In Vitro and Ultrastructural Evaluation of the Cytotoxic and Antileishmanial Activities of Thiosemicarbazone Compounds Against Promastigotes and Axenic Amastigotes of Leishmania infantum
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization and Maintenance of the Leishmania infantum Strain
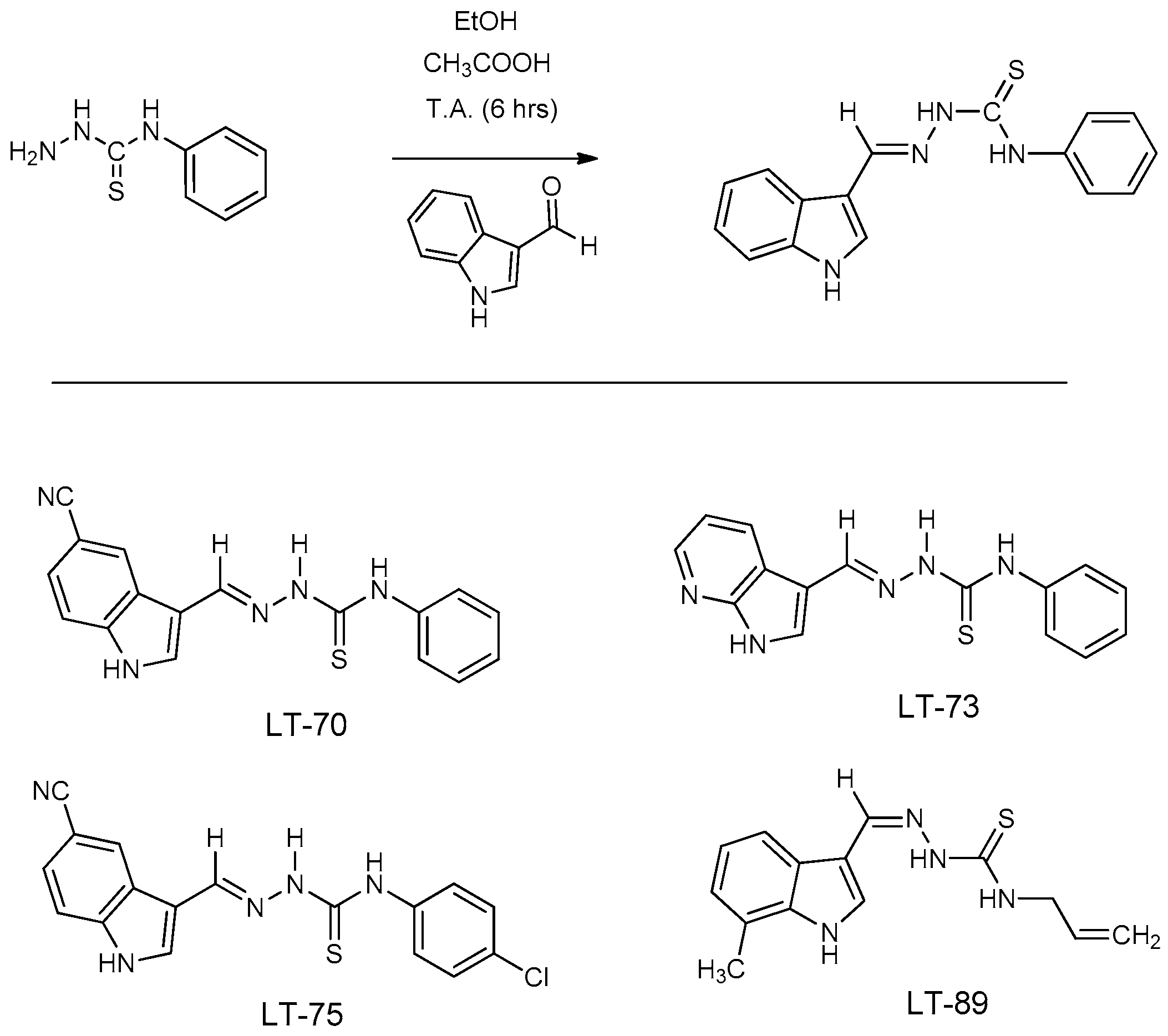
2.2. Synthesis of Indole-Thiosemicarbazone LTs Compounds
2.3. In Vitro Evaluation of Leishmanicidal Activity of Thiosemicarbazone Compounds (LT-70, LT-73, LT-75, and LT-89)
2.4. Differentiation of Promastigote Forms into Axenic Amastigote Forms
2.5. Ultrastructural Analysis of L. infantum
2.6. Cytokine Dosage of the Cellular Immune Response After Treatment of LT-73 and LT-75 Compounds
2.7. Evaluation of Mitochondrial Membrane Potential (ΔΨm)
2.8. Evaluation of Apoptosis and Necrosis in L. infantum Promastigote Forms
2.9. Statistical Analysis
3. Results
3.1. Leishmanicidal Activity of LT Compounds
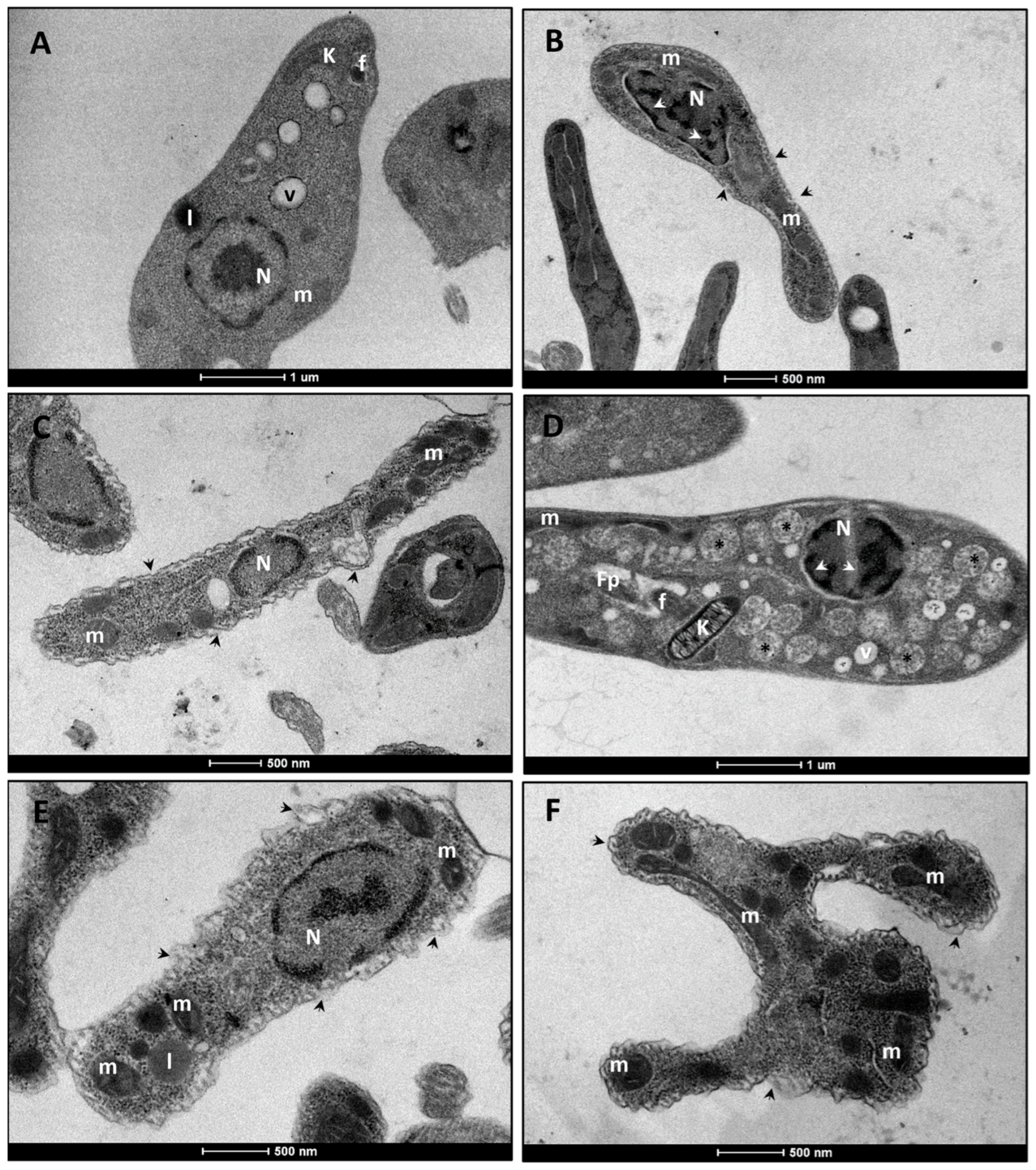
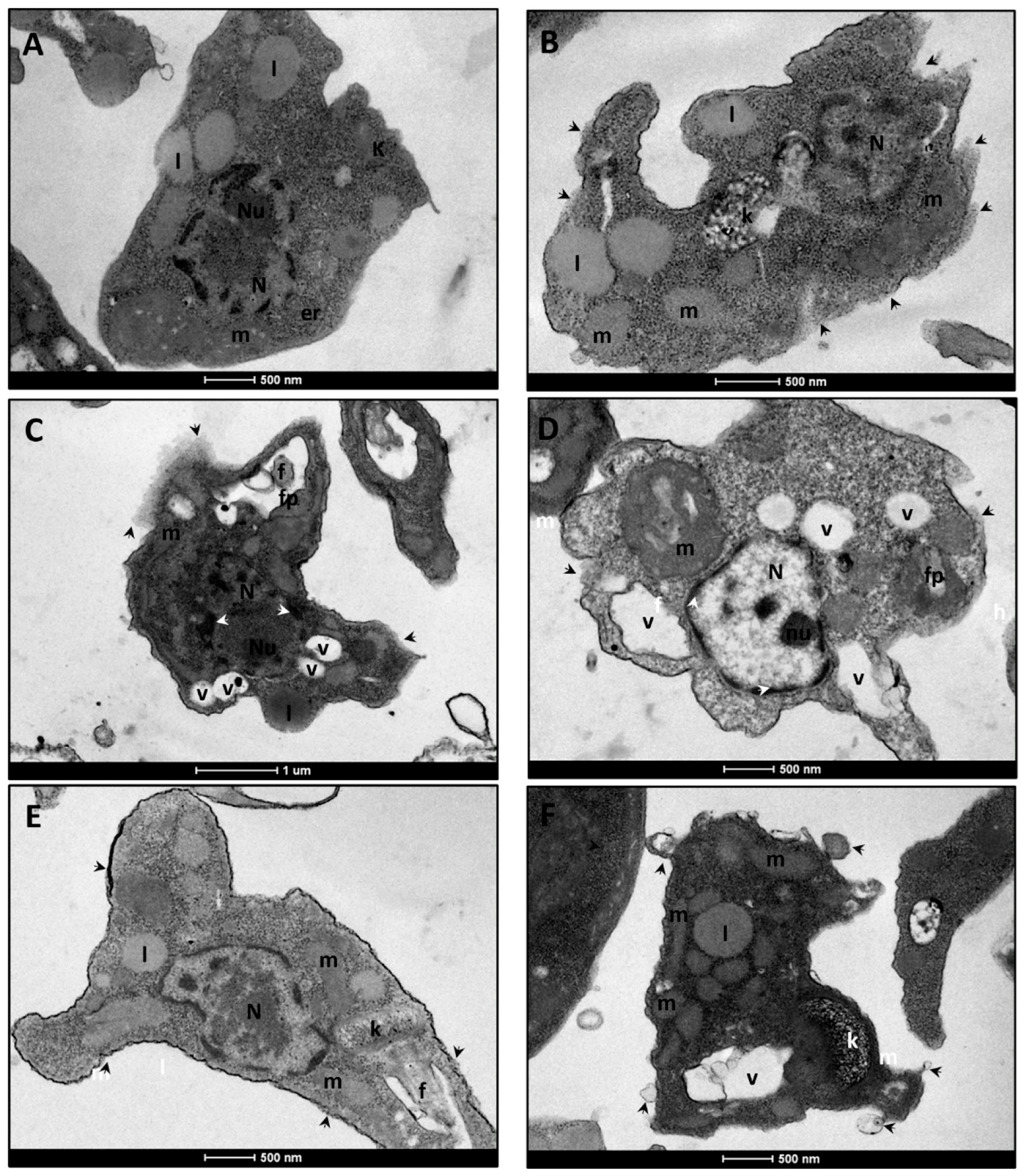
3.2. Ultrastructural Analysis of L. infantum Promastigote Forms
3.2.1. L. infantum Promastigote Forms
3.2.2. L. infantum Amastigote Forms
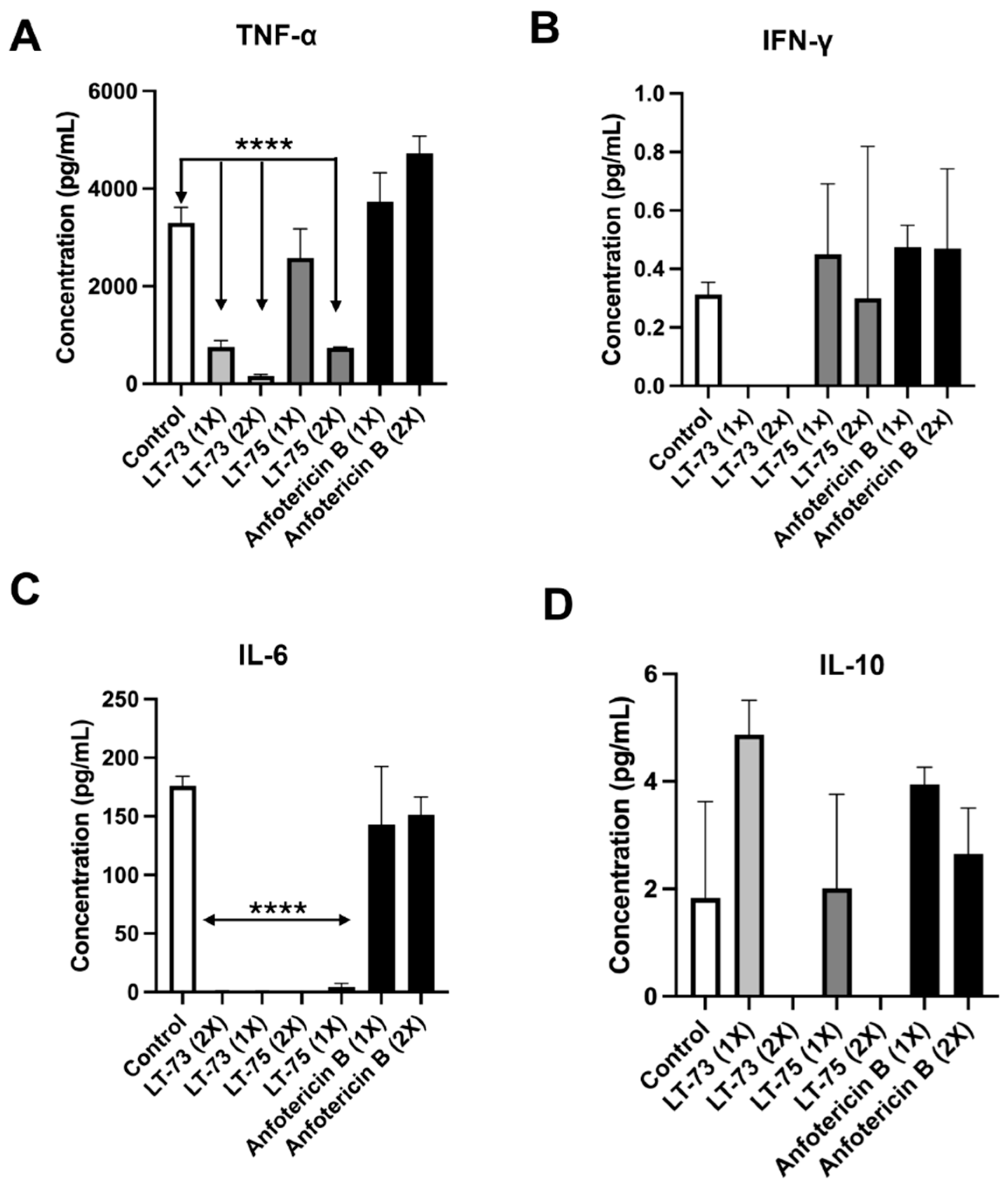
3.3. Results of Cytokine Dosage of the Cellular Immune Response After Treatment of LT-73 and LT-75 Compounds
3.4. Cell Death Analysis
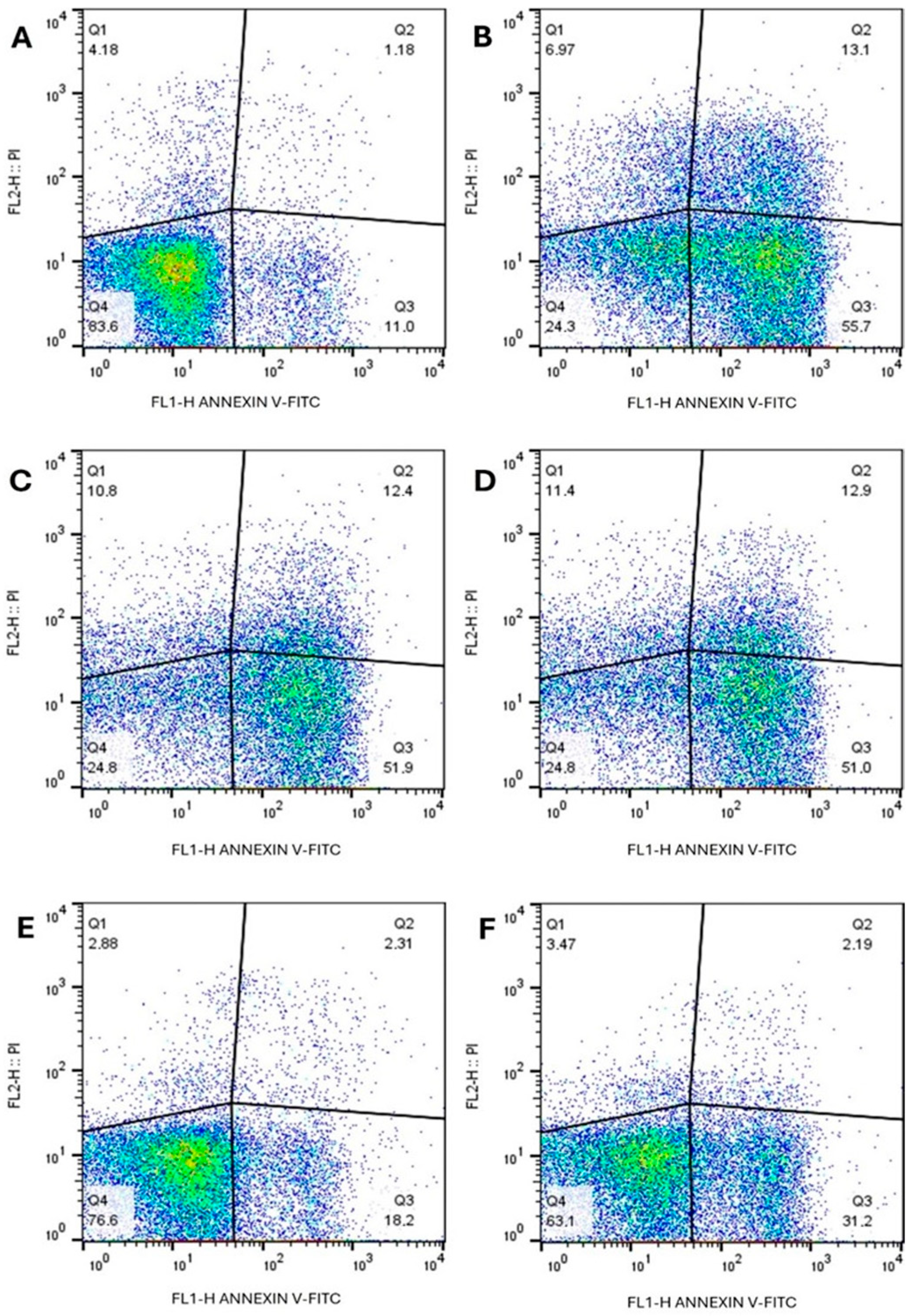
3.4.1. Annexin and Propidium Iodide
3.4.2. Assessment of Membrane Potential
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Leishmaniasis. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 6 March 2025).
- Volpedo, G.; Huston, R.H.; Holcomb, E.A.; Pacheco-Fernandez, T.; Gannavaram, S.; Bhattacharya, P.; Nakhasi, H.L.; Satoskar, A.R. From infection to vaccination: Reviewing the global burden, history of vaccine development, and recurring challenges in global leishmaniasis protection. Expert Rev. Vaccines 2021, 20, 1431–1446. [Google Scholar] [CrossRef]
- Cohen, A.; Azas, N. Challenges and tools for In Vitro Leishmania exploratory screening in the drug development process: An updated review. Pathogens 2021, 10, 1608. [Google Scholar] [CrossRef]
- De Barros, D.; Freitas, L.A.B.; dos Santos, I.R.; de Almeida, V.S.; do Amaral e Melo, R.T.; de Melo Silva, V.G.; de Fátima Maia de Santana, B.; da Conceição, J.M.; Lima Leite, A.C. An overview of the compounds tested in vivo for Leishmania spp. of the last 5 years. Curr. Med. Chem. 2021, 28, 4226–4258. [Google Scholar] [CrossRef]
- Ghorbani, M.; Farhoudi, R. Leishmaniasis in humans: Drug or vaccine therapy? Drug Des. Dev. Ther. 2018, 12, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Morelle, C.; Mukherjee, A.; Zhang, J.; Fani, F.; Khandelwal, A.; Gingras, H.; Trottier, J.; Barbier, O.; Leprohon, P.; Burke, M.D.; et al. Well-tolerated amphotericin B derivatives that effectively treat visceral leishmaniasis. ACS Infect. Dis. 2021, 7, 2472–2482. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.R.H.; Bicanic, T.; Salim, R.; Hope, W. Liposomal amphotericin B (AmBisome®): A review of the pharmacokinetics, pharmacodynamics, clinical experience and future directions. Drugs 2016, 76, 485–500. [Google Scholar] [CrossRef]
- Palić, S.; Bhairosing, P.; Beijnen, J.H.; Dorlo, T.P. Systematic review of host-mediated activity of miltefosine in leishmaniasis through immunomodulation. Antimicrob. Agents Chemother. 2019, 63, e02507-18. [Google Scholar] [CrossRef]
- Singh, R.; Kashif, M.; Srivastava, P.; Manna, P.P. Recent advances in chemotherapeutics for leishmaniasis: Importance of the cellular biochemistry of the parasite and its molecular interaction with the host. Pathogens 2023, 12, 706. [Google Scholar] [CrossRef]
- Tom, A.A.; Sunilkumar, S.A.; Thottasseri, A.A.; Kannan, T. Combating drug-resistant protozoal infections: A review of emerging therapeutics. Arch. Der Pharm. 2025, 358, e70029. [Google Scholar] [CrossRef] [PubMed]
- Hayat, F.; Moseley, E.; Salahuddin, A.; Van Zyl, R.L.; Azam, A. Antiprotozoal activity of chloroquinoline based chalcones. Eur. J. Med. Chem. 2011, 46, 1897–1905. [Google Scholar] [CrossRef]
- Bhatia, R.K. Anti-protozoal potential of heterocyclic compounds against giardiasis. Curr. Bioact. Compd. 2019, 15, 280–288. [Google Scholar] [CrossRef]
- Salat, K.; Moniczewski, A.; Librowski, T. Nitrogen, oxygen or sulfur containing heterocyclic compounds as analgesic drugs used as modulators of the nitroxidative stress. Mini Rev. Med. Chem. 2013, 13, 335–352. [Google Scholar] [CrossRef]
- Mermer, A.; Keles, T.; Sirin, Y. Recent studies of nitrogen-containing heterocyclic compounds as novel antiviral agents: A review. Bioorg. Chem. 2021, 114, 105076. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Sinha, P.K.; Rai, M.; Verma, D.K.; Nawin, K.; Alam, S.; Chakravarty, J.; Vaillant, M.; Verma, N.; Pandey, K.; et al. Comparison of short-course multidrug treatment with standard therapy for visceral leishmaniasis in India: An open-label, non-inferiority, randomised controlled trial. Lancet 2011, 377, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.R.; de Oliveira, J.F.; da Silva, A.L.; Queiroz, C.M.; Feitosa, A.P.S.; Duarte, D.M.F.A.; da Silva, A.C.; de Castro, M.C.A.B.; Pereira, V.R.A.; da Silva, R.M.F.; et al. Novel indol-3-yl-thiosemicarbazone derivatives: Obtaining, evaluation of in vitro leishmanicidal activity and ultrastructural studies. Chem. Biol. Interact. 2020, 315, 108899. [Google Scholar] [CrossRef]
- Almeida, L. Leishmanioses e Derivados de Furoxano e Benzofuroxano: Atividade Biológica In Vitro e In Vivo e Potenciais Mecanismos de Ação. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2017. Available online: http://hdl.handle.net/11449/150226 (accessed on 2 April 2022).
- Goyal, N.; Patel, M.; Batra, S. Modern drug discovery and development in the area of leishmaniasis. In Drug Discovery and Drug Development; Springer: Singapore, 2021; pp. 123–158. [Google Scholar] [CrossRef]
- Dos Santos, M.G.; Muxel, S.M.; Zampieri, R.A.; Pomorski, T.G.; Floeter-Winter, L.M. Transbilayer dynamics of phospholipids in the plasma membrane of the Leishmania genus. PLoS ONE 2013, 8, e55604. [Google Scholar] [CrossRef]
- Jacob, I.T.T.; Gomes, F.O.S.; de Miranda, M.D.S.; de Almeida, S.M.V.; da Cruz-Filho, I.J.; Peixoto, C.A.; da Silva, T.G.; Moreira, D.R.M.; de Melo, C.M.L.; de Oliveira, J.F.; et al. Anti-inflammatory activity of novel thiosemicarbazone compounds indole-based as COX inhibitors. Pharmacol. Rep. 2021, 73, 907–925. [Google Scholar] [CrossRef]
- Aliança, A.S.D.S.; Oliveira, A.R.; Feitosa, A.P.S.; Ribeiro, K.R.C.; de Castro, M.C.A.B.; Leite, A.C.L.; Alves, L.C.; Brayner, F.A. In vitro evaluation of cytotoxicity and leishmanicidal activity of phthalimido-thiazole derivatives. Eur. J. Pharm. Sci. 2017, 105, 1–10. [Google Scholar] [CrossRef]
- Oliveira, S.S.C.; Marinho, F.A.; Sangenito, L.S.; Seabra, S.H.; Menna-Barreto, R.F.; D’avila, C.M.; Santos, A.L.S.; Branquinha, M.H. Susceptibility of Leishmania amazonensis axenic amastigotes to the calpain inhibitor MDL28170. Trop. Med. Infect. Dis. 2024, 9, 259. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, C.M.; Filho, G.B.d.O.; Espíndola, J.W.P.; Nascimento, A.V.D.; Aliança, A.S.d.S.; de Lorena, V.M.B.; Feitosa, A.P.S.; da Silva, P.R.; Alves, L.C.; Leite, A.C.L.; et al. Thiosemicarbazone and thiazole: In vitro evaluation of leishmanicidal and ultrastructural activity on Leishmania infantum. Med. Chem. Res. 2020, 29, 2050–2065. [Google Scholar] [CrossRef]
- Lopes, G.D.; Zabala-Peñafiel, A.; de Albuquerque-Melo, B.C.; Souza-Silva, F.; Canto, L.M.D.; Cysne-Finkelstein, L.; Alves, C.R. Axenic amastigotes of Leishmania species as a suitable model for in vitro studies. Acta Trop. 2021, 220, 105956. [Google Scholar] [CrossRef]
- Doğan, M.; Koçyiğit, Ü.M.; Gürdere, M.B.; Ceylan, M.; Budak, Y. Synthesis and biological evaluation of thiosemicarbazone derivatives. Med. Oncol. 2022, 39, 157. [Google Scholar] [CrossRef]
- Costa, D.L.; Lima, M.S.C.; Sampaio, T.L.; Gois, R.W.; Freire, T.M.; de Assis, R.R.; de Oliveira, M.R.; Goulart, L.R.; Freitas, R.M.; Veras, P.S.T. Antileishmanial activity of antiretroviral drugs combined with miltefosine against Leishmania infantum. Parasitol. Res. 2016, 115, 3881–3887. [Google Scholar] [CrossRef]
- Vermeersch, M.; da Luz, R.I.; Tote, K.; Timmermans, J.P.; Cos, P.; Maes, L. In vitro susceptibilities of Leishmania donovani promastigote and amastigote stages to antileishmanial reference drugs: Practical relevance of stage-specific differences. Antimicrob. Agents Chemother. 2009, 53, 3855–3859. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, S.; Van Bockstal, L.; Mondelaers, A.; Caljon, G.; Maes, L. Experimental selection of paromomycin resistance in Leishmania donovani amastigotes: Stability, fitness and molecular mechanisms. Microorganisms 2021, 9, 1683. [Google Scholar] [CrossRef]
- Paris, C.; Loiseau, P.M.; Bories, C.; Bréard, J. Miltefosine induces apoptosis-like death in Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 2004, 48, 852–859. [Google Scholar] [CrossRef]
- Majoor, A.; Michel, G.; Marty, P.; Boyer, L.; Pomares, C. Leishmaniases: Strategies in Treatment Development. Parasite 2025, 32, 18. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.S.; Moreira, V.P.; Silva, E.d.S.; Cardoso, L.L.; Palmeira, P.H.d.S.; Cavalcante-Silva, L.H.A.; de Araújo, D.A.M.; Amaral, I.P.G.D.; González, E.R.P.; Keesen, T.S.L. Leishmanicidal Activity of Guanidine Derivatives against Leishmania infantum. Trop. Med. Infect. Dis. 2023, 8, 141. [Google Scholar] [CrossRef]
- Mendes, E.P.; Goulart, C.M.; Chaves, O.A.; Faiões, V.d.S.; Canto-Carvalho, M.M.; Machado, G.C.; Torres-Santos, E.C.; Echevarria, A. Evaluation of Novel Chalcone-Thiosemicarbazones Derivatives as Potential Anti-Leishmania amazonensis Agents and Its HSA Binding Studies. Biomolecules 2019, 9, 643. [Google Scholar] [CrossRef]
- DNDi. Drug Screening Manual: Hit Selection Criteria for Antileishmanial Drug Discovery; Drugs for Neglected Diseases initiative (DNDi): Geneva, Switzerland, 2009; Available online: https://www.dndi.org (accessed on 10 November 2025).
- Mollineda-Diogo, N.; Sifontes-Rodríguez, S.; Aguirre-García, M.M.; Escalona-Montaño, A.R.; Espinosa-Buitrago, T.; Mondragón-Flores, R.; Mondragón-Castelán, M.E.; Meneses-Marcel, A.; Pérez-Olvera, O.; Sánchez-Almaraz, D.A.; et al. 3-Alkoxy-1-benzyl-5-nitroindazole derivatives are potent antileishmanial compounds. Int. J. Mol. Sci. 2024, 25, 10582. [Google Scholar] [CrossRef]
- Gouveia, A.L.A.; Santos, F.A.; Alves, L.C.; Cruz-Filho, I.J.; Silva, P.R.; Jacob, I.T.; Soares, J.C.S.; Santos, D.K.; Souza, T.R.C.; Oliveira, J.F.; et al. Thiazolidine derivatives: In vitro toxicity assessment against promastigote and amastigote forms of Leishmania infantum and ultrastructural study. Exp. Parasitol. 2022, 236, 108253. [Google Scholar] [CrossRef]
- Kaczanowski, S.; Sajid, M.; Reece, S.E. Evolution of apoptosis-like programmed cell death in unicellular protozoan parasites. Parasit. Vectors 2011, 4, 44. [Google Scholar] [CrossRef]
- Minori, K.; Gadelha, F.R.; Bonsignore, R.; Alcántar, G.M.; Fontes, J.V.; Abbehausen, C.; Brioschi, M.B.; de Sousa, L.M.; Consonni, S.R.; Casini, A.; et al. An organogold compound impairs Leishmania amazonensis amastigotes survival and delays lesion progression in murine cutaneous leishmaniasis: Mechanistic insights. Biochem. Pharmacol. 2025, 232, 116716. [Google Scholar] [CrossRef] [PubMed]
- Tiuman, T.S.; Ueda-Nakamura, T.; Alonso, A.; Nakamura, C.V. Cell death in amastigote forms of Leishmania amazonensis induced by parthenolide. BMC Microbiol. 2014, 14, 152. [Google Scholar] [CrossRef] [PubMed]
- Kolodziej, H.; Kiderlen, A.F. Antileishmanial activity and immune modulatory effects of tannins and related compounds on Leishmania parasitized RAW 264.7 cells. Phytochemistry 2005, 66, 2056–2071. [Google Scholar] [CrossRef]
- Rodrigues, I.A.; Mazotto, A.M.; Cardoso, V.; Alves, R.L.; Amaral, A.C.F.; Silva, J.R.D.A.; Pinheiro, A.S.; Vermelho, A.B. Natural products: Insights into leishmaniasis inflammatory response. Mediators Inflamm. 2015, 2015, 835910. [Google Scholar] [CrossRef]
- Cristovão-Silva, A.C.; Brelaz-De-Castro, M.C.A.; da Silva, E.D.; Leite, A.C.L.; Santiago, L.B.A.A.; da Conceição, J.M.; Tiburcio, R.d.S.; de Santana, D.P.; Bedor, D.C.G.; de Carvalho, B.Í.V.; et al. Trypanosoma cruzi killing and immune response boosting by novel phenoxyhydrazine-thiazole against Chagas disease. Exp. Parasitol. 2024, 261, 108749. [Google Scholar] [CrossRef]
- da Conceição, J.M.; Santos, A.C.d.S.; Brayner, F.A.; Alves, L.C.; Pinto, A.F.; Brondani, G.L.; Filho, G.B.d.O.; Bedor, D.C.G.; da Silva, J.W.V.; Junior, P.A.S.; et al. Structural design, synthesis, and anti-Trypanosomatidae profile of new pyridyl-thiazolidinones. Eur. J. Med. Chem. 2023, 254, 115310. [Google Scholar] [CrossRef] [PubMed]
- Valigurová, A.; Kolářová, I. Unrevealing the mystery of latent Leishmaniasis: What cells can host Leishmania? Pathogens 2023, 12, 246. [Google Scholar] [CrossRef]
- Mesquita, J.T.; Pinto, E.G.; Taniwaki, N.N.; Galisteo, A.J., Jr.; Tempone, A.G. Lethal action of the nitrothiazolyl-salicylamide derivative nitazoxanide via induction of oxidative stress in Leishmania (L.) infantum. Acta Trop. 2013, 128, 666–673. [Google Scholar] [CrossRef]
- Tavares, G.S.V.; Mendonça, D.V.C.; Lage, D.P.; Granato, J.d.T.; Ottoni, F.M.; Ludolf, F.; Chávez-Fumagalli, M.A.; Duarte, M.C.; Tavares, C.A.P.; Alves, R.J.; et al. Antileishmanial activity, cytotoxicity and mechanism of action of clioquinol against Leishmania infantum and Leishmania amazonensis species. Basic Clin. Pharmacol. Toxicol. 2018, 123, 236–246. [Google Scholar] [CrossRef] [PubMed]


| Compounds | Promastigotes IC50 (µM) | % Inhibition at 20 µM | J774 CC50 (µM) * | SI (CC50/IC50) a |
|---|---|---|---|---|
| LT-70 | 12 ± 0.1 | 100% | 64 ± 5 | 5.5 |
| LT-73 | 13 ± 0.1 | 100% | >75 ** | >5.8 |
| LT-75 | 10.5 ± 1 | 100% | >75 ** | >7.1 |
| LT-89 | 14 ± 0.1 | 100% | 60 ± 4 | 4.3 |
| Amphotericin B | 0.04 ± 0 | 100% | 14 ± 0.3 | 350 |
| Compounds | Mean | Variation |
|---|---|---|
| Control | 709 ± 29 | - |
| (1× IC50) LT-73 | 658 ± 9 | −0.07 ± 0.05 |
| (2× IC50) LT-73 | 697 ± 7 | −0.02 ± 0.03 |
| (1× IC50) LT-75 | 1425 ± 106 | 1 ± 0.23 |
| (2× IC50) LT-75 | 1000 ± 21 | 0.41 ± 0.05 |
| (1× IC50) Amb B | 213 ± 1 | −0.70 ± 0.01 |
| (2× IC50) Amb B | 193 ± 3 | −0.73 ± 0.02 |
| Hydrogen Peroxide (H2O2) | 581 ± 35 | −0.18 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes Menezes da Silva, J.W.; Alves da Rocha Diniz, A.R.; Ribeiro de Araújo, A.; Gazzoni Araújo Gonçalves, G.; Leal Veras, D.; de Andrade Cavalcante, M.K.; Sandes, J.M.; da Cruz Filho, I.J.; Santa Clara Marques, D.; Alves de Lima, M.d.C.; et al. In Vitro and Ultrastructural Evaluation of the Cytotoxic and Antileishmanial Activities of Thiosemicarbazone Compounds Against Promastigotes and Axenic Amastigotes of Leishmania infantum. Trop. Med. Infect. Dis. 2025, 10, 325. https://doi.org/10.3390/tropicalmed10110325
Lopes Menezes da Silva JW, Alves da Rocha Diniz AR, Ribeiro de Araújo A, Gazzoni Araújo Gonçalves G, Leal Veras D, de Andrade Cavalcante MK, Sandes JM, da Cruz Filho IJ, Santa Clara Marques D, Alves de Lima MdC, et al. In Vitro and Ultrastructural Evaluation of the Cytotoxic and Antileishmanial Activities of Thiosemicarbazone Compounds Against Promastigotes and Axenic Amastigotes of Leishmania infantum. Tropical Medicine and Infectious Disease. 2025; 10(11):325. https://doi.org/10.3390/tropicalmed10110325
Chicago/Turabian StyleLopes Menezes da Silva, Janderson Weydson, Andréa Regina Alves da Rocha Diniz, Alberon Ribeiro de Araújo, Gabriel Gazzoni Araújo Gonçalves, Dyana Leal Veras, Marton Kaique de Andrade Cavalcante, Jana Messias Sandes, Iranildo José da Cruz Filho, Diego Santa Clara Marques, Maria do Carmo Alves de Lima, and et al. 2025. "In Vitro and Ultrastructural Evaluation of the Cytotoxic and Antileishmanial Activities of Thiosemicarbazone Compounds Against Promastigotes and Axenic Amastigotes of Leishmania infantum" Tropical Medicine and Infectious Disease 10, no. 11: 325. https://doi.org/10.3390/tropicalmed10110325
APA StyleLopes Menezes da Silva, J. W., Alves da Rocha Diniz, A. R., Ribeiro de Araújo, A., Gazzoni Araújo Gonçalves, G., Leal Veras, D., de Andrade Cavalcante, M. K., Sandes, J. M., da Cruz Filho, I. J., Santa Clara Marques, D., Alves de Lima, M. d. C., Sampaio Feitosa, A. P., Alves, L. C., & Brayner, F. A. (2025). In Vitro and Ultrastructural Evaluation of the Cytotoxic and Antileishmanial Activities of Thiosemicarbazone Compounds Against Promastigotes and Axenic Amastigotes of Leishmania infantum. Tropical Medicine and Infectious Disease, 10(11), 325. https://doi.org/10.3390/tropicalmed10110325






