Abstract
Synthesis conditions of doped polyaniline films and testing procedure affect markedly the sensor behavior and stability of them. The sensor based on such film consists of a dielectric substrate with system of metallic interdigitated electrodes on its surface. The films were deposited on system of metallic interdigitated electrodes by electrochemical synthesis from solutions of aniline with different polyoxometalates and acids. Activating additives into films were tungsten containing polyoxometalates of the eighteen series. Polyoxometalates as multielectron oxidants can alter noticeably the sensor properties of polyaniline if introduced into a conductive polymer film. Morphology of the layers surface and the infrared spectra were explored. Investigations of the behavior of polyoxometalate-polyaniline films at different temperatures, air humidity and testing procedures in clean air and in air containing ammonia were fulfilled.
1. Introduction
Conductive polymers have a number of important physicochemical properties. The films of conductive organic polymers are of great interest as sensitive layers of chemical sensors. Polyaniline (PANI) occupies a special place among conductive polymers due to the simplicity of synthesis and the possibility of controlling the structure and properties of PANI within broad limits by introducing various additives into the film [1,2]. One of the main advantages of the sensors based on films of conductive polymers consists in the possibility of the functioning at room temperature. In addition, through modifying the structure and composition of the polymer it can raise significantly the selectivity and sensitivity of such sensors with respect to various gases. An important problem, which has to be resolved for practical application at this case, is providing long-term stability of the properties of the initial polymer matrix.
Polyoxometalates is attractive as compounds with unique chemical and physical properties [3]. They can act as reversible reagent in ligand exchange reactions and redox transitions which are accompanied by change of current carriers. Polyoxometalates can catalyst different redox processes and increase gas sensitivity of matrix if introduced into one. The result of the activating additives introduction into polyaniline matrix is significant change of polymer film’s properties in gas environment.
The advantage of the method of electrochemical oxidative polymerization at potential cycling is high homogeneity of deposited PANI films and their good adhesion to electrode. The high repeatability of films’ properties is obtained due to possibility to control of basic synthesis parameters, such as current and potential. The properties of conductive polymers can be changed in wide limits by introducing into the structure of different modifying additives. There are two ways of immobilization of polyoxometalates into conductive polymer matrix. The first of them has two stages. Initially polymer film is deposited on electrode. The next modification stage consists of the electrode with film immersion into aqueous solution of polyoxometalate and potential cycling. In this work we used one-stage technique.
2. Materials and Methods
The sensor was a dielectric substrate 15.0 × 5.0 mm in size and on its surface a system of nickel with vanadium sublayer interdigitated electrodes was formed. The active area of the sensor was 4 × 4 mm and electrode gap was 0.08 mm.
The modifying additive has been put into initial solution with the monomer and activated polyaniline film is deposited on electrode’s surface. The water which bring into together with polyoxometalate doesn’t block polymerization. Films were deposited by electrochemical synthesis from solutions of aniline in hydrochloric or sulfuric acids at potentials from −0.2 to +0.7 V in reference to Ag/AgCl and were activated during the synthesis. Activating additives into films were tungsten containing polyoxometalates of the eighteen series.
Morphology of the layers surface were studied with Solver-P47 scanning probe microscope NT-MDT (Moscow, Russia), the infrared spectra were investigated by a Bruker EQUINOX 55 (Billerica, MA, USA). The conditions of film formation varied during the tests.
This sensor was put into special porous chamber connected with signal processing scheme for investigation. The transducer with tested sensor is shown in Figure 1.

Figure 1.
The transducer with sensor for test measurements.
Electrical parameters changes of the sensors were measured electronically in real time mode at different testing procedures.
3. Results
The introduction of polyoxometalate into PANI doesn’t destroy its structure. Observed IR spectra confirm this fact. There is absorption intensity at 1614 cm−1, that corresponds to valence oscillations of C=N; 1497 cm−1 corresponds to valence oscillations of C-C of aromatic ring and others absorption peaks that define the structure of polyaniline monomer. There is adsorption intensity in the range of 790–775 cm−1, that corresponds to valence oscillations of central tetrahedral PO4; 535–530 cm−1, that corresponds deformation oscillations of central tetrahedral PO4. The oscillations of heteropolyanion structure are observed at 960–939 cm−1. These results indicate that molecule structure of substance remains safe at electrochemical doping of polymer matrix.
Films’ sensitivity to ammonia was 0.1 ppm and the response time was 10 s. Films are more sensitive but not stable after 10 cycles of synthesis.
There is no difference to use hydrochloric or sulfuric acids at polyaniline polymerization to get sensitive activated films. Moreover, the films’ sensitivity to ammonia is same for films obtained at the both acids. However, there is dependence of electrical parameters change on concentration of acid in solution at polymerization. The films’ stability is achieved in 1.0 M solution of hydrochloric acid as shown in Figure 2.
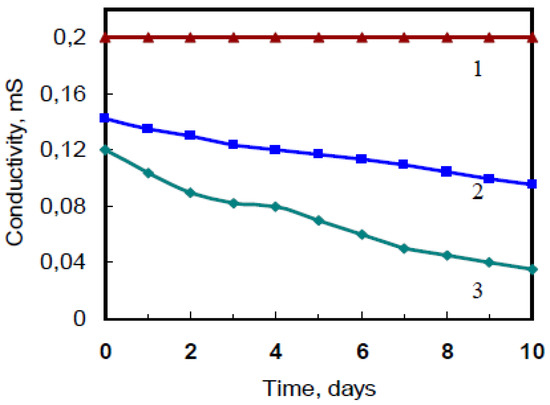
Figure 2.
The stability of PANI—H6P2W18O62 films deposited from solutions with different concentration of hydrochloric acid: 1–1.0 M; 2–0.5 M; 3–0.1 M.
The change of the activated film’s conductivity is 20–60% in the range of 15–90% relative humidity, the introduction of polyoxometalate into the PANI matrix reduces the humidity effect, which is possibly due to the stabilization of the obtained state of PANI. However, there is irreversible conductivity change at long exposition in wet air, but emeraldine form is more stable (Figure 3).
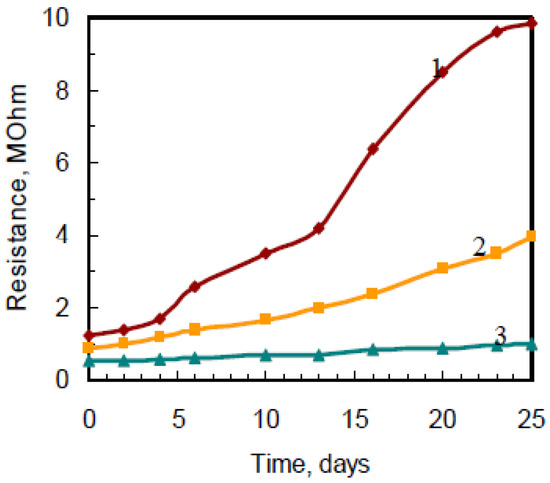
Figure 3.
Typical stability dependence of emeraldine state films activated with H6P2W18O62 on relative humidity in air at room temperature: 1–95%, 2–15%, 3–55%.
The temperature has influence which has to take into account at measurements (Figure 4).
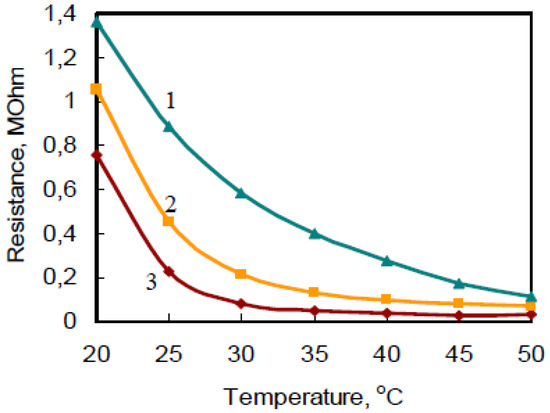
Figure 4.
Typical temperature dependence of PANI films’ resistance in the presence of NH3 with concentration 1 ppm: 1—PANI, modified with H6P2W18O62; 2—PANI, modified with (NH4)9P2W3Mo15O62; 3—unmodified PANI.
It is necessary to take into account the value of applied voltage at sensor characteristics measurements of the activated polyaniline films. The film’s behavior at applied voltage is presented in Figure 5.
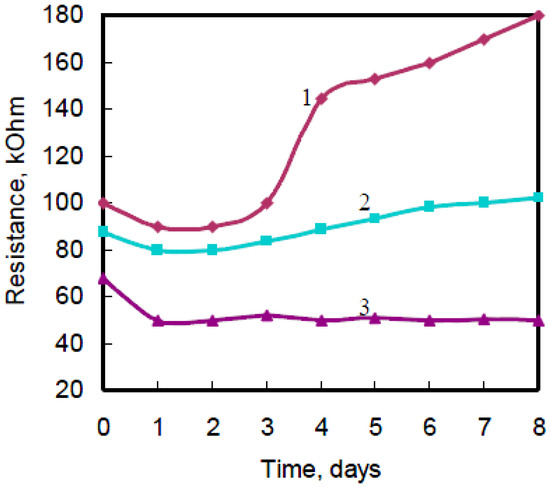
Figure 5.
Typical dependence of emeraldine state films activated with H6P2W18O62 on applied voltage at room temperature: 1–0.1 V; 2–0.05 V; 3–0.01 V.
4. Discussion
Polyoxometalate addition into film results in modification of film’s state and markedly changes its sorption characteristics. The introduction of modifying additives into PANI film raises the sensitivity to ammonia, reduces the effect of humidity on the film of the modified polymer that should be taken into account in sensor calibrating by various methods. Apparently that at higher humidity a partial reduction of the oxidized form of PANI occurs, which is showed itself in a sharp rise of the resistance.
Film resistance is stabilized at low voltages, less than 0.05 V, when there are not the losses of conjugation, crosslinking and oxidative processes in film.
It is important to do measurements at constant temperature as it has considerable effect on results. The doped PANI films show more stable characteristics in that case.
Conflicts of Interest
The authors declare no conflict of interest. “The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results”.
References
- Khan, R. Supported TritonX-100 Polyaniline Nano-Porous Electrically Active Film onto Indium-Tin-Oxide Probe for Sensors Application. Adv. Chem. Eng. Sci. 2011, 1, 140–146. [Google Scholar] [CrossRef]
- Khouchaf, A.; Takky, D.; Chbihi, M.; Benmokhtar, S. Electrocatalytic Oxidation of Methanol on Glassy Carbon Electrode Modified by Metal Ions (Copper and Nickel) Dispersed into Polyaniline Film. J. Mater. Sci. Chem. Eng. 2016, 4, 97–105. [Google Scholar] [CrossRef][Green Version]
- Briand, L.E.; Baronetti, G.T.; Thomas, H.J. The state of the art on Wells–Dawson heteropoly-compounds: A review of their properties and applications. Appl. Catal. 2003, 256, 37–50. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).