Abstract
Background: Coinfection with bacteria, fungi, and respiratory viruses has been described as a factor associated with more severe clinical outcomes in children with COVID-19. Such coinfections in children with COVID-19 have been reported to increase morbidity and mortality. Objectives: To identify the type and proportion of coinfections with SARS-CoV-2 and bacteria, fungi, and/or respiratory viruses, and investigate the severity of COVID-19 in children. Methods: For this systematic review and meta-analysis, we searched ProQuest, Medline, Embase, PubMed, CINAHL, Wiley online library, Scopus, and Nature through the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for studies on the incidence of COVID-19 in children with bacterial, fungal, and/or respiratory coinfections, published from 1 December 2019 to 1 October 2022, with English language restriction. Results: Of the 169 papers that were identified, 130 articles were included in the systematic review (57 cohort, 52 case report, and 21 case series studies) and 34 articles (23 cohort, eight case series, and three case report studies) were included in the meta-analysis. Of the 17,588 COVID-19 children who were tested for co-pathogens, bacterial, fungal, and/or respiratory viral coinfections were reported (n = 1633, 9.3%). The median patient age ranged from 1.4 months to 144 months across studies. There was an increased male predominance in pediatric COVID-19 patients diagnosed with bacterial, fungal, and/or viral coinfections in most of the studies (male gender: n = 204, 59.1% compared to female gender: n = 141, 40.9%). The majority of the cases belonged to White (Caucasian) (n = 441, 53.3%), Asian (n = 205, 24.8%), Indian (n = 71, 8.6%), and Black (n = 51, 6.2%) ethnicities. The overall pooled proportions of children with laboratory-confirmed COVID-19 who had bacterial, fungal, and respiratory viral coinfections were 4.73% (95% CI 3.86 to 5.60, n = 445, 34 studies, I2 85%, p < 0.01), 0.98% (95% CI 0.13 to 1.83, n = 17, six studies, I2 49%, p < 0.08), and 5.41% (95% CI 4.48 to 6.34, n = 441, 32 studies, I2 87%, p < 0.01), respectively. Children with COVID-19 in the ICU had higher coinfections compared to ICU and non-ICU patients, as follows: respiratory viral (6.61%, 95% CI 5.06–8.17, I2 = 0% versus 5.31%, 95% CI 4.31–6.30, I2 = 88%) and fungal (1.72%, 95% CI 0.45–2.99, I2 = 0% versus 0.62%, 95% CI 0.00–1.55, I2 = 54%); however, COVID-19 children admitted to the ICU had a lower bacterial coinfection compared to the COVID-19 children in the ICU and non-ICU group (3.02%, 95% CI 1.70–4.34, I2 = 0% versus 4.91%, 95% CI 3.97–5.84, I2 = 87%). The most common identified virus and bacterium in children with COVID-19 were RSV (n = 342, 31.4%) and Mycoplasma pneumonia (n = 120, 23.1%). Conclusion: Children with COVID-19 seem to have distinctly lower rates of bacterial, fungal, and/or respiratory viral coinfections than adults. RSV and Mycoplasma pneumonia were the most common identified virus and bacterium in children infected with SARS-CoV-2. Knowledge of bacterial, fungal, and/or respiratory viral confections has potential diagnostic and treatment implications in COVID-19 children.
Keywords:
bacterial; children; co-infection; coinfection; concurrent; COVID-19; fungal; meta-analysis; pediatric; SARS-CoV-2; viral; systematic review 1. Introduction
Although most cases of coronavirus disease 2019 (COVID-19) in pediatric populations are mild or asymptomatic [1], the clinical spectrum of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children ranges from asymptomatic to life-threatening [2,3]. Similar to adults, coinfection with bacteria, fungi, and respiratory viruses has been described as a factor associated with more severe clinical outcomes in children with COVID-19 [4,5,6,7,8,9,10,11]. Such coinfections have been reported to increase morbidity and mortality, therefore, knowledge of bacterial, fungal, and/or respiratory viral confections has potential diagnostic and treatment implications in children infected with SARS-CoV-2. Many studies have shown that COVID-19 children may develop severe diseases, requiring intensive care admission and/or mechanical ventilation because patients rapidly develop acute respiratory distress syndrome and sepsis, leading to death from multiple organ failure [12,13,14,15,16,17,18,19,20,21,22,23]. SARS-CoV-2 is hypothesized to weaken the bodies of children to bacterial, fungal, and/or respiratory viral coinfections [24], yet the mechanism of coinfection has not been fully established, but represents a threat to the respiratory epithelium favoring bacteremia, fungaemia, and/or viraemia (see Figure 1).
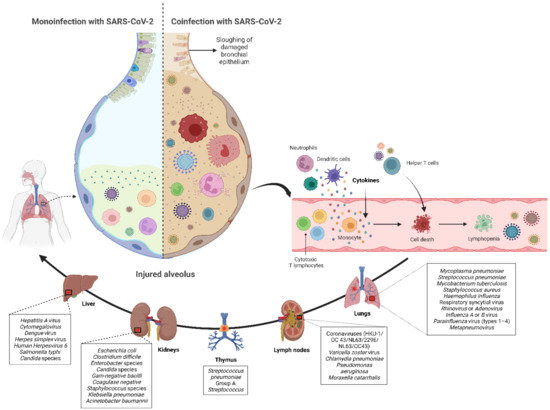
Figure 1.
Monoinfection with SARS-CoV-2 results in less severe form of COVID-19 and better prognosis. In contrast, SARS-CoV-2 coinfection with bacteria, fungi, and/or respiratory viruses may intensify the severity of COVID-19 and increase the expression of macrophages, T and B defensive cells that may cause the elevation of inflammatory cytokines such as tumor necrosis factor-alpha, interleukin-1, and interleukin-6 in the infected organs, leading to a hyperinflammatory response by recruiting immune cells.
There is a lack of systematic reviews and meta-analyses on the type and frequency of coinfection by bacterial, fungal, and/or respiratory viral infections and associated clinical outcomes among COVID-19 children. We aimed to identify the type and proportion of coinfections with SARS-CoV-2 and bacteria, fungi, and/or respiratory viruses, and investigate the severity of COVID-19 in these patients.
2. Methods
2.1. Design
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA) in conducting this systematic review and meta-analysis [25]. The following electronic databases were searched: PROQUEST, MEDLINE, EMBASE, PUBMED, CINAHL, WILEY ONLINE LIBRARY, SCOPUS, and NATURE with Full Text. We used the following keywords: (“COVID-19” OR “SARS-CoV-2” OR “Severe acute Respiratory Syndrome Coronavirus 2” OR “Coronavirus Disease 2019” OR “2019 novel coronavirus”) AND (“children” OR “child” OR “paediatric” OR “pediatric” OR “infant” OR “toddler” OR “adolescent” OR “newborn”) AND (“coinfection” OR “co-infection” OR “cocirculation” OR “co-circulation” OR “coinfected” OR “co-infected” OR “co-circulated” OR “mixed” OR “concurrent” OR “concomitant”). The search was limited to papers published in English between 1 December 2019 and 1 October 2022. Based on the title and abstract of each selected article, we selected those discussing and reporting the occurrence of bacterial, fungal, and/or respiratory viral coinfection in children with COVID-19.
2.2. Inclusion–Exclusion Criteria
Inclusion criteria were as follows: (1) published case reports, case series, and cohort studies that focused on children infected with SARS-CoV-2 and bacteria, fungi, and/or respiratory viruses; (2) studies of experimental or observational design reporting the incidence of SARS-CoV-2 infection in pediatric patients with other co-pathogens; (3) language restricted to English. The exclusion criteria were as follows: (1) editorials, commentaries, case and animal studies, reviews, and meta-analyses; (2) studies that did not report data on COVID-19 in coinfected patients; (3) studies that never reported details on identified coinfected cases with SARS-CoV-2 infection; (4) studies that reported coinfection in adult COVID-19 patients; (5) studies that reported coinfection in patients with negative SARS-CoV-2 polymerase chain reaction (PCR) tests; (6) duplicate publications.
2.3. Data Extraction
Six authors (Saad Alhumaid, Muneera Alabdulqader, Nourah Al Dossary, Zainab Al Alawi, Abdulrahman A. Alnaim, and Koblan M. Al mutared) critically reviewed all of the studies retrieved and selected those judged to be the most relevant. Data were carefully extracted from the relevant research studies independently. Articles were categorized as case report, case series, or cohort studies. The following data were extracted from selected studies: authors; publication year; study location; study design and setting; number of SARS-CoV-2 children tested for co-pathogens; number of coinfected children; age; proportion of male children; patient ethnicity; number of children with bacterial, fungal, and/or respiratory viral coinfections; total organisms identified; antimicrobials prescribed; laboratory techniques for co-pathogen detection; number of children admitted to intensive care unit (ICU), placed on mechanical ventilation, and/or suffered acute respiratory distress syndrome (ARDS); assessment of study risk of bias; and final treatment outcome (survived or died). These data are noted in Table 1.
2.4. Quality Assessment
For many selected cohort studies, the Newcastle–Ottawa scale (NOS) was used to assess the risk of bias, a tool which measures quality in the three parameters of selection, comparability, and exposure/outcome, and allocates a maximum of 4, 2, and 3 points, respectively [26]. High-quality studies are scored greater than 7 on this scale, and moderate-quality studies between 5 and 7 [26]. Otherwise, quality assessment of the selected case report and case series studies was undertaken based on the modified NOS [27]. Items related to the comparability and adjustment were removed from the NOS, and items which focused on selection and representativeness of cases, and the ascertainment of outcomes and exposure, were kept [27]. Modified NOS consists of five items, each of which requires a yes or no response to indicate whether bias is likely, and these items were applied to single-arm studies [27]. Quality of the study was considered good if all five criteria were met, moderate when four were met, and poor when three or less were met. Quality assessment was performed by six authors (Khalid Al Noaim, Mohammed A. Al Ghamdi, Suha Jafar Albahrani, Abdulaziz A. Alahmari, Sarah Mahmoud Al HajjiMohammed, and Yameen Ali Almatawah) independently, with any disagreement to be resolved by consensus.
2.5. Data Analysis
The proportion of confirmed COVID-19 children with bacterial, fungal, and/or respiratory viral coinfection were examined. This proportion was further classified based on initial presentation or during the course of the illness. A random effects DerSimonian–Laird model was used, which produces wider confidence intervals (Cis) than a fixed effect model [28]. Results are illustrated using a forest plot. The Cochran’s chi-square (χ2) and the I2 statistic provided the tools for examining statistical heterogeneity [29]. An I2 value of >50% suggested significant heterogeneity [30]. To lower the source of heterogeneity, we conducted a subgroup analysis based on children’s admission to the ICU. To estimate publication bias, funnel plots and Egger’s correlation were used, and a p-value < 0.05 was considered to indicate statistical significance. All p-values were based on two-sided tests and significance was set at a p-value less than 0.05. R version 4.1.0 with the packages finalfit and forestplot was used for all statistical analyses. Figure 1 was created with BioRender.com (agreement no. NX24IV1VNB) (accessed on 14 October 2022).
3. Results
3.1. Study Characteristics and Quality
A total of 130 publications were identified (Figure 2). After scanning titles and abstracts, 67 duplicate articles were discarded. Another 33 irrelevant articles were excluded based on the titles and abstracts. The full texts of the 378 remaining articles were reviewed, and 248 irrelevant articles were excluded. As a result, we identified 130 studies that met our inclusion criteria and reported SARS-CoV-2 infection in pediatric patients with bacterial, fungal, and viral coinfection [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140]. The detailed characteristics of the included studies are shown in Table 1. Among these, two articles were preprint versions [64,89]. There were 57 cohort [4,5,6,7,8,9,10,11,12,17,31,32,34,35,36,37,39,41,42,44,49,53,58,66,69,71,73,78,79,80,81,82,83,84,89,90,95,98,99,102,105,108,109,115,116,118,119,123,125,127,129,131,133,134,137,138,139], 52 case report [13,14,15,16,18,19,21,22,23,33,38,40,43,45,46,48,50,51,52,55,56,59,64,65,67,68,70,72,74,75,76,77,86,87,91,93,94,97,104,106,107,110,111,113,114,121,122,124,126,128,130,140], and 21 case series [20,47,54,57,60,61,62,63,85,88,92,96,100,101,103,112,117,120,132,135,136] studies. These studies were conducted in the United States (n = 23), China (n = 21), India (n = 9), Italy (n = 7), Iran (n = 7), France (n = 6), Turkey (n = 6), Spain (n = 5), Mexico (n = 4), Brazil (n = 4), Indonesia (n = 4), South Africa (n = 3), Switzerland (n = 3), Poland (n = 3), United Kingdom (n = 3), Argentina (n = 2), Saudi Arabia (n = 2), United Arab Emirates (n = 1), Portugal (n = 1), Malaysia (n = 1), Thailand (n = 1), Bulgaria (n = 1), The Netherlands (n = 1), Germany (n = 1), Lebanon (n = 1), Botswana (n = 1), Denmark (n = 1), Russia (n = 1), Pakistan (n = 1), Bangladesh (n = 1), Japan (n = 1), Greece (n = 1), and Canada (n = 1). Only four studies were conducted within multiple countries (n = 4) [53,109,118,127]. The majority of the studies were single-center [4,6,11,12,13,14,15,16,18,19,21,22,23,32,33,34,35,38,39,40,41,43,44,45,46,48,49,50,51,52,55,56,57,59,60,61,62,64,65,66,67,68,69,70,72,73,74,75,76,77,78,79,80,81,82,83,85,86,87,88,89,90,91,92,93,94,97,98,99,100,101,102,103,104,105,106,107,111,112,113,114,115,116,117,120,121,122,124,126,128,130,132,133,134,138,139,140] and only 33 studies were multicenter [5,7,8,9,10,17,20,31,36,37,42,47,53,54,58,63,71,84,95,96,108,109,110,118,119,123,125,127,129,131,135,136,137]. In some studies, concurrent infection of SARS-CoV-2 with other bacterial, fungal, and/or viral pathogens was investigated in pediatric and adult patients as the population of interest (19/130, 14.6%) [10,17,31,37,47,54,57,62,71,79,82,90,96,102,108,112,118,123,139]. The majority (n = 128) of the studies included any hospitalized patient, except for two studies that investigated potential of SARS-CoV-2 transmission in a cluster and genomic analysis of SARS-CoV-2 in a family [47,62], and two studies included only critically ill COVID-19 patients [9,18]. Eleven, four, and one studies exclusively reported on respiratory viral [10,18,31,35,61,71,73,89,101,125,140], bacterial [11,96,100,112], and fungal [90] coinfections, respectively; the remaining 114 studies reported on bacterial, fungal, and respiratory viral coinfections [4,5,6,7,8,9,12,13,14,15,16,17,19,20,21,22,23,32,33,34,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,62,63,64,65,66,67,68,69,70,72,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,91,92,93,94,95,97,98,99,102,103,104,105,106,107,108,109,110,111,113,114,115,116,117,118,119,120,121,122,123,124,126,127,128,129,130,131,132,133,134,135,136,137,138,139]. Few studies investigated the existence of COVID-19 with influenza virus type A and B only [31,101,140], Mycobacterium tuberculosis only [11,96,112], respiratory syncytial virus (RSV) only [35,61], Rhinovirus only [10,125], pneumovirus only [18], herpes simplex virus only [71], human coronavirus OC43 only [73], adenovirus only [89], Mycoplasma pneumonia only [100], and Candida species only [90]. Laboratory techniques for co-pathogen detection within studies included 52 that used real-time reverse transcription–polymerase chain reaction (RT-PCR) tests for multiple respiratory viruses [9,10,17,18,31,34,37,41,43,44,46,47,48,49,61,65,66,68,69,71,73,76,79,80,81,82,83,89,91,92,95,99,101,102,110,113,115,116,117,119,123,125,127,129,130,131,132,135,136,137,138,139], 23 that used antibody tests (immunoglobulins M and/or G) [5,8,22,23,45,52,54,56,70,72,77,78,81,85,98,100,104,108,111,120,122,133,140], 42 that used cultures (blood, urine, cerebrospinal fluid, tracheal, nasal discharge, pharyngeal swabs, wound, respiratory secretions, bronchoalveolar lavage, alveolar fluid, sputum, and pleural fluid) [5,6,11,12,13,15,16,20,21,23,38,40,42,50,51,53,55,60,63,64,74,75,84,85,87,88,90,93,96,97,105,106,107,109,112,114,118,121,124,126,128,134], 29 that used two or more laboratory methods (RT-PCR, antibody tests, and/or culture) [4,5,6,7,8,12,20,23,36,39,42,50,52,53,57,58,63,72,77,78,81,84,85,98,105,109,124,126,133], and two that did not specify their testing method [32,33]. Among the 130 included studies, 57 cohort studies were assessed using the NOS: 52 studies were found to be moderate-quality studies (i.e., NOS scores were between 5 and 7) and five studies demonstrated a relatively high quality (i.e., NOS scores > 7). All case reports and case series studies were assessed for bias using the modified NOS. Forty-nine studies were deemed to have high methodological quality, and three exhibited moderate methodological quality; Table 1.
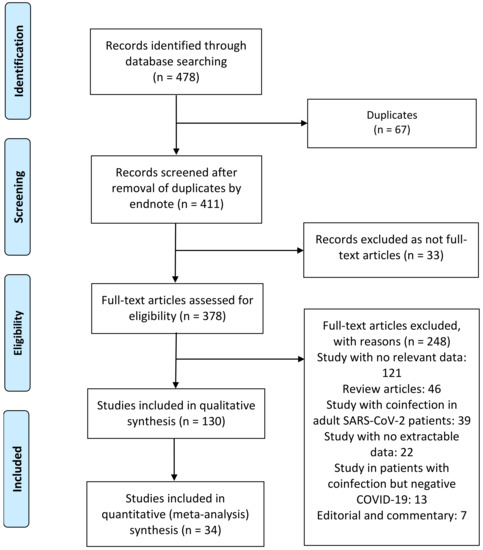
Figure 2.
Flow diagram of literature search and data extraction from studies included in the systematic review and meta-analysis.

Table 1.
Summary of the characteristics of the included studies with evidence on SARS-CoV-2 and bacterial, fungal, and/or respiratory viral coinfections in children (n = 130), 2020–2022.
Table 1.
Summary of the characteristics of the included studies with evidence on SARS-CoV-2 and bacterial, fungal, and/or respiratory viral coinfections in children (n = 130), 2020–2022.
| Author, Year, Study Location | Study Design, Setting | Number of SARS-CoV-2 Patients Tested for Co-Pathogens, n | Coinfected Patients, n | Age (Months) a | Male, n (%) AND Ethnicity, n b | Bacterial Coinfection, n | Fungal Coinfection, n | Respiratory Viral Coinfection, n | Total Organisms, n | Antimicrobials Used, n | Laboratory Techniques for Co-Pathogen Detection | Admitted to ICU, n | Mechanical Ventilation, n | ARDS, n | Assessment of Study Risk of Bias (Tool Used, Finding) and Treatment Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aggarwal et al. 2022 [31], India | Retrospective cohort, multicenter | 770 | 4 | 12, 18, 96, and 72 | 3 (75) AND 4 Indian | 0 | 0 | 6 | 3 Influenza A virus 3 Influenza B virus | 0 | RT-PCR for respiratory specimens (viruses) c | 0 | 0 | 0 | (NOS, 7) 4 survived |
| Al Mansoori et al. 2021 [32], United Arab Emirates | Retrospective cohort, single-center | 17 | 7 | Median (IQR), 84 (0–192) | Gender (not reported) AND Ethnicity (not reported) | 2 | 0 | 5 | 3 Rhinovirus 2 Group A Streptococcus 1 Enterovirus 1 Adenovirus | 7 Not reported | RT-PCR for respiratory specimens (viruses) c Not reported (Group A Streptococcus) | 0 | 0 | 0 | (NOS, 6) Treatment outcome (not reported) |
| Allen-Manzur et al. 2020 [33], Mexico | Retrospective case report, single-center | 1 | 1 | 6 | 0 (0) AND 1 Hispanic | 1 | 0 | 0 | 1 Mycobacterium bovis | 1 Not reported | RT-PCR for respiratory specimens (viruses) c Not reported (Mycobacterium bovis) | 0 | 0 | 0 | (Modified NOS, moderate) 1 survived |
| Alrayes et al. 2022 [34], United States | Retrospective cohort, single-center | 13 | 13 | Age group 0–2: 270 (71.3%) patients (RSV coinfection) | Gender (not reported) AND Ethnicity (not reported) | 0 | 0 | 15 | 13 RSV 1 Rhinovirus 1 Adenovirus | 13 Not reported | RT-PCR for respiratory specimens (viruses) c | 0 | 0 | 0 | (NOS, 7) 13 survived |
| Alvares 2021 [35], Brazil | Retrospective cohort, single-center | 32 | 6 | Median (IQR), 6 | 2 (33.3) AND 6 Hispanic | 0 | 0 | 6 | 6 RSV | 1 Not reported | Chemiluminescence for RSV | 1 | 1 | 1 Not reported | (NOS, 6) 6 survived |
| Anderson et al. 2021 [4], United States | Retrospective cohort, single-center | 29 | 10 | Age group 168 (42–198): 10 (34.4%) patients Age group 192 (168–204): 9 (31%) patients Age group 102 (72–168): 10 (34.4%) patients | Gender (not reported) AND Ethnicity (not reported) | 5 | 0 | 6 | 2 Staphylococcus aureus 2 Escherichia coli 1 Salmonella enteritis 1 Enterovirus 1 Adenovirus 2 Rhinovirus 1 Parainfluenza virus 1 EBV | 10 Not reported | RT-PCR for respiratory specimens (viruses) c PCR assays (bacteria) | 7 | 2 | 3 | (NOS, 8) 7 survived 3 died |
| Andina-Martinez et al. 2022 [36], Spain | Prospective cohort, multicenter | 9 | 2 | 1.3 and 1.8 | 1 (50) AND 2 White (Caucasian) | 1 | 0 | 1 | 1 Bordetella pertussis 1 Metapneumovirus | 2 Azithromycin | RT-PCR for respiratory specimens (viruses) c PCR assays (Mycoplasma pneumoniae, Chlamydia pneumoniae and Bordetella pertussis) | 1 | 1 | 2 Not reported | (NOS, 7) 2 survived |
| Aragón-Nogales et al. 2022 [12], Mexico | Prospective cohort, single-center | 181 | 2 | 12 and 24 | 0 (0) AND 2 Hispanic | 1 | 0 | 1 | 1 Pseudomonas aeruginosa 1 EBV | 1 Cefotaxime 1 Ceftriaxone | RT-PCR for respiratory specimens (viruses) c Blood culture (bacteria) | 2 | 2 | 2 | (NOS, 7) 2 died |
| Arguni et al. 2022 [37], Indonesia | Retrospective cohort, multicenter | 125 | 59 | Two patients: <12 months to <60 months Six patients: <60 months to <216 months | Gender (not reported) AND 8 Asian | 0 | 0 | 59 | 32 Influenza A virus 10 Adenovirus 16 Influenza B virus 1 Metapneumovirus | 59 Not reported | RT-PCR for respiratory specimens (viruses) c | 59 Not reported | 59 Not reported | 59 Not reported | (NOS, 6) Treatment outcome (not reported) |
| Arslan et al. 2021 [38], Turkey | Retrospective case report, single-center | 1 | 1 | 10 | 1 (100) AND 1 White (Caucasian) | 1 | 0 | 0 | 1 MSSA | 1 Clindamycin 1 Ceftriaxone | Blood culture (bacteria) | 0 | 0 | 0 | (Modified NOS, high) 1 survived |
| Aykac et al. 2021 [39], Turkey | Retrospective cohort, single-center | 115 | 37 | Median (IQR), 48 (12–132) | Gender (not reported) AND 37 White (Caucasian) | 37 | 0 | 4 | 37 Streptococcus pneumoniae 2 Bocavirus 1 Rhinovirus 1 Parechovirus | 7 Ceftriaxone 7 Azithromycin 7 Ampicillin/sulbactam | RT-PCR for respiratory specimens (viruses) c PCR assays (Streptococcus pneumoniae) | 1 | 1 | 1 | (NOS, 6) Treatment outcome (not reported) |
| Ayoubzadeh et al. 2021 [40], Canada | Retrospective case report, single-center | 1 | 1 | 168 | 1 (100) AND 1 Pakistani | 1 | 0 | 0 | 1 Gram-negative bacilli 1 Salmonella Typhi | 1 Meropenem 1 Ampicillin 1 Amoxicillin | Blood culture (bacteria) | 0 | 0 | 0 | (Modified NOS, high) 1 survived |
| Berksoy et al. 2021 [41], Turkey | Retrospective cohort, single-center | 128 | 21 | 1 patient: 5 Other patients: not reported | Gender (not reported) AND 21 White (Caucasian) | 0 | 0 | 23 | 9 Rhinovirus 5 Metapneumovirus 4 RSV 3 Adenovirus 2 Bocavirus | 21 Not reported | RT-PCR for respiratory specimens (viruses) c | 21 Not reported | 0 | 21 Not reported | (NOS, 6) Treatment outcome (not reported) |
| Blázquez-Gamero et al. 2021 [42], Spain | Retrospective cohort, multicenter | 27 | 2 | 1 and 3 | Gender (not reported) AND 27 White (Caucasian) | 3 | 0 | 0 | 1 Streptococcus mitis 1 Escherichia coli 1 Enterobacter cloacae | 2 Ampicillin 1 Gentamycin 1 3rd -generation cephalosporin | RT-PCR for respiratory specimens (viruses) c Blood culture (bacteria) Urine culture (bacteria) | 1 | 1 | 1 | (NOS, 7) 2 survived |
| Borocco et al. 2021 [43], France | Retrospective case report, single-center | 1 | 1 | 156 | 0 (0) AND 1 Arab | 0 | 0 | 1 | 1 EBV | 0 | RT-PCR for respiratory specimens (viruses) c | 0 | 0 | 0 | (Modified NOS, high) 1 survived |
| Brothers et al. 2021 [13], United States | Retrospective case report, single-center | 1 | 1 | 144 | 0 (0) AND 1 White (Caucasian) | 1 | 1 | 0 | 1 MSSA 1 Candida glabrata | 1 Clindamycin 1 Vancomycin 1 Cefepime 1 Fluconazole 1 Micafungin | Tracheal culture (bacteria) Urine culture (urine) | 1 | 1 | 1 | (Modified NOS, high) 1 died |
| Cason et al. 2022 [44], Italy | Retrospective cohort, single-center | 64 | 17 | Age group <24 was the most frequent) | Gender (not reported) AND 17 White (Caucasian) | 0 | 0 | 19 | 1 Other coronaviruses (229E, NL63, and OC43) 12 Rhinovirus 4 Bocavirus 2 Adenovirus | 17 Not reported | RT-PCR for respiratory specimens (viruses) c | 17 Not reported | 17 Not reported | 17 Not reported | (NOS, 6) Treatment outcome (not reported) |
| Chacón-Cruz et al. 2022 [14], Mexico | Retrospective case report, single-center | 1 | 1 | 84 | 1 (100) AND 1 Hispanic | 1 | 0 | 0 | 1 Neisseria meningitidis | 1 Amoxicillin 1 Ceftriaxone 1 Doxycycline | PCR assays (Neisseria meningitidis) | 1 Not reported | 1 Not reported | 1 Not reported | (Modified NOS, high) 1 died |
| Chen et al. 2020 [45], China | Retrospective case report, single-center | 1 | 1 | 144 | 1 (100) AND 1 Asian | 2 | 0 | 0 | 1 Mycoplasma pneumonia 1 Chlamydia pneumoniae | 1 Mezlocillin 1 Ceftizoxime 1 Amoxicillin/clavulanic acid | Serum antibody tests (IgM, IgG) | 0 | 0 | 1 | (Modified NOS, high) 1 survived |
| Choudhary et al. 2022 [5], United States | Retrospective cohort, multicenter | 947 | 235 | Age group <60: 101 (33.9%) patients (viral coinfection) Age group <60: 50 (16.8%) patients (bacterial coinfection) | Gender (not reported) AND Ethnicity (not reported) | 123 | 7 | 113 | 75 RSV 113 Viral 123 Bacterial 7 Fungal | 123 Antibiotics | RT-PCR for respiratory specimens (viruses) c Blood culture (bacteria) Serum antibody tests (IgM, IgG) | 33 | 14 | 235 Not reported | (NOS, 8) 233 survived 2 died |
| Ciuca et al. 2021 [46], Italy | Retrospective case report, single-center | 1 | 1 | 72 | 1 (100) AND 1 Black | 0 | 0 | 1 | 1 Parvovirus B19 | 1 Antibiotics | PCR assays (Parvovirus B19) | 1 | 1 | 1 | (Modified NOS, high) 1 survived |
| Danis et al. 2020 [47], France | Retrospective case series, multicenter | 12 | 1 | 108 | 1 (100) AND 1 White (Caucasian) | 0 | 0 | 2 | 1 Influenza A virus 1 Rhinovirus | 0 | RT-PCR for respiratory specimens (viruses) c | 0 | 0 | 0 | (NOS, 7) 1 survived |
| Danley and Kent 2020 [48], United States | Retrospective case report, single-center | 1 | 1 | 4 | 1 (100) AND 1 White (Caucasian) | 0 | 0 | 1 | 1 Adenovirus | 0 | RT-PCR for respiratory specimens (viruses) c | 0 | 0 | 1 | (Modified NOS, high) 1 survived |
| DeBiasi et al. 2020 [49], United States | Retrospective cohort, single-center | 63 | 4 | Median, 115.2 | Gender (not reported) AND Ethnicity (not reported) | 0 | 0 | 5 | 2 Rhinovirus 2 RSV 1 Other coronaviruses (229E, NL63, and OC43) | 4 Not reported | RT-PCR for respiratory specimens (viruses) c | 4 Not reported | 4 Not reported | 4 Not reported | (NOS, 6) Treatment outcome (not reported) |
| Demirkan and Yavuz 2021 [50], Turkey | Retrospective case reports, single-center | 2 | 2 | 84 and 156 | 0 (0) AND 2 White (Caucasian) | 0 | 2 | 0 | 2 Fungal bezoars | 1 Meropenem 2 Fluconazole | RT-PCR for respiratory specimens (viruses) c Blood culture (bacteria) | 0 | 0 | 0 | (Modified NOS, high) 2 survived |
| Dhanawade et al. 2021 [51], India | Retrospective case report, single-center | 1 | 1 | 48 | 0 (0) AND 1 Indian | 1 | 0 | 0 | 1 Mycobacterium tuberculosis | 1 Ceftriaxone 1 Antibiotics 1 Isoniazid 1 Rifampicin 1 Pyrazinamide 1 Ethionamide | CSF culture (bacteria) | 1 | 1 | 1 | (Modified NOS, high) 1 survived |
| Di Nora et al. 2022 [52], Italy | Retrospective case report, single-center | 1 | 1 | 24 | 1 (100) AND 1 White (Caucasian) | 0 | 0 | 1 | 1 Human Herpesvirus 6 | 1 Acyclovir 1 Ceftriaxone | CSF PCR assays (viruses) Serum antibody test (IgM) | 0 | 0 | 0 | (Modified NOS, high) 1 survived |
| Dikranian et al. 2022 [53], Multi-country | Retrospective cohort, multicenter | 922 | 31 | Age group ≤6: 136/820 (16.6%) Age group >120 to 180: 182/820 (22.2%) Age group >180 to 216: 189/820 (23%) | Gender (not reported) AND Ethnicity (not reported) | 0 | 0 | 30 | 10 Rhinovirus 5 RSV 2 Adenovirus 1 Coronavirus NL63 1 Parainfluenza-2 1 Parainfluenza-3 1 Parainfluenza-4 1 Metapneumovirus 8 Unspecified viruses | 31 Not reported | RT-PCR for respiratory specimens (viruses) c Blood culture (bacteria) Sputum (bacteria) | 22 | 31 Not reported | 31 Not reported | (NOS, 6) Treatment outcome (not reported) |
| Diorio et al. 2020 [6], United States | Prospective cohort, single-center | 24 | 7 | Median (IQR), 60 (30–192) | 5 (71.4) AND 3 White (Caucasian) 1 Hispanic 3 Black | 4 | 0 | 5 | 1 Parainfluenza 3 1 Parainfluenza 4 2 Escherichia coli 1 Enterovirus 1 Adenovirus 1 Rhinovirus 1 MRSA 1 Salmonella typhi | 7 Not reported | RT-PCR for respiratory specimens (viruses) c Blood culture (bacteria) Urine (culture) | 1 | 1 | 1 | (NOS, 8) 6 survived 1 died |
| Dong et al. 2020 [54], China | Retrospective case series, multicenter | 11 | 1 | 28 | 1 (100) AND 1 Asian | 0 | 0 | 1 | 1 Cytomegalovirus | 0 | Serum antibody test (IgM) | 0 | 0 | 0 | (Modified NOS, high) 1 survived |
| Essajee et al. 2020 [55], South Africa | Retrospective case report, single-center | 1 | 1 | 31 | 0 (0) AND 1 Black | 1 | 0 | 0 | 1 Mycobacterium tuberculosis | 1 Antibiotics 1 Isoniazid 1 Rifampicin 1 Pyrazinamide 1 Ethionamide | Blood culture (bacteria) | 0 | 0 | 1 | (Modified NOS, high) 1 survived |
| Ferdous et al. 2021 [56], Bangladesh | Retrospective case report, single-center | 1 | 1 | 96 | 0 (0) AND 1 Bangladeshi | 0 | 0 | 1 | 1 Dengue virus | 1 Antibiotics | Dengue NS1 antigen | 1 | 1 | 1 | (Modified NOS, high) 1 survived |
| Freij et al. 2020 [15], United States | Retrospective case report, single-center | 1 | 1 | 60 | 0 (0) AND 1 Black | 2 | 0 | 0 | 1 Mycobacterium tuberculosis 1 Group A Streptococcus | 1 Amoxicillin 1 Azithromycin | CSF culture (bacteria) | 1 | 1 | 0 | (Modified NOS, high) 1 died |
| Frost et al. 2022 [57], United States | Retrospective case series, single-center | 7 | 6 | Median (IQR), 16 (7–30) | 5 (83.3) AND 5 Hispanic | 14 | 0 | 5 | 1 Adenovirus 1 Metapneumovirus 2 Rhinovirus 1 Enterovirus 4 Streptococcus pneumoniae 5 Haemophilus influenza 3 Moraxella catarrhalis 2 Staphylococcus aureus | 7 Not reported | RT-PCR for respiratory specimens (viruses) c PCR assays (bacteria) | 0 | 0 | 0 | (Modified NOS, high) 6 survived |
| Garazzino et al. 2021 [7], Italy | Retrospective cohort, multicenter | 515 | 69 | Median (IQR), 87 (17–149) | Gender (not reported) AND 69 White (Caucasian) | 32 | 0 | 45 | 45 Unspecified viruses 32 Unspecified bacteria | 69 Not reported | RT-PCR for respiratory specimens (viruses) c PCR assays (bacteria) | 3 | 3 | 2 | (NOS, 7) 67 survived 2 died |
| Garazzino et al. 2020 [58], Italy | Retrospective cohort, multicenter | 168 | 10 | Median (IQR), 28 (4–115) | Gender (not reported) AND 10 White (Caucasian) | 1 | 0 | 10 | 3 RSV 3 Rhinovirus 2 EBV 1 Influenza A virus 1 Other coronaviruses (229E, NL63, and OC43) 1 Streptococcus pneumoniae | 10 Not reported | RT-PCR for respiratory specimens (viruses) c PCR assays (bacteria) | 2 | 2 | 2 | (NOS, 6) Treatment outcome (not reported) |
| Goussard et al. 2020 [59], South Africa | Retrospective case report, single-center | 1 | 1 | 29 | 1 (100)AND 1 Black | 1 | 0 | 0 | 1 Rifampicin-sensitive Mycobacterium tuberculosis | 1 Antibiotics 1 Isoniazid 1 Rifampicin 1 Pyrazinamide 1 Ethionamide 1 Amoxicillin/clavulanic acid | PCR assay for gastric aspirate (Mycobacterium tuberculosis) | 0 | 0 | 0 | (Modified NOS, high) 1 survived |
| Guy et al. 2022 [60], United States | Retrospective case series, single-center | 6 | 6 | Median (IQR), 144 (42–168) | 5 (83.3) AND 5 Black 1 White (Caucasian) | 5 | 0 | 0 | 1 Streptococcus intermedius 2 Prevotella species 2 Streptococcus constellatus | 4 Ceftriaxone 3 Clindamycin 2 Amoxicillin/clavulanic acid 1 Penicillin 2 Metronidazole 1 Ampicillin/sulbactam 2 Vancomycin 1 Cefdinir | Nasal discharge (culture) | 0 | 0 | 0 | (Modified NOS, high) 6 survived |
| Halabi et al. 2022 [61], United States | Retrospective and prospective case series, single-center | 18 | 18 | Median (IQR), 6 (2–36) | 11 (61.1) AND Ethnicity (not reported) | 0 | 0 | 22 | 18 RSV 3 Rhinovirus 1 Parainfluenza virus | 18 Not reported | RT-PCR for respiratory specimens (viruses) c | 9 | 2 | 2 | (Modified NOS, high) Treatment outcome (not reported) |
| Hamzavi et al. 2020 [16], Iran | Retrospective case report, single-center | 1 | 1 | 168 | 1 (100) AND 1 Persian | 1 | 0 | 0 | 1 Staphylococcus aureus | 1 Vancomycin 1 Meropenem | Blood (culture) | 1 | 1 | 1 | (Modified NOS, high) 1 died |
| Hare et al. 2021 [62], United Kingdom | Retrospective case series, single-center | 7 | 1 | 22 | 0 (0) AND 1 White (Caucasian) | 0 | 0 | 1 | 1 Rhinovirus | 0 | RT-PCR for respiratory specimens (viruses) c | 0 | 0 | 0 | (Modified NOS, high) 1 survived |
| Hashemi et al. 2021 (17], Iran | Retrospective cohort, multicenter | 105 | 5 | Age group 0 to 168: 5 (4.8%) patients (viral coinfection) | 4 (80) AND 5 Persian | 0 | 0 | 5 | 3 Metapneumovirus 1 Bocavirus 1 Influenza A virus | 5 Not reported | RT-PCR for respiratory specimens (viruses) c | 5 | 5 | 5 | (NOS, 7) 5 died |
| Hashemi et al. 2021 [18], Iran | Retrospective case reports, single-center | 3 | 3 | 13, 72, and 72 | 2 (66.6) AND 3 Persian | 0 | 0 | 3 | 3 Metapneumovirus | 3 Not reported | RT-PCR for respiratory specimens (viruses) c | 3 | 3 | 3 | (Modified NOS, high) 3 died |
| Hassoun et al. 2021 [63], United States | Retrospective case series, multicenter | 8 | 6 | Median (IQR), 1.4 (0.5–1.6) | 5 (83.3) AND 2 Black 2 White (Caucasian) 1 Hispanic 1 Indian | 1 | 0 | 6 | 5 RSV 1 Rhinovirus 1 Escherichia coli | 6 Antibiotics | RT-PCR for respiratory specimens (viruses) c Urine (culture) | 0 | 0 | 0 | (Modified NOS, high) 6 survived |
| He et al. 2020 [8], China | Retrospective cohort, multicenter | 15 | 4 | Median (IQR), 72 (36–84) | 3 (75) AND 4 Asian | 2 | 2 | 0 | 2 Unspecified bacteria 2 Unspecified fungi | 4 Antibiotics | RT-PCR for respiratory specimens (viruses) c Sputum (bacteria) G assay and GM assay (fungi) Serum antibody test (IgM) | 2 | 2 | 2 | (NOS, 7) 2 survived 2 died |
| Hertzberg et al. 2020 [64], United States | Retrospective case reports, single-center | 3 | 3 | 2, 24 and 60 | 2 (66.7) AND Ethnicity (not reported) | 1 | 0 | 2 | 2 Rhinovirus 1 Bordetella pertussis | 1 Azithromycin | RT-PCR for respiratory specimens (viruses) c Blood (culture) | 1 | 0 | 0 | (Modified NOS, moderate) 3 survived |
| Jarmoliński et al. 2021 [65], Poland | Retrospective case report, single-center | 1 | 1 | 108 | 0 (0) AND 1 White (Caucasian) | 0 | 0 | 2 | 1 Metapneumovirus 1 RSV | 1 Piperacillin/tazobactam 1 Amikacin 1 Azithromycin 1 Cefepime 1 Micafungin 1 Acyclovir | RT-PCR for respiratory specimens (viruses) c | 0 | 0 | 0 | (Modified NOS, high) 1 survived |
| Jiang et al. 2020 [66], China | Retrospective cohort, single-center | 161 | 2 | 80 and 42 | 0 (0) AND 2 Asian | 1 | 0 | 3 | 1 RSV 2 Metapneumovirus 1 Mycoplasma pneumonia | 2 Antibiotics | RT-PCR for respiratory specimens (viruses) c | 1 | 0 | 1 | (NOS, 7) 2 survived |
| Jose et al. 2021 [67], Mexico | Retrospective case report, single-center | 1 | 1 | 84 | 1 (100) AND 1 Hispanic | 0 | 0 | 1 | 1 Dengue virus | 1 Amoxicillin 1 Trimethoprim/sulfamethoxazole 1 Clindamycin 1 3rd -generation cephalosporin 1 Ceftriaxone 1 Acyclovir | RT-PCR for respiratory specimens (viruses) c DENV RTqPCR (dengue) | 1 | 1 | 1 | (Modified NOS, high) 1 survived |
| Kakuya et al. 2020 [68], Japan | Retrospective case report, single-center | 3 | 2 | 132 and 60 | 2 (100) AND 2 Asian | 0 | 0 | 2 | 1 Influenza A virus 1 Metapneumovirus | 1 Ceftriaxone | RT-PCR for respiratory specimens (viruses) c | 0 | 0 | 0 | (Modified NOS, high) 2 survived |
| Kanthimathinathan et al. 2021 [9], United Kingdom | Retrospective cohort, multicenter | 73 | 17 | Median (IQR), 120 (12–156) | Gender (not reported) AND 6 White (Caucasian) 5 Asian 4 Black | 6 | 4 | 14 | 3 Pseudomonas aeruginosa 2 Klebsiella pneumoniae 1 Acinetobacter baumannii 2 Adenovirus 2 Influenza 2 Parainfluenza 2 Rhinovirus 1 Metapneumovirus 1 RSV 4 Cytomegalovirus 4 Unspecified fungi | 3 Amoxicillin/clavulanic acid 1 Azithromycin 2 Clarithromycin 1 Piperacillin/tazobactam 1 Gentamicin | RT-PCR for respiratory specimens (viruses) c | 17 | 7 | 10 | (NOS, 8) 16 survived 1 died |
| Karaaslan et al. 2021 [69], Turkey | Retrospective cohort, single-center | 93 | 7 | Mean ± SD, 10.99 ± 6.44 | 5 (71.4) AND 7 White (Caucasian) | 1 | 0 | 7 | 2 Rhinovirus 2 Coronavirus NL63 1 Adenovirus 1 Mycoplasma pneumoniae 1 Rhinovirus 1 Adenovirus | 7 Antibiotics | RT-PCR for respiratory specimens (viruses) c | 0 | 0 | 0 | (NOS, 7) 7 survived |
| Karimi et al. 2020 [70], Iran | Retrospective case report, single-center | 1 | 1 | 144 | 1 (100) AND 1 Persian | 0 | 0 | 1 | 1 Varicella zoster virus | 1 Azithromycin | Serum antibody tests (IgM and IgG) | 0 | 0 | 0 | (Modified NOS, high) 1 survived |
| Katz et al. 2022 [71], United States | Retrospective cohort, multicenter | 16 | 2 | 72 and 120 | 1 (50) AND 2 White (Caucasian) | 0 | 0 | 2 | 2 Herpes simplex virus | 2 Not reported | RT-PCR for respiratory specimens (viruses) c | 2 Not reported | 2 Not reported | 2 Not reported | (NOS, 6) Treatment outcome (not reported) |
| Kazi et al. 2021 [72], India | Retrospective case report, single-center | 1 | 1 | 9 | 0 (0) AND 1 Indian | 0 | 0 | 1 | 1 Dengue virus | 1 Ceftriaxone 1 Vancomycin 1 Doxycycline | RT-PCR for respiratory specimens (viruses) c DENV RTqPCR (dengue) IgM antibody test from CSF (dengue) | 1 | 1 | 1 | (Modified NOS, high) 1 survived |
| Keshavarz Valian et al. 2022 [73], Iran | Retrospective cohort, single-center | 25 | 2 | Mean ± SD, 58.8 ± 51.6 | Gender (not reported) AND 2 Persian | 0 | 0 | 2 | 2 Human coronavirus OC43 | 2 Not reported | RT-PCR for respiratory specimens (viruses) c | 2 Not reported | 2 Not reported | 2 Not reported | (NOS, 6) Treatment outcome (not reported) |
| Khataniar et al. 2022 [74], India | Retrospective case report, single-center | 1 | 1 | 168 | 1 (100) AND 1 Indian | 1 | 0 | 0 | 1 Mycobacterium tuberculosis | 1 Meropenem 1 Vancomycin 1 Ceftriaxone 1 Amikacin 1 Levofloxacin 1 Isoniazid 1 Rifampicin 1 Pyrazinamide 1 Ethionamide | CSF culture (bacteria) | 1 | 1 | 1 | (Modified NOS, high) 1 survived |
| Lambrou et al. 2022 [75], Greece | Retrospective case report, single-center | 1 | 1 | 36 | 0 (0) AND 1 White (Caucasian) | 1 | 0 | 0 | 1 Escherichia hermannii | 1 Piperacillin/tazobactam 1 Amikacin 1 Teicoplanin 1 Meropenem 1 Micafungin | Blood (culture) | 0 | 0 | 0 | (Modified NOS, high) 1 survived |
| Le Glass et al. 2021 [10], France | Retrospective cohort, multicenter | 2159 | 58 | Age group <180: 25 (43.1%) patients (rhinovirus coinfection) | 33 (56.9) AND Ethnicity (not reported) | 58 Not reported | 58 Not reported | 58 | 58 Rhinovirus | 93 Not reported | RT-PCR for respiratory specimens (viruses) c | 58 Not reported | 58 Not reported | 58 Not reported | (NOS, 6) 57 survived 1 died |
| Le Roux et al. 2020 [76], France | Retrospective case report, single-center | 1 | 1 | 10 | 1 (100) AND 1 White (Caucasian) | 0 | 0 | 2 | 1 Varicella zoster virus 1 Rotavirus | 1 Amoxicillin/clavulanic acid 1 Azithromycin 1 Acyclovir | PCR | 0 | 0 | 0 | (Modified NOS, high) 1 survived |
| Leclercq et al. 2021 [77], Switzerland | Retrospective case report, single-center | 1 | 1 | 96 | 1 (100) AND 1 White (Caucasian) | 1 | 0 | 1 | 1 EBV 1 Group A Streptococcus | 1 Amoxicillin 1 Cephalosporin | RT-PCR for respiratory specimens (viruses) c Serum antibody tests (IgM, IgG) | 0 | 0 | 0 | (Modified NOS, high) 1 survived |
| Lee et al. 2022 [78], United States | Retrospective cohort, single-center | 1625 | 92 | Not reported | Gender (not reported) AND Ethnicity (not reported) | 0 | 0 | 111 | 56 RSV 38 Influenza A virus 11 Rhinovirus 2 Influenza B virus 2 Adenovirus 2 Parainfluenza virus | Not reported | RT-PCR for respiratory specimens (viruses) c Serum antibody tests (IgM, IgG) | Not reported | Not reported | Not reported | (NOS, 7) Treatment outcome (not reported) |
| Leuzinger et al. 2020 [79], Switzerland | Retrospective cohort, single-center | 16 | 4 | Age group ≤60: 2 (14.3%) patients (viral coinfection) Age group ≤192: 2 (14.3%) patients (viral coinfection) | Gender (not reported) AND 4 White (Caucasian) | 0 | 0 | 8 | 4 Rhinovirus 2 RSV 2 Parainfluenza virus (types 1–4) | 4 Not reported | RT-PCR for respiratory specimens (viruses) c | 4 Not reported | 4 Not reported | 4 Not reported | (NOS, 7) Treatment outcome (not reported) |
| Li et al. 2020 [80], China | Retrospective cohort, single-center | 40 | 15 | Mean ± SD, 61 ± 56 | Gender (not reported) AND 15 Asian | 14 | 0 | 4 | 13 Mycoplasma pneumoniae 3 Influenza A or B virus 1 Adenovirus 1 Streptococcus pneumonia | 13 Azithromycin 1 Meropenem 1 Piperacillin/tazobactam | RT-PCR for respiratory specimens (viruses) c | 1 | 1 | 1 | (NOS, 7) 15 survived |
| Li et al. 2021 [81], China | Retrospective cohort, single-center | 81 | 27 | Mean ± SD, 76.5 ± 9.6 | 15 (55.6) AND 27 Asian | 24 | 0 | 6 | 20 Mycoplasma pneumoniae 1 Influenza A virus 2 Influenza B virus 1 RSV 1 Adenovirus 1 Parainfluenza virus 2 3 Moraxella catarrhalis 1 Streptococcus pneumoniae | 27 Not reported | RT-PCR for respiratory specimens (viruses) c Sputum (bacteria) Serum antibody tests (IgM, IgG) | 1 | 1 | 1 | (NOS, 7) 27 survived |
| Lin et al. 2020 [82], China | Retrospective cohort, single-center | 92 | 1 | 36 | 0 (0) AND 1 Asian | 0 | 0 | 1 | 1 Metapneumovirus | 1 Not reported | RT-PCR for respiratory specimens (viruses) c | 1 Not reported | 1 Not reported | 1 Not reported | (Modified NOS, high) Treatment outcome (not reported) |
| Ma et al. 2020 [83], China | Retrospective cohort, single-center | 45 | 4 | 4 Not reported | Gender (not reported) AND 4 Asian | 0 | 0 | 7 | 4 Mycoplasma pneumonia 2 Parainfluenza virus 1 Adenovirus | 4 Not reported | RT-PCR for respiratory specimens (viruses) c | 3 | 3 | 3 | (NOS, 6) Treatment outcome (not reported) |
| Mania et al. 2022 [84], Poland | Retrospective cohort, multicenter | 1283 | 135 | Median (IQR), 72 (12–156) | Gender (not reported) AND 135 White (Caucasian) | 15 | 0 | 37 | 11 Streptococcus pneumoniae 2 Influenza A virus 2 Escherichia coli 1 Adenovirus 1 Rhinovirus 1 Bocavirus 1 RSV 1 Parainfluenza 1 Mycoplasma pneumoniae 1 Klebsiella oxytoca 2 Varicella zoster virus 3 Herpes simplex virus 25 Rotavirus, adenovirus, and norovirus | 135 Not reported | RT-PCR for respiratory specimens (viruses) c Blood, urine, and pharyngeal swabs (culture) | 3 | 0 | 2 | (NOS, 7) 135 survived |
| Mannheim et al. 2020 [85], United States | Retrospective case series, single-center | 10 | 4 | Median (IQR), 132 (84–192) | Gender (not reported) AND Ethnicity (not reported) | 2 | 0 | 4 | 1 Mycoplasma pneumoniae 2 Adenovirus 1 Rhinovirus 1 Escherichia coli 1 Rotavirus | 4 Not reported | RT-PCR for respiratory specimens (viruses) c Serum antibody test (IgM) Urine (culture) | 4 | 0 | 0 | (NOS, 7) 4 survived |
| Mansour et al. 2020 [86], Lebanon | Retrospective case report, single-center | 1 | 1 | 16 | 0 (0) AND 1 Arab | 1 | 0 | 0 | 1 Streptococcus pneumoniae | 1 Ceftriaxone 1 Metronidazole | Blood (culture) | 0 | 0 | 0 | (Modified NOS, high) 1 survived |
| Marsico et al. 2022 [87], Italy | Retrospective case report, single-center | 1 | 1 | <1 | 0 (0) AND 1 White (Caucasian) | 1 | 0 | 0 | 1 Multidrug-resistant Enterobacter asburiae | 1 Azithromycin 1 Vancomycin 1 Ceftazidime 1 Gentamycin 1 Meropenem 1 Aztreonam 1 Ceftazidime/avibactam 1 Fosfomycin | Blood (culture) | 1 | 1 | 1 | (Modified NOS, high) 1 survived |
| Mathur et al. 2022 [11], India | Retrospective cohort, single-center | 327 | 17 | Mean (SD), 137 (32) | 9 (52.9) AND 17 Indian | 17 | 0 | 0 | 17 Mycobacterium tuberculosis | 17 Not reported | Blood culture (bacteria) | 6 | 2 | 7 | (NOS, 7) 13 survived 4 died |
| Mithal et al. 2020 [88], United States | Retrospective case series, single-center | 18 | 2 | <3 | 1 (50) AND 2 Hispanic | 2 | 0 | 2 | 2 RSV 1 Streptococcus agalactiae 1 Klebsiella oxytoca | 1 Antibiotics | RT-PCR for respiratory specimens (viruses) c Urine (culture) | 0 | 0 | 0 | (Modified NOS, high) 2 survived |
| Mohammadi et al. 2022 [89], Iran | Retrospective cohort, single-center | 45 | 4 | 1, 36, 72, and 120 | 2 (50) AND 4 Persian | 0 | 0 | 4 | 4 Adenovirus | 0 | RT-PCR for respiratory specimens (viruses) c | 0 | 0 | 0 | (NOS, 5) 4 survived |
| Moin et al. 2021 [90], Pakistan | Retrospective cohort, single-center | 4238 | 4 | ≤ 180 (10–180) | 4 (100) AND 4 Pakistani | 0 | 4 | 0 | 1 Candida auris 1 Candida albicans 1 Candida tropicalis 1 Candida rugosa | 4 Antibiotics 4 Antifungals | Blood (culture) | 1 | 1 | 1 | (NOS, 7) 3 survived 1 died |
| Morand et al. 2020 [91], France | Retrospective case report, single-center | 1 | 1 | 55 | 0 (0) AND 1 White (Caucasian) | 0 | 0 | 1 | 1 EBV | 0 | RT-PCR for respiratory specimens (viruses) c | 0 | 0 | 0 | (Modified NOS, high) 1 survived |
| Mulale et al. 2021 [19], Botswana | Retrospective case report, single-center | 1 | 1 | 3 | 1 (100) AND 1 Black | 1 | 0 | 0 | 1 Rifampin-sensitive Mycobacterium tuberculosis | 1 Ampicillin 1 Gentamicin 1 Rifampicin 1 Isoniazid 1 Pyrazinamide 1 Ethambutol | PCR assay for gastric lavage (bacteria) | 1 | 1 | 1 | (Modified NOS, high) 1 died |
| Ng et al. 2020 [92], United Kingdom | Retrospective case series, single-center | 8 | 3 | 12, 0.5, and 10 | 1 (33.3) AND 3 White (Caucasian) | 0 | 0 | 5 | 2 Adenovirus 2 Rhinovirus 1 Other coronaviruses (229E, NL63, and OC43) | 1 Amoxicillin 1 Cefotaxime 1 Gentamicin | RT-PCR for respiratory specimens (viruses) c | 1 | 0 | 0 | (Modified NOS, high) 3 survived |
| Nieto-Moro et al. 2020 [93], Spain | Retrospective case report, single-center | 1 | 1 | 8 | 1 (100) AND 1 White (Caucasian) | 1 | 0 | 0 | 1 Streptococcus pneumoniae | 1 Azithromycin 1 Clindamycin 1 Meropenem 1 Linezolid | Blood (culture) | 1 | 0 | 1 | (Modified NOS, high) 1 survived |
| Nygaard et al. 2022 [20], Denmark | Retrospective case series, multicenter | 2 | 2 | 24 and 132 | 1 (50) AND 2 White (Caucasian) | 2 | 0 | 2 | 2 Panton-Valentine leukocidin-producing Staphylococcus aureus 1 Parainfluenza 1 Rhinovirus | 1 Meropenem 1 Clindamycin 1 Amoxicillin | Blood PCR assays (viruses) Blood, lung biopsy and CSF (culture) | 1 | 1 | 1 | (Modified NOS, high) 2 died |
| Oba et al. 2020 [94], Brazil | Retrospective case report, single-center | 1 | 1 | 2 | 0 (0) AND 1 Hispanic | 1 | 0 | 0 | 1 Clostridium difficile | 0 | Fecal PCR assays (bacteria) | 1 | 0 | 0 | (Modified NOS, high) 1 survived |
| Ogunbayo et al. 2022 [95], South Africa | Retrospective cohort, multicenter | 36 | 31 | Median (IQR), 16 (5–29) | 19 (61.3) AND 31 Black | 0 | 0 | 53 | 23 Rhinovirus 16 RSV 6 Adenovirus 8 Parainfluenza virus 3 | 31 Not reported | RT-PCR for respiratory specimens (viruses) c | 2 | 31 Not reported | 31 Not reported | (NOS, 7) Treatment outcome (not reported) |
| Palmero et al. 2020 [96], Argentina | Retrospective case series, multicenter | 4 | 4 | Range (60–192) | Gender (not reported) AND 4 Hispanic | 4 | 0 | 0 | 4 Mycobacterium tuberculosis | 4 Isoniazid 4 Rifampicin 4 Pyrazinamide 4 Ethionamide | Blood culture (bacteria) | 1 | 1 | 1 | (Modified NOS, high) 3 survived 1 died |
| Patek et al. 2020 [97], United States | Retrospective case report, single-center | 1 | 1 | 0.5 | 1 (100) AND 1 White (Caucasian) | 1 | 0 | 0 | 1 MSSA | 1 Antibiotic 1 Acyclovir | Wound (culture) | 1 | 0 | 1 | (Modified NOS, high) 1 survived |
| Peng et al. 2020 [98], China | Retrospective cohort, single-center | 75 | 42 | Mean ± SD, 72.7 ± 57.4 | Gender (not reported) AND 42 Asian | 31 | 0 | 8 | 28 Mycoplasma pneumoniae 1 Moraxella catarrhalis 1 Staphylococcus aureus 1 Streptococcus pneumoniae 3 Influenza B virus 1 Influenza A virus 2 Adenoviridae 1 Cytomegalovirus 1 RSV | 37 1st- or 2nd-generation cephalosporins 28 Azithromycin | RT-PCR for respiratory specimens (viruses) c Serum antibody test (IgM) for Mycoplasma pneumoniae (only) | 0 | 0 | 1 | (NOS, 7) 42 survived |
| Pigny et al. 2021 [99], Switzerland | Retrospective cohort, single-center | 51 | 7 | Median (IQR), 50.4 (20.4–87.6) | Gender (not reported) AND 7 White (Caucasian) | 0 | 0 | 9 | 4 Rhinovirus 2 Other coronaviruses (NL63) 2 Adenovirus 1 Metapneumovirus | 7 Not reported | RT-PCR for respiratory specimens (viruses) c | 7 Not reported | 7 Not reported | 7 Not reported | (NOS, 6) Treatment outcome (not reported) |
| Plebani et al. 2020 [100], Italy | Retrospective case series, single-center | 9 | 4 | 36, 120, 168, and 120 | 2 (50) AND 4 White (Caucasian) | 4 | 0 | 0 | 4 Mycoplasma pneumonia | 3 Ceftriaxone 1 Cefotaxime 2 Azithromycin 1 Ampicilline/sulbactam 1 Clindamycin | Serum antibody test (IgM) | 4 Not reported | 4 Not reported | 4 Not reported | (Modified NOS, high) 4 survived |
| Pokorska-Śpiewak et al. 2021 [101], Poland | Prospective case series, single-center | 15 | 1 | 1 Not reported | Gender (not reported) AND 1 White (Caucasian) | 0 | 0 | 1 | 1 Influenza A virus | 0 | RT-PCR for respiratory specimens (viruses) c | 0 | 0 | 0 | (Modified NOS, high) 1 survived |
| Pucarelli-Lebreiro et al. 2022 [102], Brazil | Prospective cohort, single-center | 105 | 9 | Median, 45 | Gender (not reported) AND 9 Hispanic | 0 | 0 | 10 | 6 RSV 1 Influenza 2 Rhinovirus 1 Norovirus | 9 Not reported | RT-PCR for respiratory specimens (viruses) c | 0 | 0 | 0 | (NOS, 7) 9 survived |
| Rastogi et al. 2022 [103], India | Retrospective case series, single-center | 19 | 1 | 108 | 0 (0) AND 1 Indian | 1 | 0 | 0 | 1 Mycobacterium tuberculosis | 1 Isoniazid 1 Rifampicin 1 Pyrazinamide 1 Ethionamide | PCR assay of bronchoalveolar lavage (bacteria) | 0 | 0 | 0 | (NOS, 7) 1 survived |
| Ratageri et al. 2021 [104], India | Retrospective case report, single-center | 1 | 1 | 96 | 1 (100) AND1 Indian | 0 | 0 | 1 | 1 Dengue virus | 0 | IgM antibody test (dengue) | 0 | 0 | 0 | (Modified NOS, high) 1 survived |
| Raychaudhuri et al. 2021 [105], India | Prospective cohort, single-center | 102 | 43 | Median (IQR), 54 (4.8–90) | 23 (53.4) AND 43 Indian | 26 | 0 | 12 | 4 MRSA 5 MSSA 3 CONS 3 Pseudomonas aeruginosa 1 Klebsiella pneumonia 7 Scrub typhus 5 Dengue 3 Salmonella typhi 1 Hepatitis A 1 EBV 2 RSV 1 Influenza A virus 1 Adenovirus 1 Rhinovirus | 38 Antibiotics | RT-PCR for respiratory specimens (viruses) c Blood, respiratory secretions, and CSF (culture) | 27 | 15 | 14 | (NOS, 8) 39 survived 4 died |
| Rebelo et al. 2022 [21], Portugal | Retrospective case report, single-center | 1 | 1 | 168 | 1 (100) AND 1 White (Caucasian) | 1 | 0 | 0 | 1 Neisseria meningitidis serogroup B | 1 Ceftriaxone 1 Meropenem 1 Vancomycin | Blood (culture) | 1 | 1 | 1 | (Modified NOS, high) 1 died |
| Said et al. 2022 [106], Saudi Arabia | Retrospective case report, single-center | 1 | 1 | 10 | Gender (not reported) AND 1 Arab | 1 | 0 | 0 | 1 Escherichia coli | 1 Antibiotic | Urine (culture) | 0 | 0 | 0 | (NOS, 6) 1 survived |
| Sanchez Solano and Sharma 2022 [107], United States | Retrospective case report, single-center | 1 | 1 | 192 | 1 (100) AND 1 White (Caucasian) | 1 | 0 | 0 | 1 MRSA | 1 Ceftriaxone 1 Vancomycin 1 Clindamycin | Bronchoalveolar lavage (culture) | 1 | 1 | 1 | (Modified NOS, high) 1 survived |
| Santoso et al. 2021 [108], Indonesia | Retrospective cohort, multicenter | 90 | 1 | 1 Not reported | Gender (not reported) AND 1 Asian | 0 | 0 | 1 | 1 Dengue virus | 1 Not reported | Dengue NS1 antigen IgM and IgG antibody tests (dengue) | 1 Not reported | 1 Not reported | 1 Not reported | (NOS, 7) Treatment outcome (not reported) |
| Schober et al. 2022 [109], Multi-country | Retrospective cohort, multicenter | 403 | 54 | 45.4 (6.4–129.2) | Gender (not reported) AND Ethnicity (not reported) | 24 | 0 | 32 | 24 Bacterial 32 Viral | 3 Azithromycin | RT-PCR for respiratory specimens (viruses) c Blood (culture) | 10 | 4 | 4 | (NOS, 7) Treatment outcome (not reported) |
| See et al. 2020 [110], Malaysia | Retrospective case reports, multicenter | 4 | 1 | 48 | 0 (0) AND 1 Asian | 0 | 0 | 1 | 1 Influenza A virus | 1 Phenoxymethylpenicillin | RT-PCR for respiratory specimens (viruses)c | 0 | 0 | 0 | (Modified NOS, high) 1 survived |
| Serrano et al. 2020 [111], Spain | Retrospective case report, single-center | 1 | 1 | 96 | 1 (100) AND 1 White (Caucasian) | 1 | 0 | 0 | 1 Mycoplasma pneumonia | 1 Not reported | IgM and IgG antibody tests (Mycoplasma pneumonia) | 0 | 0 | 0 | (Modified NOS, high) 1 survived |
| Shabrawishi et al. 2021 [112], Saudi Arabia | Retrospective case series, single-center | 7 | 1 | 168 | 0 (0) AND 1 Arab | 1 | 0 | 0 | 1 Mycobacterium tuberculosis | 1 Ceftriaxone 1 Azithromycin 1 Isoniazid 1 Rifampicin 1 Pyrazinamide 1 Ethionamide | Blood culture (bacteria) | 0 | 0 | 0 | (Modified NOS, high) 1 survived |
| Shi et al. 2020 [113], China | Retrospective case report, single-center | 1 | 1 | 3 | 1 (100) AND 1 Asian | 0 | 0 | 1 | 1 RSV | 1 Ceftizoxime | RT-PCR for respiratory specimens (viruses) c | 1 | 0 | 1 | (Modified NOS, high) 1 survived |
| Sibulo et al. 2021 [114], United States | Retrospective case report, single-center | 1 | 1 | 36 | 1 (100) AND 1 White (Caucasian) | 1 | 0 | 0 | 1 Staphylococcus epidermidis | 1 Vancomycin 1 Clindamycin 1 Piperacillin/tazobactam | Blood (culture) | 1 | 1 | 0 | (Modified NOS, high) 1 survived |
| Şık et al. 2022 [115], Turkey | Retrospective cohort, single-center | 14 | 1 | 3 | 1 (100) AND 1 White (Caucasian) | 0 | 0 | 1 | 1 Rhinovirus | RT-PCR for respiratory specimens (viruses) c | 0 | 0 | 1 | (NOS, 7) 1 survived | |
| Somasetia et al. 2020 [22], Indonesia | Retrospective case report, single-center | 1 | 1 | 72 | 1 (100) AND 1 Asian | 0 | 0 | 1 | 1 Dengue virus | 1 Antibiotics | IgM antibody test (dengue) | 1 | 1 | 1 | (Modified NOS, high) 1 died |
| Sun et al. 2020 [116], China | Retrospective cohort, single-center | 36 | 23 | Mean (range), 6.43 (2–12) | Gender (not reported) AND 23 Asian | 23 Not reported | 23 Not reported | 23 Not reported | Unspecified number of Cytomegalovirus, EBV and Mycoplasma pneumonia | 15 Cefmetazole 15 Azithromycin | RT-PCR for respiratory specimens (viruses) c | 1 | 1 | 1 | (NOS, 7) 22 survived 1 died |
| Sun et al. 2020 [117], China | Retrospective case series, single-center | 8 | 1 | 96 | 1 (100) AND 1 Asian | 0 | 0 | 1 | 1 Influenza A virus | 1 Antibiotics | RT-PCR for respiratory specimens (viruses) c | 1 | 1 | 1 | (Modified NOS, high) 1 Remained in ICU |
| Tadolini et al. 2020 [118], Multi-country | Retrospective cohort, multicenter | 49 | 1 | 3 | 1 (100) AND 1 Black | 1 | 0 | 0 | 1 Mycobacterium tuberculosis | 1 Antibiotics 1 Isoniazid 1 Rifampicin 1 Pyrazinamide 1 Ethionamide | Blood culture (bacteria) | 0 | 0 | 0 | (NOS, 7) 1 survived |
| Tagarro et al. 2021 [119], Spain | Retrospective cohort, multicenter | 41 | 2 | Median (IQR), 36 (10.8–72) | Gender (not reported) AND Ethnicity (Not reported) | 0 | 0 | 2 | 2 Influenza B virus | 2 Not reported | RT-PCR for respiratory specimens (viruses) c | 0 | 0 | 0 | (NOS, 7) 2 survived |
| Tan et al. 2020 [120], China | Retrospective case series, single-center | 10 | 3 | 24, 105, and 111 | 1 (33.3) AND 3 Asian | 4 | 0 | 0 | 3 Mycoplasma pneumonia 1 Chlamydia pneumonia | 1 Antibiotics | Serum antibody test (IgM) | 3 Not reported | 3 Not reported | 3 Not reported | (Modified NOS, high) Treatment outcome (not reported) |
| Taweevisit et al. 2022 [23], Thailand | Retrospective case report, single-center | 1 | 1 | 67 | 1 (100) AND 1 Asian | 2 | 1 | 4 | 1 Aspergillus species 1 Cytomegalovirus 1 Pseudomonas aeruginosa 1 Acinetobacter baumannii 1 Adenovirus 1 EBV 1 Herpes virus 4 | 1 Antibiotics | Alveolar fluid (culture) RT-PCR for respiratory specimens (viruses) c Serum antibody tests (IgM and IgG) | 1 | 1 | 1 | (Modified NOS, high) 1 died |
| Tchidjou et al. 2021 [121], France | Retrospective case report, single-center | 1 | 1 | 1.5 | 1 (100) AND 1 White (Caucasian) | 1 | 0 | 0 | 1 Citrobacter koseri | 1 Cefotaxime 1 Gentamycin 1 Amoxicillin/clavulanic acid | Urine (culture) | 0 | 0 | 0 | (Modified NOS, high) 1 survived |
| Tiwari et al. 2020 [122], India | Retrospective case report, single-center | 1 | 1 | 168 | 0 (0) AND 1 Indian | 0 | 0 | 1 | 1 Dengue virus | 1 Ceftriaxone 1 Azithromycin | Dengue NS1 antigen IgM antibody test (dengue) | 1 | 0 | 1 | (Modified NOS, high) 1 survived |
| Trifonova et al. 2022 [123], Bulgaria | Retrospective cohort, multicenter | 242 | 16 | All patients were <192 156 (n = 1) 36 (n = 1) | Gender (not reported) AND 16 White (Caucasian) | 16 Not reported | 16 Not reported | 2 | 2 Influenza A virus | 16 Not reported | RT-PCR for respiratory specimens (viruses) c | 1 | 0 | 0 | (NOS, 7) 16 survived |
| Vanzetti et al. 2020 [124], Argentina | Retrospective, case reports, single-center | 1 | 1 | 204 | 1 (100) AND 1 Hispanic | 1 | 0 | 0 | 1 Mycobacterium tuberculosis | 1 Isoniazid 1 Rifampicin 1 Pyrazinamide 1 Ethionamide | PCR assay (bacteria) Sputum (culture) | 0 | 0 | 0 | (Modified NOS, moderate) 1 survived |
| Varela et al. 2022 [125], Brazil | Prospective cohort, multicenter | 92 | 31 | Median (IQR), 64.8 (24–122.4) | Gender (not reported) AND 31 Hispanic | 0 | 0 | 30 | 29 Rhinovirus 1 Enterovirus | 5 Azithromycin | RT-PCR for respiratory specimens (viruses) c | 4 | 0 | 0 | (NOS, 7) 31 survived |
| Verheijen et al. 2022 [126], The Netherlands | Retrospective case report, single-center | 1 | 1 | 0.03 | 0 (0) AND 1 White (Caucasian) | 1 | 0 | 0 | 1 Staphylococcus aureus | 1 Flucloxacillin | RT-PCR for respiratory specimens (viruses) c Blood (culture) | 1 | 1 | 1 | (Modified NOS, high) 1 survived |
| Vidal et al. 2022 [127], Multi-country | Retrospective cohort, multicenter | 29 | 12 | Median, 36 | Gender (not reported) AND 12 White (Caucasian) | 0 | 0 | 12 | 12 Adenovirus | 12 Not reported | RT-PCR for respiratory specimens (viruses) c | 2 | 12 Not reported | 12 Not reported | (NOS, 7) Treatment outcome (not reported) |
| Vu et al. 2021 [128], United States | Retrospective case report, single-center | 1 | 1 | 48 | 1 (100) AND 1 White (Caucasian) | 1 | 0 | 0 | 1 Streptococcus pneumonia | 1 Cefepime 1 Vancomycin 1 Ceftriaxone 1 Amoxicillin | Pleural fluid (culture) | 1 | 1 | 1 | (Modified NOS, high) 1 survived |
| Wanga et al. 2021 [129], United States | Retrospective cohort, multicenter | 713 | 113 | Age group <12: 37 (32.4%) patients (viral coinfection) Age group 12–48: 41 (36.1%) patients (viral coinfection) | Gender (not reported) AND Ethnicity (not reported) | 113 Not reported | 113 Not reported | 113 | 113 RSV | 113 Not reported | RT-PCR for respiratory specimens (viruses) c | 113 Not reported | 113 Not reported | 113 Not reported | (NOS, 6) Treatment outcome (not reported) |
| Wehl et al. 2020 [130], Germany | Retrospective case report, single-center | 1 | 1 | 4 | Gender (not reported) AND 1 White (Caucasian) | 0 | 0 | 1 | 1 Influenza A virus | 0 | RT-PCR for respiratory specimens (viruses) c | 0 | 0 | 0 | (Modified NOS, high) 1 survived |
| Wu et al. 2020 [131], China | Retrospective cohort, multicenter | 34 | 19 | 72 (1.2–180.9) | Gender (not reported) AND 19 Asian | 16 | 0 | 10 | 16 Mycoplasma pneumoniae 3 RSV 3 EBV 3 Cytomegalovirus 1 Influenza A and B virus | 15 Azithromycin | RT-PCR for respiratory specimens (viruses) c | 1 | 0 | 1 | (NOS, 7) 19 survived |
| Xia et al. 2020 [132], China | Retrospective case series, single-center | 20 | 8 | Median, 24 | Gender (not reported) AND 8 Asian | 4 | 0 | 5 | 1 Cytomegalovirus 2 Influenza B virus 1 Influenza A virus 4 Mycoplasma pneumoniae 1 RSV | 8 Not reported | RT-PCR for respiratory specimens (viruses) c | 0 | 0 | 0 | (Modified NOS, high) 8 survived |
| Yakovlev et al. 2022 [133], Russia | Retrospective cohort, single-center | 287 | 32 | Median (IQR), 12 (8.4–30) (viral coinfection) Median (IQR), 144 (90–180) (bacterial coinfection) | Gender (not reported) AND 32 White (Caucasian) | 16 | 0 | 34 | 11 Rhinovirus 11 Other coronaviruses (HKU-1/OC 43) 9 Mycoplasma pneumoniae 7 Chlamydia pneumoniae 4 Metapneumovirus 4 Parainfluenza virus 3 4 Parainfluenza virus 4 | 32 Not reported | RT-PCR for respiratory specimens (viruses) c Serum antibody tests (IgM and IgG) | 6 | 32 Not reported | 32 Not reported | (NOS, 7) Treatment outcome (not reported) |
| Zeng et al. 2020 [134], China | Retrospective cohort, single-center | 3 | 1 | 7.75 | 1 (100) AND 1 Asian | 1 | 0 | 0 | 1 Enterobacter | 1 Antibiotics | Blood (culture) | 1 | 1 | 1 | (NOS, 7) 1 survived |
| Zhang et al. 2020 [135], China | Retrospective case series, multicenter | 34 | 16 | Median (IQR), 33 (10–94.2) | Gender (not reported) AND 16 Asian | 9 | 0 | 15 | 9 Mycoplasma pneumoniae 6 Influenza B virus 3 Influenza A virus 2 RSV 2 EBV 1 Parainfluenza virus 1 Adenovirus | 11 Antibiotics 9 Azithromycin | RT-PCR for respiratory specimens (viruses) c | 0 | 0 | 0 | (Modified NOS, high) 16 survived |
| Zhang et al. 2021 [136], United States | Retrospective case series, multicenter | 16 | 2 | Mean ± SD, 204 ± 61.3 | Gender (not reported) AND Ethnicity (not reported) | 0 | 0 | 4 | 1 Rhinovirus 1 Adenovirus 1 RSV 1 Influenza A virus | 2 Antibiotics | RT-PCR for respiratory specimens (viruses) c | 2 Not reported | 2 Not reported | 2 Not reported | (Modified NOS, high) Treatment outcome (not reported) |
| Zheng et al. 2020 [137], China | Retrospective cohort, multicenter | 25 | 3 | Median (IQR), 36 (24–108) | 2 (66.7) AND 3 Asian | 4 | 0 | 2 | 3 Mycoplasma pneumoniae 2 Influenza B virus 1 Enterobacter aerogenes | 1 Meropenem 1 Linezolid | RT-PCR for respiratory specimens (viruses) c | 1 | 1 | 1 | (NOS, 7) 3 survived |
| Zheng et al. 2020 [138], China | Retrospective cohort, single-center | 4 | 1 | 180 | 1 (100) AND 1 Asian | 0 | 0 | 1 | 1 Influenza B virus | 1 Antibiotics | RT-PCR for respiratory specimens (viruses) c | 0 | 0 | 0 | (NOS, 7) 1 survived |
| Zhu et al. 2020 [139], China | Retrospective cohort, single-center | 257 | 11 | <180 | Gender (not reported) AND 11 Asian | 20 | 2 | 3 | 6 Streptococcus pneumoniae 5 Haemophilus influenzae 3 Klebsiella pneumoniae 3 Staphylococcus aureus 2 Aspergillus 1 Metapneumovirus 1 Cytomegalovirus 1 Mycoplasma pneumonia 1 Adenovirus 1 Pseudomonas aeruginosa 1 Escherichia coli | 11 Not reported | RT-PCR for respiratory specimens (viruses) c | 0 | 0 | 0 | (NOS, 7) 11 survived |
| Zou et al. 2020 [140], China | Retrospective case report, single-center | 2 | 2 | 28 and 156 | 1 (50) AND 2 Asian | 0 | 0 | 2 | 2 Influenza A virus | 1 Cefaclor | Serum antibody tests (IgM and IgG) | 0 | 0 | 0 | (Modified NOS, high) 2 survived |
Abbreviations: ARDS, acute respiratory distress syndrome; CONS, coagulase-negative Staphylococcus species; CMV, Cytomegalovirus; COVID-19, coronavirus disease 2019; CSF, cerebrospinal fluid; EBV, Epstein–Barr virus; ICU, intensive care unit; IgG, immunoglobulin G; IgM, immunoglobulin M; IQR, interquartile range; MRSA, Methicillin-resistant Staphylococcus aureus; MSSA, Methicillin-susceptible Staphylococcus aureus; NOS, Newcastle–Ottawa scale; RT-PCR, real-time reverse transcription–polymerase chain reaction; RSV, respiratory syncytial virus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation. a Data are presented as median (25th–75th percentiles), or mean ± SD. b Patients of black ethnicity include African-American, Black African, African, and Afro-Caribbean patients. c PCR assay for multiple respiratory viruses (including influenza virus types A and B, respiratory syncytial virus type A/B, human metapneumovirus, parainfluenza virus types 1–4, other coronaviruses (229E, NL63, and OC43), metapneumovirus, rhinovirus, enterovirus, adenovirus, parechovirus, and bocavirus).
3.2. Demographic, Clinical Characteristics, and Treatment Outcomes of Children with COVID-19 and Bacterial, Fungal, and/or Respiratory Viral Coinfection
The included studies comprised a total of 17,588 children with confirmed SARS-CoV-2 infection who were tested for co-pathogens, as detailed in Table 1. Among these 17,588 COVID-19 patients, bacterial, fungal, and/or respiratory viral coinfections were reported (n = 1633, 9.3%). The median patient age ranged from 1.4 months to 144 months across studies. There was an increased male predominance in pediatric COVID-19 patients diagnosed with bacterial, fungal, and/or viral coinfections in most of the studies (male gender: n = 204, 59.1% compared to female gender: n = 141, 40.9%) [6,8,10,11,16,17,18,19,21,22,23,31,35,38,40,45,46,47,48,52,54,57,59,60,61,63,64,67,68,69,70,71,74,76,77,81,90,93,95,97,104,105,107,111,113,114,117,118,121,124,128,134,137,138]. The majority of the cases belonged to White (Caucasian) (n = 441, 53.3%) [6,7,9,13,20,21,36,38,39,41,42,44,47,48,50,52,58,60,62,63,65,69,71,75,76,77,79,84,87,91,93,97,99,100,101,107,111,114,115,121,123,126,127,128,130,133], Asian (n = 205, 24.8%) [8,9,22,23,37,45,54,65,66,68,80,81,82,83,98,108,110,113,116,117,120,131,132,134,135,137,138,139,140], Indian (n = 71, 8.6%) [11,31,51,63,72,74,103,104,105,122], and Black (n = 51, 6.2%) [6,9,15,19,46,55,59,60,63,95,118] ethnicities.
COVID-19 children coinfected with bacteria, fungi, and/or respiratory viruses were reported to have received antibiotics in 77 studies [5,8,9,12,13,14,15,16,19,20,21,22,23,36,38,39,40,42,45,46,50,51,52,55,56,59,60,63,64,65,66,67,68,69,70,72,74,75,76,77,80,86,87,88,90,92,93,96,97,98,100,103,105,106,107,109,110,112,113,114,116,117,118,120,121,122,124,125,126,128,131,134,135,136,137,138,140]. The most prescribed antibiotics were azithromycin (n = 109) [9,15,36,39,64,65,70,76,80,87,93,98,100,109,112,116,122,125,131,135], 1st/2nd/3rd generation of cephalosporins (n = 66) [12,13,42,45,60,65,67,77,87,98,100,113,116,121,128,140], ceftriaxone (n = 29) [12,14,21,38,39,51,52,60,67,68,72,74,86,100,107,112,122,128], isoniazid (n = 13) [19,51,55,59,74,96,103,112,118,124], pyrazinamide (n = 13) [19,51,55,59,74,96,103,112,118,124], rifampicin (n = 13) [19,51,55,59,74,96,103,112,118,124], ethionamide (n = 12) [51,55,59,74,96,103,112,118,124], meropenem (n = 11) [16,20,21,40,50,74,75,80,87,93,137], vancomycin (n = 11) [13,16,21,60,72,74,87,107,114,128], amoxicillin/clavulanic acid (n = 9) [9,45,59,60,76,121], amoxicillin (n = 8) [14,15,20,40,67,77,92,128], clindamycin (n = 8) [13,20,38,60,67,93,100,107,114], ampicillin/sulbactam (n = 7) [39,60,100], and gentamycin (n = 6) [9,19,42,87,92,121]. There were children who were admitted to the intensive care unit (n = 214, 18.6%) [4,5,6,7,8,9,11,12,13,15,16,18,19,20,21,22,23,35,36,39,42,46,51,53,56,58,61,63,64,66,67,72,74,80,81,83,84,85,87,90,92,93,94,95,96,97,105,107,109,113,114,116,117,122,123,125,126,127,128,131,133,134,137], intubated and placed on mechanical ventilation (n = 98, 9.2%) [4,5,6,7,8,9,11,12,13,15,16,17,18,19,20,21,22,23,35,36,39,42,46,51,56,58,61,67,72,74,80,81,83,87,90,96,105,107,109,114,116,117,126,128,134,137], and suffered acute respiratory distress syndrome (n = 100, 12.5%) [4,6,7,8,9,11,12,13,16,17,18,19,20,21,22,23,39,42,45,46,48,51,55,56,58,61,66,67,72,74,80,81,83,84,87,90,93,96,97,98,105,107,109,113,115,116,117,122,126,128,131,134,137].
Clinical treatment outcomes for the COVID-19 children who were coinfected with bacteria, fungi, and/or respiratory viruses and died was documented in 43 (4.4%) cases [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,90,96,105,116], while 931 (95.6%) of the COVID-19 cases recovered [4,5,6,7,8,9,10,11,31,33,34,35,36,38,40,42,43,45,46,47,48,50,51,52,54,55,56,57,59,60,62,63,64,65,66,67,68,69,70,72,74,75,76,77,80,81,84,85,86,87,88,89,90,91,92,93,94,95,96,97,100,101,102,103,104,105,106,107,110,111,112,113,114,115,116,118,119,121,122,123,124,125,126,128,130,131,132,134,135,137,138,139,140], and final treatment outcome was reported in one patient who remained in the intensive care unit (n = 1, %) [117].
3.3. Meta-Analysis of Bacterial, Fungal, and Respiratory Viral Coinfections in Children with SARS-CoV-2
The overall pooled proportions of COVID-19 children who had laboratory-confirmed bacterial, fungal, and respiratory viral coinfections were 4.73% (95% CI 3.86 to 5.60, n = 445, 34 studies, I2 85%, p < 0.01), 0.98% (95% CI 0.13 to 1.83, n = 17, six studies, I2 49%, p < 0.08), and 5.41% (95% CI 4.48 to 6.34, n = 441, 32 studies, I2 87%, p < 0.01), respectively; (Figure 3, Figure 4 and Figure 5).
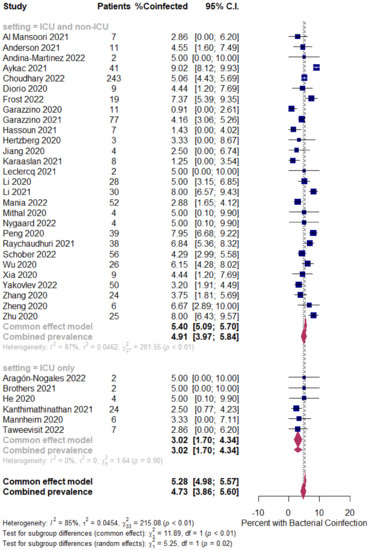
Figure 3.
Pooled estimate for the prevalence of bacterial coinfections in children with COVID-19 stratified by the ICU admission (ICU and non-ICU compared to ICU only). [4,5,6,7,8,9,12,13,20,23,32,36,39,57,58,63,64,66,69,77,80,81,84,85,88,98,105,109,131,132,133,135,137,139].
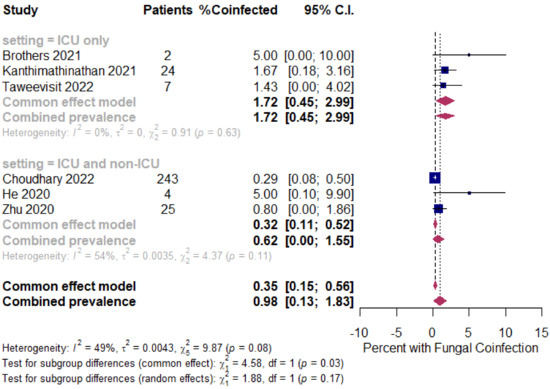
Figure 4.
Pooled estimate for the prevalence of fungal coinfections in children with COVID-19 stratified by the ICU admission (ICU and non-ICU compared to ICU only). [5,8,9,13,23,139].
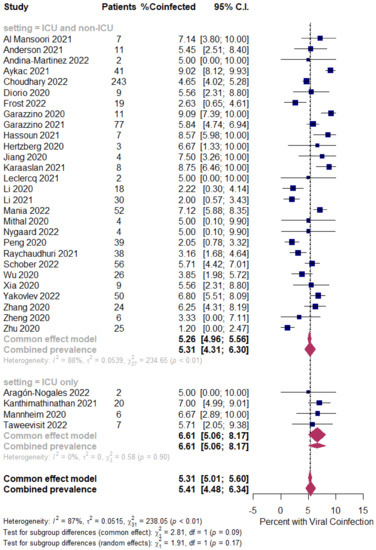
Figure 5.
Pooled estimate for the prevalence of respiratory viral coinfections in children with COVID-19 stratified by the ICU admission (ICU and non-ICU compared to ICU only). [4,5,6,7,9,12,20,23,32,36,39,57,58,63,64,66,69,77,80,81,84,85,88,98,105,109,131,132,133,135,137,139].
In bacterial coinfected COVID-19 children, subgroup analysis showed some difference in the rates between all patients (patients in the ICU and non-ICU group or ICU only group); the ICU and non-ICU group showed a prevalence of 4.91% (95% CI 3.97 to 5.84, n = 431, 28 studies, I2 87%, p < 0.01), while the ICU only group showed a prevalence of 3.02% (95% CI 1.70 to 4.34, n = 14, six studies, I2 0%, p = 0.90), respectively; Figure 3.
In fungal coinfected COVID-19 children, subgroup analysis showed almost a threefold increase in the rates between all patients (patients in the ICU and non-ICU group or ICU only group); the ICU only group showed a prevalence of 1.72% (95% CI 0.45 to 2.99, n = 11, three studies, I2 0%, p = 0.63), while the ICU and non-ICU group showed a prevalence of 0.62% (95% CI 0.00 to 1.55, n = 6, three studies, I2 54%, p = 0.11), respectively; Figure 4.
However, in the respiratory viral coinfected COVID-19 children, subgroup analysis showed a slight difference in the rates between all patients (patients in the ICU and non-ICU group or ICU only group); the ICU and non-ICU group showed a prevalence of 5.31% (95% CI 4.31 to 6.30, n = 418, 28 studies, I2 88%, p < 0.01), while the ICU only group showed a prevalence of 6.61% (95% CI 5.06 to 8.17, n = 23, four studies, I2 0%, p = 0.90), respectively; Figure 5.
Funnel plots for possible publication bias for the pooled effect size to determine the prevalence of bacterial, fungal, and/or fungal coinfections in children with COVID-19 appeared asymmetrical on visual inspection, and Egger’s tests confirmed asymmetry with p-values < 0.05; Figure 6, Figure 7 and Figure 8.
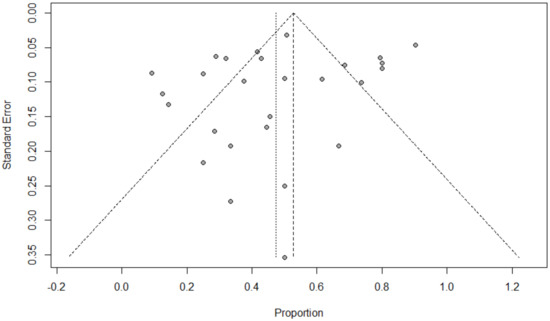
Figure 6.
Funnel plot to evaluate publication bias for the pooled effect size to estimate the prevalence of bacterial coinfections in children with COVID-19 based on ICU admission.
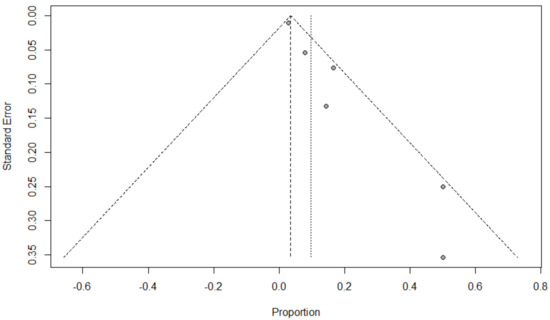
Figure 7.
Funnel plot to evaluate publication bias for the pooled effect size to estimate the prevalence of fungal coinfections in children with COVID-19 based on ICU admission.
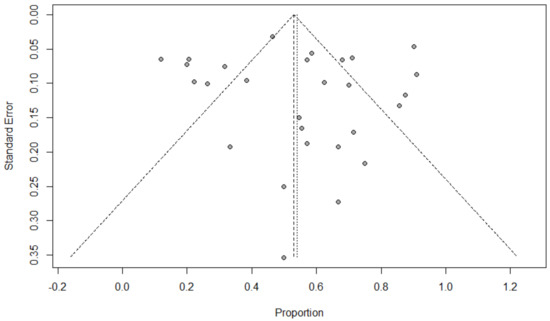
Figure 8.
Funnel plot to evaluate publication bias for the pooled effect size to estimate the prevalence of respiratory viral coinfections in children with COVID-19 based on ICU admission.
3.4. Bacterial, Fungal, and Respiratory Viral Co-Pathogens in COVID-19 Children
Specific bacterial co-pathogens were reported in 71/130 (54.6%) studies, which is about 31.8% of the reported coinfections. The most common bacteria were Mycoplasma pneumoniae (n = 120), Streptococcus pneumoniae (n = 65), Mycobacterium tuberculosis (n = 31), Staphylococcus aureus (n = 12), Escherichia coli (n = 11), Haemophilus influenza (n = 10), Chlamydia pneumoniae (n = 9), and Pseudomonas aeruginosa (n = 9) (Table 2).

Table 2.
Proportion of all identified bacterial co-pathogens in children with COVID-19 (N = 520).
Fungal co-pathogens were reported in 8/130 (6.1%) studies, which is equal to only 1.4% of the reported coinfections. The most common fungal organisms were Aspergillus species (n = 3), fungal bezoars (n = 2), Candida albicans (n = 1), Candida auris (n = 1), Candida glabrata (n = 1), Candida rugosa (n = 1), and Candida tropicalis (n = 1) (Table 3).

Table 3.
Proportion of all identified fungal co-pathogens in children with COVID-19 (N = 23).
Respiratory viral co-pathogens were reported in 88/130 (67.7%) studies, representing about 66.8% of the reported coinfections. The most common respiratory viruses were RSV (n = 342), Rhinovirus (n = 209), Influenza A virus (n = 80), Adenovirus (n = 60), Parainfluenza virus (types 1–4) (n = 29), Influenza B virus (n = 28), Metapneumovirus (n = 27), EBV (n = 14), Cytomegalovirus (n = 12), Dengue virus (n = 12), Coronaviruses (HKU-1/OC 43) (n = 11), and Bocavirus (n = 10) (Table 4).

Table 4.
Proportion of all identified respiratory viral co-pathogens in children with COVID-19 (N = 1090).
4. Discussion
This systematic review and meta-analysis included 17,588 laboratory-confirmed COVID-19 children from 130 observational studies to estimate the prevalence of coinfections with bacteria, fungi, and/or respiratory viruses. Children with SARS-CoV-2 infection had the following prevalence of pathogen coinfections: bacterial (4.7%, 95% CI 3.8–5.6), fungal (0.9%, 95% CI 0.1–1.8), and respiratory viral (5.4%, 95% CI 4.4–6.3). COVID-19 children had higher fungal and respiratory viral coinfections in ICU units (1.7%, 95% CI 0.4–2.9 and 6.6%, 95% CI 5–8.1, respectively) than mixed ICU and non-ICU patients. However, bacterial coinfection was lower in children infected with SARS-CoV-2 in ICU group (3%, 95% CI 1.7–4.3). Children with COVID-19 seem to have a distinctly lower susceptibility to bacterial, fungal, and/or respiratory viral coinfections than adults. Our study documents that 4.7% (bacteria), 0.9% (fungal), and 5.4% (viral) of the pediatric COVID-19 population harbor microbiologically confirmed coinfections, which is much lower than the recent systematic review and meta-analysis, including 72 studies, conducted from 1 December 2019 to 31 March 2021, portraying coinfection rates of 15.9% (bacterial), 3.7% (fungal), and 6.6% (viral) in the adult COVID-19 population [141]. Lower rates of bacterial, fungal, and/or respiratory viral coinfection in children with SARS-CoV-2 infection compared to the adult COVID-19 population may have different explanations. Immunologically, children seem to have an immature receptor system, immune-system-specific regulatory mechanisms, and possible cross-protection from other common bacterial, fungal, and viral infections occurring in children [142,143]. A growing body of evidence suggests that children’s immune systems can neutralize SARS-CoV-2 because their T cells are relatively naïve and mostly untrained, and thus might have a greater capacity to respond to new viruses and eliminate SARS-CoV-2 before it replicates in large numbers [144,145,146]. Children are also the main reservoir for seasonal coronaviruses, and some researchers have suggested that antibodies for these coronaviruses might confer some protection against SARS-CoV-2 [143,146]. Moreover, children are more protected at the cellular level, as the expression of angiotensin-converting enzyme 2, which is the receptor that SARS-CoV-2 uses for host entry, is less frequently expressed in the epithelial cells of the nasal passages and lungs of younger children [147]. Otherwise, differences can be explained by the numerous different study designs to a large extent, as well as selection bias, consideration of respiratory and extra-respiratory pathogens, microbiological investigations employed, use of culture and non-culture methods, time of specimen collection, exclusion/inclusion of contaminants, climate, temporal variations in microbial epidemiology and the study population itself.
Three previous systematic reviews and meta-analyses reported on bacterial, fungal, and respiratory vial coinfections; however, these studies included mixed populations of adults and children, included a smaller number of studies (with most data for adults and very few pediatric patients), and sensitivity analysis to study the proportion of coinfection in COVID-19 children was not conducted [148,149,150]. To the best of our knowledge, this is the first and largest systematic review and meta-analysis to report exclusively on bacterial, fungal, and respiratory viral coinfection in children with COVID-19, and we pooled evidence from 130 studies, including at least Mycoplasma pneumoniae, Streptococcus pneumoniae, Mycobacterium tuberculosis, Staphylococcus aureus, RSV, rhinovirus, influenza A or B virus, adenovirus, parainfluenza virus, and metapneumovirus due to their virulence and prevalence, in an attempt to avoid measurement bias. Of the 98.6% who had additional respiratory viruses or bacteria detected, we found that the most common identified virus and bacterium in children with COVID-19 were RSV (n = 342, 31.4%) and Mycoplasma pneumonia (n = 120, 23.1%), in line with findings in two previous systematic reviews and meta-analyses, which reported that RSV and Mycoplasma pneumonia were the most commonly isolated co-pathogens in the adult population with SARS-CoV-2 infection [148,150]. RSV and Mycoplasma pneumonia cause acute respiratory tract illness in people of all ages, and all children are infected with RSV by 2 years of age [151], while approximately one-half of patients infected with Mycoplasma pneumonia are <6 years old [school-age years) [152]. RSV is the most common cause of lower respiratory tract infection in children <1 year of age [153], and bronchiolitis (up to 80% of which is caused by RSV) is a leading cause of hospital admission [154] and an important cause of death in infants and young children [155]. Mycoplasma pneumonia is the second most common cause of respiratory tract infections, and upper and lower respiratory tracts may be affected [156]. This pathogen causes a wide spectrum of illness, ranging from asymptomatic to severe community-acquired pneumonia or extrapulmonary manifestations necessitating ICU admission [157,158]. Several countries have reported that there has been a suppression of RSV and Mycoplasma pneumonia circulation, and their typical seasonality, since early 2020 due to the preventive infection control measures and non-pharmaceutical interventions against SARS-CoV-2 [159,160,161,162,163,164]. However, RSV and Mycoplasma pneumonia activity rebounded in early–mid 2021 at a fast pace, as public health restrictions and social distancing regulations were relaxed; higher hospitalization rates were reported, and most of the hospitalized children required ICU admission [165,166,167]. Although two recent studies demonstrated no association between SARS-CoV-2 and RSV coinfection and clinical severity (need or use of supplemental oxygen, ICU admission, mechanical ventilation, and mortality), the evidence was only based on three small studies [167,168]. In contrast, evidence of clinical severity regarding cases coinfected with SARS-CoV-2 and Mycoplasma pneumonia is well-established, and several studies reported such coinfection as being associated with an increase in inpatient mortality, length of hospital stay, and need for mechanical ventilation [69,100,169,170]. In children, both RSV and Mycoplasma pneumonia are similar to SARS-CoV-2; as potential triggers for a cytokine storm, leading to the development of Multisystem Inflammatory Syndrome in Children (MIS-C), they appear to play a role in the pathogenesis, and may contribute to the subsequent clinical severity of COVID-19. The cytokines tumor necrosis factor-alpha, interleukin-8, interleukin-6, and interleukin-1 beta were detected in the airway secretions of children infected with RSV and Mycoplasma pneumonia, which may act as a double whammy of respiratory pathogens and correlate with severe pathogenesis [171,172,173,174]. As coinfection with either the highly contagious RSV or Mycoplasma pneumonia and SARS-CoV-2 can modify the disease course and contribute to severity, and can cause serious compilations in children, especially those with high-risk comorbidities, healthcare workers need to consider RSV or Mycoplasma pneumonia and SARS-CoV-2 coinfection in the differential diagnosis of acute febrile illness in the endemic areas.
It is noteworthy that in the studies where the laboratory techniques for co-pathogen detection were described, a high number of bacterial and viral coinfections in children infected with SARS-CoV-2 included in our review were diagnosed serologically through the detection of immunoglobulins M and/or G. One of the easiest, most convenient, and fastest point-of-care testing to diagnose COVID-19 and other bacterial, fungal, and/or respiratory co-pathogens is by rapid serology tests; however, serology testing has been associated with many false-positive antibody test results for COVID-19 and mixed pathogens [111,175,176]. Therefore, application of serologic laboratory techniques for co-pathogen detection across all studies was likely to reveal an even higher overall coinfection proportion and high rates of anti-infective use for admitted children with SARS-CoV-2 infection to treat documented or presumed bacterial, fungal, and/or respiratory viral coinfections [177,178,179]. In line with previous studies, we identified high anti-infective use in pediatric patients with COVID-19 [177,180,181]. As the prevalence of bacterial, fungal, or respiratory viral coinfections in children with COVID-19 is not high, and anti-infectives likely provide minimal benefit as an empirical treatment, clinicians should prescribe anti-infectives wisely, and only in cases with an objective diagnosis of coinfection, as injudicious use of anti-infectives is associated with unintended consequences, such as adverse events, toxicity, resistance, Clostridioides difficile infections, risk of emergence and transmission of multidrug-resistant organisms, morbidity, and death [182,183,184,185,186,187]. Undoubtedly, coinfection in children with COVID-19 is likely to be an important modifier in the development of these abovementioned unintended consequences; however, the degree to which co-pathogens interact with SARS-CoV-2 remains unclear in many cases, and even where we know that interactions are occurring, the mechanisms are often poorly defined [188,189].
The combined pooled prevalence for fungal coinfections reported in our review in COVID-19 children is very low (0.98%). In general, very low numbers of fungal species, out of thousands of fungi, are pathogenic [190], and fungal infections in children, other than those caused by Candida species, are uncommon [191]. This can be explained by the strong natural immunity towards fungi in healthy children, and almost every invasive fungal infection that occurs in children is opportunistic [192]. In line with previous studies, all children infected with SARS-CoV-2 who were coinfected with fungi had recognized risk factors for fungaemia, such as use of central lines, malignancy, renal failure, mechanical ventilation, immunosuppression, neutropenia, solid organ transplant recipients, and use of broad-spectrum parenteral antibiotics and corticosteroids [193,194]. Fungal infections in children can be curbed by early diagnosis and timely treatment with the optimal prescription of antifungals based on culture and susceptibility tests, along with adopting appropriate hygienic and sanitization measures [195,196].
Limitations of the Study
We acknowledge that our study is not without some limitations. First, while all of the evidence discussed was based on many cohorts and case series, and some case reports, many of these were small and performed in single centers, and not necessarily generalizable to children infected with SARS-CoV-2 who had bacterial, fungal, or respiratory viral coinfections. Second, almost all studies included in this review were retrospective in design, except seven prospective studies, which could have introduced potential reporting bias due to reliance on obtaining illness histories regarding the identified pediatric cases with COVID-19 and coinfection from household members or contacts and clinical case records. Third, to asses factors associated with the clinical severity in children infected with SARS-CoV-2 who have coinfections, a larger cohort of patients is needed. Last, the study was not registered in Prospero, an international prospective register of systematic reviews, as this might have added extra work and the merit was mostly limited to the avoidance of duplication.
5. Conclusions
Children with COVID-19 seem to have distinctly lower rates of bacterial, fungal, and/or respiratory viral coinfections than adults. RSV and Mycoplasma pneumonia were the most common identified virus and bacterium in children infected with SARS-CoV-2. Knowledge of bacterial, fungal, and/or respiratory viral confections has potential diagnostic and treatment implications in COVID-19 children.
Author Contributions
S.A., M.A., N.A.D., Z.A.A. and A.A.A. (Abdulrahman A. Alnaim) contributed equally to the systematic review. S.A., A.A.M. and A.A.R. were the core team leading the systematic review. S.A., M.A., N.A.D., Z.A.A., A.A.A. (Abdulrahman A. Alnaim) and K.M.A.M. identified and selected the studies. K.A.N., M.A.A.G., S.J.A., A.A.A. (Abdulaziz A. Alahmari), S.M.A.H.M. and Y.A.A. (Yameen Ali Almatawah) conducted the quality assessment of the studies. S.A., O.M.B., A.A.A. (Ahmed Abdulwhab Alismaeel), S.K.A., S.A.A., Z.R.A., N.A.A.B., H.Y.A., J.A.A., Q.A.A., S.M.A., H.A.A., T.N.A. and Y.A.A. (Yousif Ahmad Alabdulaly) collected the data. S.A., M.A., Z.A.A., A.A.A. (Abdulrahman A. Alnaim), K.A.N., M.A.A.G., S.J.A. and A.A.A. (Abdulaziz A. Alahmari) drafted the manuscript. The corresponding author attests that all listed authors meet authorship criteria, and that no others meeting the criteria have been omitted. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This review is exempt from ethics approval because we collected and synthesized data from previous clinical studies in which informed consent had already been obtained by the investigators.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank authors and their colleagues who contributed to the availability of evidence needed to compile this article. We would also like to thank the reviewers for their helpful and valuable comments and suggestions for improving the paper.
Conflicts of Interest
The authors declare that they have no competing interest.
Abbreviations
ARDS, acute respiratory distress syndrome; CMV, Cytomegalovirus; CONS, coagulase-negative Staphylococcus species; COVID-19, coronavirus disease 2019; CSF, cerebrospinal fluid; EBV, Epstein–Barr virus; ICU, intensive care unit; IgG, immunoglobulin G; IgM, immunoglobulin M; MRSA, Methicillin-resistant Staphylococcus aureus; MSSA, Methicillin-susceptible Staphylococcus aureus; NOS, Newcastle–Ottawa scale; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RSV, respiratory syncytial virus; RT-PCR, real-time reverse transcription–polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
References
- Rubenstein, S.; Grew, E.; Clouser, K.; Kwok, A.; Veerapandiyan, A.; Kornitzer, J.; Pecor, K.; Ming, X. COVID-19 in Pediatric Inpatients: A Multi-Center Observational Study of Factors Associated with Negative Short-Term Outcomes. Children 2021, 8, 951. [Google Scholar] [CrossRef] [PubMed]
- Fainardi, V.; Meoli, A.; Chiopris, G.; Motta, M.; Skenderaj, K.; Grandinetti, R.; Bergomi, A.; Antodaro, F.; Zona, S.; Esposito, S. Long COVID in Children and Adolescents. Life 2022, 12, 285. [Google Scholar] [CrossRef] [PubMed]
- Jugulete, G.; Pacurar, D.; Pavelescu, M.L.; Safta, M.; Gheorghe, E.; Borcoș, B.; Pavelescu, C.; Oros, M.; Merișescu, M. Clinical and Evolutionary Features of SARS-CoV-2 Infection (COVID-19) in Children, a Romanian Perspective. Children 2022, 9, 1282. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.M.; Diorio, C.; Goodwin, E.C.; McNerney, K.O.; Weirick, M.E.; Gouma, S.; Bolton, M.J.; Arevalo, C.P.; Chase, J.; Hicks, P. Severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) antibody responses in children with multisystem inflammatory syndrome in children (MIS-C) and mild and severe coronavirus disease 2019 (COVID-19). J. Pediatr. Infect. Dis. Soc. 2021, 10, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, R.; Webber, B.J.; Womack, L.S.; Dupont, H.K.; Chiu, S.K.; Wanga, V.; Gerdes, M.E.; Hsu, S.; Shi, D.S.; Dulski, T.M. Factors Associated with Severe Illness in Patients Aged< 21 Years Hospitalized for COVID-19. Hosp. Pediatr. 2022, 12, 760–783. [Google Scholar]
- Diorio, C.; Henrickson, S.E.; Vella, L.A.; McNerney, K.O.; Chase, J.; Burudpakdee, C.; Lee, J.H.; Jasen, C.; Balamuth, F.; Barrett, D.M. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS–CoV-2. J. Clin. Investig. 2020, 130, 5967–5975. [Google Scholar] [CrossRef]
- Garazzino, S.; Lo Vecchio, A.; Pierantoni, L.; Calò Carducci, F.I.; Marchetti, F.; Meini, A.; Castagnola, E.; Vergine, G.; Donà, D.; Bosis, S.; et al. Epidemiology, Clinical Features and Prognostic Factors of Pediatric SARS-CoV-2 Infection: Results from an Italian Multicenter Study. Front. Pediatr. 2021, 9, 649358. [Google Scholar] [CrossRef]
- He, B.; Wang, J.; Wang, Y.; Zhao, J.; Huang, J.; Tian, Y.; Yang, C.; Zhang, H.; Zhang, M.; Gu, L. The metabolic changes and immune profiles in patients with COVID-19. Front. Immunol. 2020, 11, 2075. [Google Scholar] [CrossRef]
- Kanthimathinathan, H.K.; Buckley, H.; Lamming, C.; Davis, P.; Ramnarayan, P.; Feltbower, R.; Draper, E.S. Characteristics of severe acute respiratory syndrome coronavirus-2 infection and comparison with influenza in children admitted to UK PICUs. Crit. Care Explor. 2021, 3, e0362. [Google Scholar] [CrossRef]
- Le Glass, E.; Hoang, V.T.; Boschi, C.; Ninove, L.; Zandotti, C.; Boutin, A.; Bremond, V.; Dubourg, G.; Ranque, S.; Lagier, J.-C. Incidence and outcome of coinfections with SARS-CoV-2 and rhinovirus. Viruses 2021, 13, 2528. [Google Scholar] [CrossRef]
- Mathur, S.B.; Saxena, R.; Pallavi, P.; Jain, R.; Mishra, D.; Jhamb, U. Effect of Concomitant Tuberculosis Infection on COVID-19 Disease in Children: A Matched, Retrospective Cohort Study. J. Trop. Pediatr. 2022, 68, fmac056. [Google Scholar] [CrossRef] [PubMed]
- Aragón-Nogales, R.; Zurita-Cruz, J.; Vázquez-Rosales, G.; Arias-Flores, R.; Gómez-González, C.; Montaño-Luna, V.; Sámano-Aviña, M.; Pacheco-Rosas, D.; Flores-Ruiz, E.; Villasís-Keever, M. Clinical presentation of pediatric patients with symptomatic SARS-CoV-2 infection during the first months of the COVID-19 pandemic in a single center in Mexico City. Front. Pediatr. 2022, 10, 912784. [Google Scholar] [CrossRef] [PubMed]
- Brothers, E.M.; Lidsky, K.; Simmons, J.; Nakagawa, T. A Child With COVID-19, Type 1 Diabetes, and Candida glabrata: A Case Report and Literature Review. Clin. Pediatr. 2021, 60, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Cruz, E.; Lopatynsky, E.Z.; Machado-Contreras, J.R.; Gatica-Herrera, R.; Zazueta, O.E. Fatal Pediatric Meningococcal Invasive Disease Caused by Neisseria meningitidis Serogroup C and Co-Infected With SARS-CoV-2: Report of a Case in Tijuana, Mexico. Cureus 2022, 14, e22100. [Google Scholar] [CrossRef]
- Freij, B.J.; Gebara, B.M.; Tariq, R.; Wang, A.-M.; Gibson, J.; El-Wiher, N.; Krasan, G.; Patek, P.M.; Levasseur, K.A.; Amin, M. Fatal central nervous system co-infection with SARS-CoV-2 and tuberculosis in a healthy child. BMC Pediatr. 2020, 20, 429. [Google Scholar] [CrossRef]
- Hamzavi, S.S.; Gholami, M.A.; Dashti, A.S. A Case of COVID 19 and Staphylococcus Coinfection. Arch. Iran. Med. 2020, 23, 568–569. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Safamanesh, S.; Ghasemzadeh-moghaddam, H.; Ghafouri, M.; Azimian, A. High prevalence of SARS-CoV-2 and influenza A virus (H1N1) coinfection in dead patients in Northeastern Iran. J. Med. Virol. 2021, 93, 1008–1012. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Safamanesh, S.; Ghasemzadeh-Moghaddam, H.; Ghafouri, M.; Mohajerzadeh-Heydari, M.; Namdar-Ahmadabad, H.; Azimian, A. Report of death in children with SARS-CoV-2 and human metapneumovirus (hMPV) coinfection: Is hMPV the trigger? J. Med. Virol. 2021, 93, 579. [Google Scholar] [CrossRef]
- Mulale, U.K.; Kashamba, T.; Strysko, J.; Kyokunda, L.T. Fatal SARS-CoV-2 and Mycobacterium tuberculosis coinfection in an infant: Insights from Botswana. BMJ Case Rep. CP 2021, 14, e239701. [Google Scholar] [CrossRef]
- Nygaard, U.; Petersen, A.; Larsen, A.R.; Rytter, M.J.H.; Hartling, U.; Kirkby, N.; Hansen, R.N.; Nielsen, A.B.; Lundstrøm, K.; Holm, M. Fatal SARS-CoV-2-Associated Panton-Valentine Leukocidin-producing Staphylococcal Bacteremia: A Nationwide Multicenter Cohort Study. Pediatr. Infect. Dis. J. 2022, 41, e142–e145. [Google Scholar] [CrossRef]
- Rebelo, A.; Dias, D.I.; Sousa, E.; Alves, J.F.; Pinto, M.; Pereira, M.; Menezes, F. Fatal meningococaemia in a SARS-CoV-2-positive adolescent. J. Paediatr. Child Health 2022, 58, 354. [Google Scholar] [CrossRef] [PubMed]
- Somasetia, D.H.; Malahayati, T.T.; Andriyani, F.M.; Setiabudi, D.; Nataprawira, H.M. A fatal course of multiple inflammatory syndrome in children coinfection with dengue. A case report from Indonesia. IDCases 2020, 22, e01002. [Google Scholar] [CrossRef] [PubMed]
- Taweevisit, M.; Chindamporn, A.; Sujjavorakul, K.; Samransamruajkit, R.; Thorner, P.S. Multisystem inflammatory syndrome in children (MIS-C) showing disseminated aspergillosis, cytomegalovirus reactivation and persistent SARS-COV-2: Case report with autopsy review. Pathol. Res. Pract. 2022, 238, 154106. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Randolph, A.G.; Novak, T.; Walker, T.C.; Loftis, L.L.; Zinter, M.S.; Irby, K.; Khurana, S. Systemic and lower respiratory tract immunity to SARS-CoV-2 Omicron and variants in pediatric severe COVID-19 and Mis-C. Vaccines 2022, 10, 270. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2011; pp. 1–12. [Google Scholar]
- Bazerbachi, F.; Sawas, T.; Vargas, E.J.; Prokop, L.J.; Chari, S.T.; Gleeson, F.C.; Levy, M.J.; Martin, J.; Petersen, B.T.; Pearson, R.K. Metal stents versus plastic stents for the management of pancreatic walled-off necrosis: A systematic review and meta-analysis. Gastrointest. Endosc. 2018, 87, 30–42.e15. [Google Scholar] [CrossRef]
- DerSimonian, R.; Kacker, R. Random-effects model for meta-analysis of clinical trials: An update. Contemp. Clin. Trials 2007, 28, 105–114. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Aggarwal, N.; Potdar, V.; Vijay, N.; Mukhopadhyay, L.; Borkakoty, B.; Manjusree, S.; Choudhary, M.L.; Chowdhury, D.; Verma, R.; Bhardwaj, S.D. SARS-CoV-2 and Influenza Virus Co-Infection Cases Identified through ILI/SARI Sentinel Surveillance: A Pan-India Report. Viruses 2022, 14, 627. [Google Scholar] [CrossRef]
- Al Mansoori, L.; Al Kaabi, S.; Nair, S.C.; Al Katheeri, M.; Ghatasheh, G.; Al Dhanhani, H.; Al Kaabi, A. Epidemiological characteristics of children with coronavirus at a joint commission-accredited hospital in the United Arab Emirates. J. Fam. Med. Prim. Care 2021, 10, 2348. [Google Scholar] [CrossRef] [PubMed]
- Allen-Manzur, J.G.; Espinosa-Padilla, S.E.; Bustamante, J.; Blancas-Galicia, L.; Mendieta-Flores, E. Disseminated infection caused by the bacillus Calmette-Guérin vaccine and SARS-CoV-2 coinfection in a patient with IL-12 receptor β1 subunit deficiency. Rev. Alerg. Mex. 2020, 67, 401–407. [Google Scholar] [PubMed]
- Alrayes, T.; Wait, A.; Spencer, P.; Merolla, D.M.; Lampe, K.; Salimnia, H.; Kannikeswaran, N. Features of an Atypical RSV Surge During the COVID-19 Pandemic. Clin. Pediatr. 2022, 00099228221124677. [Google Scholar] [CrossRef] [PubMed]
- Alvares, P.A. SARS-CoV-2 and respiratory syncytial virus coinfection in hospitalized pediatric patients. Pediatr. Infect. Dis. J. 2021, 40, e164–e166. [Google Scholar] [CrossRef]
- Andina-Martinez, D.; Alonso-Cadenas, J.A.; Cobos-Carrascosa, E.; Bodegas, I.; Oltra-Benavent, M.; Plazaola, A.; Epalza, C.; Jimenez-García, R.; Moraleda, C.; Tagarro, A. SARS-CoV-2 acute bronchiolitis in hospitalized children: Neither frequent nor more severe. Pediatr. Pulmonol. 2022, 57, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Arguni, E.; Supriyati, E.; Hakim, M.S.; Daniwijaya, E.W.; Makrufardi, F.; Rahayu, A.; Rovik, A.; Saraswati, U.; Oktoviani, F.N.; Prastiwi, N. Co-infection of SARS-CoV-2 with other viral respiratory pathogens in Yogyakarta, Indonesia: A cross-sectional study. Ann. Med. Surg. 2022, 77, 103676. [Google Scholar] [CrossRef] [PubMed]
- Arslan, S.Y.; Bal, Z.S.; Ozenen, G.G.; Bilen, N.M.; Kurugol, Z.; Ozkinay, F. Cervical abscess caused by methicillin-susceptible Staphylococcus aureus in an infant infected with SARS-CoV-2: Diagnostic dilemma. J. Infect. Chemother. 2021, 27, 1092–1096. [Google Scholar] [CrossRef] [PubMed]
- Aykac, K.; Ozsurekci, Y.; Cura Yayla, B.C.; Evren, K.; Lacinel Gurlevik, S.; Oygar, P.D.; Yucel, M.; Karakoc, A.E.; Alp, A.; Cengiz, A.B. Pneumococcal carriage in children with COVID-19. Hum. Vaccines Immunother. 2021, 17, 1628–1634. [Google Scholar] [CrossRef]
- Ayoubzadeh, S.I.; Isabel, S.; Coomes, E.A.; Morris, S.K. Enteric fever and COVID-19 co-infection in a teenager returning from Pakistan. J. Travel Med. 2021, 28, taab019. [Google Scholar] [CrossRef]
- Berksoy, E.; Kanik, A.; Cicek, A.; Bardak, Ş.; Elibol, P.; Demir, G.; Yilmaz, N.; Nalbant, T.; Gökalp, G.; Yilmaz Çiftdoğan, D. Clinical and laboratory characteristics of children with SARS-CoV-2 infection. Pediatr. Pulmonol. 2021, 56, 3674–3681. [Google Scholar] [CrossRef]
- Blázquez-Gamero, D.; Epalza, C.; Cadenas, J.A.A.; Gero, L.C.; Calvo, C.; Rodríguez-Molino, P.; Méndez, M.; Santos, M.d.M.; Fumadó, V.; Guzmán, M.F. Fever without source as the first manifestation of SARS-CoV-2 infection in infants less than 90 days old. Eur. J. Pediatr. 2021, 180, 2099–2106. [Google Scholar] [CrossRef] [PubMed]
- Borocco, C.; Lafay, C.; Plantard, I.; Gottlieb, J.; Koné-Paut, I.; Galeotti, C. SARS-CoV-2-associated Henoch–Schönlein purpura in a 13-year-old girl. Arch. Pédiatrie 2021, 28, 573–575. [Google Scholar] [CrossRef] [PubMed]
- Cason, C.; Zamagni, G.; Cozzi, G.; Tonegutto, D.; Ronfani, L.; Oretti, C.; De Manzini, A.; Barbi, E.; Comar, M.; Amaddeo, A. Spread of Respiratory Pathogens During the COVID-19 Pandemic Among Children in the Northeast of Italy. Front. Microbiol. 2022, 308. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-R.; Zou, H.; Xue, M.; Chen, Z.-B.; Chen, W.-X. A case of childhood COVID-19 infection with pleural effusion complicated by possible secondary mycoplasma pneumoniae infection. Pediatr. Infect. Dis. J. 2020, 39, e135. [Google Scholar] [CrossRef] [PubMed]
- Ciuca, C.; Fabi, M.; Di Luca, D.; Niro, F.; Ghizzi, C.; Donti, A.; Balducci, A.; Rocca, A.; Zarbo, C.; Gargiulo, G.D. Myocarditis and coronary aneurysms in a child with acute respiratory syndrome coronavirus 2. ESC Heart Fail. 2021, 8, 761–765. [Google Scholar] [CrossRef]
- Danis, K.; Epaulard, O.; Bénet, T.; Gaymard, A.; Campoy, S.; Botelho-Nevers, E.; Bouscambert-Duchamp, M.; Spaccaferri, G.; Ader, F.; Mailles, A. Cluster of coronavirus disease 2019 (COVID-19) in the French Alps, February 2020. Clin. Infect. Dis. 2020, 71, 825–832. [Google Scholar] [CrossRef]
- Danley, K.; Kent, P. 4-month-old boy coinfected with COVID-19 and adenovirus. BMJ Case Rep. CP 2020, 13, e236264. [Google Scholar] [CrossRef]
- DeBiasi, R.L.; Song, X.; Delaney, M.; Bell, M.; Smith, K.; Pershad, J.; Ansusinha, E.; Hahn, A.; Hamdy, R.; Harik, N. Severe coronavirus disease-2019 in children and young adults in the Washington, DC, metropolitan region. J. Pediatr. 2020, 223, 199–203.e1. [Google Scholar] [CrossRef]
- Demirkan, H.; Yavuz, S. COVID-19 complicated with acute renal failure due to mycotic bezoars in two children. Arch. Esp. Urol. 2021, 74, 712–715. [Google Scholar]
- Dhanawade, S.S.; Kurade, A.V. Tuberculous Meningitis and COVID-19 Coinfection: A Diagnostic Challenge. Pediatr. Infect. Dis. 2021, 3, 79–80. [Google Scholar] [CrossRef]
- Di Nora, A.; Pizzo, F.; Costanza, G.; Ruggieri, M.; Falsaperla, R. Human herpes 6 encephalitis in co-infection with Covid-19. Acta Neurol. Belg. 2022, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Dikranian, L.; Barry, S.; Ata, A.; Chiotos, K.; Gist, K.; Bhalala, U.; Danesh, V.; Heavner, S.; Gharpure, V.; Bjornstad, E.C. SARS-CoV-2 With Concurrent Respiratory Viral Infection as a Risk Factor for a Higher Level of Care in Hospitalized Pediatric Patients. Pediatr. Emerg. Care 2022, 38, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Cao, Y.y.; Lu, X.x.; Zhang, J.j.; Du, H.; Yan, Y.q.; Akdis, C.A.; Gao, Y.d. Eleven faces of coronavirus disease 2019. Allergy 2020, 75, 1699–1709. [Google Scholar] [CrossRef] [PubMed]
- Essajee, F.; Solomons, R.; Goussard, P.; Van Toorn, R. Child with tuberculous meningitis and COVID-19 coinfection complicated by extensive cerebral sinus venous thrombosis. BMJ Case Rep. 2020, 13, e238597. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, A.; Hossain, M.M.; Afrin, M.; Shirin, M. Dengue With COVID-19: Associated with Co-infection and Multiple Organ Dysfunction in a Child. Cureus 2021, 13, e20763. [Google Scholar] [CrossRef]
- Frost, H.M.; Sebastian, T.; Keith, A.; Kurtz, M.; Dominguez, S.R.; Parker, S.K.; Jenkins, T.C. COVID-19 and Acute Otitis Media in Children: A Case Series. J. Prim. Care Community Health 2022, 13, 2351. [Google Scholar] [CrossRef]
- Garazzino, S.; Montagnani, C.; Donà, D.; Meini, A.; Felici, E.; Vergine, G.; Bernardi, S.; Giacchero, R.; Vecchio, A.L.; Marchisio, P. Multicentre Italian study of SARS-CoV-2 infection in children and adolescents, preliminary data as of 10 April 2020. Eurosurveillance 2020, 25, 2000600. [Google Scholar] [CrossRef]
- Goussard, P.; Solomons, R.S.; Andronikou, S.; Mfingwana, L.; Verhagen, L.M.; Rabie, H. COVID-19 in a child with tuberculous airway compression. Pediatr. Pulmonol. 2020, 55, 2201–2203. [Google Scholar] [CrossRef]
- Guy, K.; Lelegren, M.; Shomaker, K.; Han, J.; Lam, K. Management of complicated acute sinusitis in the setting of concurrent COVID-19. Am. J. Otolaryngol. 2022, 43, 103603. [Google Scholar] [CrossRef]
- Halabi, K.C.; Wang, H.; Leber, A.L.; Sánchez, P.J.; Ramilo, O.; Mejias, A. Respiratory Syncytial Virus and SARS-CoV-2 Coinfections in Children. Pediatr. Pulmonol. 2022. [Google Scholar] [CrossRef]
- Hare, D.; Gonzalez, G.; Dean, J.; McDonnell, K.; Carr, M.J.; De Gascun, C.F. Genomic epidemiological analysis of SARS-CoV-2 household transmission. Access Microbiol. 2021, 3, 000252. [Google Scholar] [CrossRef]
- Hassoun, A.; Dahan, N.; Kelly, C. A case series of SARS-CoV-2 RT-PCR-Positive hospitalized infants 60 Days of age or younger from 2 New York city pediatric emergency departments. Clin. Pediatr. 2021, 60, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Hertzberg, E.; Lim, C.A.; Eiting, E.; Yung, S.; Nunez, J.; Calderon, Y.; Barnett, B. Respiratory Viral Co-infection with Novel Coronavirus in Children: A Case Series. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Jarmoliński, T.; Matkowska-Kocjan, A.; Rosa, M.; Olejnik, I.; Gorczyńska, E.; Kałwak, K.; Ussowicz, M. SARS-CoV-2 viral clearance during bone marrow aplasia after allogeneic hematopoietic stem cell transplantation—A case report. Pediatr. Transplant. 2021, 25, e13875. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Liu, P.; Xiong, G.; Yang, Z.; Wang, M.; Li, Y.; Yu, X.-j. Coinfection of SARS-CoV-2 and multiple respiratory pathogens in children. Clin. Chem. Lab. Med. 2020, 58, 1160–1161. [Google Scholar] [CrossRef] [PubMed]
- Jose, P.-M.M.; Paola, Z.-S.; Eduardo, D.-G.; Arturo, S.-M.M.O.; Fernando, B.-G. A case of coinfection of a pediatric patient with acute SARS-COV-2 with MIS-C and severe DENV-2 in Mexico: A case report. BMC Infect. Dis. 2021, 21, 1072. [Google Scholar] [CrossRef] [PubMed]
- Kakuya, F.; Okubo, H.; Fujiyasu, H.; Wakabayashi, I.; Syouji, M.; Kinebuchi, T. The first pediatric patients with coronavirus disease 2019 (COVID-19) in Japan; The risk of co-infection with other respiratory viruses. Jpn. J. Infect. Dis. 2020, 181, 377–380. [Google Scholar] [CrossRef]
- Karaaslan, A.; Çetin, C.; Akın, Y.; Tekol, S.D.; Söbü, E.; Demirhan, R. Coinfection in SARS-CoV-2 infected children patients. J. Infect. Dev. Ctries. 2021, 15, 761–765. [Google Scholar] [CrossRef]
- Karimi, A.; Tabatabaei, S.R.; Khalili, M.; Sadr, S.; Alibeik, M.; Omidmalayeri, S.; Fahimzad, S.A.; Ghanaiee, R.M.; Armin, S. COVID-19 and chickenpox as a viral co-infection in a 12-year-old patient, a case report. Arch. Pediatr. Infect. Dis. 2020, 8, e105591. [Google Scholar] [CrossRef]
- Katz, J.; Yue, S.; Xue, W. Herpes simplex and herpes zoster viruses in COVID-19 patients. Ir. J. Med Sci. 2021, 191, 1093–1097. [Google Scholar] [CrossRef]
- Kazi, M.A.; Ghosh, S.; Roychowdhury, S.; Giri, P.P.; Sarkar, M. A Case Study of Dual Infection of Dengue and COVID-19: Presenting as Multiorgan Dysfunction in an Infant. J. Trop. Pediatr. 2020, 67, fmaa080. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz Valian, N.; Pourakbari, B.; Asna Ashari, K.; Hosseinpour Sadeghi, R.; Mahmoudi, S. Evaluation of human coronavirus OC43 and SARS-COV-2 in children with respiratory tract infection during the COVID-19 pandemic. J. Med. Virol. 2022, 94, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Khataniar, H.; Sunil, D.; Lalitha, A. A case report on disseminated tuberculosis in the setting of coronavirus disease 2019: Cause or consequence? Emerg. Crit. Care Med. 2022, 2, 175–178. [Google Scholar] [CrossRef]
- Lambrou, M.; Antari, V.; Totikidis, G.; Papadimitriou, E.; Roilides, E.; Papakonstantinou, E. Coinfections and pulmonary embo-lism in a patient with onset of Leukemia concomitantly with COVID19-Case report. J. Clin. Case Rep. Med. Imag. Health Sci. 2022, 1. Available online: https://jmedcasereportsimages.org/articles/JCRMHS-1004.pdf (accessed on 14 October 2022).
- Le Roux, P.; Millardet, E.; Duquenoy, A.; Labbé, F.; Vandendriessche, A. Pleuropneumonia resulting from varicella and COVID-19 co-infection in a 10-month-old infant. Arch. Pédiatrie 2020, 27, 509–510. [Google Scholar] [CrossRef]
- Leclercq, C.; Toutain, F.; Baleydier, F.; L’Huillier, A.G.; Wagner, N.; Lironi, C.; Calza, A.-M.; Ansari, M.; Blanchard-Rohner, G. Pediatric acute B-cell lymphoblastic leukemia developing following recent SARS-CoV-2 infection. J. Pediatr. Hematol. Oncol. 2021, 43, e1177–e1180. [Google Scholar] [CrossRef]
- Lee, B.R.; Harrison, C.J.; Myers, A.L.; Jackson, M.A.; Selvarangan, R. Differences in pediatric SARS-CoV-2 symptomology and Co-infection rates among COVID-19 Pandemic waves. J. Clin. Virol. 2022, 154, 105220. [Google Scholar] [CrossRef]
- Leuzinger, K.; Roloff, T.; Gosert, R.; Sogaard, K.; Naegele, K.; Rentsch, K.; Bingisser, R.; Nickel, C.H.; Pargger, H.; Bassetti, S. Epidemiology of severe acute respiratory syndrome coronavirus 2 emergence amidst community-acquired respiratory viruses. J. Infect. Dis. 2020, 222, 1270–1279. [Google Scholar] [CrossRef]
- Li, H.; Chen, K.; Liu, M.; Xu, H.; Xu, Q. The profile of peripheral blood lymphocyte subsets and serum cytokines in children with 2019 novel coronavirus pneumonia. J. Infect. 2020, 81, 115–120. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Wang, F.; Lu, X.; Du, H.; Xu, J.; Han, F.; Zhang, L.; Zhang, M. Co-infections of SARS-CoV-2 with multiple common respiratory pathogens in infected children: A retrospective study. Medicine 2021, 100, e24315. [Google Scholar] [CrossRef]
- Lin, D.; Liu, L.; Zhang, M.; Hu, Y.; Yang, Q.; Guo, J.; Guo, Y.; Dai, Y.; Xu, Y.; Cai, Y. Co-infections of SARS-CoV-2 with multiple common respiratory pathogens in infected patients. Sci. China Life Sci. 2020, 63, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-L.; Xia, S.-Y.; Wang, M.; Zhang, S.-M.; Wen-Hui, D.; Chen, Q. Clinical features of children with SARS-CoV-2 infection: An analysis of 115 cases. Chin. J. Contemp. Pediatr. 2020, 22, 290–293. [Google Scholar]
- Mania, A.; Pokorska-Śpiewak, M.; Figlerowicz, M.; Pawłowska, M.; Mazur-Melewska, K.; Faltin, K.; Talarek, E.; Zawadka, K.; Dobrzeniecka, A.; Ciechanowski, P. Pneumonia, gastrointestinal symptoms, comorbidities, and coinfections as factors related to a lengthier hospital stay in children with COVID-19—Analysis of a paediatric part of Polish register SARSTer. Infect. Dis. 2022, 54, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Mannheim, J.; Gretsch, S.; Layden, J.E.; Fricchione, M.J. Characteristics of hospitalized pediatric coronavirus disease 2019 cases in Chicago, Illinois, March–April 2020. J. Pediatr. Infect. Dis. Soc. 2020, 9, 519–522. [Google Scholar] [CrossRef]
- Mansour, A.; Atoui, R.; Kanso, K.; Mohsen, R.; Fares, Y.; Fares, J. First Case of an Infant with COVID-19 in the Middle East. Cureus 2020, 12, e7520. [Google Scholar] [CrossRef]
- Marsico, C.; Capretti, M.G.; Aceti, A.; Vocale, C.; Carfagnini, F.; Serra, C.; Campoli, C.; Lazzarotto, T.; Corvaglia, L. Severe neonatal COVID-19: Challenges in management and therapeutic approach. J. Med. Virol. 2022, 94, 1701–1706. [Google Scholar] [CrossRef]
- Mithal, L.B.; Machut, K.Z.; Muller, W.J.; Kociolek, L.K. SARS-CoV-2 infection in infants less than 90 days old. J. Pediatr. 2020, 224, 150–152. [Google Scholar] [CrossRef]
- Mohammadi, M.; Bid-Hendi, S.; Baghershiroodi, M.; Chehrazi, M.; Yahyapour, Y.; GouranOurimi, A.; Sadeghi, F. Detection of Human Adenovirus among Iranian Pediatric Hospitalized Patients Suspected to COVID-19 Epidemiology and Comparison of Clinical Features. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Moin, S.; Farooqi, J.; Rattani, S.; Nasir, N.; Zaka, S.; Jabeen, K.C. Auris and non-C. auris candidemia in hospitalized adult and pediatric COVID-19 patients; single center data from Pakistan. Med. Mycol. 2021, 59, 1238–1242. [Google Scholar] [CrossRef]
- Morand, A.; Roquelaure, B.; Colson, P.; Amrane, S.; Bosdure, E.; Raoult, D.; Lagier, J.-C.; Fabre, A. Child with liver transplant recovers from COVID-19 infection. A case report. Arch. Pédiatrie 2020, 27, 275–276. [Google Scholar] [CrossRef]
- Ng, K.F.; Bandi, S.; Bird, P.W.; Tang, J.W.-T. COVID-19 in neonates and infants: Progression and recovery. Pediatr. Infect. Dis. J. 2020, 39, e140–e142. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Moro, M.; Ecclesia, F.G.; Tomé-Masa, I.; Caro-Patón, G.D.L.; Leoz-Gordillo, I.; Cabrero-Hernández, M.; García-Salido, A. SARS-CoV-2 and Streptococcus pneumoniae coinfection as a cause of severe pneumonia in an infant. Pediatr. Pulmonol. 2020, 55, 2198–2200. [Google Scholar] [CrossRef] [PubMed]
- Oba, J.; Silva, C.A.; Toma, R.K.; Carvalho WBd Delgado, A.F. COVID-19 and coinfection with Clostridioides (Clostridium) difficile in an infant with gastrointestinal manifestation. Einstein 2020, 18. [Google Scholar] [CrossRef] [PubMed]
- Ogunbayo, A.E.; Mogotsi, M.T.; Sondlane, H.; Nkwadipo, K.R.; Sabiu, S.; Nyaga, M.M. Pathogen Profile of Children Hospitalised with Severe Acute Respiratory Infections during COVID-19 Pandemic in the Free State Province, South Africa. Int. J. Environ. Res. Public Health 2022, 19, 10418. [Google Scholar] [CrossRef]
- Palmero, D.; Levi, A.; Casco, N.; González, N.; González, C.; Pizarro, M.; Poropat, A.; Tullas, M.; Jajati, M. COVID-19 y tuberculosis en 5 hospitales de la Ciudad de Buenos Aires. Rev. Am. Med. Respir. 2020, 251–254. [Google Scholar]
- Patek, P.; Corcoran, J.; Adams, L.; Khandhar, P. SARS-CoV-2 infection in a 2-week-old male with neutropenia. Clin. Pediatr. 2020, 59, 918–920. [Google Scholar] [CrossRef]
- Peng, H.; Gao, P.; Xu, Q.; Liu, M.; Peng, J.; Wang, Y.; Xu, H. Coronavirus disease 2019 in children: Characteristics, antimicrobial treatment, and outcomes. J. Clin. Virol. 2020, 128, 104425. [Google Scholar] [CrossRef]
- Pigny, F.; Wagner, N.; Rohr, M.; Mamin, A.; Cherpillod, P.; Posfay-Barbe, K.M.; Kaiser, L.; Eckerle, I.; L’Huillier, A.G. Viral co-infections among SARS-CoV-2-infected children and infected adult household contacts. Eur. J. Pediatr. 2021, 180, 1991–1995. [Google Scholar] [CrossRef]
- Plebani, A.; Meini, A.; Cattalini, M.; Lougaris, V.; Bugatti, A.; Caccuri, F.; Caruso, A. Mycoplasma infection may complicate the clinical course of SARS-Co-V-2 associated Kawasaki-like disease in children. Clin. Immunol. 2020, 221, 108613. [Google Scholar] [CrossRef]
- Pokorska-Śpiewak, M.; Talarek, E.; Popielska, J.; Nowicka, K.; Ołdakowska, A.; Zawadka, K.; Kowalik-Mikołajewska, B.; Tomasik, A.; Dobrzeniecka, A.; Lipińska, M.; et al. Comparison of clinical severity and epidemiological spectrum between coronavirus disease 2019 and influenza in children. Sci. Rep. 2021, 11, 5760. [Google Scholar] [CrossRef]
- Pucarelli-Lebreiro, G.; Venceslau, M.T.; Cordeiro, C.C.; Maciel, F.Q.; Anachoreta, T.D.; de Abreu, T.F.; Frota, A.C.C.; Castiñeiras, T.M.P.P.; da Costa, A.M.; Lopes, A.C.d.L.; et al. Clinical Manifestations and Complications of Children With COVID-19 Compared to Other Respiratory Viral Infections: A Cohort Inpatient Study from Rio de Janeiro, Brazil. Front. Pediatr. 2022, 10, 934648. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Gala, F.; Kulkarni, S.; Gavali, V. Neurological and Neuroradiological Patterns with COVID-19 Infection in Children: A Single Institutional Study. Indian J. Radiol. Imaging 2022, 3. [Google Scholar] [CrossRef]
- Ratageri, V.H.; Pawar, G.R.; Nikhil, G.; George, S.S. Co-Infection of Dengue Fever with COVID-19 in a Child with MIS-C. Indian J. Pediatr. 2021, 88, 485. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, D.; Sarkar, M.; Roy, A.; Roy, D.; Datta, K.; Sengupta, T.; Hazra, A.; Mondal, R. Covid-19 and Co-Infection in Children: The Indian Perspectives. J. Trop. Pediatr. 2021, 67, fmab073. [Google Scholar] [CrossRef]
- Said, K.B.; Alsolami, A.; Moussa, S.; Alfouzan, F.; Bashir, A.I.; Rashidi, M.; Aborans, R.; Taha, T.E.; Almansour, H.; Alazmi, M.; et al. COVID-19 Clinical Profiles and Fatality Rates in Hospitalized Patients Reveal Case Aggravation and Selective Co-Infection by Limited Gram-Negative Bacteria. Int. J. Environ. Res. Public Heal. 2022, 19, 5270. [Google Scholar] [CrossRef]
- Sanchez Solano, N.; Sharma, P. MRSA and COVID-19 Co-Infection in a Pediatric Patient with Tracheitis: A Rare Association. In Proceedings of the C62. Expanding Our Insight Into COVID-19, San Francisco, CA, USA, 17 May 2022; p. A4553. [Google Scholar]
- Santoso, M.S.; Masyeni, S.; Haryanto, S.; Yohan, B.; Hibberd, M.L.; Sasmono, R.T. Assessment of dengue and COVID-19 antibody rapid diagnostic tests cross-reactivity in Indonesia. Virol. J. 2021, 18, 54. [Google Scholar] [CrossRef]
- Schober, T.; Caya, C.; Barton, M.; Bayliss, A.; Bitnun, A.; Bowes, J.; Brenes-Chacon, H.; Bullard, J.; Cooke, S.; Dewan, T. Risk factors for severe PCR-positive SARS-CoV-2 infection in hospitalised children. BMJ Paediatr. Open 2022, 6. [Google Scholar] [CrossRef]
- See, K.; Liew, S.M.; Ng, D.C.; Chew, E.; Khoo, E.M.; Sam, C.; Sheena, D.; Filzah, Z.Z.; Chin, S.; Lee, P. COVID-19: Four paediatric cases in Malaysia. Int. J. Infect. Dis. 2020, 94, 125–127. [Google Scholar] [CrossRef]
- Serrano, J.M.; García-Gil, M.F.; Monferrer, J.C.; Manrique, B.A.; Prieto-Torres, L.; García, M.G.; Ochoa, C.M.; Ara-Martín, M. COVID-19 and Mycoplasma pneumoniae: SARS-CoV-2 false positive or coinfection? Int. J. Dermatol. 2020, 59, 1282–1283. [Google Scholar] [CrossRef]
- Shabrawishi, M.; AlQarni, A.; Ghazawi, M.; Melibari, B.; Baljoon, T.; Alwafi, H.; Samannodi, M. New disease and old threats: A case series of COVID-19 and tuberculosis coinfection in Saudi Arabia. Clin. Case Rep. 2021, 9, e04233. [Google Scholar] [CrossRef]
- Shi, B.; Xia, Z.; Xiao, S.; Huang, C.; Zhou, X.; Xu, H. Severe pneumonia due to SARS-CoV-2 and respiratory syncytial virus infection: A case report. Clin. Pediatr. 2020, 59, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Sibulo, L.; Kogel, W.; Landolt, L.; Seeni, S.; Markel, J.; Mlady, A. Anesthetic Management of a Child with Propionic Acidemia Complicated by Bacteremia and Severe Acute Respiratory Syndrome Coronavirus 2. J. Med. Cases 2021, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Şık, N.; Başerdem, K.A.Ç.; Başerdem, O.; Appak, Ö.; Sayıner, A.A.; Yılmaz, D.; Duman, M. Distribution of Viral Respiratory Pathogens During the COVID-19 Pandemic: A Single-Center Pediatric Study from Turkey. Turk. Arch. Pediatr. 2022, 57, 354. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Chen, X.; Li, H.; Lu, X.-X.; Xiao, H.; Zhang, F.-R.; Liu, Z.-S. SARS-CoV-2 infection in infants under 1 year of age in Wuhan City, China. World J. Pediatr. 2020, 16, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Li, H.; Lu, X.-X.; Xiao, H.; Ren, J.; Zhang, F.-R.; Liu, Z.-S. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: A single center’s observational study. World J. Pediatr. 2020, 16, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Tadolini, M.; Codecasa, L.R.; García-García, J.-M.; Blanc, F.-X.; Borisov, S.; Alffenaar, J.-W.; Andréjak, C.; Bachez, P.; Bart, P.-A.; Belilovski, E. Active tuberculosis, sequelae and COVID-19 co-infection: First cohort of 49 cases. Eur. Respir. J. 2020, 56, 2001398. [Google Scholar] [CrossRef] [PubMed]
- Tagarro, A.; Epalza, C.; Santos, M.; Sanz-Santaeufemia, F.J.; Otheo, E.; Moraleda, C.; Calvo, C. Screening and severity of coronavirus disease 2019 (COVID-19) in children in Madrid, Spain. JAMA Pediatr. 2021, 175, 316–317. [Google Scholar] [CrossRef]
- Tan, Y.-p.; Tan, B.-y.; Pan, J.; Wu, J.; Zeng, S.-z.; Wei, H.-y. Epidemiologic and clinical characteristics of 10 children with coronavirus disease 2019 in Changsha, China. J. Clin. Virol. 2020, 127, 104353. [Google Scholar] [CrossRef]
- Tchidjou, H.K.; Romeo, B. Infant Case of Co-infection with SARS-CoV-2 and Citrobacter koseri Urinary Infection. J. Trop. Pediatr. 2021, 67, fmaa032. [Google Scholar] [CrossRef]
- Tiwari, L.; Shekhar, S.; Bansal, A.; Kumar, P. COVID-19 with dengue shock syndrome in a child: Coinfection or cross-reactivity? BMJ Case Rep. CP. 2020, 13, e239315. [Google Scholar] [CrossRef]
- Trifonova, I.; Christova, I.; Madzharova, I.; Angelova, S.; Voleva, S.; Yordanova, R.; Tcherveniakova, T.; Krumova, S.; Korsun, N. Clinical significance and role of coinfections with respiratory pathogens among individuals with confirmed severe acute respiratory syndrome coronavirus-2 infection. Front. Public Health 2022, 2855. [Google Scholar] [CrossRef] [PubMed]
- Vanzetti, C.P.; Salvo, C.P.; Kuschner, P.; Brusca, S.; Solveyra, F.; Vilela, A. Coinfección tuberculosis y COVID-19. Medicina 2020, 80, 100–103. [Google Scholar] [PubMed]
- Varela, F.H.; Sartor, I.T.S.; Polese-Bonatto, M.; Azevedo, T.R.; Kern, L.B.; Fazolo, T.; de David, C.N.; Zavaglia, G.O.; Fernandes, I.R.; Krauser, J.R.M. Rhinovirus as the main co-circulating virus during the COVID-19 pandemic in children. J. Pediatr. 2022, 98, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Verheijen, A.C.; Janssen, E.E.; van der Putten, M.E.; van Horck, M.W.; van Well, G.T.; Van Loo, I.H.; Hütten, M.C.; Van Mechelen, K. Management of severe neonatal respiratory distress due to vertical transmission of severe acute respiratory syndrome coronavirus 2: A case report. J. Med. Case Rep. 2022, 16, 140. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.R.; Vaughan, A.; Innocenti, F.; Colombe, S.; Nerlander, L.; Rachwal, N.; Ciancio, B.C.; Mougkou, A.; Carvalho, C.; Delgado, E. Hepatitis of unknown aetiology in children–epidemiological overview of cases reported in Europe, 1 January to 16 June 2022. Eurosurveillance 2022, 27, 2200483. [Google Scholar]
- Vu, K.C.; Heresi, G.P.; Chang, M.L. SARS-CoV-2 and Streptococcus pneumoniae Coinfection in a Previously Healthy Child. Case Rep. Pediatr. 2021, 2021, 8907944. [Google Scholar] [CrossRef]
- Wanga, V.; Gerdes, M.E.; Shi, D.S.; Choudhary, R.; Dulski, T.M.; Hsu, S.; Idubor, O.I.; Webber, B.J.; Wendel, A.M.; Agathis, N.T. Characteristics and clinical outcomes of children and adolescents aged <18 years hospitalized with COVID-19—Six hospitals, United States, July–August 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 1766. [Google Scholar]
- Wehl, G.; Laible, M.; Rauchenzauner, M. Co-infection of SARS CoV-2 and influenza A in a pediatric patient in Germany. Klin. Pädiatrie 2020, 232, 217–218. [Google Scholar] [CrossRef]
- Wu, Q.; Xing, Y.; Shi, L.; Li, W.; Gao, Y.; Pan, S.; Wang, Y.; Wang, W.; Xing, Q. Coinfection and other clinical characteristics of COVID-19 in children. Pediatrics 2020, 146, e20200961. [Google Scholar] [CrossRef]
- Xia, W.; Shao, J.; Guo, Y.; Peng, X.; Li, Z.; Hu, D. Clinical and CT features in pediatric patients with COVID-19 infection: Different points from adults. Pediatr. Pulmonol. 2020, 55, 1169–1174. [Google Scholar] [CrossRef]
- Yakovlev, A.S.; Belyaletdinova, I.K.; Mazankova, L.N.; Samitova, E.R.; Osmanov, I.M.; Gavelya, N.V.; Volok, V.P.; Kolpakova, E.S.; Shishova, A.A.; Dracheva, N.A. SARS-CoV-2 infection in children in Moscow in 2020: Clinical features and impact on circulation of other respiratory viruses: SARS-CoV-2 infection in children in Moscow in 2020. Int. J. Infect. Dis. 2022, 116, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Xia, S.; Yuan, W.; Yan, K.; Xiao, F.; Shao, J.; Zhou, W. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020, 174, 722–725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gu, J.; Chen, Q.; Deng, N.; Li, J.; Huang, L.; Zhou, X. Clinical and epidemiological characteristics of pediatric SARS-CoV-2 infections in China: A multicenter case series. PLoS Med. 2020, 17, e1003130. [Google Scholar] [CrossRef]
- Zhang, D.D.; Acree, M.E.; Ridgway, J.P.; Shah, N.; Hazra, A.; Ravichandran, U.; Kumar, M. Characterizing coinfection in children with COVID-19: A dual center retrospective analysis. Infect. Control. Hosp. Epidemiol. 2021, 42, 1160–1162. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Liao, C.; Fan, Q.-h.; Chen, H.-b.; Zhao, X.-g.; Xie, Z.-g.; Li, X.-l.; Chen, C.-x.; Lu, X.-x.; Liu, Z.-s. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr. Med. Sci. 2020, 40, 275–280. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, H.; Su, Z.; Li, W.; Yang, D.; Deng, F.; Chen, J. Co-infection of SARS-CoV-2 and influenza virus in early stage of the COVID-19 epidemic in Wuhan, China. J. Infect. 2020, 81, e128–e129. [Google Scholar] [CrossRef]
- Zhu, X.; Ge, Y.; Wu, T.; Zhao, K.; Chen, Y.; Wu, B.; Zhu, F.; Zhu, B.; Cui, L. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020, 285, 198005. [Google Scholar] [CrossRef]
- Zou, B.; Ma, D.; Li, Y.; Qiu, L.; Chen, Y.; Hao, Y.; Luo, X.; Shu, S. Are they just two children COVID-19 cases confused with flu? Front. Pediatr. 2020, 8, 341. [Google Scholar] [CrossRef]
- Alhumaid, S.; Al Mutair, A.; Al Alawi, Z.; Alshawi, A.M.; Alomran, S.A.; Almuhanna, M.S.; Almuslim, A.A.; Bu Shafia, A.H.; Alotaibi, A.M.; Ahmed, G.Y. Coinfections with bacteria, fungi, and respiratory viruses in patients with SARS-CoV-2: A systematic review and meta-analysis. Pathogens 2021, 10, 809. [Google Scholar] [CrossRef]
- Lyu, J.; Miao, T.; Dong, J.; Cao, R.; Li, Y.; Chen, Q. Reflection on lower rates of COVID-19 in children: Does childhood immunizations offer unexpected protection? Med. Hypotheses 2020, 143, 109842. [Google Scholar] [CrossRef]
- Sinaei, R.; Pezeshki, S.; Parvaresh, S.; Sinaei, R. Why COVID-19 is less frequent and severe in children: A narrative review. World J. Pediatr. 2021, 17, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Loske, J.; Röhmel, J.; Lukassen, S.; Stricker, S.; Magalhães, V.G.; Liebig, J.; Chua, R.L.; Thürmann, L.; Messingschlager, M.; Seegebarth, A. Pre-activated antiviral innate immunity in the upper airways controls early SARS-CoV-2 infection in children. Nat. Biotechnol. 2022, 40, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; Connors, T.J.; Zhu, Y.; Baldwin, M.R.; Lin, W.-H.; Wontakal, S.; Szabo, P.A.; Wells, S.B.; Dogra, P.; Gray, J. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat. Immunol. 2021, 22, 25–31. [Google Scholar] [CrossRef]
- Nogrady, B. How kids’ immune systems can evade COVID. Nature 2020, 588, 382–383. [Google Scholar] [CrossRef] [PubMed]
- Bunyavanich, S.; Do, A.; Vicencio, A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA 2020, 323, 2427–2429. [Google Scholar] [CrossRef] [PubMed]
- Musuuza, J.S.; Watson, L.; Parmasad, V.; Putman-Buehler, N.; Christensen, L.; Safdar, N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251170. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Committee on Infectious Diseases. From the American Academy of Pediatrics: Policy statements--Modified recommendations for use of palivizumab for prevention of respiratory syncytial virus infections. Pediatrics 2009, 124, 1694–1701. [Google Scholar] [CrossRef]
- Gordon, O.; Oster, Y.; Michael-Gayego, A.; Marans, R.S.; Averbuch, D.; Engelhard, D.; Moses, A.E.; Nir-Paz, R. The clinical presentation of pediatric Mycoplasma pneumoniae infections—A single center cohort. Pediatr. Infect. Dis. J. 2019, 38, 698–705. [Google Scholar] [CrossRef]
- Hall, C.B.; Weinberg, G.A.; Iwane, M.K.; Blumkin, A.K.; Edwards, K.M.; Staat, M.A.; Auinger, P.; Griffin, M.R.; Poehling, K.A.; Erdman, D. The burden of respiratory syncytial virus infection in young children. New Engl. J. Med. 2009, 360, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Meissner, H.C. Viral bronchiolitis in children. New Engl. J. Med. 2016, 374, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef]
- Marino, S.; Pavone, P.; Marino, L.; Nunnari, G.; Ceccarelli, M.; Coppola, C.; Distefano, C.; Falsaperla, R. SARS-CoV-2: The Impact of Co-Infections with Particular Reference to Mycoplasma pneumonia—A Clinical Review. Microorganisms 2022, 10, 1936. [Google Scholar] [CrossRef] [PubMed]
- Sauteur, P.M.M.; Theiler, M.; Buettcher, M.; Seiler, M.; Weibel, L.; Berger, C. Frequency and clinical presentation of mucocutaneous disease due to Mycoplasma pneumoniae infection in children with community-acquired pneumonia. JAMA Dermatol. 2020, 156, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Youn, Y.-S.; Lee, K.-Y. Mycoplasma pneumoniae pneumonia in children. Korean J. Pediatr. 2012, 55, 42. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Ai, T.; Luo, J.; Liu, H. Effect of COVID-19 on childhood Mycoplasma pneumoniae infection in Chengdu, China. BMC Pediatr. 2021, 21, 202. [Google Scholar] [CrossRef]
- Sauteur, P.M.M.; Beeton, M.L.; Uldum, S.A.; Bossuyt, N.; Vermeulen, M.; Loens, K.; Pereyre, S.; Bébéar, C.; Keše, D.; Day, J. Mycoplasma pneumoniae detections before and during the COVID-19 pandemic: Results of a global survey, 2017 to 2021. Eurosurveillance 2022, 27, 2100746. [Google Scholar] [CrossRef]
- Casalegno, J.-S.; Ploin, D.; Cantais, A.; Masson, E.; Bard, E.; Valette, M.; Fanget, R.; Targe, S.C.; Myar-Dury, A.-F.; Doret-Dion, M. Characteristics of the delayed respiratory syncytial virus epidemic, 2020/2021, Rhône Loire, France. Eurosurveillance 2021, 26, 2100630. [Google Scholar] [CrossRef]
- Tempia, S.; Walaza, S.; Bhiman, J.N.; McMorrow, M.L.; Moyes, J.; Mkhencele, T.; Meiring, S.; Quan, V.; Bishop, K.; McAnerney, J.M. Decline of influenza and respiratory syncytial virus detection in facility-based surveillance during the COVID-19 pandemic, South Africa, January to October 2020. Eurosurveillance 2021, 26, 2001600. [Google Scholar] [CrossRef]
- Huang, Q.S.; Wood, T.; Jelley, L.; Jennings, T.; Jefferies, S.; Daniells, K.; Nesdale, A.; Dowell, T.; Turner, N.; Campbell-Stokes, P. Impact of the COVID-19 nonpharmaceutical interventions on influenza and other respiratory viral infections in New Zealand. Nat. Commun. 2021, 12, 1001. [Google Scholar] [CrossRef] [PubMed]
- Eden, J.-S.; Sikazwe, C.; Xie, R.; Deng, Y.-M.; Sullivan, S.G.; Michie, A.; Levy, A.; Cutmore, E.; Blyth, C.C.; Britton, P.N. Off-season RSV epidemics in Australia after easing of COVID-19 restrictions. Nat. Commun. 2022, 13, 2884. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-Y.; Tsai, W.-C.; Lee, H.-F.; Ho, T.-S.; Huang, L.-M.; Shen, C.-F.; Liu, C.-C.; Alliance TPID. The epidemiology, clinical characteristics, and macrolide susceptibility of Mycoplasma pneumoniae pneumonia in children in Southern Taiwan, 2019–2020. J. Microbiol. Immunol. Infect. 2022, 55, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Agha, R.; Avner, J.R. Delayed seasonal RSV surge observed during the COVID-19 pandemic. Pediatrics 2021, 148, e2021052089. [Google Scholar] [CrossRef] [PubMed]
- Foley, D.A.; Phuong, L.K.; Peplinski, J.; Lim, S.M.; Lee, W.H.; Farhat, A.; Minney-Smith, C.A.; Martin, A.C.; Mace, A.O.; Sikazwe, C.T. Examining the interseasonal resurgence of respiratory syncytial virus in Western Australia. Arch. Dis. Child. 2022, 107, e1–e7. [Google Scholar] [CrossRef]
- Cheng, Y.; Cheng, Y.; Dai, S.; Hou, D.; Ge, M.; Zhang, Y.; Fan, L.; Pei, Y.; Yu, L.; Xue, G. The Prevalence of Mycoplasma Pneumoniae Among Children in Beijing Before and During the COVID-19 Pandemic. Front. Cell. Infect. Microbiol. 2022, 457. [Google Scholar] [CrossRef]
- Swets, M.C.; Russell, C.D.; Harrison, E.M.; Docherty, A.B.; Lone, N.; Girvan, M.; Hardwick, H.E.; Visser, L.G.; Openshaw, P.J.; Groeneveld, G.H. SARS-CoV-2 co-infection with influenza viruses, respiratory syncytial virus, or adenoviruses. Lancet 2022, 399, 1463–1464. [Google Scholar] [CrossRef]
- Li, Y. The role of respiratory co-infection with influenza or respiratory syncytial virus in the clinical severity of COVID-19 patients: A systematic review and meta-analysis. Authorea Prepr. 2022, 12, 05040. [Google Scholar] [CrossRef]
- Li, A.; Zhou, X.; Lu, W.; Zhou, Y.; Liu, Q. COVID-19 in two infants in China. Immun. Inflamm. Dis. 2020, 8, 380–383. [Google Scholar] [CrossRef]
- Rangroo, R.; Young, M.; Davis, A.; Pack, S.; Thakore, S.; Schepcoff, A.; Oyesanmi, O. The Severity of the Co-infection of Mycoplasma pneumoniae in COVID-19 Patients. Cureus 2022, 14, e24563. [Google Scholar] [CrossRef]
- Zhang, Y.; Mei, S.; Zhou, Y.; Huang, M.; Dong, G.; Chen, Z. Cytokines as the good predictors of refractory Mycoplasma pneumoniae pneumonia in school-aged children. Sci. Rep. 2016, 6, 37037. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hooper, W.C.; Phillips, D.J.; Talkington, D.F. Cytokines in Mycoplasma pneumoniae infections. Cytokine Growth Factor Rev. 2004, 15, 157–168. [Google Scholar] [CrossRef] [PubMed]
- McNamara, P.; Flanagan, B.; Selby, A.; Hart, C.; Smyth, R. Pro-and anti-inflammatory responses in respiratory syncytial virus bronchiolitis. Eur. Respir. J. 2004, 23, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.A.; Arredondo, S.M.; Bono, M.R.; Gaggero, A.A.; Díaz, P.V. T helper 1/T helper 2 cytokine imbalance in respiratory syncytial virus infection is associated with increased endogenous plasma cortisol. Pediatrics 2006, 117, e878–e886. [Google Scholar] [CrossRef]
- Yan, G.; Lee, C.K.; Lam, L.T.; Yan, B.; Chua, Y.X.; Lim, A.Y.; Phang, K.F.; Kew, G.S.; Teng, H.; Ngai, C.H. Covert COVID-19 and false-positive dengue serology in Singapore. Lancet Infect. Dis. 2020, 20, 536. [Google Scholar] [CrossRef]
- Luvira, V.; Leaungwutiwong, P.; Thippornchai, N.; Thawornkuno, C.; Chatchen, S.; Chancharoenthana, W.; Tandhavanant, S.; Muangnoicharoen, S.; Piyaphanee, W.; Chantratita, N. False Positivity of Anti-SARS-CoV-2 Antibodies in Patients with Acute Tropical Diseases in Thailand. Trop. Med. Infect. Dis. 2022, 7, 132. [Google Scholar] [CrossRef]
- Grau, S.; Hernández, S.; Echeverría-Esnal, D.; Almendral, A.; Ferrer, R.; Limón, E.; Horcajada, J.P.; (Vincat-Proa), O.B.O.T.C.I.C.A.S.P. Antimicrobial Consumption among 66 Acute Care Hospitals in Catalonia: Impact of the COVID-19 Pandemic. Antibiotics 2021, 10, 943. [Google Scholar] [CrossRef]
- Lai, C.-C.; Wang, C.-Y.; Hsueh, P.-R. Co-infections among patients with COVID-19: The need for combination therapy with non-anti-SARS-CoV-2 agents? J. Microbiol. Immunol. Infect. 2020, 53, 505–512. [Google Scholar] [CrossRef]
- Mazumder, P.; Kalamdhad, A.; Chaminda, G.T.; Kumar, M. Coalescence of co-infection and antimicrobial resistance with SARS-CoV-2 infection: The blues of post-COVID-19 world. Case Stud. Chem. Environ. Eng. 2021, 3, 100093. [Google Scholar] [CrossRef]
- Silva, A.R.O.d.S.; Salgado, D.R.; Nagem, L.P.L.; Castanheira, D.; Emmerick, I.C.M.; Lima, E.D.C. Increased use of antibiotics in the intensive care unit during coronavirus disease (COVID-19) pandemic in a brazilian hospital. Front. Pharmacol. 2021, 12, 778386. [Google Scholar] [CrossRef]
- Mah-E-Muneer, S.; Hassan, M.Z.; Biswas, M.A.A.J.; Rahman, F.; Akhtar, Z.; Das, P.; Islam, M.A.; Chowdhury, F. Use of antimicrobials among suspected COVID-19 patients at selected hospitals, Bangladesh: Findings from the first wave of COVID-19 pandemic. Antibiotics 2021, 10, 738. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Khan, M.; Saleem, W.; Karobari, M.I.; Mohamed, R.N.; Heboyan, A.; Rabaan, A.A.; Mutair, A.A.; Alhumaid, S.; Alsadiq, S.A. Evaluation of bi-lateral co-infections and antibiotic resistance rates among COVID-19 patients. Antibiotics 2022, 11, 276. [Google Scholar] [CrossRef] [PubMed]
- Thoma, R.; Seneghini, M.; Seiffert, S.N.; Vuichard Gysin, D.; Scanferla, G.; Haller, S.; Flury, D.; Boggian, K.; Kleger, G.-R.; Filipovic, M. The challenge of preventing and containing outbreaks of multidrug-resistant organisms and Candida auris during the coronavirus disease 2019 pandemic: Report of a carbapenem-resistant Acinetobacter baumannii outbreak and a systematic review of the literature. Antimicrob. Resist. Infect. Control. 2022, 11, 12. [Google Scholar] [PubMed]
- Luo, Y.; Grinspan, L.T.; Fu, Y.; Adams-Sommer, V.; Willey, D.K.; Patel, G.; Grinspan, A.M. Hospital-onset Clostridioides difficile infections during the COVID-19 pandemic. Infect. Control. Hosp. Epidemiol. 2021, 42, 1165–1166. [Google Scholar] [CrossRef] [PubMed]
- Temperoni, C.; Caiazzo, L.; Barchiesi, F. High prevalence of antibiotic resistance among opportunistic pathogens isolated from patients with COVID-19 under mechanical ventilation: Results of a single-center study. Antibiotics 2021, 10, 1080. [Google Scholar] [CrossRef]
- Martinez-Guerra, B.A.; Gonzalez-Lara, M.F.; de-Leon-Cividanes, N.A.; Tamez-Torres, K.M.; Roman-Montes, C.M.; Rajme-Lopez, S.; Villalobos-Zapata, G.I.; Lopez-Garcia, N.I.; Martínez-Gamboa, A.; Sifuentes-Osornio, J. Antimicrobial resistance patterns and antibiotic use during hospital conversion in the COVID-19 pandemic. Antibiotics 2021, 10, 182. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, J.; Jiang, Y.; Shao, Y. High concentration and high dose of disinfectants and antibiotics used during the COVID-19 pandemic threaten human health. Environ. Sci. Eur. 2021, 33, 11. [Google Scholar] [CrossRef]
- Bassetti, M.; Kollef, M.H.; Timsit, J.-F. Bacterial and fungal superinfections in critically ill patients with COVID-19. Intensive Care Med. 2020, 46, 2071–2074. [Google Scholar] [CrossRef]
- Hoque, M.N.; Akter, S.; Mishu, I.D.; Islam, M.R.; Rahman, M.S.; Akhter, M.; Islam, I.; Hasan, M.M.; Rahaman, M.M.; Sultana, M. Microbial co-infections in COVID-19: Associated microbiota and underlying mechanisms of pathogenesis. Microb. Pathog. 2021, 156, 104941. [Google Scholar] [CrossRef]
- Pana, Z.D.; Vikelouda, K.; Roilides, E. Rare fungal infections in children: An updated review of the literature. Curr. Fungal Infect. Rep. 2014, 8, 21–36. [Google Scholar] [CrossRef]
- Noni, M.; Stathi, A.; Velegraki, A.; Malamati, M.; Kalampaliki, A.; Zachariadou, L.; Michos, A. Rare invasive yeast infections in greek neonates and children, a retrospective 12-year study. J. Fungi 2020, 6, 194. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Jain, S.; Rawat, S. Emerging fungal infections among children: A review on its clinical manifestations, diagnosis, and prevention. J. Pharm. Bioallied Sci. 2010, 2, 314. [Google Scholar] [CrossRef] [PubMed]
- Zaoutis, T.E.; Prasad, P.A.; Localio, A.R.; Coffin, S.E.; Bell, L.M.; Walsh, T.J.; Gross, R. Risk factors and predictors for candidemia in pediatric intensive care unit patients: Implications for prevention. Clin. Infect. Dis. 2010, 51, e38–e45. [Google Scholar] [CrossRef] [PubMed]
- Santolaya, M.E.; Alvarado, T.; Queiroz-Telles, F.; Colombo, A.L.; Zurita, J.; Tiraboschi, I.N.; Cortes, J.A.; Thompson, L.; Guzman, M.; Sifuentes, J. Active surveillance of candidemia in children from Latin America: A key requirement for improving disease outcome. Pediatr. Infect. Dis. J. 2014, 33, e40–e44. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).