Abstract
Background: Muscle and bone show reciprocal interactions and are associated in a muscle-bone unit. The muscle-bone unit has been investigated to a very limited extent in soccer players. The objective of this work was to investigate in detail the muscle-bone unit in male youth elite soccer players. Methods: Bone mineral and lean mass were measured with dual-energy X-ray absorptiometry (DXA). The functional muscle-bone unit (fMBU) and the muscle-to-bone ratio (MBR) were calculated from the DXA output in a sample of players aged 14–19 (n = 193) playing in the youth squads of an Italian Serie A team. Results: Statistically significant (p < 0.05) correlations were found between lean mass variables and bone mineral content and density, also after adjusting for age, body mass, stature, maturity, and ethnicity (White/Black). fMBU and MBR were statistically significantly associated with age, body mass, stature, maturity, and ethnicity. Linear regression showed that body lean mass was the strongest predictor for bone mineral content and density. Age was a statistically significant predictor for fMBU and MBR. Playing position did not show any statistically significant relationship with bone mineral content and density, as well as fMBU or MBR. Centiles for fMBU and MBR were calculated as a reference. Conclusions: This work is the first detailed characterization of the muscle-to-bone relationship in soccer players. It is expected to be of use for sport scientists and the wide community of sportsmen and professionals involved in soccer.
1. Introduction
Muscle and bone are closely associated since early development and represent a single functional system. They derive from the same progenitors [], work in strict interdependence, forming the muscle-bone unit [], and affect each other both anatomically and biochemically in a muscle-bone crosstalk []. Initially, the crosstalk between muscle and bone was attributed essentially to mechanotransduction. Further work showed that bone and muscle are secretory tissues, which are able to interact with one another using myokines and osteokines affecting distant bones and muscles, respectively. Physical activity plays a crucial role in muscle-bone crosstalk [,], essentially through mechanical stress [], since the forces produced by skeletal muscles are more prominent than those associated with gravity [], although exercise also has a potential to affect myokines and osteokines.
It has been shown that sports practice exerts a powerful osteogenic stimulus [], especially during growth []. Soccer is a dynamic sport [] characterized by high-intensity intermittent efforts involving high loading impact at body weight on the bone, e.g., rapid accelerations and decelerations, frequent changes in direction, and frequent jumps. In adults, soccer participation at a professional level has been shown to improve bone quality compared to nonsporting controls [] and a different body weight-loading sport, such as elite running []. In youth, soccer has been shown to exert a positive effect on both bone mineral content (BMC) and bone mineral density (BMD) [] even after controlling for several confounding variables []. Further, soccer may be superior to other sports in accruing bone mineral [,]. Besides being a key period for bone formation [], adolescence, i.e., the period of maximal growth velocity, is also crucial to muscle mass accrual []. There is evidence that body lean soft tissue, mainly skeletal muscle mass, is a mediator of improved bone mineral density in sporting adolescents []. However, the status of the muscle-bone unit in youth elite soccer players has been investigated to a very limited extent [].
When investigating the muscle-bone unit in a sporting population of growing individuals, maturity should be considered, as people of the same chronological age showing differences in stature and body mass [] are comprised therein. In the context of youth soccer, the accurate determination of maturity through sophisticated laboratory techniques is not practical. The maturity offset (MO) [] is a practical tool to reliably estimate maturity from simple anthropometric measurements [MO = −7.999994 + (0.0036124 x (age x height))] [] and has been used in different sporting populations [,]; the equation estimates time before or after peak height velocity. Ethnicity (Eth) should also be considered when investigating the muscle–bone relationship in soccer players because of its capacity to affect body composition, including BMC and aBMD [,,,], and the muscle-bone unit []. Furthermore, the playing position (PP) may induce differences in the physiological and metabolic demands placed on the player that are distinctive to the position [], thereby affecting body composition and, possibly, the muscle–bone relationship.
Dual-energy X-ray absorptiometry (DXA) is a reliable technique to assess BMC and BMD in youth [], yielding, at the same time, accurate measurement of fat-free soft tissue mass (FFSTM). In general terms, the DXA outcome measures of bone mineral and lean (muscle) mass have been used as surrogates of bone and muscle strength, respectively [].
Several DXA-derived body lean mass variables and indices could be used as determinants of bone mineral status. The Appendicular (upper limbs + lower limbs) FFSTM has been shown to represent a reliable proxy for body skeletal muscle mass [,], which, in turn, is associated with bone mineral [,,] in the muscle-bone unit. The total body less head (TBLH) FFSTM incorporates Appendicular FFSTM and trunk lean soft tissue, representing a proxy of body mass, which, in turn, is associated with bone mineral status [,]. The stature-normalized FFSTM (fat-free mass index, FFMI = FFSTM (kg)/stature2 (m)) represents an absolute value, which is useful to compare individuals and groups [,] and is associated with bone mineral status, especially during growth [].
Several DXA-derived variables have been used to assess the muscle–bone relationship. The functional muscle-bone unit (fMBU, BMC [g]/FFSTM [g]) [] “could reflect the mass-related aspect of the mechanical equilibrium achieved by the system [i.e., the bone-muscle unit] as a function of its strain-related point” []. A similar index, the muscle-to-bone ratio (MBR, FFSTM/BMC), has been used to evaluate the relationships between lean muscle mass and bone mass in athletes [,,]. Total body MBR has been previously explored in soccer players [,] using anthropometry and equations. In this work, we chose to use DXA for the estimation of MBR because it allows for a more direct measurement of muscle- and bone-related variables vs. anthropometry, also at the regional level.
In this cross-sectional study, a sample of youth elite soccer players was recruited to define the relationship between muscle mass and bone characteristics at the organismic and regional levels and to explore the effect thereupon of relevant variables such as chronological age, maturity, Eth, and PP.
2. Materials and Methods
2.1. Participants
A convenience sample of male elite soccer players aged 14–19 years was collected over several years from the youth squads of a club competing in the Italian Serie A for multiple investigations. Players trained 4/5 times a week for two hours (<18 y) or 5/6 times a week for two hours (<20 y) and had a championship match one time a week during the season. The study was conducted according to the Helsinki Declaration and approved by the local ethics committee (2019-UNIVRCLR-0422326). The sample size for linear regression was calculated a priori with G*Power 3.1 []. Setting a medium effect size (Cohen’s f2) of 0.15 with alpha = 0.01, power = 0.95, and a maximum number of predictors = 5, the calculated total sample size was n = 180. Where needed, participants were subdivided into the following PPs: Goalkeeper (GK, n = 19), Defender (D, n = 60), Midfielder (M, n = 76), and Forward (F, n = 38). Considering the aims of this work, further fractionation of the study sample into sub-positions, e.g., central and lateral defender, was deemed not essential. All data were collected in the summer, before starting conditioning for the competitive season. The inclusion criteria were as follows: 14 y < age <20 y, no acute illness of any kind, no major injuries in the last six months, and no medications potentially affecting body composition or bone in the previous twelve months.
2.2. Procedures
Body mass was measured using an electronic scale (Tanita electronic scale BWB-800 MA, Wunder SA.BI. Srl, Milano, Italy) and recorded to the nearest 0.1 kg. A Harpenden stadiometer (Holtain Ltd., Crymych, Pembs, UK) was used to measure stature at the nearest mm of the participant without shoes and with minimal clothing. The body mass index (BMI) was calculated as weight (kg)/height (m2). For body composition evaluation, FFSTM, BMC, and areal BMD (aBMD) were measured using a total body DXA scanner (QDR Horizon, Hologic, Marlborough, MA, USA; fan-beam technology, software version 13.6.05). Possible baseline drift was assessed daily against a reference phantom supplied by the manufacturer. Scans were performed in the late morning in a post-absorptive state. Participants were instructed to avoid vigorous exercise for at least 24 h before measurement. The protocol of Nana et al. [] was adopted for measurements. All other procedures were according to the manufacturer’s recommendations. All scans were taken of the whole body (WB). In vivo short-term precision was calculated by repeated (n = 2) scanning of 30 subjects with repositioning []; precision (percent coefficient of variation) was 0.59%, 0.75%, and 0.68% for FFSTM, BMC, and aBMD, respectively. The Hologic software provides readings for the WB and the trunk, the entire arm (left and right), the entire leg (left and right), and the head. In this work, BMC and aBMD of the total body less head region (TBLH) was considered in the analysis because the skull contains a large amount of total body mineral and is insensitive to physical activity []. The WB values for BMC and aBMD were also reported to compare with the previous literature. FFSTM, BMC, and aBMD were also calculated for the upper limbs together (Arms), lower limbs together (Legs), and the four limbs together (Appendicular). The same operator performed all scans and regional measurements to ensure consistency. The fMBU was expressed as fMBUtot, i.e., the ratio of TBLH BMC to WB FFSTM [,], and fMBUapp, i.e., the ratio of Appendicular BMC to Appendicular FFSTM. The MBR was calculated at the WB and regional (Arms, Legs, and Trunk) level.
Stature and body mass may represent confounding variables for bone measurements because DXA is a projectional technique. DXA-derived measurements do not adequately correct for body and/or bone size []. Accordingly, the bone mineral was also expressed as bone mineral apparent density (BMAD, g/cm3) according to the formula proposed by Katzman et al. []: BMAD = BMC/(bone area2/body stature), thereby reducing the risk of overestimating players with higher stature and the risk of underestimating those with shorter stature [].
Fractional age (FA) was calculated (YEARFRAC function in Microsoft Excel) and used throughout the analysis to fully exploit the information potential of chronological age in a sample of growing individuals.
2.3. Statistical Analysis
The normality of data was assessed using the Shapiro–Wilk test. Data are presented as mean ± standard deviation. Correlation and partial correlation (PC) analysis was conducted by calculating Pearson’s r and r(PC). The strength of correlation was rated as per Hopkins [], i.e., small (0–0.30), moderate (0.31–0.49), large (0.50–0.69), very large (0.70–0.89), and almost perfect (0.90–1).
One-way ANOVA in the general linear model was used to assess differences in fMBU and MBR in the four PPs (GK, D, M, F) and the effect of covariates. The Levene test assessed homogeneity of variance. Heteroscedasticity was evaluated with the Breusch–Pagan test. In case of significance, post hoc analysis with Bonferroni’s correction was conducted to assess differences between individual PPs. The effect size (partial η2) was calculated and rated according to Cohen [] as small (0.01), medium (0.06), and large (0.14).
Linear regression analysis was carried out with bone mineral variable as the dependent variable and TBLH FFSTM, Appendicular FFSTM, or FFMI as the independent variable, together with FA, MO, Eth, and PP. The goodness-of-fit of models were assessed by calculating the adjusted coefficient of determination (AdjR2) and the standard error of the estimate (SEE). The standardized partial regression coefficient β assessed the independent effect of each predictive variable in bivariate regression. All covariates and predictors were selected after checking for multicollinearity.
Statistical significance was set at p ≤ 0.05. The IBM SPSS statistical package (v. 26, Armonk, NY, USA) was used for all analyses.
3. Results
3.1. Characteristics of the Study Sample
The final dataset included 193 players; therefore, the study was enough powered. The mean age of participants was 17.3 ± 1.06 y. The mean body mass and stature were 71.6 ± 6.96 kg and 179.7 ± 6.14 cm, respectively. The mean BMI was 22.1 ± 1.57 kg/m2. Most participants were White (n = 172); the rest were Black (n = 21).
3.2. Correlation of Muscle and Bone Variables
The results of bivariate and adjusted correlation analysis between lean mass and bone variables are shown in Table 1 and Figure 1. Very large (r from 0.70 to 0.79), statistically significant correlations were found for Appendicular FFSTM and TBLH FFSTM, and WB, TBLH, Appendicular, and Arms BMC. Appendicular FFSTM and TBLH FFSTM showed large (r from 0.53 to 0.69), statistically significant correlations with Pelvis and Legs BMC and Arms aBMD. The correlation of FFMI with bone variables was moderate to large (r from 0.21 to 0.54), being statistically significant in most instances. No statistically significant correlation was found between Appendicular FFSTM, TBLH FFSTM, and FFMI (collectively “lean mass variables” from here on) and Appendicular and TBLH BMAD. Adjusting correlation for several confounding variables (body mass, stature, FA, Eth, and MO for lean mass variables and body mass, FA, Eth, and MO for BMAD) resulted in a general reduction in the r value. However, all the lean mass variables statistically significantly correlated with TBLH BMC, Appendicular BMC, and aBMD, and Arms and Legs BMC and aBMD. No statistically significant bivariate or adjusted correlation was found for BMAD.

Table 1.
Bivariate and partial correlations between Appendicular fat-free soft tissue mass (App FFSTM, g), total body less head (TBLH) FFSTM (g), and fat-free mass index (FFMI, g/cm2) and bone mineral variables in 193 male youth elite soccer players.
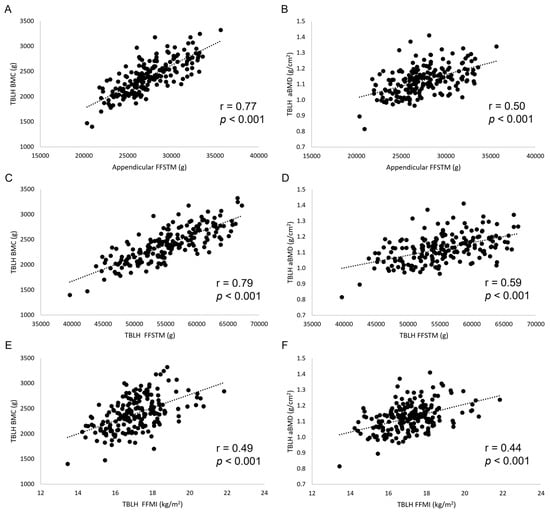
Figure 1.
Scatterplots (A–F) showing the relationship between lean mass and bone mineral variables. TBLH, total body less head; BMC, bone mineral content.
The mean values for fMBU in the youth elite soccer players sample were as follows: fMBUtot (g/g), 0.0410 ± 0.00351; fMBUapp (g/g), 0.0579 ± 0.00621. The mean values for MBR were as follows: MBRWB (kg/kg), 23.3 ± 1.73; MBRarms (kg/kg), 18.7 ± 1.59; MBRlegs (kg/kg), 17.1 ± 1.97; MBRtrunk (kg/kg), 35.1 ± 5.56.
Table 2 shows the results of correlation analysis between fMBU and MBR, and body mass, stature, MO, and Eth, as well as FA (for the latter, both bivariate and adjusted correlation coefficients are presented; see table for details).

Table 2.
Correlation coefficient between muscle-bone unit indices and body mass, stature, maturity offset (MO), ethnicity (Eth), and fractional age (FA). The adjusted correlation coefficient for FA is also shown.
FA showed statistically significant, small to moderate correlations with all muscle-bone indices (Figure 2). A similar pattern was found for MO (except for MBRtrunk) at a correlation strength from small to moderate. A decreasing number of statistically significant, small correlations was found for body mass, stature, and Eth. After adjusting for all the other biological variables, Eth was the only variable to show a small, statistically significant correlation with a muscle-bone unit index (fMBUtot: r = 0.168, p = 0.021; MBRWB: r = −0.172, p = 0.018).
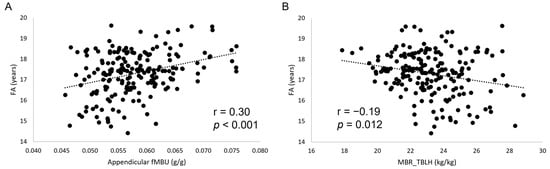
Figure 2.
Scatterplots (A,B) showing the relationship between fractional age (FA) and muscle-bone indices. fMBU, functional muscle-bone index; MBR, muscle-to-bone ratio.
3.3. Results of ANOVA
One-way ANOVA conducted for fMBU and MBR with PP as the main factor showed no statistically significant difference for any index (F(3,192) from 0.192 to 1.089, p from 0.902 to 0.351, effect size from 0.003 to 0.017, observed power from 0.094 to 0.265). Similar results were obtained when the same analysis was carried out with FA, Eth, MO, body mass, and stature as covariates (Table 3). The effect size was medium for all variables.

Table 3.
Results of one-way ANOVA conducted with PP as the main factor and fractional age, ethnicity, maturity offset, body mass, and stature as the covariates for functional muscle-bone unit (fMBU) at whole body (tot), total body less head (TBLH), and Appendicular (App), as well as muscle-to-bone ratio (MBR) at TBLH, upper limbs (Arms), lower limbs (Legs), and Trunk. Partial η2, effect size.
3.4. Centiles for Bone-Muscle Indices
Centiles for all muscle-bone indices are presented in Table 4. Please note that data are presented of the White players only, because the number of Black players (n = 21) prevented meaningful calculation of centiles.

Table 4.
Centiles for functional muscle-bone unit (fMBU) at whole body (tot), total body less head (TBLH), and Appendicular (App), as well as muscle-to-bone ratio (MBR) at TBLH, upper limbs (Arms), lower limbs (Legs), and Trunk in White youth elite soccer players.
3.5. Results of Linear Regression Analysis
Results of linear regression analysis carried out in the whole sample (n = 193) using TBLH FFSTM, Appendicular FFSTM, and FFMI together with FA, MO, Eth, and PP as the predictors of bone mineral variables are summarized in Table 5, Table 6 and Table 7. All models were statistically significant (p ≤ 0.05). The Durbin–Watson statistic was <2.5, and the variance inflation factor was <5.0.

Table 5.
Linear regression of total body less head (TBLH, g) and fat-free soft tissue mass (FFSTM, g) together with fractional age (FA), maturity offset (MO), ethnicity (Eth), and playing position (PP) on bone mineral variables in the whole sample of elite youth soccer players (n = 193). The standardized regression coefficient (β) and its p value, as well as the overall adjusted coefficient of determination (AdjR2) and standard error of the estimate (SEE), are reported. Non-statistically significant p values are in bold.

Table 6.
Linear regression of Appendicular fat-free soft tissue mass (App FFSTM, g) together with fractional age (FA), maturity offset (MO), ethnicity (Eth), and playing position (PP) on bone mineral variables in the whole sample of elite youth soccer players (n = 193). The standardized regression coefficient (β) and its p value, as well as the overall adjusted coefficient of determination (AdjR2) and standard error of the estimate (SEE), are reported. Non-statistically significant p values are in bold.

Table 7.
Linear regression of Appendicular fat-free mass index (App FFMI, kg/m2) together with fractional age (FA), maturity offset (MO), ethnicity (Eth), and playing position (PP) on bone mineral variables in the whole sample of elite youth soccer players (n = 193). The standardized regression coefficient (β) and its p value, as well as the overall adjusted coefficient of determination (AdjR2) and standard error of the estimate (SEE), are reported. Non-statistically significant p values are in bold.
In regression analysis, TBLH FFSTM (Table 5) showed the highest, statistically significant β coefficient for a large majority of bone mineral variables in comparison with FA, MO, Eth, and PP; FA showed the highest, statistically significant β coefficient for some aBMD variables and Trunk BMC. Similar findings were found when Appendicular FFSTM was used in analysis (Table 6). When the stature-adjusted lean mass index FFMI was used in regression analysis (Table 7), MO showed the highest, statistically significant β coefficient for most bone mineral variables. FFMI and FA showed the highest, statistically significant β coefficient for a few aBMD and BMAD variables, respectively. PP showed not statistically significant β coefficients.
Regression analysis carried out for functional muscle-bone indices with FA, body mass, stature, Eth, and PP as the independent variables yielded the results presented in Table 8. All models were statistically significant (p ≤ 0.05). The Durbin-Watson statistic was always <2.5, and the variance inflation factor was <5.0.

Table 8.
Linear regression of fractional age (FA), body mass, stature, ethnicity (Eth), and playing position (PP) on muscle-to-bone indices in the whole sample of elite youth soccer players (n = 193). The standardized regression coefficient (β) and its p value, as well as the overall adjusted coefficient of determination (AdjR2) and standard error of the estimate (SEE), are reported. Non-statistically significant p values are in bold.
FA was the only predictor showing a statistically significant β coefficient for all indices. Eth showed a statistically significant β coefficient for fMBUWB and MBRTBLH, both of which were lower than those for FA. Stature, body mass, and PP showed no statistically significant β coefficient for all indices.
4. Discussion
In this work, we investigated the muscle-bone unit in a large sample of youth elite soccer players by means of DXA to characterize the functional relationship of muscle and bone mineral mass and density in this sporting population and the possible effect on it of several confounding biological variables.
The first finding of this work was that, in youth elite soccer players, BMC and aBMD statistically significantly correlate with body lean mass at the WB, TBLH, and regional levels. While a positive relationship between lean mass and bone is well documented [] and may be easily explained by their consensual accretion during growth, information on the correlation between lean mass and bone mineral mass or density is scant. Capozza et al. [] found a correlation (r = 0.71) between lean mass and BMC at WB in males, which is superimposable to that found in the current study (Table 1). In another study [] it was found that, in young adults, a statistically significant correlation exists between FFMI and WB BMC (r = 0.61), aBMD (r = 0.60), LS BMC (r = 0.35), and LS BMD (r = 0.33), which is consistent with findings in this work (Table 1). As expected, the correlation of bone variables with the stature-adjusted index FFMI was generally lower than that shown by absolute lean mass variables (TBLH FFSTM and Appendicular FFSTM) because stature is in a linear relationship with muscle mass []. After adjusting for several confounding factors (Table 1), the correlation between TBLH BMC, Appendicular BMC, and aBMD, and Appendicular FFSTM, TBLH FFSTM, and FFMI remained statistically significant, suggesting an independent reciprocal influence between bone and lean mass in the studied population. The lack of statistically significant bivariate and adjusted correlation between BMAD and lean mass (Table 1) further underlines the role of stature in mediating the relationship between lean mass and bone mineral.
The results of the correlation analysis presented in Table 2 showed a small, statistically significant r value between biological variables (body mass, stature, MO, Eth, and FA) and fMBU (mind that fMBUtot is calculated as TBLH BMC/WB FFSTM to avoid the confounding effect of head BMC, which represents a large proportion of WB BMC and may increase in a non-linear relationship with TBLH BMC), as well as MBR. These findings indicate that, at the organismic level, chronological age, body size, and maturity are associated with an increase in the amount of BMC accrued per unit lean mass. Interestingly, Eth was also positively associated with both muscle-bone indices. Since players participating in this study were White and Black (coded as “dummy” variables in the dataset), it is apparent that Black ethnicity plays a role in increasing the amount of BMC accrued per unit lean mass in youth elite soccer players. This point will be further discussed in the following. At the regional (Appendicular, Trunk, Arms, And Legs) level, FA showed a statistically significant r value for all muscle-bone indices (4/4), followed by MO (3/4), and body mass and stature (2/4). These data suggest that among the considered biological variables, chronological age is the only one able to act on the amount of BMC accrued per unit lean mass at all sites in the body. Playing position, a soccer-specific variable, showed no statistically significant correlation with any muscle-bone index. Therefore, PP seems not involved in mediating BMC accrual per unit lean mass in this sporting population. This conclusion is supported by ANOVA for muscle-bone indices conducted with PP as the factor and FA, body mass, stature, MO, and Eth as covariates (Table 3), showing no statistically significant difference for any index. Regression analysis (Table 8) confirmed that FA is the main factor determining the muscle-bone index at the organismic and regional level, with a minor role played by Eth at the organismic level only, whereas no significant role is played by body size (body mass and stature) or PP.
Table 4 presents the centiles for the muscle-bone indices in the White players. The age distribution of participants prevented the calculation of meaningful centiles for year age. However, it should be considered that the sample of White players was relatively homogeneous in terms of body size (mean BMI = 22.1 ± 1.55 kg/m2), and all participants showed positive MO values (mean: 3.2 ± 0.79 y). Given the lack of an established optimum range of muscle-bone indices in soccer players, the presented data can be practical for both athletes and coaches.
The relative importance of each lean mass variable (TBLH FFSTM, Appendicular FFSTM, and FFMI) and other variables of interest (FA, MO, Eth, and PP) in predicting bone mineral variables was assessed in linear regression analysis by calculating the standardized partial regression coefficient β. Considering the statistically significant β coefficients in Table 5, Table 6 and Table 7, TBLH FFSTM showed the highest values in comparison with Appendicular FFSTM and FFMI. Appendicular FFSTM, in turn, showed higher values than FFMI. This indicates that the overall lean mass (Appendicular + Trunk) and not skeletal muscle mass or stature-normalized WB lean mass better explain BMC and aBMD. This is possibly due to the positive action body mass exerts, per se, on bone mineral []. The role of lean mass in determining bone mineral variables in youth soccer players is confirmed by previous findings in Brazilian male players who were 12 to 18 y of age (n = 148) [], where DXA-measured lean mass was the most important predictor for TBLH BMC (R2 = 0.524), LS BMC (R2 = 0.492), and LS BMD (R2 = 0.513). In the present study, FA showed some statistically significant β coefficients in the models, including TBLH FFSTM and Appendicular FFSTM. Most β coefficients for FA were higher than those for TBLH, FFSTM, and Appendicular FFSTM in predicting aBMD at several sites. This is consistent with the soccer players still growing []. Instead, in the model with FFMI, FA showed higher β coefficients values than FFMI for almost all BMC variables. Since FFMI is a stature-adjusted index, this reveals a relevant stature-independent role of chronological age in bone mineral accrual in growing elite soccer players, as confirmed by previous findings in the general population []. Maturity offset, which incorporates the product of age and stature, showed a limited number of statistically significant β coefficients in the models, including TBLH FFSTM, or Appendicular FFSTM. In both models, MO showed the highest β coefficient among all predictive variables for Trunk BMC and aBMD. In the model including FFMI, MO showed the highest β coefficient for most BMC and aBMD variables, while FA showed negative coefficients. This indicates that MO should be given attention when investigating the relationships between bone mineral variables and lean mass variables in growing soccer players. Eth showed several low, albeit statistically significant, positive β coefficients, especially in the model including TBLH FFSTM. This finding could be explained by the higher average lean mass found in Black vs. White people []. A previous work using DXA [] did not find a statistically significant difference in lean mass between Caucasian and non-Caucasian adult elite soccer players. However, in the study of Sutton et al. [], the non-Caucasian group included players of African-Caribbean and Asian descent and mixed race, which may be non-comparable with the Black players investigated in the present work. PP did not show any statistically significant β coefficient in any model, showing that the different positions on the field do not affect, per se, the muscle-bone unit. A difference in body composition is often found in soccer players between goalkeepers and outfield players, both in adult [] and youth soccer players [,,]. However, such a difference typically involves FM and %FM, thereby limiting its impact on bone mineral variables.
The mean value of fMBUtot was in line with those found in the adolescent general population []. This may be due, at least in part, to the fact that the ratio between two variables (muscle mass and skeletal mass) growing roughly in a similar way tends to be similar across a large interval of values. However, the mean fMBUapp value was closer to that of young adults in the general population [], suggesting that elite soccer practice is highly effective in promoting bone mineral accrual per unit muscle mass in the limbs of youth players. This finding is supported by previous findings [] showing increased BMC and BMD in youth soccer players. Interestingly, the mean MBR values found in elite youth soccer players at TBLH, Arms, Legs, and Trunk were obviously higher than in 20-year-old track and field athletes [], as well as younger (20.1 y) [] and older (24.2 y) [] American football players, suggesting that those sports promote lower and higher bone mineral accrual per unit muscle mass vs. soccer, respectively. Estimation of β coefficients in linear regression (Table 8) showed a main role for FA in determining fMBU and MBR. This finding is supported by data in the general healthy population showing a steady increase in fMBU in male adolescents []. Interestingly, Eth showed statistically significant β coefficients for fMBUtot and MBRWB, indicating that Eth is able to differentially modulate the age-associated changes in the muscle-bone unit. Previous work in the general population [] (age: 5–35 y) showed no differences in the muscle-bone unit according to race. However, participants in the current study were highly fitted sporting adolescents and it is reasonable to infer that an effect of Eth on the muscle-bone unit only emerges upon long periods of intense physical activity. Further investigation with a larger number of soccer players is needed to confirm such a hypothesis. Playing position did not show a statistically significant effect on any index (Table 8), confirming the results presented in Table 5, Table 6 and Table 7 and suggesting that soccer practice is associated with similar accumulation of bone mass per unit increase in muscle mass independently of the role played on the field. This suggestion is supported by findings obtained at the WB level using anthropometry by Bernal-Orozco et al. [] showing very limited statistically significant differences among PPs in young (<20 y) Mexican soccer players.
This paper has strengths that should be underlined. First, to the best of our knowledge, this paper is the first to present both WB and regional data on the muscle-bone unit in soccer players. The study included a large number of participants, thereby offering a broad overview of muscle-bone characteristics with the production of reference data. Second, the use of DXA for body composition analysis guaranteed accurate measurement of the lean and bone mineral components at the WB and regional level, which is impossible using anthropometry. Third, the influence of PP on the muscle–bone relationship was explored.
This study has limitations. First, it was cross-sectional and not longitudinal, thereby limiting insights into the biology of the muscle-bone unit in adolescent elite soccer players. Second, dietary information was not available for the young participants that would have added sound information to the paper with special reference to mineral accrual. Third, performance metrics were not investigated, so the association of the muscle-bone unit and sport-specific variables could not be assessed.
5. Conclusions
In conclusion, the novelty of this work is the extensive characterization of the relationship between lean mass and bone in youth elite soccer players. It was shown that both muscle mass and lean mass are independent determinants of the bone mineral content and density, albeit stature plays an important role in mediating such a relationship. fMBU and MBR were especially modulated by chronological age, and Black ethnicity showed a positive effect on BMC accrual per unit lean mass. Reference values for fMBU and MBR were provided. The playing position did not affect the muscle-to-bone ratio to any extent. Findings presented herein should be of use for evaluating and monitoring youth soccer players.
Author Contributions
Conceptualization, C.M. and C.Z.; methodology, C.M., V.C., and C.Z.; formal analysis, V.C., and C.Z.; investigation, V.C. and C.M.; resources, C.M. and C.Z.; data curation, V.C., C.M., and C.Z.; writing—original draft preparation, C.Z.; writing—review and editing, C.M. and V.C.; visualization, V.C.; supervision, C.Z.; project administration, C.M.; funding acquisition, C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board at the University of Verona (2019-UNIVRCLR-0422326), approved on 13 November 2019.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| FA | Fractional age |
| BMC | Bone mineral content |
| BMD | Bone mineral density |
| MO | Maturity offset |
| PP | Playing position |
| GK | Goalkeeper |
| D | Defender |
| M | Midfielder |
| F | Forward |
| DXA | Dual-energy X-ray absorptiometry |
| FFSTM | Fat-free soft tissue mass |
| FFMI | Fat-free mass index |
| fMBU | Functional muscle-bone unit |
| MBR | Muscle-to-bone ratio |
| Eth | Ethnicity |
| BMI | Body mass index |
| aBMD | Areal bone mineral density |
| WB | Whole body |
| TBLH | Total body less head region |
| Arms | upper limbs together |
| Legs | lower limbs together |
| Appendicular | Four limbs together |
| BMAD | Bone mineral apparent density |
| AdjR2 | Coefficient of determination |
| SEE | Standard error of the estimate |
| PC | Partial correlation |
References
- Buckingham, M.; Bajard, L.; Chang, T.; Daubas, P.; Hadchouel, J.; Meilhac, S.; Montarras, D.; Rocancourt, D.; Relaix, F. The Formation of Skeletal Muscle: From Somite to Limb. J. Anat. 2003, 202, 59–68. [Google Scholar] [CrossRef]
- Schoenau, E. The “Functional Muscle-Bone Unit”: A Two-Step Diagnostic Algorithm in Pediatric Bone Disease. Pediatr. Nephrol. 2005, 20, 356–359. [Google Scholar] [CrossRef]
- Brotto, M.; Bonewald, L. Bone and Muscle: Interactions beyond Mechanical. Bone 2015, 80, 109–114. [Google Scholar] [CrossRef]
- Nichols, D.L.; Sanborn, C.F.; Essery, E.V. Bone Density and Young Athletic Women. An Update. Sports Med. 2007, 37, 1001–1014. [Google Scholar] [CrossRef]
- Karsenty, G.; Mera, P. Molecular Bases of the Crosstalk between Bone and Muscle. Bone 2018, 115, 43–49. [Google Scholar] [CrossRef]
- Isaacson, J.; Brotto, M. Physiology of Mechanotransduction: How Do Muscle and Bone “Talk” to One Another? Clin. Rev. Bone Miner. Metab. 2014, 12, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Schönau, E.; Werhahn, E.; Schiedermaier, U.; Mokow, E.; Schiessl, H.; Scheidhauer, K.; Michalk, D. Influence of Muscle Strength on Bone Strength during Childhood and Adolescence. Horm. Res. 1996, 45 (Suppl. 1), 63–66. [Google Scholar] [CrossRef] [PubMed]
- Dolan, E.; Varley, I.; Ackerman, K.E.; Pereira, R.M.R.; Elliott-Sale, K.J.; Sale, C. The Bone Metabolic Response to Exercise and Nutrition. Exerc. Sport Sci. Rev. 2020, 48, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Moshage, S.G.; Kersh, M.E. Play During Growth: The Effect of Sports on Bone Adaptation. Curr. Osteoporos. Rep. 2020, 18, 684–695. [Google Scholar] [CrossRef]
- Cometti, G.; Maffiuletti, N.A.; Pousson, M.; Chatard, J.C.; Maffulli, N. Isokinetic Strength and Anaerobic Power of Elite, Subelite and Amateur French Soccer Players. Int. J. Sports Med. 2001, 22, 45–51. [Google Scholar] [CrossRef]
- Wittich, A.; Mautalen, C.; Oliveri, B.; Bagur, A.; Somoza, F.; Rotemberg, E. Professional Football (Soccer) Players Have a Markedly Greater Skeletal Mineral Content, Density and Size Than Age- and BMI-Matched Controls. Calcif. Tissue Int. 1998, 63, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Fredericson, M.; Chew, K.; Ngo, J.; Cleek, T.; Kiratli, J.; Cobb, K. Regional Bone Mineral Density in Male Athletes: A Comparison of Soccer Players, Runners and Controls. Br. J. Sports Med. 2007, 41, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Berges, G.; Matute-Llorente, Á.; González-Agüero, A.; Gómez-Bruton, A.; Gómez-Cabello, A.; Vicente-Rodríguez, G.; Casajús, J.A. Soccer Helps Build Strong Bones during Growth: A Systematic Review and Meta-Analysis. Eur. J. Pediatr. 2018, 177, 295–310. [Google Scholar] [CrossRef]
- Agostinete, R.R.; Fernandes, R.A.; Narciso, P.H.; Maillane-Vanegas, S.; Werneck, A.O.; Vlachopoulos, D. Categorizing 10 Sports According to Bone and Soft Tissue Profiles in Adolescents. Med. Sci. Sports Exerc. 2020, 52, 2673–2681. [Google Scholar] [CrossRef]
- Vlachopoulos, D.; Barker, A.R.; Williams, C.A.; Arngrímsson, S.A.; Knapp, K.M.; Metcalf, B.S.; Fatouros, I.G.; Moreno, L.A.; Gracia-Marco, L. The Impact of Sport Participation on Bone Mass and Geometry in Male Adolescents. Med. Sci. Sports Exerc. 2017, 49, 317–326. [Google Scholar] [CrossRef]
- Chevalley, T.; Rizzoli, R. Acquisition of Peak Bone Mass. Best Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101616. [Google Scholar] [CrossRef]
- Wheeler, M.D. Physical Changes of Puberty. Endocrinol. Metab. Clin. N. Am. 1991, 20, 1–14. [Google Scholar] [CrossRef]
- Maillane-Vanegas, S.; Agostinete, R.R.; Lynch, K.R.; Ito, I.H.; Luiz-de-Marco, R.; Rodrigues-Junior, M.A.; Turi-Lynch, B.C.; Fernandes, R.A. Bone Mineral Density and Sports Participation. J. Clin. Densitom. 2020, 23, 294–302. [Google Scholar] [CrossRef]
- Anliker, E.; Sonderegger, A.; Toigo, M. Side-to-Side Differences in the Lower Leg Muscle-Bone Unit in Male Soccer Players. Med. Sci. Sports Exerc. 2013, 45, 1545–1552. [Google Scholar] [CrossRef]
- Iuliano-Burns, S.; Mirwald, R.L.; Bailey, D.A. Timing and Magnitude of Peak Height Velocity and Peak Tissue Velocities for Early, Average, and Late Maturing Boys and Girls. Am. J. Hum. Biol. 2001, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mirwald, R.L.; Baxter-Jones, A.D.G.; Bailey, D.A.; Beunen, G.P. An Assessment of Maturity from Anthropometric Measurements. Med. Sci. Sports Exerc. 2002, 34, 689–694. [Google Scholar] [CrossRef]
- Moore, S.A.; McKay, H.A.; Macdonald, H.; Nettlefold, L.; Baxter-Jones, A.D.G.; Cameron, N.; Brasher, P.M.A. Enhancing a Somatic Maturity Prediction Model. Med. Sci. Sports Exerc. 2015, 47, 1755–1764. [Google Scholar] [CrossRef]
- Malina, R.M.; Martinho, D.V.; Valente-Dos-Santos, J.; Coelho-E-Silva, M.J.; Kozieł, S.M. Growth and Maturity Status of Female Soccer Players: A Narrative Review. Int. J. Environ. Res. Public Health 2021, 18, 1448. [Google Scholar] [CrossRef] [PubMed]
- Malina, R.M.; Claessens, A.L.; Van Aken, K.; Thomis, M.; Lefevre, J.; Philippaerts, R.; Beunen, G.P. Maturity Offset in Gymnasts: Application of a Prediction Equation. Med. Sci. Sports Exerc. 2006, 38, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Bachrach, L.K.; Hastie, T.; Wang, M.C.; Narasimhan, B.; Marcus, R. Bone Mineral Acquisition in Healthy Asian, Hispanic, Black, and Caucasian Youth: A Longitudinal Study. J. Clin. Endocrinol. Metab. 1999, 84, 4702–4712. [Google Scholar] [CrossRef] [PubMed]
- Barondess, D.A.; Nelson, D.A.; Schlaen, S.E. Whole Body Bone, Fat, and Lean Mass in Black and White Men. J. Bone Miner. Res. 1997, 12, 967–971. [Google Scholar] [CrossRef]
- Travison, T.G.; Chiu, G.R.; McKinlay, J.B.; Araujo, A.B. Accounting for Racial/Ethnic Variation in Bone Mineral Content and Density: The Competing Influences of Socioeconomic Factors, Body Composition, Health and Lifestyle, and Circulating Androgens and Estrogens. Osteoporos. Int. 2011, 22, 2645–2654. [Google Scholar] [CrossRef]
- Zemel, B.S.; Kalkwarf, H.J.; Gilsanz, V.; Lappe, J.M.; Oberfield, S.; Shepherd, J.A.; Frederick, M.M.; Huang, X.; Lu, M.; Mahboubi, S.; et al. Revised Reference Curves for Bone Mineral Content and Areal Bone Mineral Density According to Age and Sex for Black and Non-Black Children: Results of the Bone Mineral Density in Childhood Study. J. Clin. Endocrinol. Metab. 2011, 96, 3160–3169. [Google Scholar] [CrossRef]
- Leonard, M.B.; Elmi, A.; Mostoufi-Moab, S.; Shults, J.; Burnham, J.M.; Thayu, M.; Kibe, L.; Wetzsteon, R.J.; Zemel, B.S. Effects of Sex, Race, and Puberty on Cortical Bone and the Functional Muscle Bone Unit in Children, Adolescents, and Young Adults. J. Clin. Endocrinol. Metab. 2010, 95, 1681–1689. [Google Scholar] [CrossRef]
- Di Salvo, V.; Baron, R.; Tschan, H.; Calderon Montero, F.J.; Bachl, N.; Pigozzi, F. Performance Characteristics According to Playing Position in Elite Soccer. Int. J. Sports Med. 2007, 28, 222–227. [Google Scholar] [CrossRef]
- Guss, C.E.; McAllister, A.; Gordon, C.M. DXA in Children and Adolescents. J. Clin. Densitom. 2021, 24, 28–35. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Smith, R.; Aulet, M.; Bensen, B.; Lichtman, S.; Wang, J.; Pierson, R.N. Appendicular Skeletal Muscle Mass: Measurement by Dual-Photon Absorptiometry. Am. J. Clin. Nutr. 1990, 52, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Wang, Z.; Heymsfield, S.B.; Baumgartner, R.N.; Gallagher, D. Total-Body Skeletal Muscle Mass: Estimation by a New Dual-Energy X-Ray Absorptiometry Method. Am. J. Clin. Nutr. 2002, 76, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, J.; Ren, W.; Li, X.; Luo, M.; Hu, Y. The Relationship between Skeletal Muscle Mass and Bone Mass at Different Sites in Older Adults. Ann. Nutr. Metab. 2023, 79, 256–262, Erratum in Ann. Nutr. Metab. 2023, 79, 405. [Google Scholar] [CrossRef]
- Cristi-Montero, C.; Peña-Jorquera, H.; Landaeta-Díaz, L.; Mello, J.B.; Araya-Quintanilla, F.; Brand, C.; Reuter, C.; Jorquera, C.; Ferrari, G. The Inverse Relationship between Fatness and Bone Mineral Content Is Mediated by the Adolescent Appendicular Skeletal Muscle Mass Index: The Cogni-Action Project. Front. Nutr. 2022, 9, 1040116. [Google Scholar] [CrossRef]
- Han, H.; Chen, S.; Wang, X.; Jin, J.; Li, X.; Li, Z. Association between Muscle Strength and Mass and Bone Mineral Density in the US General Population: Data from NHANES 1999–2002. J. Orthop. Surg. Res. 2023, 18, 397. [Google Scholar] [CrossRef]
- Iwaniec, U.T.; Turner, R.T. Influence of Body Weight on Bone Mass, Architecture and Turnover. J. Endocrinol. 2016, 230, R115–R130. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.C.; Kurokawa, C.S.; Nga, H.S.; Moretto, M.R.; Dalmas, J.C.; Goldberg, T.B.L. Bone Metabolism Biomarkers, Body Weight, and Bone Age in Healthy Brazilian Male Adolescents. J. Pediatr. Endocrinol. Metab. 2012, 25, 479–484. [Google Scholar] [CrossRef]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of Sarcopenia among the Elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef]
- Kyle, U.G.; Schutz, Y.; Dupertuis, Y.M.; Pichard, C. Body Composition Interpretation: Contributions of the Fat-Free Mass Index and the Body Fat Mass Index. Nutrition 2003, 19, 597–604. [Google Scholar] [CrossRef]
- Ivuskans, A.; Lätt, E.; Mäestu, J.; Saar, M.; Purge, P.; Maasalu, K.; Jürimäe, T.; Jürimäe, J. Bone Mineral Density in 11–13-Year-Old Boys: Relative Importance of the Weight Status and Body Composition Factors. Rheumatol. Int. 2013, 33, 1681–1687. [Google Scholar] [CrossRef]
- Goodman, C.A.; Hornberger, T.A.; Robling, A.G. Bone and Skeletal Muscle: Key Players in Mechanotransduction and Potential Overlapping Mechanisms. Bone 2015, 80, 24–36. [Google Scholar] [CrossRef]
- Duran, I.; Martakis, K.; Bossier, C.; Stark, C.; Rehberg, M.; Semler, O.; Schoenau, E. Interaction of Body Fat Percentage and Height with Appendicular Functional Muscle-Bone Unit. Arch. Osteoporos. 2019, 14, 65. [Google Scholar] [CrossRef]
- Dengel, D.R.; Evanoff, N.G. Positional Differences in Muscle-to-Bone Ratio in National Football League Players. Int. J. Sports Med. 2023, 44, 720–727. [Google Scholar] [CrossRef]
- Gomez-Bruton, A.; Gonzalez-Aguero, A.; Matute-Llorente, A.; Lozano-Berges, G.; Gomez-Cabello, A.; Moreno, L.A.; Casajus, J.A.; Vicente-Rodríguez, G. The Muscle-Bone Unit in Adolescent Swimmers. Osteoporos. Int. 2019, 30, 1079–1088. [Google Scholar] [CrossRef]
- Westerberg, H.; Stanforth, P.R.; Carbuhn, A.; Bosch, T.; Dengel, D.R. Muscle-to-Bone and Soft Tissue-to-Bone Ratios in Track and Field Athletes. Int. J. Sports Med. 2025, 46, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Orozco, M.F.; Posada-Falomir, M.; Quiñónez-Gastélum, C.M.; Plascencia-Aguilera, L.P.; Arana-Nuño, J.R.; Badillo-Camacho, N.; Márquez-Sandoval, F.; Holway, F.E.; Vizmanos-Lamotte, B. Anthropometric and Body Composition Profile of Young Professional Soccer Players. J. Strength Cond. Res. 2020, 34, 1911–1923. [Google Scholar] [CrossRef]
- Brocherie, F.; Girard, O.; Forchino, F.; Al Haddad, H.; Dos Santos, G.A.; Millet, G.P. Relationships between Anthropometric Measures and Athletic Performance, with Special Reference to Repeated-Sprint Ability, in the Qatar National Soccer Team. J. Sports Sci. 2014, 32, 1243–1254. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical Power Analyses Using G*Power 3.1: Tests for Correlation and Regression Analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Nana, A.; Slater, G.J.; Stewart, A.D.; Burke, L.M. Methodology Review: Using Dual-Energy X-Ray Absorptiometry (DXA) for the Assessment of Body Composition in Athletes and Active People. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 198–215. [Google Scholar] [CrossRef] [PubMed]
- Hangartner, T.N.; Warner, S.; Braillon, P.; Jankowski, L.; Shepherd, J. The Official Positions of the International Society for Clinical Densitometry: Acquisition of Dual-Energy X-Ray Absorptiometry Body Composition and Considerations Regarding Analysis and Repeatability of Measures. J. Clin. Densitom. 2013, 16, 520–536. [Google Scholar] [CrossRef]
- Taylor, A.; Konrad, P.T.; Norman, M.E.; Harcke, H.T. Total Body Bone Mineral Density in Young Children: Influence of Head Bone Mineral Density. J. Bone Miner. Res. 1997, 12, 652–655. [Google Scholar] [CrossRef]
- Crabtree, N.J.; Högler, W.; Cooper, M.S.; Shaw, N.J. Diagnostic Evaluation of Bone Densitometric Size Adjustment Techniques in Children with and without Low Trauma Fractures. Osteoporos. Int. 2013, 24, 2015–2024. [Google Scholar] [CrossRef]
- Crabtree, N.J.; Arabi, A.; Bachrach, L.K.; Fewtrell, M.; El-Hajj Fuleihan, G.; Kecskemethy, H.H.; Jaworski, M.; Gordon, C.M.; International Society for Clinical Densitometry. Dual-Energy X-Ray Absorptiometry Interpretation and Reporting in Children and Adolescents: The Revised 2013 ISCD Pediatric Official Positions. J. Clin. Densitom. 2014, 17, 225–242. [Google Scholar] [CrossRef]
- Prentice, A.; Parsons, T.; Cole, T. Uncritical Use of Bone Mineral Density in Absorptiometry May Lead to Size-Related Artifacts in the Identification of Bone Mineral Determinants. Am. J. Clin. Nutr. 1994, 60, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Katzman, D.K.; Bachrach, L.K.; Carter, D.R.; Marcus, R. Clinical and Anthropometric Correlates of Bone Mineral Acquisition in Healthy Adolescent Girls. J. Clin. Endocrinol. Metab. 1991, 73, 1332–1339. [Google Scholar] [CrossRef]
- Hopkins, W.G. A Scale of Magnitudes for Effect Statistics. Available online: http://www.sportsci.org/resource/stats/ (accessed on 4 November 2025).
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988. [Google Scholar]
- Locquet, M.; Beaudart, C.; Durieux, N.; Reginster, J.-Y.; Bruyère, O. Relationship between the Changes over Time of Bone Mass and Muscle Health in Children and Adults: A Systematic Review and Meta-Analysis. BMC Musculoskelet. Disord. 2019, 20, 429. [Google Scholar] [CrossRef] [PubMed]
- Capozza, R.F.; Cointry, G.R.; Cure-Ramírez, P.; Ferretti, J.L.; Cure-Cure, C. A DXA Study of Muscle-Bone Relationships in the Whole Body and Limbs of 2512 Normal Men and Pre- and Post-Menopausal Women. Bone 2004, 35, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Khawaja, A.; Sabbagh, P.; Prioux, J.; Zunquin, G.; Baquet, G.; Maalouf, G.; Hage, Z.E.; Antoun, A.; El Hage, R. The Relationships Between Skeletal Muscle Index and Bone Variables in a Group of Young Adults. J. Clin. Densitom. 2021, 24, 78–87. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Wang, Z.M.; Ross, R. Skeletal Muscle Mass and Distribution in 468 Men and Women Aged 18–88 Yr. J. Appl. Physiol. 2000, 89, 81–88. [Google Scholar] [CrossRef]
- Bergamo, R.R.; Páscoa, M.A.; Hespanhol, J.E.; de Moraes, A.M.; Guerra-Júnior, G. Positive Association of Lean Mass and Negative Association of Protein Intake on Bone Mass and Bone Geometry of Adolescent Soccer Players. Nutrition 2023, 105, 111857. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.R.; Heyward, V.H. Measures of Body Composition in Blacks and Whites: A Comparative Review. Am. J. Clin. Nutr. 2000, 71, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
- Sutton, L.; Wallace, J.; Goosey-Tolfrey, V.; Scott, M.; Reilly, T. Body Composition of Female Wheelchair Athletes. Int. J. Sports Med. 2009, 30, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.-W.; Kim, M.-Y.; Lee, L.-K.; Park, B.-S.; Yang, S.-M.; Jeon, H.-J.; Lee, W.-D.; Kim, J.-H.; Lee, J.-U.; Kwak, T.-Y.; et al. Somatotype and Body Composition Analysis of Korean Youth Soccer Players According to Playing Position for Sports Physiotherapy Research. J. Phys. Ther. Sci. 2015, 27, 1013–1017. [Google Scholar] [CrossRef]
- Dengel, D.R.; Studee, H.R.; Juckett, W.T.; Bosch, T.A.; Carbuhn, A.F.; Stanforth, P.R.; Evanoff, N.G. Muscle-to-Bone Ratio in NCAA Division I Collegiate Football Players by Position. J. Strength Cond. Res. 2024, 38, 1607–1612. [Google Scholar] [CrossRef]
- Płudowski, P.; Matusik, H.; Olszaniecka, M.; Lebiedowski, M.; Lorenc, R.S. Reference Values for the Indicators of Skeletal and Muscular Status of Healthy Polish Children. J. Clin. Densitom. 2005, 8, 164–177. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).