Abstract
Cystic echinococcosis is a zoonosis caused by the ingestion of food or water contaminated by Echinococcus eggs. E. granulosus is the most common causative agent of cystic echinococcosis that still has a relevant incidence in Italy, especially on the islands of Sicily and Sardinia. We report the case of a 64-year-old man with disseminated abdominal cystic echinococcosis (liver, spleen, peritoneum). The patient was asymptomatic and non-eligible for surgical treatment. Treatment with albendazole 400 mg/twice daily was started in 2012 for 15 cycles (each cycle consisted of three 28-day treatments at 14-day intervals) over 10 years for a total of 1260 days of treatment. Serum anti-Echinococcus antibody titers and imaging (echography, TC) were evaluated to monitor the evolution of the disease. Imaging techniques documented the regression of all cyst lesions, but it was less evident for the peritoneal localizations that still are in follow-up. In this case, the prolonged treatment with albendazole was effective, safe and free of side effects. Until today, the patient displays a good clinical condition.
1. Introduction
Cystic echinococcosis is a zoonosis caused more frequently by the ingestion of food or water contaminated by the larvae of Echinococcus granulosus or multilocularis of the Taeniidae family [1]. In Italy, the annual incidence of cystic echinococcosis is 1.6 for 100,000 inhabitants [2], but higher incidences were reported especially in Sardinia and Sicily islands [3]. The abdominal disseminated echinococcosis has an even less frequent occurrence that, in most cases, is due to the symptomatic or silent peritoneal rupture of a hepatic Echinococcus cyst (HEC) [4]. Mebendazole and albendazole (ABZ) are the two anthelmintic drugs used for medical treatment, with the latter being acknowledged to have better performance and safety profile. Therapy with ABZ is recommended for uncomplicated abdominal HEC with a diameter <5 cm, disseminated infection and peri-interventional prophylaxis of secondary echinococcosis [5,6]. ABZ inhibits both microtubule assembly and the activity of helminthic fumarate reductase leading to cell death. The most frequent side effects of ABZ include abdominal pain, anemia, bone marrow suppression with cytopenia, alopecia, increased reversible serum concentrations of aspartate (AST) and alanine transaminase (ALT) and gamma-glutamyl transferase (GGT) that may occur due to drug toxicity or to parasite death [7]. It was reported that treatment with only ABZ is successful in about 40% of cases of hepatic HEC [8]. However, no clinical trial has evaluated the long-term efficacy and safety of ABZ in patients with disseminated abdominal cystic echinococcosis. We describe the case of a patient with disseminated abdominal echinococcosis who was successfully treated with cycles of ABZ therapy for ten years.
2. Case Description
Mr P.R., a 64-year-old bricklayer, had his first visit in 2012 at the diabetes outpatients section of the Internal Medicine department of the University Hospital Policlinico “P. Giaccone” of Palermo (Sicily, Italy). He had well-controlled type 2 diabetes on metformin treatment (2 g/day). Abdomen ultrasound (US) and CT examinations performed in 2011 demonstrated disseminated cystic echinococcosis with hepatic (n = 3), splenic (n = 1), and peritoneum localizations (n = 2) of the cysts. In particular, the exams showed a partially exophytic cystic lesion with signs of rupture along the lower hepatic margin of the VI segment. So, it was concluded that the HEC rupture occurred asymptomatically and was probably responsible for the abdominal dissemination of the Echinococcus. Given the patient’s job, it was hypothesized that the HEC rupture was a consequence of abdominal trauma. After the exclusion of pulmonary involvement by CT scan of the thorax, the medical and surgical team defined the patient as non-eligible for surgical treatment, and the drug treatment with ABZ (400 mg/twice daily for three cycles of 28 days each, with a 14-day pause) was undertaken following the doses and protocol in use in 2012. Cautiously, metformin was discontinued and replaced with basal insulin (8–12 IU/day) and diet.
A follow-up was started monitoring, in particular, anti-Echinococcus granulosus antibody titers (ELISA; regional reference center for diseases transmitted by arthropods—Sicily Region, Italy), US and CT imaging of the abdomen (Table 1; Figure 1 and Figure 2). Serological tests are problematic primarily for the diagnosis of cystic echinococcosis [9]; however, despite its low sensitivity, the ELISA assay is the best laboratory procedure, and we used serially detected antibodies against Echinococcus granulosus to improve the quality of follow-up to support our clinical strategy. Due to the slow but favorable response of the HEC, it was decided to continue the treatment with ABZ until a possible definitive regression of the disease was confirmed. No safety problems occurred until the second month of therapy with ABZ when increased serum concentrations of AST, ALT and GGT were observed (Table 1). Although it was hypothesized that the abnormal values of AST, ALT and GGT were due to the toxic effects of ABZ on Echinococcus, given the persistence of high hepatic blood tests after the second cycle of ABZ, it was decided to suspend the treatment and to adopt a “watch and wait” strategy for that year.

Table 1.
Evolution of laboratory tests and cycles of albendazole treatment from 2012 to 2021.
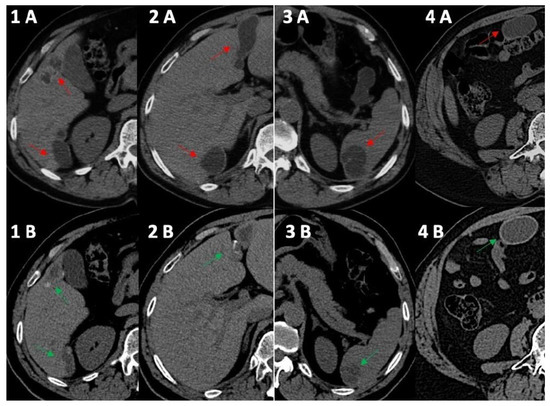
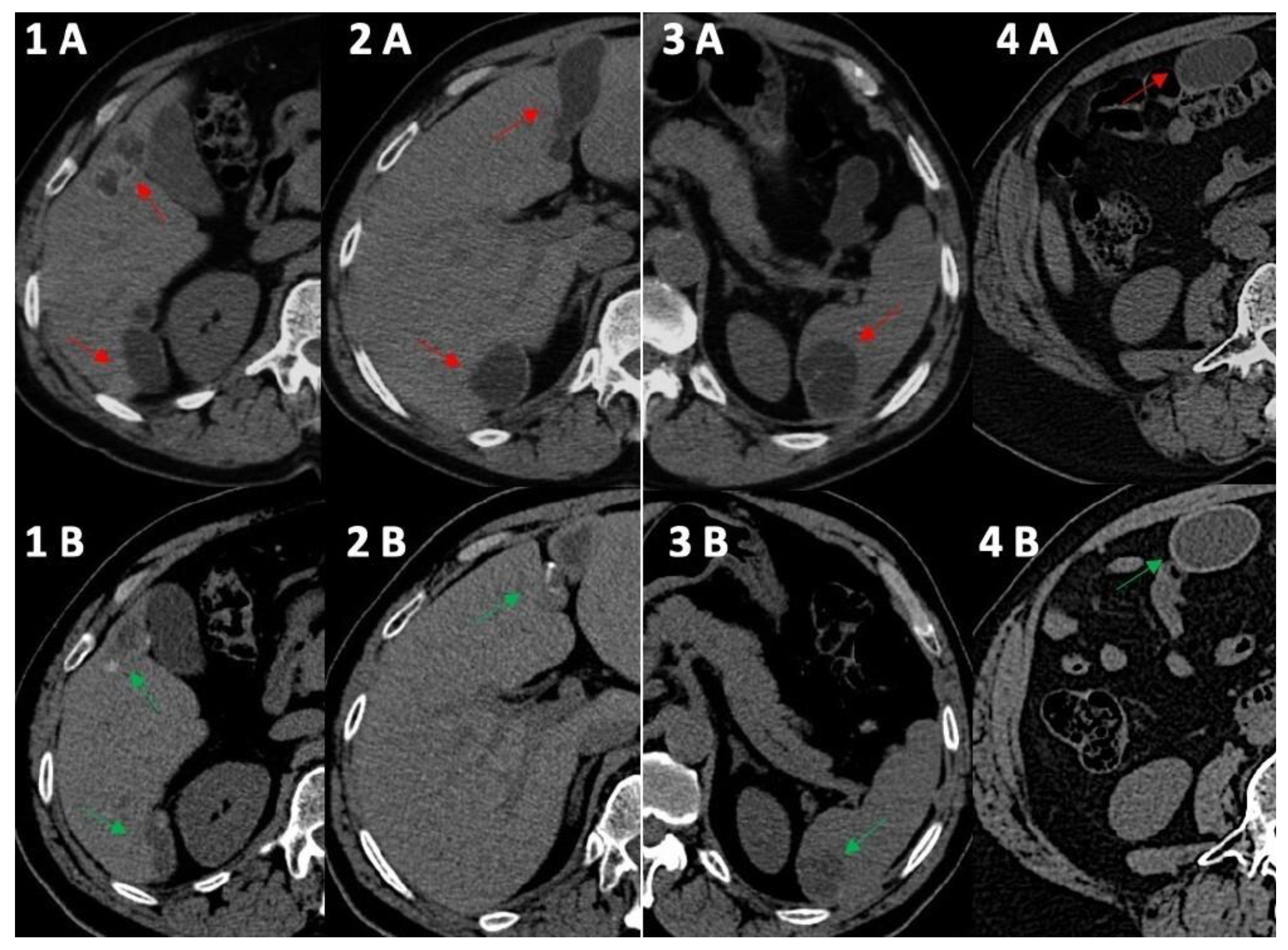
Figure 1.
Computed tomography comparative imaging of some Echinococcus cysts in 2011 (A, red arrow) and in 2021 (B, green arrow). The cysts are located in liver segments 5 ((1A) 15 × 35 mm vs. (1B) 15 × 20 mm) and 6 (1–(2A) 41 × 45 mm vs. (1B) 10 × 36 mm), in segments 3–4 ((2A) 20 × 68 mm vs. 2B 19 × 45 mm), in the upper pole of the spleen ((3A) 30 × 40 mm vs. (3B) 30 × 30 mm), and in the right inframesocolic space ((4A) 41 × 22 mm vs. 4B 40 × 26 mm). In 2021, CT scans (1B,2B,3B) showed some initial signs of cystic wall calcifications that were not present in 2011 (1A, 2A, 3A). Uncertainty persisted for the right inframesocolic cyst (4B), but in 2023 (Figure 2, #3), US demonstrated signs of intracystic degenerative content (Figure 2—n3).
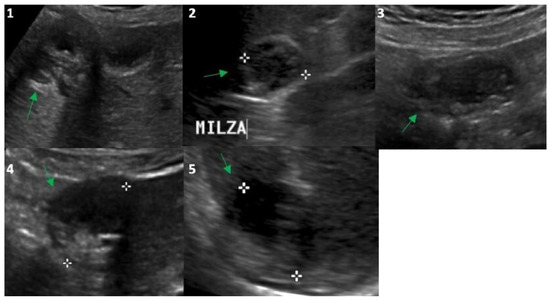
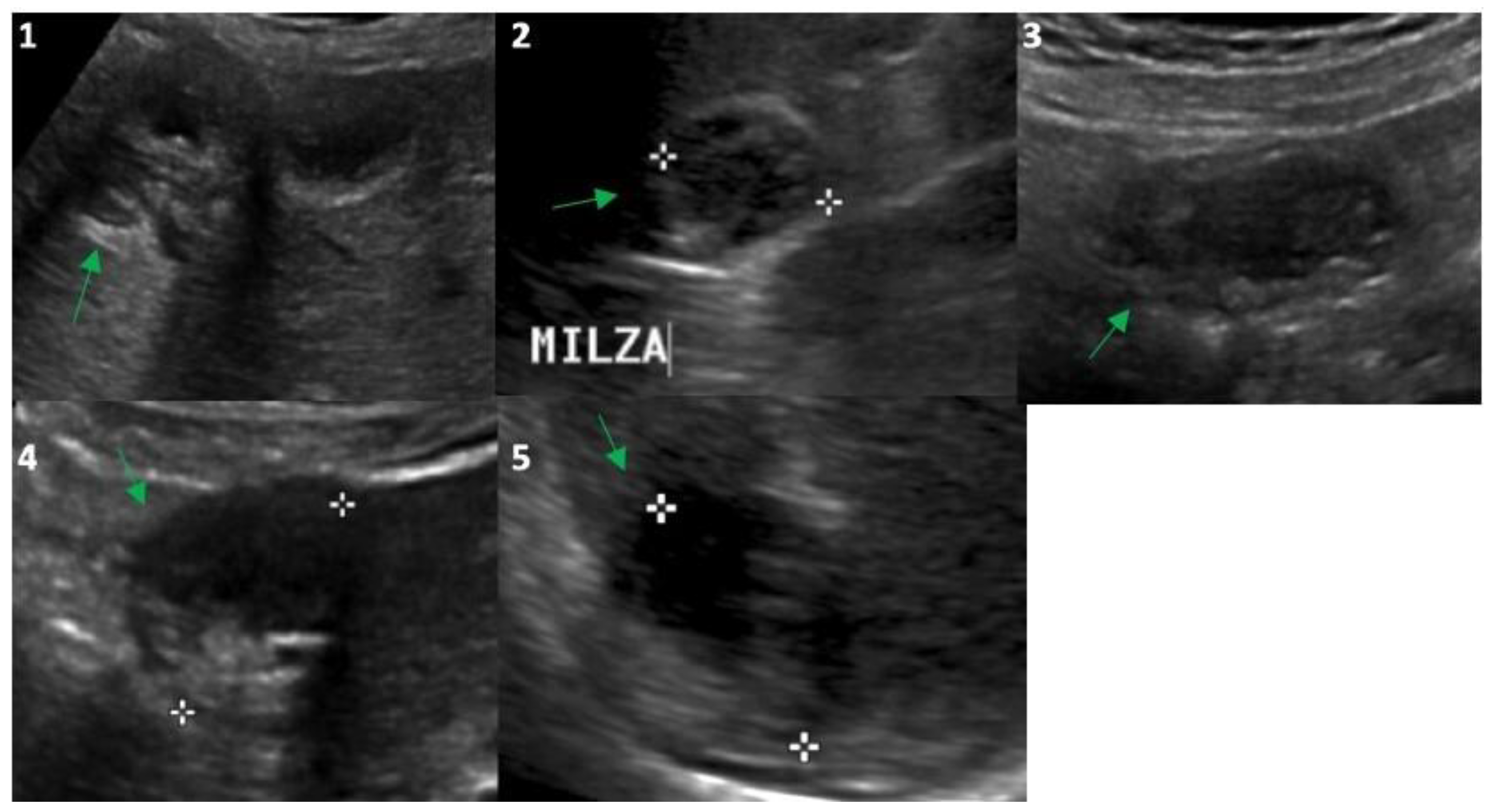
Figure 2.
Ultrasound evaluation in 2023 of the Echinococcus cysts (green arrows) presented in Figure 1: segment 5 of the liver (frame (1); 25 mm), upper pole of the spleen (frame (2); 26 mm), right inframesocolic space (frame (3); 36 mm), segment 3–4 (frame (4); 30 mm) and 6 (frame (5); 30 mm) of the liver. White asterisks correspond to the points of the echo-calipers used to measure the size of the cysts.
In 2014, the anti-Echinococcus titers were still elevated, serum AST, ALT and GGT were normalized and imaging of the cysts suggested possible activity especially for the peritoneal localizations; therefore, treatment with ABZ was re-started and continued until 2021. Normal hepatic blood test results were observed until the end of treatment with ABZ in 2021 (Table 1) and a final (2021) hepatic FibroScan demonstrated normal stiffness values (May 2021, 2.8 kPa). We observed no significant change in the eosinophil count for the whole duration of the follow-up (Table 1), thus excluding a significant relationship between eosinophilia in this case of cystic echinococcosis [10]. Finally, at the end of 2021, the patient had received a total of 15 complete cycles with ABZ that equaled 1260 days of treatment. All cystic lesions progressively showed US [11] and CT signs of regression (Figure 1 and Figure 2) with some uncertainty for peritoneal lesions, which are still in follow-up in 2023. In particular, based on WHO-IWGE US classification [11], in 2023, all cysts were defined as inactive (CE4–5; Figure 2, frame 1, 2, 4, 5) with the exception of the peritoneal lesion that was considered transitional (CE3; Figure 2, frame 3).
Subsequent measurements of anti-Echinococcus antibody titers reported values of 1/200 or were undetectable. In fact, after treatment with ABZ, the patient displayed good health with adequate glycemic control and nutritional state (body weight at the last visit: 71.5 kg, +2.4 kg from 2021), including normal body composition (BIA-101 Akern, Italy) and bioelectrical phase angle (7.7°).
3. Discussion
According to the WHO, cystic echinococcosis is a Neglected Tropical Disease (NTD), relatively neglected by scientific research and public/private funding, compared to the magnitude of the health problem [12]. Cystic echinococcosis sometimes causes severe illness or death, especially when the clinical picture of the disseminated form occurs [13]. In that case, we have no clear indications about the most efficacious strategy to follow. As described in our case, the rupture of a hepatic cyst is the most frequent cause of disseminated abdominal echinococcosis. The rupture of a cyst is often a clinically dramatic event. In fact, data reported in the literature show that all cases of spontaneous intraperitoneal ruptured hepatic echinococcosis were treated by emergency surgery, except one case that was treated successfully by initial conservative measures, followed by elective radical surgery two months later [14]. Therefore, our case report was a particularly complex picture of disseminated echinococcosis following a clinically silent rupture of a hepatic cyst that had a favorable conclusion, demonstrating that long-term treatment with ABZ is possible, at least in the absence of symptoms due to mass effects. Our observation is in agreement with Zavoikin and colleagues who reported a follow-up of 117 patients with pulmonary echinococcosis in which ABZ administered from 3 months to 11 years was well tolerated and effective [15]. Also, cystic echinococcosis of the bone, which is a rare but very destructive form of the disease, requires chronic treatment. A recent European report by Cattaneo et al. [16] retrospectively identified 32 cases until 2018. All these patients underwent surgical treatment that was followed by lifelong treatment with albendazole (continuous treatment or on/off protocols). Overall, they report that the treatment was well tolerated and no patient had to stop treatment. However, to our knowledge, this is the first time that prolonged (10-year) treatment with ABZ has been reported in a case of disseminated abdominal cystic echinococcosis. It is also interesting to note that the treatment was well tolerated despite the patient being elderly and with a significant comorbidity such as diabetes. Given the evolution of serum concentrations of AST, ALT and GGT throughout the 10 years of treatment, we hypothesize that the initial high concentrations of these variables were a consequence of focal inflammatory damage due to the Echinococcus death and not to the direct hepatotoxic effect of ABZ, since it occurred only after the first two cycles of treatment. The peritoneal lesions produced some uncertainty about their evolution due to the fact that this site is possibly less reachable by the drug and imaging was less clear than for other classical localizations in the liver. However, the evolution of serum anti-Echinococcus antibody titers and the monitoring of cysts with echo imaging made it possible to conclude that ABZ was efficacious even in these problematic sites. Another point of interest is that after CT definition, the US method was an adequate technique of imaging for monitoring the evolution of cysts; therefore, after 2021, we decided to monitor the patient exclusively with echography, an inexpensive, easily repeatable, accurate and safe method [17]. Disseminated Echinoccoccosis is quite common and our case report demonstrates that treatment is complex despite effective, so we conclude that clinical trials with standardized diagnostic and therapeutic procedures are warranted to define the treatment of this parasitosis.
4. Conclusions
Cystic echinococcosis is a common endemic disease and disseminated abdominal cystic echinococcosis can cause severe illness or death. The case we describe suggests that long-term ABZ treatment might be a safe and effective treatment in patients with disseminated abdominal cystic echinococcosis. Clinical trials are warranted to define the most appropriate treatment of this parasitosis.
Author Contributions
Conceptualization, C.B., C.R., P.B. and S.B.; methodology, P.B., M.L. and S.B.; software, M.L. and P.B.; formal analysis, C.B., C.R., P.B. and S.B.; investigation C.B., R.C., M.L. and S.B.; resources, S.B.; data curation, C.B., R.C. and S.B.; writing—original draft preparation, C.B., C.R. and S.B.; writing—review and editing, C.B., C.R.; P.B., R.C., M.L. and S.B.; visualization, C.B., C.R., P.B., R.C. and S.B.; supervision, S.B.; funding acquisition, S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Informed consent was obtained from the patient involved in the study.
Data Availability Statement
No data to share besides those reported in this case report. Specific and motivated requests can be addressed to Silvio Buscemi, silvio.buscemi@unipa.it until 31 December 2024.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Agudelo Higuita, N.I.; Brunetti, E.; McCloskey, C. Cystic Echinococcosis. J. Clin. Microbiol. 2016, 54, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Deplazes, P.; Rinaldi, L.; Alvarez Rojas, C.A.; Torgerson, P.R.; Harandi, M.F.; Romig, T.; Antolova, D.; Schurer, J.M.; Lahmar, A.; Cringoli, G.; et al. Global Distribution of Alveolar and Cystic Echinococcosis. Adv. Parasitol. 2017, 95, 315–493. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, F.; Bortoletti, G.; Conchedda, M.; Palmas, C.; Ecca, A.R. Idatidosi cistica umana in Italia: Un problema di salute pubblica? Passato e presente [Human cystic hydatidosis in Italy: A public health emergency? Past to present]. Parassitologia 2004, 46, 39–43. [Google Scholar] [PubMed]
- Majbar, M.A.; Souadka, A.; Sabbah, F.; Raiss, M.; Hrora, A.; Ahallat, M. Peritoneal echinococcosis: Anatomoclinical features and surgical treatment. World J. Surg. 2012, 36, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Teggi, A.; Lastilla, M.G.; De Rosa, F. Therapy of human hydatid disease with mebendazole and albendazole. Antimicrob. Agents Chemother. 1993, 37, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Junghanss, T.; da Silva, A.M.; Horton, J.; Chiodini, P.L.; Brunetti, E. Clinical management of cystic echinococcosis: State of the art, problems, and perspectives. Am. J. Trop. Med. Hyg. 2008, 79, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Salinas, J.C.; Torcal, J.; Lozano, R.; Sousa, R.; Morandeira, A.; Cabezali, R. Intracystic infection of liver hydatidosis. Hepatogastroenterology 2000, 47, 1052–1055. [Google Scholar] [PubMed]
- Horton, R.J. Albendazole in treatment of human cystic echinococcosis14: 12 years of experience. Acta Trop. 1997, 64, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Mihmanli, M.; Idiz, U.O.; Kaya, C.; Demir, U.; Bostanci, O.; Omeroglu, S.; Bozkurt, E. Current status of diagnosis and treatment of hepatic echinococcosis. World J. Hepatol. 2016, 8, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Collado-Aliaga, J.; Romero-Alegría, Á.; Alonso-Sardón, M.; López-Bernus, A.; Galindo-Pérez, I.; Muro, A.; Velasco-Tirado, V.; Muñoz Bellido, J.L.; Belhassen-García, M.; Pardo-Lledias, J. Eosinophilia and cystic echinococcosis: What is the relationship? Trans. R. Soc. Trop. Med. Hyg. 2020, 114, 6–22. [Google Scholar] [CrossRef] [PubMed]
- Mirabile, E.; Solomon, N.; Fields, P.J.; Macpherson, C.N.L. Progress towards international adoption of the World Health Organization ultrasound classification of cystic echinococcosis. Acta Trop. 2019, 189, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Casulli, A. New global targets for NTDs in the WHO roadmap 2021–2030. PLoS Negl. Trop. Dis. 2021, 15, e0009373. [Google Scholar] [CrossRef]
- Mandal, S.; Mandal, M.D. Human cystic echinococcosis: Epidemiologic, zoonotic, clinical, diagnostic and therapeutic aspects. Asian Pac. J. Trop. Med. 2012, 5, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Obeid, M.; Mansour, S.; Damouny, M.; Farah, A.; Halloun, K.; Marjiyeh, R.; Ghalia, J.; Khuri, S. A conservative management of spontaneously ruptured liver hydatid cyst. Gastroenterol. Res. 2021, 14, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Zavoikin, V.D.; Zelya, O.P.; Tumolskaya, N.I. Clinical tolerance and efficacy of anti-parasitic treatment with albendazole in patients with alveolar echinococcosis: Long-term follow-up observation in 117 patients. Parasitol. Res. 2021, 120, 3603–3610. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, L.; Manciulli, T.; Cretu, C.M.; Giordani, M.T.; Angheben, A.; Bartoloni, A.; Zammarchi, L.; Bartalesi, F.; Richter, J.; Chiodini, P.; et al. Cystic echinococcosis of the bone: A European multicenter study. Am. J. Trop. Med. Hyg. 2019, 100, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, I.; Saíz, A.; Arrazola, J.; Ferreirós, J.; Pedrosa, C.S. Hydatid disease: Radiologic and pathologic features and complications. Radiographics 2000, 20, 795–817. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).