Abstract
Background: Heart failure with reduced ejection fraction (HFrEF) markedly impairs quality of life (QoL) and life expectancy. The main therapeutic goals are to reduce mortality, improve functional capacity, and enhance QoL. Exercise training is an evidence-based, non-pharmacological component of standard care that improves functional capacity and clinical outcomes in HFrEF. This review examines the effects of endurance and resistance training on peak oxygen uptake (VO2peak), ventilatory efficiency (VE/VCO2 slope), health-related QoL, and cardiovascular outcomes in patients with HFrEF. Methods: A structured narrative review was conducted using comprehensive searches of PubMed, EMBASE, and the Cochrane Library for English-language studies published between January 2004 and October 2024. Eligible studies included adult HFrEF populations undergoing aerobic and/or resistance training with reported effects on VO2peak, ventilatory efficiency, QoL, or clinical outcomes. Given the heterogeneity of interventions, comparators, and outcome metrics, data were synthesized descriptively. Results: Across 18 studies (plus one sub-analysis) including 3401 patients, 17 trials assessed VO2peak and 16 reported significant improvements, with an average increase of approximately 2 mL·kg−1·min−1. Six studies assessed ventilatory efficiency, and five demonstrated reductions in VE/VCO2 slope averaging 4.4 units. Eleven studies analyzed QoL, and nine reported significant improvements corresponding to an ≈5-point decrease in the Minnesota Living with Heart Failure Questionnaire (MLHFQ). In the largest trial, exercise training was associated with modest but statistically significant reductions in all-cause mortality or hospitalization (HR 0.89) and cardiovascular mortality or heart-failure hospitalization (HR 0.85) after adjustment for baseline prognostic factors. Conclusions: Structured exercise training improves aerobic capacity, ventilatory efficiency, and QoL in patients with HFrEF, with supportive evidence for reduced morbidity and mortality. These findings underscore the value of structured exercise as a core component of modern HFrEF management.
1. Introduction
Heart failure with reduced ejection fraction (HFrEF) is a prevalent condition that substantially impairs quality of life (QoL). Globally, heart failure (HF) affects an estimated 56 million people, with prevalence rates ranging from 1 to 3% in the general population and rising sharply with age. Although age-adjusted incidence has declined modestly in some high-income regions, the absolute burden of HF continues to increase due to population aging and the growing prevalence of cardiometabolic risk factors. Approximately half of all individuals with HF have HFrEF, which occurs more frequently in men, whereas HF with preserved ejection fraction (HFpEF) is more common in women [1,2,3,4].
Coronary artery disease (CAD) and hypertension are the leading causes of HFrEF. Other relevant etiologies include valvular heart disease, genetic and acquired cardiomyopathies, myocarditis, and cardiotoxic agents such as alcohol or chemotherapeutics [5]. While treatment advancements have improved prognosis, mortality and QoL remain concerning [6,7,8]. Women generally have better survival rates [9]. Patients often face frequent hospitalizations, averaging once per year, many due to non-cardiovascular causes [10]. Recent data suggest increased admissions driven by diabetes, obesity, and impaired kidney function [11]. As the population ages and comorbidities rise, hospitalizations for HFrEF are expected to increase significantly [1].
Pharmacotherapy is essential for treating HFrEF, prioritized alongside non-pharmacological interventions before device therapy. Guideline-directed medical therapy includes an angiotensin-converting enzyme inhibitor (ACEi), or an angiotensin II receptor blocker (ARB), or preferably an angiotensin receptor-neprilysin inhibitor (ARNI), together with a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a sodium-glucose cotransporter-2 inhibitor (SGLT2i). These therapies collectively reduce neurohormonal activation, improve cardiac remodeling, and substantially lower mortality and hospitalization risk. The main treatment goals are reducing mortality, preventing hospitalizations, and improving the functional capacity and QoL [1]. Structured exercise training is the most evidence-based non-pharmacological strategy for improving both physiological and patient-centered outcomes in HFrEF [1,12]. In the general population, broad evidence supports reduced mortality risk through regular physical activity [13,14,15]. Regular physical activity also improves QoL and favorably modifies cardiovascular risk factors [16,17].
Cardiorespiratory fitness (CRF) is strongly and inversely associated with mortality across age, sex, and racial groups, with low fitness conferring greater risk than any single cardiac risk factor [18,19,20]. Aerobic capacity (VO2peak) is the most accurate marker of CRF [20], and both VO2peak and ventilatory efficiency (VE/VCO2 slope) are independent prognostic indicators in HF [21,22]. Chronic HF impairs oxygen transport pathways, leading to exercise intolerance and reduced QoL. Structured exercise training mitigates these deficits by enhancing skeletal-muscle oxygen extraction and metabolic efficiency, thereby improving aerobic capacity and functional performance. Higher VO2peak also reflects more efficient muscle metabolism and vascular function, which supports better exercise tolerance and QoL [23].
Earlier meta-analyses have consistently demonstrated beneficial effects of exercise in HF. Gomes-Neto et al. (2019) reported significant improvements in strength and QoL and modest increases in VO2peak (+2.9 mL·kg−1·min−1) in patients with HFrEF, but no consistent changes in ventilatory efficiency [24]. Edwards and O’Driscoll (2022), who evaluated both HFpEF and HFrEF, found improvements in VO2peak, exercise tolerance, ventricular function, and QoL, but no significant effects on hospitalization or mortality [25].
These analyses provided valuable quantitative summaries but did not explore the underlying physiological and mechanistic pathways linking exercise adaptations to clinical prognosis [24,25].
Despite robust evidence supporting exercise in HF, uncertainty remains regarding the comparative effectiveness of specific training modalities, variability across patient subgroups, and the extent to which physiological improvements translate into clinical outcomes. Optimal exercise prescriptions for different HFrEF phenotypes also remain incompletely defined. Therefore, the central question of this review is how endurance and resistance training influence aerobic capacity, ventilatory efficiency, QoL, and clinical outcomes in patients with HFrEF. We focused on VO2peak, VE/VCO2 slope, QoL, and clinical events, as these outcomes represent the most robust and consistently reported prognostic markers in contemporary HFrEF exercise trials.
This structured narrative review synthesizes contemporary trials conducted within the modern era of HFrEF management, integrating recent evidence on aerobic and resistance training to clarify their effects on functional capacity, ventilatory efficiency, and patient-centered outcomes. By drawing on findings from recent randomized and observational studies, it highlights the central role of structured exercise and cardiac rehabilitation in HFrEF care and identifies characteristics of exercise interventions that may offer the greatest prognostic and QoL benefits. These insights may support more personalized and effective exercise prescriptions in clinical practice.
2. Methods
2.1. Search Strategy
Comprehensive searches were conducted in PubMed, EMBASE, and the Cochrane Library for studies published from January 2004 to 6 October 2024. These databases were selected for their broad and complementary coverage of biomedical, cardiovascular, and rehabilitation research and are commonly used in exercise-cardiology reviews. The 20-year time window was chosen to capture research conducted within the contemporary era of HF management, reflecting modern pharmacotherapy and exercise-rehabilitation practices.
Searches were limited to English-language publications due to resource and translation constraints inherent to a structured narrative review; therefore, some non-English-language studies may not have been identified. Reference lists of prior reviews and included studies were screened manually; however, the review was not designed to re-evaluate all primary studies included in earlier meta-analyses. The aim was to identify contemporary trials reporting one or more of four key outcomes—VO2peak, ventilatory efficiency, QoL, and clinical events—within modern HFrEF management. The grey literature (trial registries, conference abstracts, unpublished data) was not systematically searched.
The same search strategy was applied across all three databases (PubMed, EMBASE, Cochrane Library). The identical Boolean search string used in all databases was as follows: (‘endurance training’ OR ‘strength training’ OR ‘exercise’ OR ‘rehabilitation’) AND (‘cardiovascular outcomes’ OR ‘VO2’ OR ‘quality of life’) AND ‘heart failure’ AND ‘reduced ejection fraction’.
2.2. Inclusion Criteria
Studies were eligible for inclusion if they
- -
- involved adults (≥18 years) with HFrEF (LVEF ≤ 40–45%);
- -
- investigated aerobic and/or resistance training, alone or in combination, compared with usual care or standard therapy;
- -
- reported outcomes related to VO2peak, VE/VCO2 slope, QoL, or clinical endpoints (e.g., hospitalization or mortality).
Randomized controlled and observational studies were both included to provide a comprehensive overview of intervention effects.
2.3. Study Selection and Data Extraction
Study screening and selection were conducted using Rayyan (Rayyan.ai, Rayyan Systems Inc., Cambridge, MA, USA, accessed October 2024). From each included study, data were extracted on study design, sample size, baseline characteristics, intervention details (type, intensity, and duration), comparator group, outcomes assessed (VO2peak, VE/VCO2 slope, QoL, and mortality), follow-up duration, and main statistical findings. Extraction followed a standardized template to ensure consistency across studies. No authors were contacted for additional data.
After removing duplicates, 129 records were screened by title and abstract. Eighty-five were excluded as not relevant to the research question or not meeting inclusion criteria. The remaining 44 articles were assessed in full text; 25 were excluded (most commonly for wrong population, intervention, outcome, or study design). Nineteen studies were included in the final review (Figure 1). Title, abstract, and full-text screening were conducted by the first author, and the co-authors verified the final selection for accuracy and consistency.
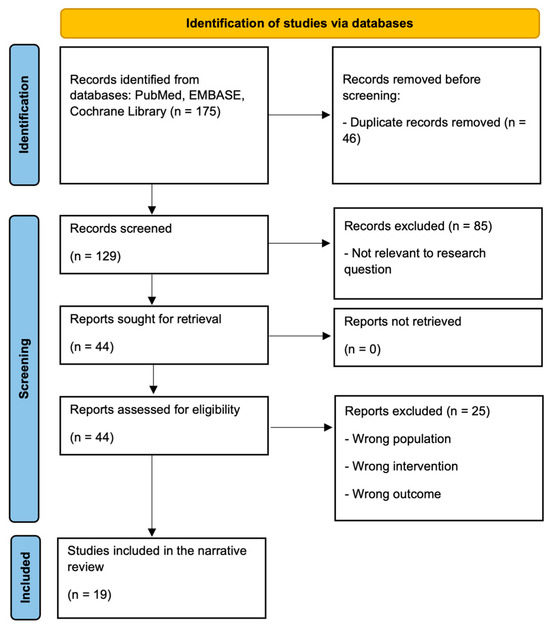
Figure 1.
Flow diagram of study selection.
2.4. Synthesis
Substantial diversity in study design, patient characteristics, intervention modalities (aerobic, resistance, combined, or interval training), and training intensity and duration precluded a quantitative meta-analysis. Consequently, findings were summarized descriptively and grouped according to the primary outcomes of interest (VO2peak, VE/VCO2 slope, and QoL). This structured narrative framework allowed the integration of physiological, clinical, and mechanistic evidence to clarify how exercise training influences prognosis in HFrEF.
3. Results
3.1. Study Selection and Overview
Systematic searches of PubMed, EMBASE, and the Cochrane Library yielded 175 records. After removal of duplicates, 129 articles were screened by title and abstract, and 44 were assessed in full text. Eighteen studies (plus one sub-analysis) met the inclusion criteria and examined the effects of exercise training on VO2peak, ventilatory efficiency, and/or QoL in adults with HFrEF (Figure 1). Altogether, the included trials enrolled 3401 participants published between 2004 and 2024. Most studies were randomized controlled trials (n = 16) and two were prospective cohort studies. Participants generally had an LVEF of 30–40% and NYHA class II–IV (Table 1). Detailed narrative summaries of all included studies are presented in the Appendix A.

Table 1.
Clinical Trials on Exercise in Patients with HFrEF (Significant results in bold, non-significant results in italics).
3.2. Study and Intervention Heterogeneity
Considerable heterogeneity was observed across the included studies. Sample sizes ranged from 19 to 2331 participants, and exercise protocols differed markedly in content, intensity, duration, and supervision. Interventions included center-based programs, hybrid supervised and home-based models, with program durations between 7 and 24 weeks. Exercise frequency ranged from two to five sessions per week, session duration from 20 to 60 min, and intensity from 40 to 90% of VO2peak or heart-rate reserve.
The modalities comprised moderate continuous training (MCT), high-intensity interval training (HIIT), combined endurance and resistance programs, and progressive strength or functional programs. HIIT protocols were typically conducted for 12–16 weeks, MCT for 12–24 weeks, and combined or functional programs for 8–16 weeks.
Overall, the studies differed in participant age, HF severity, training dose, and outcome definitions—factors that contribute to variability in reported effects.
3.3. Summary of Main Outcomes
3.3.1. Cardiorespiratory Fitness (VO2peak)
Seventeen of the nineteen included studies reported VO2peak outcomes, and sixteen demonstrated significant improvements. Across trials, VO2peak increased by +0.6 to +3.8 mL·kg−1·min−1 after 7–24 weeks of training, with a median gain of approximately +2.0 mL·kg−1·min−1 (Table 1).
Aerobic endurance training: In the largest trial (HF-ACTION, O’Connor et al. [26]), 2331 patients with chronic HF (LVEF ≤ 35%) participated in a 12-week aerobic training program consisting of supervised sessions three times per week followed by a home-based phase. The intervention led to modest but statistically significant improvements in VO2peak of +0.6 mL·kg−1·min−1 after 3 months and +0.7 mL·kg−1·min−1 after 12 months. In contrast, a 16-week MCT program in older patients (≥60 years, LVEF ≈ 31%) did not improve mean VO2peak, although 26% achieved gains ≥ 10% [32].
Casillas et al. reported that VO2peak increased significantly only in the concentric endurance-training group over a 7-week period, rising by 2.0 mL·kg−1·min−1 [36]. Similarly, Alves et al. found a VO2peak increase of +3.8 mL·kg−1·min−1 after 12 weeks of moderate endurance training in HFrEF patients with permanent atrial fibrillation (AF) [41].
Interval training: Overall, HIIT tended to produce the largest short-term VO2peak gains (≈+2 to +3.5 mL·kg−1·min−1).
Ellingsen et al. [28] compared HIIT, MCT, and regular exercise recommendations over 12 weeks, reporting VO2peak changes of +1.4, +0.8, and −1.0 mL·kg−1·min−1, respectively, with effects not maintained at 1-year follow-up. In other 12-week programs, Huang et al. [30] found a +2.2 mL·kg−1·min−1 increase with modified HIIT, and Fu et al. [31] observed a gain of ≈+2.6 mL·kg−1·min−1 with interval training at 40% and 80% of VO2peak. Fernandes-Silva et al. [34] reported larger improvements of +3.5 mL·kg−1·min−1 in patients with low inflammatory biomarkers, and Alshamari et al. [35] showed VO2peak increases of +3.1 mL·kg−1·min−1 with HIIT and +1.6 mL·kg−1·min−1 with HIIT plus strength training.
In patients receiving CRT, HIIT increased VO2peak by +1.7 mL·kg−1·min−1 compared with +0.6 mL·kg−1·min−1 in the CRT-only group [38]. Both HIIT and MCT improved VO2peak in the study by Sales et al., accompanied by reductions in muscle sympathetic nerve activity [39].
Combined aerobic and resistance and multi-component programs: Overall, combined aerobic-resistance programs yielded improvements comparable to those achieved with HIIT (≈+1.5 to +3 mL·kg−1·min−1).
Rengo et al. [33] reported a +2.0 mL·kg−1·min−1 increase in VO2peak after 36 sessions of combined endurance and strength training. Similarly, Antunes-Correa et al. [37] found that a 16-week cycling and strength exercise program led to a +2.7 mL·kg−1·min−1 gain in VO2peak.
Fabri et al. [40] observed significant aerobic improvements after 12 weeks of supervised combined training, corresponding to an increase of ≈+2 METs (≈+7 mL·kg−1·min−1). Guimarães et al. [42] reported a VO2peak rise from +1.6 mL·kg−1·min−1 following 12 weeks of combined endurance and resistance training.
In a randomized study by Andrade et al. [43], moderate endurance and resistance training over 12 weeks increased VO2peak by +2.7 mL·kg−1·min−1 in the center-based group compared with +0.8 mL·kg−1·min−1 in the home-based group. The smallest trial, by Giuliano et al. [44], reported a VO2peak increase of +2.4 mL·kg−1·min−1 after 4 weeks of low-load, high-repetition exercises followed by 4 weeks of combined training, compared with only +0.2 mL·kg−1·min−1 after 8 weeks of combined training alone.
3.3.2. Ventilatory Efficiency (VE/VCO2 Slope)
Six studies evaluated changes in the VE/VCO2 slope; four reported a significant reduction, with an average decrease of approximately 4.4 units, and one study showed a non-significant trend toward improvement.
Huang et al. [30] reported a decrease in the VE/VCO2 slope from 32.4 to 30.0 after 12 weeks of moderate HIIT. Alshamari et al. [35] found a non-significant trend toward improvement following HIIT. Alves et al. [41] reported a decrease from 38.5 to 32.1 after 12 weeks of endurance training in HFrEF patients with permanent AF.
Antunes-Correa et al. [37] observed decreases from 38.1 to 34.4 in men and from 40.0 to 35.4 in women after four months of combined endurance and strength training. Guimarães et al. [42] also found a significant decrease after 12 weeks of combined training, whereas Andrade et al. [43] did not observe a significant change in a smaller sample of 23 patients after a similar intervention lasting 12 weeks.
3.3.3. Quality of Life (QoL)
Eleven studies assessed QoL using validated questionnaires, mainly the Minnesota Living with Heart Failure Questionnaire (MLHFQ) [29,31,32,35,37,41,43], the Kansas City Cardiomyopathy Questionnaire (KCCQ) [27], and the SF-36 or related scales [33,40]. Nine studies reported statistically significant within-group improvements, averaging a reduction of approximately 5 points in MLHFQ scores (Table 1).
Aerobic endurance training: In the HF-ACTION trial [27], aerobic exercise training was associated with a KCCQ increase of +5.2 points in the exercise group, compared to 3.3 in controls, with improvements sustained over 2.5 years. Brubaker et al. [32], which included older patients, found no significant group difference, as the control group improved more (MLHFQ −6 vs. −4.6). Alves et al. 2022 [41] reported a 16-point MLHFQ reduction after 12 weeks of endurance training in patients with AF.
Interval training: Fu et al. [31] reported significant QoL improvements after 12 weeks of aerobic interval training, with MLHFQ decreasing by 18 points, and SF-36 Physical and Mental scores increasing by 9 and 12 points, respectively. Alshamari et al. [35] found significant MLHFQ reductions after 12 weeks, −12 points with HIIT and −11 points with HIIT plus strength training. Santa-Clara et al. [38] observed improved QoL after CRT implantation in both groups, without additional benefit of HIIT over usual care.
Combined training: Dalal et al. (REACH-HF) [29] reported a mean reduction of −5.7 in MLHFQ after 12 weeks of home-based aerobic training. Rengo et al. [33] found that SF-36 scores increased from 57 to 69 and depressive symptoms (PHQ-9) decreased from 5 to 3. Fabri et al. [40] reported improvement in six or more SF-36 domains after combined training. Andrade et al. [43] observed a reduction in MLHFQ from 35 to 22 after 12 weeks of center-based exercise with no change in the home-based group.
3.3.4. Mortality and Hospitalizations
HF-ACTION was the only trial powered for clinical events. Unadjusted analyses showed a non-significant trend toward reduced mortality and hospitalization with exercise training. However, when models were adjusted for key baseline predictors of prognosis—including exercise capacity, LVEF, depressive symptoms, and atrial arrhythmias—exercise training was associated with modest but statistically significant reductions in all-cause mortality or hospitalization (HR 0.89) and cardiovascular mortality or HF hospitalization (HR 0.85) [26].
3.3.5. Additional Findings
- -
- Home-based vs. center-based training: Both groups improved VO2peak, with the center-based group showing larger increases (+2.7 vs. +0.8 mL·kg−1·min−1) and greater QoL improved (MLHFQ −13 vs. −1) [43].
- -
- Low-mass, high-repetitiontraining: The PRIME protocol increased VO2peak by +2.4 mL·kg−1·min−1, whereas combined training alone improved VO2peak by only +0.2 mL·kg−1·min−1 [44].
- -
- Inflammatory Biomarkers: Improvements in VO2peak (+3.5 mL·kg−1·min−1) only in low-inflammation groups [34].
- -
- Muscle and Vascular Function: Exercise decreased muscle sympathetic nerve activity, improved forearm blood flow, and reduced vascular resistance; HIIT induced larger effects than MCT [37,39,42].
- -
- NYHA class: Dyspnea improved by 0.8 points after 4 months of supervised training in both men and women, with no significant change in untrained controls [37].
- -
- Echocardiography: LVEF improved in some studies, including 39% to 44% with supervised combined training and 31% to 36% in patients with permanent AF [40,41], while older adults showed no change [32]. HIIT and MCT reduced left ventricular end-diastolic diameter (LVEDD, −2.8 mm and −1.2 mm, respectively) [28]. Left atrial dimensions also decreased in HFrEF patients with permanent AF [41].
- -
- In older patients (≥60 years): VO2peak did not improve significantly overall (mean change −0.2 mL·kg−1·min−1), although 26% of participants achieved ≥ 10% individual improvements [32].
- -
- Patients with HFrEF and permanent AF: Aerobic training increased VO2peak by +3.8 mL·kg−1·min−1, along with improvements in HR and QoL [41].
- -
- Post-cardiac resynchronization therapy (CRT): CRT combined with HIIT, as well as HIIT alone, improved exercise capacity, QoL, and LVEF [38].
4. Discussion
4.1. Overview
Across the included studies, structured exercise training consistently improved aerobic capacity, ventilatory efficiency, and health-related QoL in patients with HFrEF. Mechanistic patterns observed across trials help explain these benefits. Subgroup differences, including attenuated responses in older adults or those with elevated inflammation and larger gains in patients with permanent AF, further highlight how individual patient characteristics influence training responsiveness.
4.2. Aerobic Capacity (VO2peak)
Most trials (16 of 17) showed meaningful increases in VO2peak, typically around +2 mL·kg−1·min−1 after 8–24 weeks of structured exercise training [26,28,30,31,33,34,35,36,37,39,40,41,42,43,44]. This magnitude is clinically relevant, as VO2peak is a strong prognostic marker in HF and even a 1 mL·kg−1·min−1 increase has been associated with a 10–15% reduction in mortality risk [45,46,47,48]. Small absolute gains may be particularly important for patients with values below the 12–14 mL·kg−1·min−1 threshold commonly used to identify advanced HF or transplant candidacy [49]. A 2 mL·kg−1·min−1 improvement corresponds to roughly 0.6 METs, a change known to carry meaningful reductions in long-term mortality risk [18,19].
However, improvements were not uniform across patient groups. In older patients (≥60 years), only a subset (26%) achieved a ≥10% increase in VO2peak, indicating a heterogeneous response to exercise training [32]. This attenuated effect, particularly beyond age 70, is well described in the literature and reflects age-related limitations in cardiac output, peripheral oxygen extraction, and overall physiological adaptability [50,51]. Evidence in older adults shows that endurance training produces the largest gains in VO2peak, with combined aerobic-resistance programs also providing meaningful improvements. Accordingly, individualized or multimodal training approaches that incorporate resistance work are recommended to optimize adaptations in this population [52].
Similarly, patients with high inflammatory markers showed no improvement [34]. Elevated inflammation is associated with blunted training responsiveness, including smaller gains in aerobic capacity and muscle remodeling. Chronic low-grade inflammation impairs muscle function and limits physiological adaptation, although exercise—particularly aerobic or combined aerobic-resistance training—can still reduce systemic inflammatory markers [53,54].
Notably, the largest increase was observed in the HFrEF group with permanent AF (+3.8 mL·kg−1·min−1) [41]. Exercise training has been shown to significantly improve cardiopulmonary function in AF, particularly when training intensity exceeds 50%. These adaptations—such as greater stroke volume, increased cardiac output, enhanced myocardial perfusion, and more efficient oxygen transport and utilization—may help explain the pronounced VO2peak response in this group [55].
4.3. Ventilatory Efficiency—VE/VCO2 Slope
The VE/VCO2 slope is a robust prognostic marker in HF with steeper slopes (>34–36) consistently associated with higher mortality and hospitalization risk, and each 1-unit increase is linked to a 4–10% rise in adverse events [21,22,49,56,57,58,59]. Conversely, reductions in VE/VCO2 reflect improved ventilatory efficiency and are associated with better survival [58]. Across the included trials, exercise training reduced the VE/VCO2 slope by approximately 4.4 units [30,37,41,42,43], a change considered clinically meaningful. These improvements likely arise from enhanced cardiac output and pulmonary perfusion—reducing ventilation–perfusion mismatch and physiological dead space—as well as attenuation of excessive ventilatory drive mediated by heightened chemo- and ergoreflex activation. Improved skeletal-muscle metabolism further lowers afferent signaling and delays early lactic acidosis, collectively reducing the ventilatory requirement for a given CO2 output [60].
Moderate-intensity continuous training, HIIT, and combined endurance-strength programs can enhance ventilatory efficiency. Variability in VE/VCO2 responses may largely reflect the small sample sizes of several trials, which limits statistical power, rather than true differences between training modalities. Notably, the largest improvement was observed in HFrEF patients with permanent AF (−6.4 units) [41], mirroring the disproportionately high VO2peak gains seen in this subgroup. Given that AF impairs cardiopulmonary coupling through irregular ventricular filling, these patients may derive greater benefit from training via increases in cardiac output and improved rate control [55]. The absence of significant change reported by Andrade et al. [43] may relate more to the small sample size (n = 23) than to the shorter training duration of three 30-min sessions per week over 12 weeks.
4.4. Quality of Life (QoL)
Across the 11 studies assessing QoL, exercise training consistently improved health-related QoL in patients with HFrEF. Most trials demonstrated significantly greater gains in the exercise groups compared with controls, with average improvements of approximately 5 points in MLHFQ scores. A 5-point change is widely accepted in clinical research and practice as the benchmark for a clinically relevant difference in MLHFQ scores [61]. Benefits typically appeared early during training and were sustained when programs incorporated a structured transition to home-based exercise [27].
Training setting and modality influenced the magnitude of improvement. Center-based CR produced the largest QoL gains, likely due to enhanced supervision, structured progression, and social interaction, whereas home-based programs also improved QoL but to a lesser extent [43]. HIIT-based interventions tended to yield some of the most pronounced improvements (−12 to −18 MLHFQ points) [31,35].
Two studies did not show superior QoL benefits of exercise over control. In patients recently receiving CRT, QoL improved in both groups, likely because CRT itself produces substantial symptomatic relief by improving cardiac output and reducing ventricular dissynchrony, thereby overshadowing any additional benefit of exercise [38]. In older adults, both groups experienced QoL improvements, which may reflect optimization of usual care, increased clinical contact, and nonspecific participation effects. Notably, this was also the only study that failed to demonstrate a meaningful improvement in VO2peak, consistent with the well-described blunted physiological training response in older adults and helping explain the limited between-group differences in QoL [32].
Mechanistically, improvements in QoL appear to arise from several core components of structured exercise trainings. Gains in exercise capacity were strongly associated with improvements across emotional, social, and physical QoL domains, indicating that enhanced functional status—and better symptom control such as reduced dyspnea—play a central role [37]. The inpatient rehabilitation environment also provides intensified social support through continuous interaction with healthcare staff and peers, alongside temporary relief from home and caregiving responsibilities—factors that particularly benefit patients who enter rehabilitation with lower social or emotional well-being. In addition, the structured psychosocial and educational components of rehabilitation help reduce emotional distress and strengthen coping, further contributing to the observed QoL improvements [62].
4.5. Mortality and Hospitalization Rates
HF-ACTION remains the only large trial powered for clinical events. Although unadjusted results showed only a non-significant trend, adjusted analyses identified modest reductions in all-cause mortality or hospitalization (HR 0.89) and in cardiovascular mortality or HF hospitalization (HR 0.85). These findings suggest that improvements in functional capacity and ventilatory efficiency may translate into clinically meaningful reductions in morbidity, particularly in patients who maintain long-term adherence [26].
4.6. Additional Physiological Effects of Exercise Training
Exercise training elicited meaningful improvements in neurovascular regulation. Both HIIT and combined aerobic-resistance training reduced muscle sympathetic nerve activity and vascular resistance while enhancing peripheral blood flow [37,39,42]. These adaptations improve exercise tolerance and symptom burden. No consistent reductions in inflammatory biomarkers were observed [34], suggesting that anti-inflammatory effects may require longer interventions or be restricted to specific subgroups [53,54].
4.7. Cardiac Effects of Exercise Training
Several studies demonstrated favorable cardiac remodeling, including increases in LVEF [40,41], reductions in LVEDD [28], and decreased left atrial dimensions in patients with permanent AF [41]. These changes were not observed in older patients, consistent with reduced physiological plasticity in advanced age [32]. Overall, cardiac remodeling effects of exercise are modest but clinically meaningful when combined with optimized pharmacotherapy.
4.8. Mode of Exercise
HIIT and modified HIIT improved both VO2peak and ventilatory efficiency in several studies [28,30], although HIIT was not superior to MCT for altering left ventricular remodeling or aerobic capacity [28]. HIIT may be more effective in improving neuromuscular and vascular parameters [39]. When combined with resistance training, HIIT enhanced muscular strength, endurance, and anaerobic threshold [34]. High-intensity intermittent exercise tailored to individual capacity represents an efficient training option for many patients with HFrEF [63].
Low-mass, high-repetition strength training (PRIME-HF) also improved aerobic capacity in older patients and may represent a practical alternative exercise option for this population [44].
4.9. Supervised vs. Unsupervised Exercise and Cardiac Rehabilitation
Home-based training is safe and improves exercise capacity and QoL [29,43], but supervised programs more effectively enhance physical capacity, exercise tolerance, and QoL [28,40,42]. Supervision may improve adherence and ensure adequate training intensity, both of which are strong predictors of benefit.
Long-term maintenance remains challenging, underscoring the importance of transitioning from supervised to self-managed exercise [28]. HF-ACTION demonstrated that hybrid models combining supervised initiation with structured home-based continuation can sustain improvements in VO2peak and QoL [26]. Home-based programs also offer cost-effectiveness advantages [29].
Older adults derive substantial benefit from multidomain rehabilitation after acute cardiac decompensation, showing improvements in frailty, physical function, and QoL [64]. Improving access to rehabilitation through systematic in-hospital referral and close outpatient follow-up may increase participation rates [33].
4.10. Subgroup Variability
Exercise responses varied across subgroups. Younger patients and those with lower baseline inflammatory status experienced greater gains, whereas older adults exhibited more heterogeneous responses, potentially due to age-related physiological constraints or insufficient training intensity [32,34,50]. HFrEF patients with permanent AF or those post-CRT showed significant improvements in aerobic capacity, cardiac function, and QoL, although HIIT did not provide additional QoL benefit compared with CRT alone [38,41]. Further research is required to define optimal training regimens for these subgroups.
4.11. Clinical Implications
These findings support integrating structured exercise training into standard HFrEF care. A supervised period of combined endurance and strength training, followed by a transition to self-managed exercise, appears to offer the most sustainable benefits.
4.12. Comparison with Previous Reviews
A 2022 meta-analysis evaluating exercise across both HF phenotypes reported significant improvements in cardiorespiratory fitness, QoL, and selected cardiac parameters [25]. A 2024 network meta-analysis of 82 trials identified high-intensity aerobic interval and MCT as the most effective modalities for improving VO2peak, LVEF, and QoL, with combined aerobic-resistance programs offering additional but smaller benefits [65].
The present review builds on previous work by focusing exclusively on patients with HFrEF and by restricting the analysis to four clinically robust outcomes—VO2peak, VE/VCO2 slope, QoL, and cardiovascular events. It incorporates studies published through 2024, including modern HIIT protocols, combined training models, and trials involving distinct patient subgroups. In contrast to quantitative meta-analyses, this review provides a structured and transparent synthesis of evidence across multiple training modalities and patient cohorts, linking physiological mechanisms to clinically meaningful endpoints. This approach offers an updated and practical perspective that may support more individualized exercise prescription in contemporary HFrEF management.
4.13. Limitations
Several limitations should be acknowledged. Many included studies had small sample sizes, short intervention and follow-up periods, and substantial heterogeneity in exercise modality, training intensity, and outcome definitions, which limits comparability across trials. As a structured narrative review, this work did not involve dual independent screening, a formal risk-of-bias assessment, or meta-analytic pooling, and the findings should therefore be interpreted qualitatively. The literature search was conducted systematically across major databases, although the grey literature was not searched, and only English-language publications were included. In addition, this review focused on contemporary studies reporting VO2peak, ventilatory efficiency, QoL, and clinical events, and did not attempt to re-evaluate all studies examined in earlier meta-analyses. These methodological considerations should be taken into account when interpreting the results.
4.14. Future Research
Future studies should investigate the long-term effects of exercise interventions, identify optimal training regimens for diverse patient subgroups, and clarify predictors of responsiveness. Large, multicenter trials comparing HIIT, MCT, and combined modalities- with extended follow-up would help refine exercise prescription. Personalized approaches incorporating age, comorbidity burden, inflammatory status, and functional capacity may enhance the effectiveness of training programs.
5. Conclusions
Structured exercise training provides consistent improvements in VO2peak, ventilatory efficiency, and QoL in patients with HFrEF, supporting its integration into standard care as recommended by current guidelines. Training responses vary across subgroups: older adults and individuals with elevated inflammation show attenuated gains, highlighting the need for optimized protocols and strategies to address inflammation, whereas patients with permanent AF demonstrate substantial potential for improvement.
Further research should clarify which patient characteristics predict improvements in ventilatory efficiency and how different training modalities influence VE/VCO2 outcomes. An effective transition from supervised to home-based training is essential, with supervised programs particularly beneficial for patients with reduced well-being or poorer baseline QoL. Continued refinement of individualized exercise prescriptions will help maximize the therapeutic impact of structured exercise in HFrEF.
Author Contributions
Conceptualization, M.S. and M.P.; methodology, M.S. and M.P.; data curation and literature search, M.S.; validation, M.S. and M.P.; formal analysis, M.S.; investigation, M.S.; writing—original draft preparation, M.S.; writing—review and editing, M.S., M.P., T.R. and J.O.; visualization, H.B.d.S.; supervision, M.P. and T.R.; project administration, M.S.; funding acquisition, none. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AF | Atrial Fibrillation |
| COM | Combined Training |
| COMBO | Combined Moderate-Intensity Aerobic and Resistance Training |
| CON | Concentric Training |
| CPT | Combined Physical Training |
| CR | Cardiac Rehabilitation |
| CRF | Cardiorespiratory Fitness |
| CRT | Cardiac Resynchronization Therapy |
| ECC | Eccentric Training |
| ET | Exercise Training |
| FBF | Forearm Blood Flow |
| HF | Heart Failure |
| HFrEF | Heart Failure with reduced Ejection Fraction |
| HIIT | High-Intensity Interval Training |
| KCCQ | Kansas City Cardiomyopathy Questionnaire |
| LVEDD | Left Ventricular End-Diastolic Dimension |
| LVEF | Left Ventricular Ejection Fraction |
| MCT | Moderate Continuous Training |
| METs | Metabolic Equivalents |
| MLHFQ | Minnesota Living with HF Questionnaire |
| MOS-SF-36 | Medical Outcomes Study Short Form 36 |
| MSNA | Muscle Sympathetic Nerve Activity |
| NYHA | New York Heart Association |
| PHQ-9 | Patient Health Questionnaire-9 |
| PRIME | Progressive Resistance Intensity Modulated Exercise |
| QoL | Quality of Life |
| RCT | Randomized Controlled Trial |
| RRE | Regular Exercise Regimen |
| VO2peak | Peak Oxygen Uptake |
Appendix A. Short Summary of All the Included Studies
- -
- The HF-ACTION study [26] enrolled 2331 patients with chronic HF (LVEF ≤ 35%). These patients participated in a 12-week aerobic exercise training program, which included three supervised sessions per week, gradually transitioning to home-based exercise five times a week. The study aimed to assess the impact of exercise on clinical outcomes. While initial findings showed a non-significant reduction in all-cause mortality or hospitalization, further analysis revealed modest yet significant improvements in cardiovascular outcomes, specifically in cardiovascular mortality or HF hospitalization. Participants in the exercise group experienced modest improvements in VO2peak over time. Specifically, there was a small but significant increase in VO2peak at both 3 and 12 months, indicating improved aerobic capacity due to the exercise intervention.
- -
- The sub-analysis of the HF-ACTION study by Flynn et al. [27] explored how exercise training impacts the QoL in patients with chronic HF. It revealed that the Kansas City Cardiomyopathy Questionnaire (KCCQ) scores improved significantly at 3 months for those in the exercise group, with an increase of 5.21 points compared to 3.28 points in the usual care group. This improvement was both modest and statistically significant, with the benefits sustained over a median follow-up of 2.5 years.
- -
- The study by Ellingsen et al. 2017 [28] involved 261 patients with HFrEF. It compared high-intensity interval training (HIIT) with moderate continuous training (MCT) and regular exercise recommendations (RRE). HIIT consisted of four 4-min intervals at 90–95% of maximal heart rate, while MCT involved continuous exercise at 60–70% of maximal heart rate. Patients following RRE received home exercise advice with occasional sessions. The study found no significant difference in left ventricular remodeling between HIIT and MCT. Both HIIT and MCT improved VO2peak more than RRE but were similar to each other. However, these improvements were not maintained at the 52-week follow-up.
- -
- Dalal et al. [29] included 216 participants with HFrEF, divided into 107 in the intervention group and 109 in the control group. The home-based cardiac rehabilitation program (REACH-HF) involved chair-based exercises or progressive walking training, performed at least three times per week over 12 weeks. The intervention significantly improved QoL, with a mean improvement of −5.7 points on the Minnesota Living with HF Questionnaire (MLHFQ) compared to the control group.
- -
- Huang et al. 2014 [30]. The study involved 68 HFrEF patients (LVEF ≤ 40%), split into 35 for modified high-intensity interval training (mHIT) and 33 for usual care. The intervention lasted 12 weeks, with the first 4 weeks focused on continuous aerobic training and the next 8 weeks on 3-min intervals at 40% and 80% VO2 reserve, conducted 3 days per week for 50 min per session under supervision. Results for the mHIT group showed significant improvements: cardiac power output increased from 1151 ± 573 to 1306 ± 596 L/min/mmHg, ventilation efficiency improved with a VE/VO2 reduction from 32.4 ± 4.6 to 30.0 ± 4.0, and both cardiac output and stroke volume were enhanced during exercise. VO2peak increased from 16.4 ± 0.6 to 18.6 ± 0.9 mL·kg−1·min−1, indicating improved aerobic capacity.
- -
- The study by Fu et al. [31] examined aerobic interval training (AIT) effects on 60 HFrEF patients, defined as LVEF < 30%. The AIT regimen involved 3-min intervals at 40% and 80% VO2peak for 30 min per session, three days a week, over 12 weeks. This training led to significant increases in VO2peak and improvements in QoL, as measured by enhanced Short Form-36 scores and reduced Minnesota Living with HF questionnaire scores.
- -
- Brubaker et al. 2009 [32]. The study included 59 patients aged 60 and older with HFrEF and a median LVEF of 31% (LVEF < 45%). They were randomized into two groups: 30 in the exercise training (ET) group and 29 in the control group. The ET group participated in a 16-week program of walking and stationary cycling, three times a week, at moderate intensity regulated by heart rate and perceived exertion. Results showed no significant changes in VO2peak or QoL between the groups, although some individuals in the ET group experienced improvements.
- -
- The observational study by Rengo et al. [33] involved 49 patients with HFrEF. The intervention included aerobic and strength training sessions, typically conducted three times a week, up to 36 sessions. Patients engaged in activities such as treadmill walking, elliptical training, cycling, and resistance exercises. Outcomes showed significant improvements: VO2peak increased from 14.4 to 16.4 mL·kg−1·min−1, and QoL scores, measured by MOS-SF-36, improved from 57 to 69, while PHQ-9 scores for depressive symptoms decreased from 5 to 3.
- -
- In the study by Fernandes-Silva et al. [34], 44 HF patients were divided into two groups: 28 in the exercise group and 16 in the control group. The exercise group participated in a 12-week program with three sessions per week, totaling 36 sessions. This included both aerobic interval training and moderate continuous training. The results showed that aerobic exercise significantly improved peak oxygen uptake (VO2peak) in patients with low inflammatory biomarkers. Those with higher inflammation levels did not experience the same degree of improvement.
- -
- The study by Alshamari et al. [35] involved 44 patients with chronic HF to assess exercise interventions. The HIIT group (19 patients) underwent HIIT training, while the COM group (25 patients) added strength training. Both groups showed significant improvements in functional capacity and QoL. VO2peak increased for the HIIT group, from 18.4 to 21.5 mL·kg−1·min−1, and for the COM group, from 18.5 to 20.1 mL·kg−1·min−1. QoL scores improved, with the HIIT group decreasing from 33 to 21 and the COM group from 23 to 12. The COM group also benefited from enhanced muscle strength and endurance.
- -
- In a study by Casillas et al. [36], 42 chronic HF patients were randomized into eccentric (ECC) and concentric (CON) training groups for 20 sessions over 7 weeks. The ECC group used an ergocycle at low perceived exertion, while the CON group trained at a heart rate corresponding to the first ventilatory threshold. Both groups improved peak work rate (+20%, p < 0.01), but VO2peak increased significantly only in the CON group (+13.5%, p < 0.01), indicating better cardiovascular outcomes with concentric training.
- -
- Antunes-Correa 2010 [37]: Over 4 months, participants in the exercise program engaged in cycling and strengthening exercises tailored to their anaerobic thresholds. This regimen significantly improved VO2peak and decreased the VE/VCO2 slope, enhancing aerobic capacity and ventilatory efficiency in both men and women. QoL, measured by NYHA class, improved for both genders. Additionally, muscle sympathetic nerve activity (MSNA) and forearm vascular resistance (FVR) decreased, while forearm blood flow (FBF) increased. The benefits were consistent across genders.
- -
- The study by Santa-Clara et al. [38] explored HIIT post-cardiac resynchronization therapy (CRT) in chronic HF patients. Over 24 weeks, HIIT led to an 8.6% increase in VO2peak, compared to 4.9% in the control group, with no significant difference between them. Both groups showed similar improvements in cardiovascular outcomes like left ventricular ejection fraction. HIIT enhanced exercise performance but did not significantly affect functional capacity or QoL compared to CRT alone.
- -
- The study by Sales et al. [39] involved 30 patients with HF and reduced ejection fraction, divided into three groups: HIIT (n = 11), moderate-intensity continuous training (MICT, n = 11), and no training (NT, n = 8). Both HIIT and MICT were performed on a cycle ergometer, three times a week for 12 weeks. HIIT-Sessions were conducted at a heart rate 5% below the respiratory compensation point (RCP). MICT-Intensity was set between the anaerobic threshold (AT) and RCP. Outcomes showed significant improvements in VO2peak for both HIIT and MICT. Additionally, HIIT led to greater reductions in muscle sympathetic nerve activity and improved peripheral vascular function.
- -
- The study by Fabri et al. [40] involved 28 patients with HFrEF. The intervention included a trained group of 17 patients who underwent 12 weeks of supervised combined physical training, while the nontrained group of 11 patients followed unsupervised, physician-prescribed regular exercise. VO2peak was assessed using METS, showing significant improvement in the trained group. Additionally, there was an enhanced QoL in areas such as physical functioning, vitality, and mental health.
- -
- The study by Alves et al. [41] investigated the effects of aerobic exercise training on patients with HF and permanent AF. It involved a 12-week program with 26 participants split into trained and untrained groups. Results showed that the exercise-trained group experienced improvements in oxygen consumption, heart rate, and cardiac function, as well as enhanced QoL. The untrained group showed no significant changes.
- -
- The study by Guimaraes et al. [42] involved 24 patients with HFrEF. The intervention was a 12-week supervised exercise program with three sessions per week, including a 5-min warm-up, 30-min endurance exercise on a cycle ergometer, resistance exercises, and a 5-min cool-down with stretching. Results showed a significant increase in VO2peak from 15.5 to 17.1 mL·kg−1·min−1 and a significant decrease in the VE/VCO2 slope in the exercise group. Additionally, there was improved muscle oxygenation, increased muscle blood flow, and reduced muscle sympathetic nerve activity.
- -
- The study by Andrade et al. 2021 [43] involved 23 patients with chronic HF and reduced ejection fraction. Participants were randomized into two groups: a home-based group (n = 11) engaged in walking and resistance exercises and a center-based group (n = 12) involved in supervised cycling and resistance exercises over a 12-week period. Both groups showed improvements in VO2peak, with the center-based group experiencing a more substantial increase (19%). While there were no significant changes in the VE/VCO2 slope between the groups, QoL, as measured by the Minnesota Living with HF Questionnaire (MLHF), improved significantly in the center-based group, with scores decreasing from 35 to 22. Additionally, the center-based group showed a greater improvement in inspiratory muscle strength and a significant increase in daily steps compared to the home-based group.
- -
- The study by Giuliano et al. [44] involved 19 older adults with HF with reduced ejection fraction (HFrEF). Participants were divided into two groups: the PRIME group (9 patients) underwent 4 weeks of low-mass, high-repetition exercises followed by 4 weeks of combined moderate-intensity aerobic and resistance training (COMBO). The COMBO group (10 patients) participated in 8 weeks of combined training. Outcomes showed a significant increase in VO2peak by 2.4 mL·kg−1·min−1 in the PRIME group, while the COMBO group had a minimal change of 0.2 mL·kg−1·min−1.
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Brouwers, F.P.; de Boer, R.A.; van der Harst, P.; Voors, A.A.; Gansevoort, R.T.; Bakker, S.J.; Hillege, H.L.; van Veldhuisen, D.J.; van Gilst, W.H. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur. Heart J. 2013, 34, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.; Brouwers, F.P.; Voors, A.A.; Hillege, H.L.; de Boer, R.A.; Gansevoort, R.T.; van der Harst, P.; Rienstra, M.; van Gelder, I.C.; van Veldhuisen, D.J.; et al. Sex differences in new-onset heart failure. Clin. Res. Cardiol. 2015, 104, 342–350. [Google Scholar] [CrossRef]
- Fonarow, G.C.; Ahmad, F.S.; Ahmad, T.; Albert, N.M.; Alexander, K.M.; Baker, W.L.; Bozkurt, B.; Breathett, K.; Carter, S.; Cheng, R.K.; et al. HF STATS 2025: Heart Failure Epidemiology and Outcomes Statistics an Updated 2025 Report from the Heart Failure Society of America. J. Card. Fail. 2025. [Google Scholar] [CrossRef]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858, Erratum in Lancet 2019, 393, E44. https://doi.org/10.1016/S0140-6736(19)31047-5. [Google Scholar] [CrossRef]
- Tsao, C.W.; Lyass, A.; Enserro, D.; Larson, M.G.; Ho, J.E.; Kizer, J.R.; Gottdiener, J.S.; Psaty, B.M.; Vasan, R.S. Temporal Trends in the Incidence of and Mortality Associated with Heart Failure with Preserved and Reduced Ejection Fraction. JACC Heart Fail. 2018, 6, 678–685. [Google Scholar] [CrossRef]
- Gerber, Y.; Weston, S.A.; Redfield, M.M.; Chamberlain, A.M.; Manemann, S.M.; Jiang, R.; Killian, J.M.; Roger, V.L. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern. Med. 2015, 175, 996–1004. [Google Scholar] [CrossRef]
- Heiat, A.; Gross, C.P.; Krumholz, H.M. Representation of the elderly, women, and minorities in heart failure clinical trials. Arch. Intern. Med. 2002, 162, 1682–1688. [Google Scholar] [CrossRef]
- Motiejūnaitė, J.; Akiyama, E.; Cohen-Solal, A.; Maggioni, A.P.; Mueller, C.; Choi, D.-J.; Kavoliūnienė, A.; Čelutkienė, J.; Parenica, J.; Lassus, J.; et al. The association of long-term outcome and biological sex in patients with acute heart failure from different geographic regions. Eur. Heart J. 2020, 41, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Barasa, A.; Schaufelberger, M.; Lappas, G.; Swedberg, K.; Dellborg, M.; Rosengren, A. Heart failure in young adults: 20-year trends in hospitalization, aetiology, and case fatality in Sweden. Eur. Heart J. 2014, 35, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Mosterd, A.; Hoes, A.W.; CliniMosterd, A.; Hoes, A.W. Clinical epidemiology of heart failure. Heart 2007, 93, 1137–1146. [Google Scholar] [CrossRef]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.-P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef]
- Wen, C.P.; Wu, X. Stressing harms of physical inactivity to promote exercise. Lancet 2012, 380, 192–193. [Google Scholar] [CrossRef]
- Lee, I.M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T.; Lancet Physical Activity Series Working Group. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef]
- Nguyen, S.; Bellettiere, J.; Anuskiewicz, B.; Di, C.; Carlson, J.; Natarajan, L.; LaMonte, M.J.; LaCroix, A.Z. Prospective Associations of Accelerometer-Measured Machine-Learned Sedentary Behavior with Death Among Older Women: The OPACH Study. J. Am. Heart Assoc. 2024, 13, e031156. [Google Scholar] [CrossRef] [PubMed]
- Kirk-Sanchez, N.J.; McGough, E.L. Physical exercise and cognitive performance in the elderly: Current perspectives. Clin. Interv. Aging 2013, 9, 51–62. [Google Scholar] [CrossRef]
- Biddle, S.J.; Ciaccioni, S.; Thomas, G.; Vergeer, I. Physical activity and mental health in children and adolescents: An updated review of reviews and an analysis of causality. Psychol. Sport Exerc. 2019, 42, 146–155. [Google Scholar] [CrossRef]
- Kokkinos, P.; Faselis, C.; Samuel, I.B.H.; Pittaras, A.; Doumas, M.; Murphy, R.; Heimall, M.S.; Sui, X.; Zhang, J.; Myers, J. Cardiorespiratory Fitness and Mortality Risk Across the Spectra of Age, Race, and Sex. J. Am. Coll. Cardiol 2022, 80, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Prakash, M.; Froelicher, V.; Do, D.; Partington, S.; Atwood, J.E. Exercise Capacity and Mortality among Men Referred for Exercise Testing. N. Engl. J. Med. 2002, 346, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Blair, S.N.; Arena, R.; Church, T.S.; Despres, J.P.; Franklin, B.A.; Haskell, W.L.; Kaminsky, L.A.; Levine, B.D.; Lavie, C.J.; et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e653–e699. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, G.V.; da Silva, M.S.V.; D’AVila, V.M.; Ferreira, S.M.A.; Silva, C.P.; Bocchi, E.A. Peak VO2 and VE/VCO2 slope in betablockers era in patients with heart failure: A Brazilian experience. Arq. Bras Cardiol. 2008, 91, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Haykowsky, M.J.; Tomczak, C.R.; Scott, J.M.; Paterson, D.I.; Kitzman, D.W. Determinants of exercise intolerance in patients with heart failure and reduced or preserved ejection fraction. J. Appl. Physiol. 2015, 119, 739–744. [Google Scholar] [CrossRef]
- Hirai, D.M.; Musch, T.I.; Poole, D.C. Exercise training in chronic heart failure: Improving skeletal muscle O2 transport and utilization. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1419–H1439. [Google Scholar] [CrossRef]
- Gomes-Neto, M.; Durães, A.R.; Conceição, L.S.R.; Roever, L.; Silva, C.M.; Alves, I.G.N.; Ellingsen, Ø.; Carvalho, V.O. Effect of combined aerobic and resistance training on peak oxygen consumption, muscle strength and health-related quality of life in patients with heart failure with reduced left ventricular ejection fraction: A systematic review and meta-analysis. Int. J. Cardiol. 2019, 293, 165–175. [Google Scholar] [CrossRef]
- Edwards, J.J.; O’Driscoll, J.M. Exercise Training in Heart failure with Preserved and Reduced Ejection Fraction: A Systematic Review and Meta-Analysis. Sports Med. Open 2022, 8, 76. [Google Scholar] [CrossRef]
- O’cOnnor, C.M.; Whellan, D.J.; Lee, K.L.; Keteyian, S.J.; Cooper, L.S.; Ellis, S.J.; Leifer, E.S.; Kraus, W.E.; Kitzman, D.W.; Blumenthal, J.A.; et al. Efficacy and safety of exercise training in patients with chronic heart failure HF-ACTION randomized controlled trial. JAMA 2009, 301, 1439–1450. [Google Scholar] [CrossRef]
- Flynn, K.E.; Piña, I.L.; Whellan, D.J.; Lin, L.; Blumenthal, J.A.; Ellis, S.J.; Fine, L.J.; Howlett, J.G.; Keteyian, S.J.; Kitzman, D.W.; et al. Effects of exercise training on health status in patients with chronic heart failure HF-ACTION randomized controlled trial. JAMA 2009, 301, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Ellingsen, Ø.; Halle, M.; Conraads, V.; Støylen, A.; Dalen, H.; Delagardelle, C.; Larsen, A.-I.; Hole, T.; Mezzani, A.; Van Craenenbroeck, E.M.; et al. High-Intensity Interval Training in Patients with Heart Failure with Reduced Ejection Fraction. Circulation 2017, 135, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Dalal, H.M.; Taylor, R.S.; Jolly, K.; Davis, R.C.; Doherty, P.; Miles, J.; van Lingen, R.; Warren, F.C.; Green, C.; Wingham, J.; et al. The effects and costs of home-based rehabilitation for heart failure with reduced ejection fraction: The REACH-HF multicentre randomized controlled trial. Eur. J. Prev. Cardiol. 2019, 26, 262–272, Erratum in Eur. J. Prev. Cardiol. 2020, 27, NP17. [Google Scholar] [CrossRef]
- Huang, S.-C.; Wong, M.-K.; Lin, P.-J.; Tsai, F.-C.; Fu, T.-C.; Wen, M.-S.; Kuo, C.-T.; Wang, J.-S. Modified high-intensity interval training increases peak cardiac power output in patients with heart failure. Eur. J. Appl. Physiol. 2014, 114, 1853–1862. [Google Scholar] [CrossRef]
- Fu, T.-C.; Yang, N.-I.; Wang, C.-H.; Cherng, W.-J.; Chou, S.-L.; Pan, T.-L.; Wang, J.-S. Aerobic Interval Training Elicits Different Hemodynamic Adaptations between Heart Failure Patients with Preserved and Reduced Ejection Fraction. Am. J. Phys. Med. Rehabil. 2016, 95, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, P.H.; Moore, J.B.; Stewart, K.P.; Wesley, D.J.; Kitzman, D.W. Endurance exercise training in older patients with heart failure: Results from a randomized, controlled, single-blind trial. J. Am. Geriatr. Soc. 2009, 57, 1982–1989. [Google Scholar] [CrossRef]
- Rengo, J.L.M.; Savage, P.D.M.; Barrett, T.; Ades, P.A. Cardiac Rehabilitation Participation Rates and Outcomes for Patients with Heart Failure. J. Cardiopulm. Rehabil. Prev. 2018, 38, 38–42. [Google Scholar] [CrossRef]
- Fernandes-Silva, M.M.; Guimarães, G.V.; Rigaud, V.O.; Lofrano-Alves, M.S.; E Castro, R.; Cruz, L.G.d.B.; A Bocchi, E.; Bacal, F. Inflammatory biomarkers and effect of exercise on functional capacity in patients with heart failure: Insights from a randomized clinical trial. Eur. J. Prev. Cardiol. 2017, 24, 808–817. [Google Scholar] [CrossRef]
- Alshamari, M.; Kourek, C.; Sanoudou, D.; Delis, D.; Dimopoulos, S.; Rovina, N.; Nanas, S.; Karatzanos, E.; Philippou, A. Does the Addition of Strength Training to a High-Intensity Interval Training Program Benefit More the Patients with Chronic Heart Failure? Rev. Cardiovasc. Med. 2023, 24, 29. [Google Scholar] [CrossRef]
- Casillas, J.M.; Besson, D.; Hannequin, A.; Gremeaux, V.; Morisset, C.; Tordi, N.; Laurent, Y.; Laroche, D. Effects of an eccentric training personalized by a low rate of perceived exertion on the maximal capacities in chronic heart failure: A randomized controlled trial. Eur. J. Phys. Rehabil. Med 2015, 52, 159–168. [Google Scholar]
- Antunes-Correa, L.M.; Melo, R.C.; Nobre, T.S.; Ueno, L.M.; Franco, F.G.; Braga, A.M.; Rondon, M.U.; Brum, P.C.; Barretto, A.C.; Middlekauff, H.R.; et al. Impact of gender on benefits of exercise training on sympathetic nerve activity and muscle blood flow in heart failure. Eur. J. Heart Fail. 2010, 12, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Santa-Clara, H.; Abreu, A.; Melo, X.; Santos, V.; Cunha, P.; Oliveira, M.; Pinto, R.; Carmo, M.M.; Fernhall, B. High-intensity interval training in cardiac resynchronization therapy: A randomized control trial. Eur. J. Appl. Physiol. 2019, 119, 1757–1767. [Google Scholar] [CrossRef]
- Sales, A.R.; Azevedo, L.F.; Silva, T.O.; Rodrigues, A.G.; Oliveira, P.A.; Jordão, C.P.; Andrade, A.C.; Urias, U.; Guimaraes, G.V.; Bocchi, E.A.; et al. High-Intensity Interval Training Decreases Muscle Sympathetic Nerve Activity and Improves Peripheral Vascular Function in Patients with Heart Failure with Reduced Ejection Fraction. Circ. Heart Fail. 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Fabri, T.; Catai, A.M.; Ribeiro, F.H.O.; Junior, J.A.A.; Milan-Mattos, J.; Rossi, D.A.A.; Coneglian, R.C.; Borra, R.C.; Bazan, S.G.Z.; Hueb, J.C.; et al. Impact of a supervised twelve-week combined physical training program in heart failure patients: A randomized trial. Cardiol. Res. Pr. 2019, 2019, 1–6. [Google Scholar] [CrossRef]
- Alves, L.S.; Bocchi, E.A.; Chizzola, P.R.; Castro, R.E.; Salemi, V.M.C.; de Melo, M.D.T.; Andreta, C.R.d.L.; Guimarães, G.V. Exercise training in heart failure with reduced ejection fraction and permanent atrial fibrillation: A randomized clinical trial. Heart Rhythm. 2022, 19, 1058–1066. [Google Scholar] [CrossRef]
- Guimarães, G.V.; Ribeiro, F.; Castro, R.E.; Roque, J.M.; Machado, A.D.T.; Antunes-Correa, L.M.; Ferreira, S.A.; Bocchi, E.A. Effects of the exercise training on skeletal muscle oxygen consumption in heart failure patients with reduced ejection fraction. Int. J. Cardiol. 2021, 343, 73–79. [Google Scholar] [CrossRef]
- de Andrade, G.N.; Umeda, I.I.K.; Fuchs, A.R.C.N.; Mastrocola, L.E.; Rossi-Neto, J.M.; Moreira, D.A.R.; de Oliveira, P.A.; de André, C.D.S.; Cahalin, L.P.; Nakagawa, N.K. Home-based training program in patients with chronic heart failure and reduced ejection fraction: A randomized pilot study. Clinics 2021, 76, e2550. [Google Scholar] [CrossRef]
- Giuliano, C.; Levinger, I.; Vogrin, S.; Neil, C.J.; Allen, J.D. PRIME-HF: Novel Exercise for Older Patients with Heart Failure. A Pilot Randomized Controlled Study. J. Am. Geriatr. Soc. 2020, 68, 1954–1961. [Google Scholar] [CrossRef]
- Kitzman, D.W. Exercise training in heart failure with preserved ejection fraction: Beyond proof-of-concept. J. Am. Coll. Cardiol. 2011, 58, 1792–1794. [Google Scholar] [CrossRef]
- Tucker, W.J.; Nelson, M.D.; I Beaudry, R.; Halle, M.; Sarma, S.; Kitzman, D.W.; La Gerche, A.; Haykowksy, M.J. Impact of Exercise Training on Peak Oxygen Uptake and its Determinants in Heart Failure with Preserved Ejection Fraction. Card. Fail. Rev. 2016, 2, 95–101, Erratum in Card. Fail. Rev. 2018, 4, 62. [Google Scholar] [CrossRef] [PubMed]
- Keteyian, S.J.; Patel, M.; Kraus, W.E.; Brawner, C.A.; McConnell, T.R.; Piña, I.L.; Leifer, E.S.; Fleg, J.L.; Blackburn, G.; Fonarow, G.C.; et al. Variables measured during cardiopulmonary exercise testing as predictors of mortality in chronic systolic heart failure. J. Am. Coll. Cardiol. 2016, 67, 780–789, Erratum in J. Am. Coll. Cardiol. 2016, 67, 1979–1980. [Google Scholar] [CrossRef] [PubMed]
- Mancini, D.M. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 1991, 83, 778–786. [Google Scholar] [CrossRef]
- Cahalin, L.P.; Chase, P.; Arena, R.; Myers, J.; Bensimhon, D.; Peberdy, M.A.; Ashley, E.; West, E.; Forman, D.E.; Pinkstaff, S.; et al. A meta-analysis of the prognostic significance of cardiopulmonary exercise testing in patients with heart failure. Heart Fail. Rev. 2013, 18, 79–94. [Google Scholar] [CrossRef]
- Woo, J.S.; Derleth, C.; Stratton, J.R.; Levy, W.C. The influence of age, gender, and training on exercise efficiency. J. Am. Coll. Cardiol. 2006, 47, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, S.A.; Wiswell, R.A. Rate and Mechanism of Maximal Oxygen Consumption Decline with Aging: Implications for Exercise Training. Sports Med. 2003, 33, 877–888. [Google Scholar] [CrossRef]
- López-González, M.; Priego-Jiménez, S.; López-Requena, A.; De Miguel-Brox, M.; Álvarez-Bueno, C. Effect of exercise interventions on oxygen uptake in healthy older adults: A network meta-analysis of randomized controlled trials. Exp. Gerontol. 2025, 212, 112962. [Google Scholar] [CrossRef]
- Kunz, H.E.; Lanza, I.R. Age-associated inflammation and implications for skeletal muscle responses to exercise. Exp. Gerontol. 2023, 177, 112177. [Google Scholar] [CrossRef]
- You, T.; Arsenis, N.C.; Disanzo, B.L.; LaMonte, M.J. Effects of exercise training on chronic inflammation in obesity: Current evidence and potential mechanisms. Sports Med. 2013, 43, 243–256. [Google Scholar] [CrossRef]
- Shuai, Z.; Jie, M.S.; Wen, X.K.; Xu, H.; Yuan, L. Effects of exercise intervention on exercise capacity and cardiopulmonary function in patients with atrial fibrillation: A randomized controlled trial systematic review and meta-analysis. Med Clin. 2025, 164, 106908. [Google Scholar] [CrossRef]
- Chua, T.P.; Ponikowski, P.; Harrington, D.; Anker, S.D.; Webb-Peploe, K.; Clark, A.L.; A Poole-Wilson, P.; Coats, A.J. Clinical correlates and prognostic significance of the ventilatory response to exercise in chronic heart failure. J. Am. Coll. Cardiol. 1997, 29, 1585–1590. [Google Scholar] [CrossRef] [PubMed]
- Kleber, F.X.; Vietzke, G.; Wernecke, K.D.; Bauer, U.; Opitz, C.; Wensel, R.; Sperfeld, A.; Gläser, S. Impairment of ventilatory efficiency in heart failure: Prognostic impact. Circulation 2000, 101, 2803–2809. [Google Scholar] [CrossRef] [PubMed]
- Arena, R.; Myers, J.; Abella, J.; Peberdy, M.A.; Bensimhon, D.; Chase, P.; Guazzi, M. Development of a ventilatory classification system in patients with heart failure. Circulation 2007, 115, 2410–2417. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Castro, R.R.; Caron, J.P.; Bay, C.P.; Hainer, J.; Opotowsky, A.R.; Mehra, M.R.; Maron, B.A.; Di Carli, M.F.; Groarke, J.D.; et al. Usefulness of ventilatory inefficiency in predicting prognosis across the heart failure spectrum. ESC Heart Fail. 2022, 9, 293–302. [Google Scholar] [CrossRef]
- Neder, J.A.; Phillips, D.B.; O’DOnnell, D.E.; Dempsey, J.A. Excess ventilation and exertional dyspnoea in heart failure and pulmonary hypertension. Eur. Respir. J. 2022, 60, 2200144. [Google Scholar] [CrossRef]
- de Tejada, M.G.-S.; Bilbao, A.; Ansola, L.; Quirós, R.; García-Perez, L.; Navarro, G.; Escobar, A. Responsiveness and minimal clinically important difference of the Minnesota living with heart failure questionnaire. Health Qual. Life Outcomes 2019, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Jellestada, L.; Auschraa, B.; Zuccarella-Hackla, C.; Principa, M.; von Känela, R.; Eulera, S.; Hermannb, M. Sex and age as predictors of health-related quality of life change in Phase II cardiac rehabilitation. Eur. J. Prev. Cardiol. 2023, 30, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Gayda, M.; Normandin, E.; Meyer, P.; Juneau, M.; Haykowsky, M.; Nigam, A. Central hemodynamic responses during acute high-intensity interval exercise and moderate continuous exercise in patients with heart failure. Appl. Physiol. Nutr. Metab. 2012, 37, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Mentz, R.J.; Whellan, D.J.; Reeves, G.R.; Pastva, A.M.; Duncan, P.; Upadhya, B.; Nelson, M.B.; Chen, H.; Reed, S.D.; Rosenberg, P.B.; et al. Rehabilitation Intervention in Older Patients with Acute Heart Failure with Preserved Versus Reduced Ejection Fraction. JACC Heart Fail. 2021, 9, 747–757. [Google Scholar] [CrossRef]
- Li, Y.; He, W.; Jiang, J.; Zhang, J.; Ding, M.; Li, G.; Luo, X.; Ma, Z.; Li, J.; Ma, Y.; et al. Non-Pharmacological Interventions in Patients with Heart Failure with Reduced Ejection Fraction: A Systematic Review and Network Meta-analysis. Arch. Phys. Med. Rehabil. 2024, 105, 963–974. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).