Audit of Clinical Care Received by COVID-19 Patients Treated at a Tertiary Care Hospital of Nepal in 2021
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting
2.2.1. General Setting
2.2.2. Specific Setting
2.2.3. COVID-19 Management in STIDH
2.2.4. Recording of Data
2.2.5. Guidelines for Management of Care
2.3. Study Population
2.4. Data Variables and Sources
2.5. Data Management and Analysis
3. Results
3.1. Eligibility of Patients for Admission and Their Socio-Demographic/Clinical Characteristics
3.2. Management of COVID-19 Patients
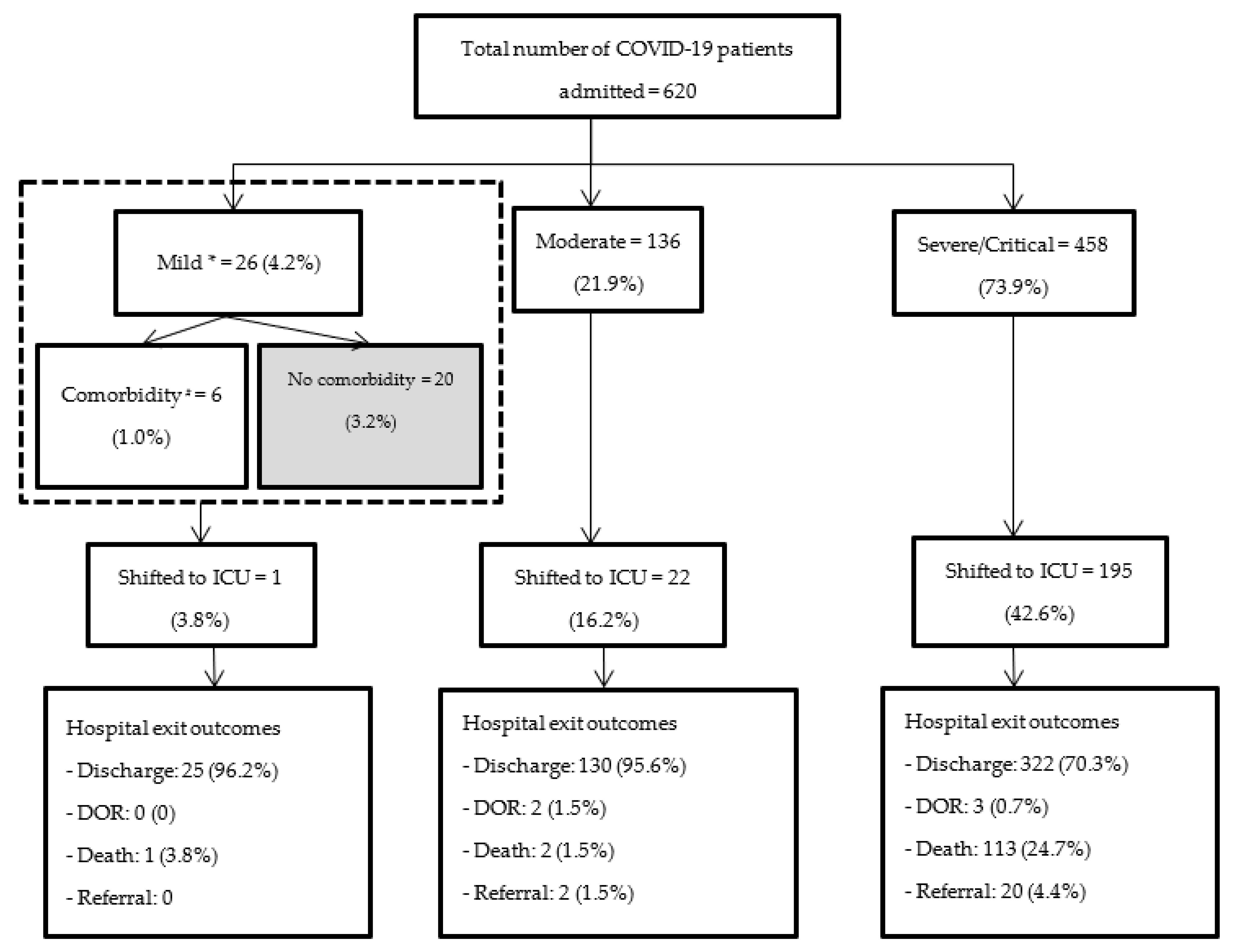
3.3. Hospital Exit Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Open Access Statement and Disclaimer
References
- Fong, S.J.; Dey, N.; Chaki, J. An Introduction to COVID-19. Artif. Intell. Coronavirus Outbreak 2021, 23, 1–22. [Google Scholar] [CrossRef]
- Nepal Daily Situation Report of COVID-19 in Nepal, MOHP Health Emergency Operation Center—Health Emergency Operation Center. Available online: http://heoc.mohp.gov.np/ (accessed on 18 March 2022).
- MOHP COVID19-Dashboard. Available online: https://covid19.mohp.gov.np/ (accessed on 18 March 2022).
- Zar, H.J.; Dawa, J.; Fischer, G.B.; Castro-Rodriguez, J.A. Challenges of COVID-19 in Children in Low- and Middle-Income Countries. Paediatr. Respir. Rev. 2020, 35, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Poudel, K.; Subedi, P. Impact of COVID-19 Pandemic on Socioeconomic and Mental Health Aspects in Nepal. Int. J. Soc. Psychiatry 2020, 66, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Maskey, U.; Sedhai, Y.R.; Atreya, A.; Kidwai, A. Nepal’s COVID-19 Crisis: A Global Call to Arms. Acta Biomed. 2021, 92, e2021421. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tao, Z.W.; Wang, L.; Yuan, M.L.; Liu, K.; Zhou, L.; Wei, S.; Deng, Y.; Liu, J.; Liu, H.G.; et al. Analysis of Factors Associated with Disease Outcomes in Hospitalized Patients with 2019 Novel Coronavirus Disease. Chin. Med. J. 2020, 133, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Gallo Marin, B.; Aghagoli, G.; Lavine, K.; Yang, L.; Siff, E.; Chiang, S.; Salazar-Mather, T.; Dumenco, L.; Savaria, M.; Aung, S.; et al. Predictors of COVID-19 Severity: A Literature Review. Rev. Med. Virol. 2021, 31, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, D.; Zou, B.; Yang, H.; Hui, W.; Rui, F.; Yee, N.; Liu, C.; Nerurkar, S.; Kai, J.; et al. Epidemiology of COVID-19: A Systematic Review and Meta-Analysis of Clinical Characteristics, Risk Factors, and Outcomes. J. Med. Virol. 2021, 93, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Katzenschlager, S.; Zimmer, A.; Gottschalk, C.; Grafeneder, J.; Schmitz, S.; Kraker, S.; Ganslmeier, M.; Muth, A.; Seitel, A.; Maier-Hein, L.; et al. Can We Predict the Severe Course of COVID-19—A Systematic Review and Meta-Analysis of Indicators of Clinical Outcome? PLoS ONE 2021, 16, e0255154. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Zhou, Y.; Wang, F.; Wang, H.; Zhang, M.; Pan, X.; Zhao, Q.; Liu, J. Clinical Characteristics, Laboratory Outcome Characteristics, Comorbidities, and Complications of Related COVID-19 Deceased: A Systematic Review and Meta-Analysis. Aging Clin. Exp. Res. 2020, 32, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.; Patel, U.; Mehta, D.; Patel, N.; Kelkar, R.; Akrmah, M.; Gabrilove, J.; Sacks, H. Biomarkers and Outcomes of COVID-19 Hospitalisations: Systematic Review and Meta-Analysis. BMJ Evid Based Med. 2021, 26, 107–108. [Google Scholar] [CrossRef] [PubMed]
- Moghadas, S.M.; Vilches, T.N.; Zhang, K.; Wells, C.R.; Shoukat, A.; Singer, B.H.; Meyers, L.A.; Neuzil, K.M.; Langley, J.M.; Fitzpatrick, M.C.; et al. The Impact of Vaccination on COVID-19 Outbreaks in the United States. Clin. Infect. Dis. 2021, 73, 2257–2264. [Google Scholar] [CrossRef] [PubMed]
- Bastola, A.; Shrestha, S.; Nepal, R.; Maharjan, K.; Shrestha, B.; Chalise, B.S.; Thapa, P.; Balla, P.; Sapkota, A.; Shah, P. Clinical Mortality Review of COVID-19 Patients at Sukraraj Tropical and Infectious Disease Hospital, Nepal; A Retrospective Study. Trop. Med. Infect. Dis. 2021, 6, 137. [Google Scholar] [CrossRef] [PubMed]
- Capalbo, C.; Aceti, A.; Simmaco, M.; Bonfini, R.; Rocco, M.; Ricci, A.; Napoli, C.; Rocco, M.; Alfonsi, V.; Teggi, A.; et al. The Exponential Phase of the COVID-19 Pandemic in Central Italy: An Integrated Care Pathway. Int. J. Environ. Res. Public Health 2020, 17, 3792. [Google Scholar] [CrossRef] [PubMed]
- Nepal | Facts, History & News. Available online: https://www.infoplease.com/world/countries/nepal (accessed on 27 September 2021).
- Census Nepal 2021. Available online: https://censusnepal.cbs.gov.np/Home/Index/EN (accessed on 26 September 2021).
- WHO (World Health Organization). Clinical Management Clinical Management Living Guidance COVID-19. 2021, pp. 16–44. Available online: https://apps.who.int/iris/handle/10665/338882 (accessed on 18 March 2022).
- Nepal Medical Council. Interim Clinical Guidance for Care of Patients with COVID-19 in Healthcare Settings Nepal Medical Council. 2020, pp. 3–31. Available online: https://nmc.org.np/files/4/Interim%20clinical%20guidance%20for%20care%20of%20patients%20with%20COVID-19%20in%20healthcare%20settings.pdf (accessed on 18 March 2022).
- WHO (World Health Organization). Therapuetics and COVID-19. 2021, pp. 5–48. Available online: https://apps.who.int/iris/handle/10665/340374 (accessed on 18 March 2022).
- Dakroub, F.; Fakhredine, S.; Yassine, M.; Dayekh, A.; Jaber, R.; Fadel, A.; Akl, H.; Maatouk, A. A Retrospective Analysis of 902 Hospitalized COVID-19 Patients in Lebanon: Clinical Epidemiology and Risk Factors. J. Clin. Virol. Plus 2021, 1, 100048. [Google Scholar] [CrossRef] [PubMed]
- Az, A.; Sogut, O.; Akdemir, T.; Ergenc, H.; Dogan, Y.; Cakirca, M. Impacts of Demographic and Clinical Characteristics on Disease Severity and Mortality in Patients with Confirmed COVID-19. Int. J. Gen. Med. 2021, 14, 2989–3000. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.P.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic Prescribing in Patients with COVID-19: Rapid Review and Meta-Analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Navas-Blanco, J.R.; Dudaryk, R. Management of Respiratory Distress Syndrome Due to COVID-19 Infection. BMC Anesthesiol. 2020, 20, 177. [Google Scholar] [CrossRef] [PubMed]
- Izcovich, A.; Ragusa, M.A.; Tortosa, F.; Marzio, M.A.L.; Agnoletti, C.; Bengolea, A.; Ceirano, A.; Espinosa, F.; Saavedra, E.; Sanguine, V.; et al. Prognostic Factors for Severity and Mortality in Patients Infected with COVID-19: A Systematic Review. PLoS ONE 2020, 15, e0269291. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbrouckef, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Bull. World Health Organ 2007, 85, 867–872. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Mild | Moderate | Severe |
|---|---|---|---|
| Admission | N/Y * | Y | Y |
| Laboratory investigations | |||
| Hemoglobin | N | Y | Y |
| Total leucocyte count | N | Y | Y |
| Platelet count | N | Y | Y |
| D-dimer | N | Y | Y |
| C-reactive protein | N | Y | Y |
| Random blood sugar | N | Y | Y |
| Serum creatinine | N | Y | Y |
| Blood culture | N | N | Y |
| Sputum culture | N | N | Y |
| Medication | |||
| Remdesivir | N | N | Y |
| Corticosteroids | N | N | Y |
| Anticoagulants | N | Y | Y |
| Antibiotics | N | N | Y/N ** |
| Oxygen support | |||
| Invasive mechanical ventilation | N | N | Y/N ^ |
| Non-invasive ventilation | N | N | Y/N ^ |
| Low-flow O2 device | N | N | Y |
| Characteristics | Mild ^ | Moderate ^ | Severe/Critical ^ | Total | ||||
|---|---|---|---|---|---|---|---|---|
| n | (%) * | n | (%) * | n | (%) * | n | (%) * | |
| Total | 26 | (100) | 136 | (100) | 458 | (100) | 620 | (100) |
| Age (in years) | ||||||||
| ≤19 | 2 | (7.7) | 3 | (2.2) | 0 | (0) | 5 | (0.8) |
| 20–39 | 12 | (46.2) | 53 | (39.0) | 80 | (17.5) | 145 | (23.4) |
| 40–59 | 9 | (34.6) | 49 | (36.0) | 216 | (47.2) | 274 | (44.2) |
| ≥60 | 3 | (11.5) | 31 | (22.8) | 162 | (35.4) | 196 | (31.6) |
| Sex | ||||||||
| Male | 16 | (61.5) | 87 | (64.0) | 294 | (64.2) | 397 | (64.0) |
| Female | 10 | (38.5) | 49 | (36.0) | 164 | (35.8) | 223 | (36.0) |
| Presence of symptoms # | ||||||||
| Cough | 26 | (100) | 118 | (86.8) | 404 | (88.2) | 548 | (88.4) |
| Fever | 24 | (92.3) | 128 | (94.1) | 358 | (78.2) | 510 | (82.3) |
| Shortness of breath | 9 | (34.6) | 103 | (75.7) | 390 | (85.2) | 502 | (81.0) |
| Myalgia | 15 | (57.7) | 64 | (47.1) | 81 | (17.7) | 160 | (25.8) |
| Chest pain | 6 | (23.1) | 26 | (19.1) | 42 | (9.2) | 74 | (11.9) |
| Sore throat | 8 | (30.8) | 12 | (8.8) | 11 | (2.4) | 31 | (5.0) |
| Running nose | 0 | (0) | 1 | (0.7) | 10 | (2.2) | 11 | (1.8) |
| Fatigue | 3 | (11.5) | 31 | (22.8) | 68 | (14.8) | 102 | (16.5) |
| Headache | 12 | (46.2) | 42 | (30.9) | 149 | (32.5) | 203 | (32.7) |
| Loss of appetite | 3 | (11.5) | 35 | (25.7) | 90 | (19.7) | 128 | (20.6) |
| Anosmia | 4 | (15.4) | 31 | (22.8) | 45 | (9.8) | 80 | (12.9) |
| Loss of taste | 4 | (15.4) | 27 | (19.9) | 48 | (10.5) | 79 | (12.7) |
| Diarrhea | 2 | (7.7) | 31 | (22.8) | 36 | (7.9) | 69 | (11.1) |
| Hemoptysis | 0 | (0) | 5 | (3.7) | 16 | (3.5) | 21 | (3.4) |
| Comorbidities | ||||||||
| Diabetes mellitus | 4 | (15.4) | 14 | (10.3) | 77 | (16.8) | 95 | (15.3) |
| Hypertension | 5 | (19.2) | 23 | (16.9) | 123 | (26.9) | 151 | (24.4) |
| COPD | 0 | (0) | 1 | (0.7) | 28 | (6.1) | 29 | (4.7) |
| Hypothyroidism | 0 | (0) | 6 | (4.4) | 24 | (5.2) | 30 | (4.8) |
| Asthma | 0 | (0) | 2 | (1.5) | 1 | (0.2) | 3 | (0.5) |
| PTB | 0 | (0) | 1 | (0.7) | 9 | (2.0) | 10 | (1.6) |
| None | 20 | (76.9) | 98 | (72.1) | 262 | (57.2) | 380 | (61.3) |
| Saturation at admission | ||||||||
| ≥94% | 24 | (92.3) | 43 | (31.6) | 0 | (0) | 67 | (10.8) |
| 86–93% | 2 | (7.7) | 93 | (68.4) | 102 | (22.3) | 197 | (31.8) |
| 70–85% | 0 | (0) | 0 | (0) | 231 | (50.4) | 231 | (37.3) |
| <70% | 0 | (0) | 0 | (0) | 121 | (26.4) | 121 | (19.5) |
| Not available | 0 | (0) | 0 | (0) | 4 | (0.9) | 4 | (0.7) |
| Duration from diagnosis to admission (in days) | ||||||||
| ≤0 | 4 | (15.4) | 38 | (27.9) | 132 | (28.8) | 174 | (28.1) |
| 1–3 | 12 | (46.2) | 50 | (36.8) | 155 | (33.8) | 217 | (35.0) |
| 4–7 | 9 | (34.6) | 34 | (25.0) | 122 | (26.6) | 165 | (26.6) |
| 8–14 | 1 | (9.6) | 13 | (9.6) | 38 | (8.3) | 52 | (8.4) |
| ≥15 | 0 | (0) | 1 | (0.7) | 11 | (2.4) | 12 | (1.9) |
| Vaccine status | ||||||||
| Received | 6 | (23.1) | 25 | (81.6) | 57 | (12.5) | 88 | (14.2) |
| Not received | 20 | (76.9) | 111 | (18.4) | 401 | (87.5) | 532 | (85.8) |
| Laboratory Parameters | Mild, N = 26 | Moderate, N = 136 | Severe/Critical, N = 458 | Total, N = 620 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tested | Abnormal | Tested | Abnormal | Tested | Abnormal | Tested | Abnormal | |||||||||
| n | (%) * | N | (%) # | N | (%) * | N | (%) # | n | (%) * | n | (%) # | n | (%) * | n | (%) # | |
| Hemoglobin | 25 | (96.2) | 0 | (0) | 127 | (93.4) | 2 | (1.6) | 430 | (93.9) | 15 | (3.5) | 582 | (93.9) | 17 | (2.9) |
| Total leucocyte count | 25 | (96.2) | 6 | (24.0) | 127 | (93.4) | 34 | (26.8) | 430 | (93.9) | 115 | (26.7) | 582 | (93.9) | 155 | (26.6) |
| Platelet count | 25 | (96.2) | 0 | (0) | 127 | (93.4) | 1 | (0.8) | 430 | (93.9) | 0 | (0) | 582 | (93.9) | 1 | (0.2) |
| D-dimer | 19 | (73.1) | 6 | (31.6) | 87 | (64.0) | 36 | (41.4) | 368 | (80.3) | 212 | (57.6) | 474 | (76.5) | 254 | (53.6) |
| C-reactive protein | 23 | (88.5) | 17 | (73.9) | 96 | (70.6) | 76 | (79.2) | 311 | (67.9) | 291 | (93.6) | 430 | (69.4) | 384 | (89.3) |
| Random blood sugar | 26 | (100) | 2 | (7.7) | 136 | (100) | 18 | (13.2) | 458 | (100) | 137 | (29.9) | 620 | (100) | 157 | (25.3) |
| Serum creatinine | 24 | (92.3) | 0 | (0) | 120 | (88.2) | 2 | (0.2) | 424 | (92.6) | 27 | (6.3) | 568 | (91.6) | 29 | (5.1) |
| Blood culture | 1 | (3.8) | 0 | (0) | 19 | (14.0) | 0 | (0) | 162 | (35.4) | 10 | (6.2) | 182 | (29.4) | 10 | (5.5) |
| Sputum culture | 1 | (3.8) | 0 | (0) | 20 | (14.7) | 4 | (20.0) | 168 | (36.7) | 44 | (26.2) | 189 | (30.5) | 48 | (25.4) |
| Treatment | Mild, N = 26 | Moderate, N = 136 | Severe/Critical, N = 458 | Total, N = 620 | ||||
| n | (%) * | n | (%) * | n | (%) * | N | (%) * | |
| Medication | ||||||||
| Remdesivir | 1 | (3.8) | 19 | (14.0) | 116 | (25.3) | 136 | (21.9) |
| Corticosteroids # | 8 | (30.8) | 101 | (74.3) | 458 | (100) | 567 | (91.5) |
| Dexamethasone | 8 | (30.8) | 101 | (74.3) | 440 | (96.1) | 549 | (88.5) |
| Methylprednisolone | 0 | (0) | 3 | (2.2) | 67 | (14.6) | 70 | (11.3) |
| Anticoagulant agent $ | 11 | (42.3) | 106 | (77.9) | 458 | (100) | 575 | (92.7) |
| UFH | 8 | (30.8) | 84 | (61.8) | 399 | (87.1) | 491 | (79.2) |
| Enoxaparin | 0 | (0) | 8 | (5.9) | 214 | (46.7) | 222 | (35.8) |
| Aspirin | 10 | (38.5) | 60 | (44.1) | 322 | (70.3) | 392 | (63.2) |
| Antibiotics | 6 | (23.1) | 43 | (31.6) | 387 | (84.5) | 436 | (70.3) |
| Oxygen support | ||||||||
| Invasive mechanical ventilation | 0 | (0) | 1 | (0.7) | 7 | (1.5) | 8 | (1.3) |
| Non-invasive ventilation | 1 | (3.8) | 1 | (0.7) | 149 | (32.5) | 151 | (24.4) |
| Low-flow O2 device | 4 | (15.4) | 107 | (78.7) | 302 | (66.0) | 413 | (66.6) |
| No support given | 21 | (80.8) | 27 | (19.9) | 0 | (0.0) | 48 | (7.7) |
| Median days of oxygen support (IQR) | 0 | (0) | 2 | (2) | 7 | (10) | 5 | (8) |
| Median days of hospital stay (IQR) | 4 | (2) | 5 | (3) | 9 | (10) | 7 | (7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandal, S.K.; Neupane, J.; Kumar, A.M.V.; Davtyan, H.; Thekkur, P.; Jayaram, A.; Chalise, B.S.; Rawal, M.; Paudel, M.; Baral, B.; et al. Audit of Clinical Care Received by COVID-19 Patients Treated at a Tertiary Care Hospital of Nepal in 2021. Trop. Med. Infect. Dis. 2022, 7, 381. https://doi.org/10.3390/tropicalmed7110381
Mandal SK, Neupane J, Kumar AMV, Davtyan H, Thekkur P, Jayaram A, Chalise BS, Rawal M, Paudel M, Baral B, et al. Audit of Clinical Care Received by COVID-19 Patients Treated at a Tertiary Care Hospital of Nepal in 2021. Tropical Medicine and Infectious Disease. 2022; 7(11):381. https://doi.org/10.3390/tropicalmed7110381
Chicago/Turabian StyleMandal, Shrawan Kumar, Jenish Neupane, Ajay M. V. Kumar, Hayk Davtyan, Pruthu Thekkur, Anup Jayaram, Bimal Sharma Chalise, Manisha Rawal, Manu Paudel, Bishwodip Baral, and et al. 2022. "Audit of Clinical Care Received by COVID-19 Patients Treated at a Tertiary Care Hospital of Nepal in 2021" Tropical Medicine and Infectious Disease 7, no. 11: 381. https://doi.org/10.3390/tropicalmed7110381
APA StyleMandal, S. K., Neupane, J., Kumar, A. M. V., Davtyan, H., Thekkur, P., Jayaram, A., Chalise, B. S., Rawal, M., Paudel, M., Baral, B., Shah, R. K., Maharjan, K., Shrestha, S., Bhandari, L., K.C., N., Gautam, N., Sunny, A. K., Thakur, N., Subeedee, K. C., ... Bastola, A. (2022). Audit of Clinical Care Received by COVID-19 Patients Treated at a Tertiary Care Hospital of Nepal in 2021. Tropical Medicine and Infectious Disease, 7(11), 381. https://doi.org/10.3390/tropicalmed7110381










