Strategies for Tuberculosis Prevention in Healthcare Settings: A Narrative Review
Abstract
1. Introduction
2. Primary Prevention
2.1. Basics of Primary Prevention
2.2. Healthcare Worker Behaviors and Barriers Towards Infection Control
2.3. Suggested Solutions
2.4. Role of Vaccines
3. Secondary Prevention
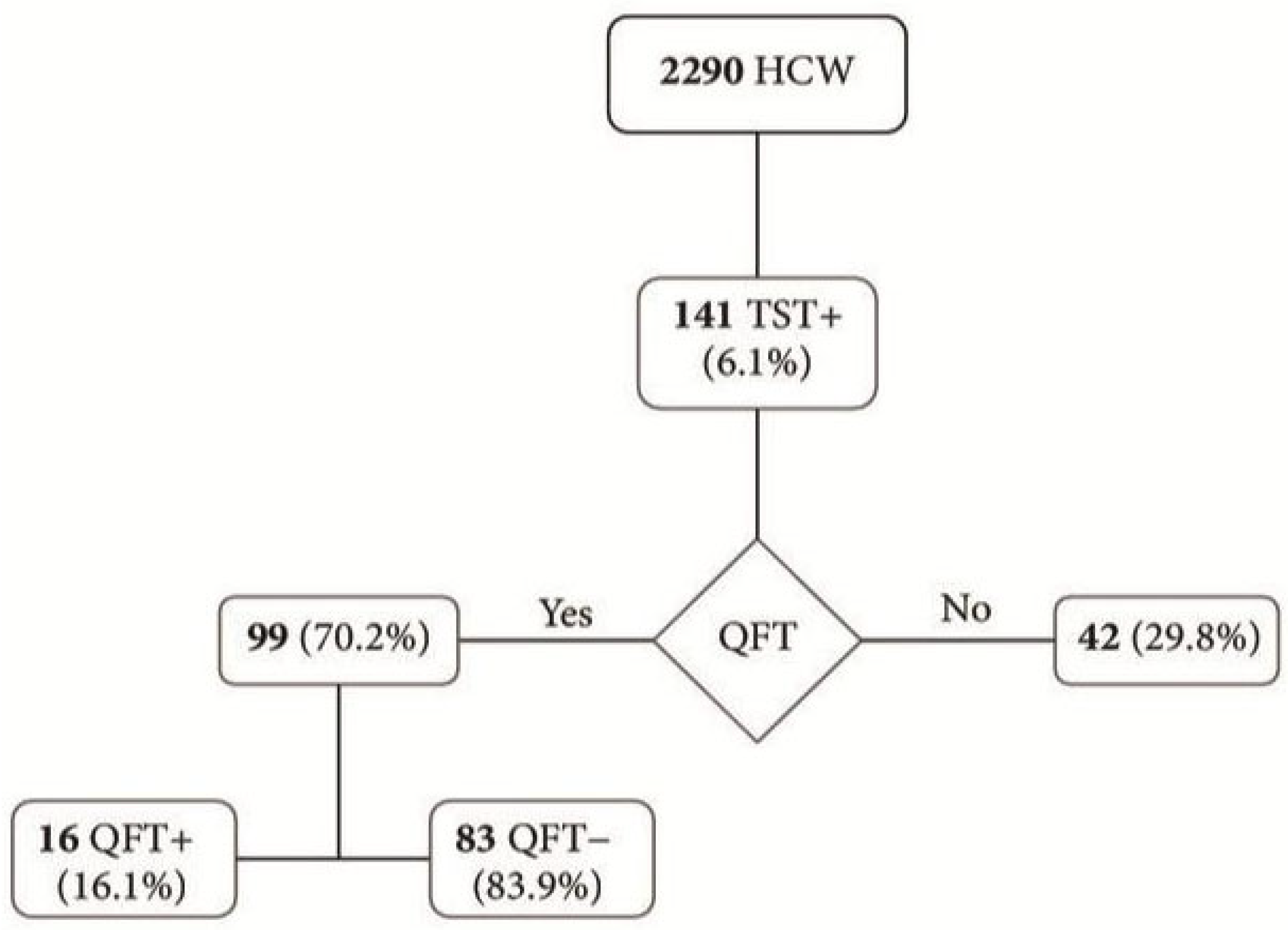
3.1. Routine Screening
3.2. Early Diagnosis
3.3. Role of Rapid Diagnostics
4. Tertiary Prevention
4.1. Ensuring Adherence to Treatment
4.2. Drug-Resistant TB
5. Cost-Effectiveness and Real-World Applicability
6. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TB | tuberculosis |
| HCW | healthcare workers |
| LTBI | latent tuberculosis infection |
| TST | tuberculin skin test |
| IGRA | interferon-gamma release assay |
| HEPA | high-efficiency particulate air |
| UVGI | Ultra-Violet Germicidal Irradiation |
| PPE | personal protective equipment |
| BCG | Baccillus Calmette-Guerin |
| QFT | QuantiFERON-TB Gold |
| CAD | computer-aided detection |
| AI | artificial intelligence |
| WGS | whole-genome sequencing |
| DOT | Directly Observed Therapy |
| VOT | Video-Observed Therapy |
| SAT | self-administered therapy |
| XDR-TB | Extensively Drug-Resistant Tuberculosis |
| TPT | targeted tuberculosis preventive therapy |
References
- Martinez, L.; Verma, R.; Croda, J.; Horsburgh, C.R., Jr.; Walter, K.S.; Degner, N.; Middelkoop, K.; Koch, A.; Hermans, S.; Warner, D.F.; et al. Detection, survival and infectious potential of Mycobacterium tuberculosis in the environment: A review of the evidence and epidemiological implications. Eur. Respir. J. 2019, 53, 1802302. [Google Scholar] [CrossRef] [PubMed]
- Uden, L.; Barber, E.; Ford, N.; Cooke, G.S. Risk of tuberculosis infection and disease for healthcare workers: An updated meta-analysis. Open Forum Infect. Dis. 2017, 4, ofx137. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global TB Report 2024; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Reported Tuberculosis Cases in Healthcare Workers, United States, 2023; CDC: Atlanta, GA, USA, 2024. [Google Scholar]
- Du, J.; Zhang, L.; Liu, Y.; Shu, W.; Ma, Y.; Gao, J.; Li, L. Determination of the optimal time for N95 respirator for aerosol infection control. Medicine 2020, 99, e23709. [Google Scholar] [CrossRef]
- Lee, J.Y. Tuberculosis infection control in health-care facilities: Environmental control and personal protection. Tuberc. Respir. Dis. 2016, 79, 234–240. [Google Scholar] [CrossRef]
- Pai, M.; Behr, M.A.; Dowdy, D. Tuberculosis. Nat. Rev. Dis. Primers 2016, 2, 16076. [Google Scholar] [CrossRef]
- Dorman, S.E.; Schumacher, S.G.; Alland, D.; Nabeta, P.; Armstrong, D.T.; King, B.; Hall, S.L.; Chakravorty, S.; Cirillo, D.M.; Tukvadze, N.; et al. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: A prospective multicentre diagnostic accuracy study. Lancet Infect. Dis. 2018, 18, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Aranas, L.L.; Alam, K.; Gyawali, P.; Alam, R.M. Drug-resistant tuberculosis stigma among healthcare workers: A scoping review. Inquiry 2023, 60, 469580231180754. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Bhatia, V.; Sharma, M.; Mandal, P.P.; Arinaminpathy, N. The potential impact of preventive therapy against tuberculosis in the WHO South-East Asian Region: A modelling approach. BMC Med. 2020, 18, 163. [Google Scholar] [CrossRef]
- Harries, A.D.; Kumar, A.M.V.; Satyanarayana, S.; Thekkur, P.; Lin, Y.; Dlodlo, R.A.; Khogali, M.; Zachariah, R. The Growing Importance of Tuberculosis Preventive Therapy and How Research and Innovation Can Enhance Its Implementation on the Ground. Trop. Med. Infect. Dis. 2020, 5, 61. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tanimura, T.; Jaramillo, E.; Weil, D.; Raviglione, M.; Lonnroth, K. Financial burden for tuberculosis patients in low- and middle-income countries: A systematic review. Eur. Respir. J. 2014, 43, 1763–1775. [Google Scholar] [CrossRef]
- Jiang, W.-X.; Li, Z.-P.; Zhao, Q.; Gao, M.-Q.; Long, Q.; Wang, W.-B.; Huang, F.; Wang, N.; Tang, S.-L. Impacts of a comprehensive tuberculosis control model on the quality of clinical services and the financial burden of treatment for patients with drug-resistant tuberculosis in China: A mixed-methods evaluation. Infect. Dis. Poverty 2021, 10, 54. [Google Scholar] [CrossRef]
- Assefa, D.G.; Dememew, Z.G.; Zeleke, E.D.; Manyazewal, T.; Bedru, A. Financial burden of tuberculosis diagnosis and treatment for patients in Ethiopia: A systematic review and meta-analysis. BMC Public Health 2024, 24, 260. [Google Scholar] [CrossRef] [PubMed]
- Escombe, A.R.; Moore, D.A.J.; Gilman, R.H.; Navincopa, M.; Ticona, E.; Mitchell, B.; Noakes, C.; Martínez, C.; Sheen, P.; Ramirez, R.; et al. Upper-room ultraviolet light and negative air ionization to prevent tuberculosis transmission. PLoS Med. 2015, 6, e1000043. [Google Scholar] [CrossRef] [PubMed]
- CDC. Clinical Testing Guidance for Tuberculosis: Health Care Personnel. TB Prevention in Health Care Settings. 2024. Available online: https://www.cdc.gov/tb-healthcare-settings/hcp/screening-testing/index.html (accessed on 25 July 2025).
- Wisconsin Department of Health Services. Tuberculosis Precautions; Wisconsin Department of Health Services: Madison, WI, USA, 2024. Available online: https://www.dhs.wisconsin.gov/tb/precautions.htm (accessed on 25 July 2025).
- Houghton, C.; Meskell, P.; Delaney, H.; Smalle, M.; Glenton, C.; Booth, A.; Chan, X.H.S.; Devane, D.; Biesty, L.M. Barriers and facilitators to healthcare workers’ adherence with infection prevention and control (IPC) guidelines for respiratory infectious diseases: A rapid qualitative evidence synthesis. Cochrane Database Syst. Rev. 2020, 4, CD013582. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Kallon, I.I.; Colvin, C.J.; Grant, A.D. Barriers and facilitators of tuberculosis infection prevention and control in low- and middle-income countries from the perspective of healthcare workers: A systematic review. PLoS ONE 2020, 15, e0241039. [Google Scholar] [CrossRef]
- Sissolak, D.; Marais, F.; Mehtar, S. TB infection prevention and control experiences of South African nurses--a phenomenological study. BMC Public Health 2011, 11, 262. [Google Scholar] [CrossRef]
- Nathavitharana, R.R.; Bond, P.; Dramowski, A.; Kotze, K.; Lederer, P.; Oxley, I.; Peters, J.A.; Rossouw, C.; van der Westhuizen, H.M.; Willems, B.; et al. Agents of change: The role of healthcare workers in the prevention of nosocomial and occupational tuberculosis. Presse Medicale 2017, 46, e53–e62. [Google Scholar] [CrossRef]
- Umoya Omuhle [Internet]. Available online: https://www.lshtm.ac.uk/uo (accessed on 20 July 2025).
- McCreesh, N.; Karat, A.S.; Baisley, K.; Diaconu, K.; Bozzani, F.; Govender, I.; Beckwith, P.; Yates, T.A.; Deol, A.K.; Houben, R.M.G.J.; et al. Modelling the effect of infection prevention and control measures on rate of Mycobacterium tuberculosis transmission to clinic attendees in primary health clinics in South Africa. BMJ Glob. Health 2021, 6, e007124. [Google Scholar] [CrossRef]
- Munro, S.; Lewin, S.; Swart, T.; Volmink, J. A review of health behaviour theories: How useful are these for developing interventions to promote long-term medication adherence for TB and HIV/AIDS? BMC Public Health 2007, 7, 104. [Google Scholar] [CrossRef]
- Ward, D.J. The role of education in the prevention and control of infection: A review of the literature. Nurse Educ. Today 2011, 31, 9–17. [Google Scholar] [CrossRef]
- Qu, M.; Zhou, X.; Li, H. BCG vaccination strategies against tuberculosis: Updates and perspectives. Hum. Vaccines Immunother. 2021, 17, 5284–5295. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.; Cords, O.; Liu, Q.; Acuna-Villaorduna, C.; Bonnet, M.; Fox, G.J.; Carvalho, A.C.C.; Chan, P.C.; Croda, J.; Hill, P.C.; et al. Infant BCG vaccination and risk of pulmonary and extrapulmonary tuberculosis throughout the life course: A systematic review and individual participant data meta-analysis. Lancet. Glob. Health 2022, 10, e1307–e1316. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Pan, C.; Cheng, P.; Wang, J.; Zhao, G.; Wu, X. Peptide-Based Vaccines for Tuberculosis. Front. Immunol. 2022, 13, 830497. [Google Scholar] [CrossRef]
- Vasiliu, A.; Martinez, L.; Gupta, R.K.; Hamada, Y.; Ness, T.; Kay, A.; Bonnet, M.; Sester, M.; Kaufmann, S.H.E.; Lange, C.; et al. Tuberculosis prevention: Current strategies and future directions. Clin. Microbiol. Infect. 2024, 30, 1123–1130. [Google Scholar] [CrossRef]
- Napoli, C.; Ferretti, F.; Di Ninno, F.; Orioli, R.; Marani, A.; Sarlo, M.G.; Prestigiacomo, C.; De Luca, A.; Orsi, G.B. Screening for tuberculosis in health care workers: Experience in an Italian teaching hospital. Biomed Res. Int. 2017, 2017, 7538037. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.M.; Madhukeshwar, A.K.; Naik, P.R.; Nayarmoole, B.M.; Satyanarayana, S. Magnitude and reasons for gaps in tuberculosis diagnostic testing and treatment initiation: An operational research study from Dakshina Kannada, South India. J. Epidemiol. Glob. Health 2020, 10, 326–336. [Google Scholar] [CrossRef]
- World Health Organization. WHO Operational Handbook on Tuberculosis Module 2: Screening—Systematic Screening for Tuberculosis Disease. Available online: https://www.who.int/publications/i/item/9789240022614 (accessed on 20 July 2025).
- World Health Organization. WHO Consolidated Guidelines on Tuberculosis: Module 1: Prevention: Tuberculosis Preventive Treatment. Available online: https://www.who.int/publications/i/item/9789240001503 (accessed on 20 July 2025).
- Navarro, C.E.; Benjumea-Bedoya, D.; Estupinan-Bohorquez, A.F.; Florez, I.D. Cost-effectiveness analysis comparing QuantiFERON test and tuberculin skin test for the diagnosis of latent tuberculosis infection in immunocompetent children under 15 years of age in Colombia. BMJ Open 2025, 15, e087333. [Google Scholar] [CrossRef]
- Yasaratna, N.R.; Weerasinghe, M.C. Cost-Effectiveness Analysis of a Latent Tuberculosis Screening Program Among Healthcare Personnel. J. Health Care Organ. Provis. Financ. 2025, 62, 1–7. [Google Scholar] [CrossRef]
- Coppeta, L.; Somma, G.; Baldi, S.; Tursi, E.; D’alessandro, I.; Torrente, A.; Perrone, S.; Pietroiusti, A. Cost-effectiveness of annual screening for tuberculosis among Italian healthcare workers: A retrospective study. Int. J. Environ. Res. Public Health 2020, 17, 1697. [Google Scholar] [CrossRef]
- Yayan, J.; Franke, K.J.; Berger, M.; Windisch, W.; Rasche, K. Early detection of tuberculosis: A systematic review. Pneumonia 2024, 16, 11. [Google Scholar] [CrossRef]
- Raviglione, M.; Uplekar, M.; Vincent, C.; Pablos-Méndez, A. Rebalancing the global battle against tuberculosis. Lancet Glob. Health 2014, 2, e71–e72. [Google Scholar] [CrossRef] [PubMed]
- Baussano, I.; Bugiani, M.; Carosso, A.; Mairano, D.; Pia Barocelli, A.; Tagna, M.; Cascio, V.; Piccioni, P.; Arossa, W. Risk of tuberculin conversion among healthcare workers and the adoption of preventive measures. Occup. Environ. Med. 2007, 64, 161–166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Getahun, H.; Matteelli, A.; Abubakar, I. Management of latent tuberculosis infection: WHO guidelines. Eur. Respir. J. 2022, 44, 132–146. [Google Scholar]
- Ibrahim, H.U.; Garba, R.M.; Ahmad, J.I.; Abdulsalam, K.; Jibril, Y.N.; Dankishiya, F.S.; Ibrahim, A.; Garzali, I.U.; Galadanci, A.A.; Suleiman, A.K.; et al. Cost-effectiveness of different tuberculosis diagnostic approaches in Nigeria based on decision analytical modelling. BMJ Glob. Health 2025, 10, e019270. [Google Scholar]
- d’Elbée, M.; Harker, M.; Mafirakureva, N.; Nanfuka, M.; Ton Nu Nguyet, M.H.; Taguebue, J.V.; Moh, R.; Khosa, C.; Mustapha, A.; Mwanga-Amumpere, J.; et al. Cost-effectiveness and budget impact of decentralising childhood tuberculosis diagnosis in six high tuberculosis incidence countries: A mathematical modelling study. eClinicalMedicine 2024, 70, 102528. [Google Scholar] [CrossRef]
- Kay, A.W.; Ness, T.; Verkuijl, S.E.; Viney, K.; Brands, A.; Masini, T.; González Fernández, L.; Eisenhut, M.; Detjen, A.K.; Mandalakas, A.M.; et al. Xpert MTB/RIF Ultra assay for tuberculosis disease and rifampicin resistance in children: A systematic review. Cochrane Database Syst. Rev. 2022, 9, CD013359. [Google Scholar]
- Hansun, S.; Argha, A.; Bakhshayeshi, I.; Wicaksana, A.; Alinejad-Rokny, H.; Fox, G.J.; Liaw, S.-T.; Celler, B.G.; Marks, G.B. Diagnostic Performance of Artificial Intelligence–Based Methods for Tuberculosis Detection: Systematic Review. J. Med. Internet Res. 2025, 27, e69068. [Google Scholar] [CrossRef]
- Qin, Z.Z.; Ahmed, S.; Sarker, M.S.; Paul, K.; Adel, A.S.S.; Naheyan, T.; Barrett, R.; Banu, S.; Creswell, J. Tuberculosis detection from chest x-rays for triaging in a high tuberculosis-burden setting: An evaluation of five artificial intelligence algorithms. Lancet Digit. Health 2021, 3, e543–e554. [Google Scholar] [CrossRef]
- Walker, T.M.; Miotto, P.; Köser, C.U.; Fowler, P.W.; Knaggs, J.; Iqbal, Z.; Hunt, M.; Chindelevitch, L.; Farhat, M.R.; Cirillo, D.M.; et al. The 2021 WHO catalogue of Mycobacterium tuberculosis complex mutations associated with drug resistance: A genotypic analysis. Lancet Microbe 2022, 3, e265–e273. [Google Scholar] [CrossRef]
- Garg, T.; John, S.; Abdulkarim, S.; Ahmed, A.D.; Kirubi, B.; Rahman, M.T.; Ubochioma, E.; Creswell, J. Implementation costs and cost-effectiveness of ultraportable chest X-ray with artificial intelligence in active case finding for tuberculosis in Nigeria. PLOS Digit Health 2025, 4, e0000894. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brümmer, L.E.; Thompson, R.R.; Malhotra, A.; Shrestha, S.; Kendall, E.A.; Andrews, J.R.; Phillips, P.; Nahid, P.; Cattamanchi, A.; Marx, F.M.; et al. Cost-effectiveness of Low-complexity Screening Tests in Community-based Case-finding for Tuberculosis. Clin. Infect. Dis. 2024, 78, 154–163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burzynski, J.; Mangan, J.M.; Lam, C.K.; Macaraig, M.; Salerno, M.M.; deCastro, B.R.; Goswami, N.D.; Lin, C.Y.; Schluger, N.W.; Vernon, A. In-person vs electronic directly observed therapy for tuberculosis treatment adherence: A randomized noninferiority trial. JAMA Netw. Open 2022, 5, e2144210. [Google Scholar] [CrossRef]
- Amimo, F.; Lambert, B.; Magit, A. What does the COVID-19 pandemic mean for HIV, tuberculosis, and malaria control? Trop. Med. Health 2020, 48, 32. [Google Scholar] [CrossRef]
- Belknap, R.; Holland, D.; Feng, P.J.; Millet, J.P.; Caylà, J.A.; Martinson, N.A.; Wright, A.; Chen, M.P.; Moro, R.N.; Scott, N.A.; et al. Self-administered Versus Directly Observed Once-Weekly Isoniazid and Rifapentine Treatment of Latent Tuberculosis Infection: A Randomized Trial. Ann. Intern. Med. 2017, 167, 689–697. [Google Scholar] [CrossRef]
- Tiberi, S.; Utjesanovic, N.; Galvin, J.; Centis, R.; D’Ambrosio, L.; van den Boom, M.; Zumla, A.; Migliori, G.B. Drug resistant TB—Latest developments in epidemiology, diagnostics and management. Int. J. Infect. Dis. 2022, 124 (Suppl. S1), S20–S25. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2021, Section 3.4: Drug-Resistant TB—Treatment and Treatment Coverage. Available online: https://www.who.int/publications/digital/global-tuberculosis-report-2021/tb-diagnosis-treatment/drug-resistant-treatment (accessed on 20 July 2025).
- Nasiri, M.J.; Zangiabadian, M.; Arabpour, E.; Amini, S.; Khalili, F.; Centis, R.; D’Ambrosio, L.; Denholm, J.T.; Schaaf, H.S.; van den Boom, M.; et al. Delamanid-containing regimens and multidrug-resistant tuberculosis: A systematic review and meta-analysis. Int. J. Infect. Dis. 2022, 124 (Suppl. S1), S90–S103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matteelli, A.; Lovatti, S.; Rossi, B.; Rossi, L. Update on multidrug-resistant tuberculosis preventive therapy toward the global tuberculosis elimination. Int. J. Infect. Dis. 2025, 155, 107849. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daradkeh, A.F.; Alawyia, B.; Ballas, H.; Spernovasilis, N.; Alon-Ellenbogen, D. Strategies for Tuberculosis Prevention in Healthcare Settings: A Narrative Review. Trop. Med. Infect. Dis. 2025, 10, 316. https://doi.org/10.3390/tropicalmed10110316
Daradkeh AF, Alawyia B, Ballas H, Spernovasilis N, Alon-Ellenbogen D. Strategies for Tuberculosis Prevention in Healthcare Settings: A Narrative Review. Tropical Medicine and Infectious Disease. 2025; 10(11):316. https://doi.org/10.3390/tropicalmed10110316
Chicago/Turabian StyleDaradkeh, Ahmad Faris, Basil Alawyia, Hassan Ballas, Nikolaos Spernovasilis, and Danny Alon-Ellenbogen. 2025. "Strategies for Tuberculosis Prevention in Healthcare Settings: A Narrative Review" Tropical Medicine and Infectious Disease 10, no. 11: 316. https://doi.org/10.3390/tropicalmed10110316
APA StyleDaradkeh, A. F., Alawyia, B., Ballas, H., Spernovasilis, N., & Alon-Ellenbogen, D. (2025). Strategies for Tuberculosis Prevention in Healthcare Settings: A Narrative Review. Tropical Medicine and Infectious Disease, 10(11), 316. https://doi.org/10.3390/tropicalmed10110316






