox, used as a standard for antioxidant capacity, was also mixed with 100 μM luminol to create a calibration curve.
3. Results and Discussion
3.1. ECL Intensity Measurements Using In Situ ROS Generating Bipotential System
Linear sweep voltammetry was found to be more suitable than chronoamperometry for the imaging of luminescence (Figure 3). The comb-shaped screen-printed electrode was able to detect up to 10 µM hydrogen peroxide by mixing with 50 µM of luminol. From these results, it is expected that increasing the number of combs could further enhance the ECL intensity.
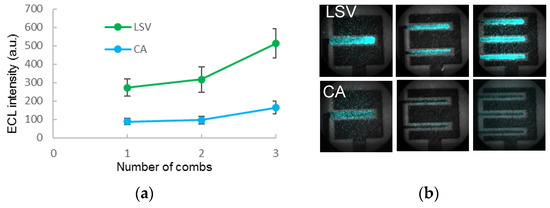
Figure 3.
(a) ECL intensities of 50 µM luminol from LSV and CA; (b) Images of luminol ECL on different comb-shaped electrodes by LSV (top) and CA (bottom).
3.2. Antioxidant Detection Using In Situ ROS Generating Bipotential System
Ascorbic acid was measured through quenching of luminescence induced by in situ generated ROS without external addition of hydrogen peroxide. Quenching was observed in the range from 1 mM to 50 µM of ascorbic acid (Figure 4). Quenching effect was also observed by chlorogenic acid.
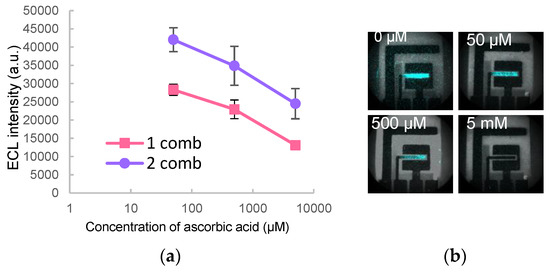
Figure 4.
(a) ECL quenching effect of ascorbic acid; (b) Images of the quenching of luminol ECL by different concentrations of ascorbic acid (shown in top left of image).
In addition, the quenching effects of antioxidants in real beverage samples (Green tea, apple juice etc.) were measured. As the samples were increasingly diluted, recovery of the quenched luminescence was observed (Figure 5). Clear differences were observed among the luminescence quenching from different samples. These results suggest that this system may be used for sensing of other antioxidants through their reactions with ROS.
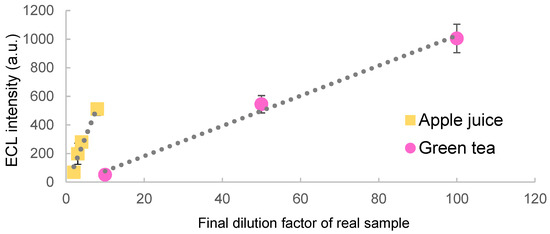
Figure 5.
ECL quenching effect of real beverage samples.
4. Conclusions
In this work, comb-shaped screen-printed bi-potential electrodes were used for the in situ electrochemical generation of ROS. This was then applied for the rapid and facile detection of antioxidants based on the quenching of luminol ECL. This proposed method was successfully used to detect several common antioxidants as well as antioxidant activity in real beverage samples.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Devasagayam, T.P.A.; Tilak, J.C.; Boloor, K.K.; Sane, K.S.; Ghaskadbi, S.S.; Lele, R.D. Free radicals and antioxidants in human health: Current status and future prospects. J. Assoc. Physicians India 2004, 52, 794–804. [Google Scholar] [PubMed]
- Dematteis, M.; Pépin, J.; Lévy, P.; Khaw, S.K. Plasma ascorbic acid in heart disease. Lancet 2001, 358, 71–72. [Google Scholar]
- Gardner, P.T.; Mcphail, D.B.; Duthie, G.G. Electron Spin Resonance Spectroscopic Assessment of the Antioxidant Potential of Teas in Aqueous and Organic Media. J. Sci. Food Agric. 1998, 76, 257–262. [Google Scholar] [CrossRef]
- Smit, M.H.; Cass, A.E. Cyanide detection using a substrate-regenerating, peroxidase-based biosensor. Anal. Chem. 1990, 62, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).