Abstract
In 2012, a novel superconductor BaTi2Sb2O was found in the layered titanium pnictide oxides ATi2Pn2O. A related superconductor BaTi2Bi2O was subsequently discovered in 2013. The structure of these materials consists of alternate stacking of superconducting Ti2Pn2O layers and Ba blocking layers, which is somewhat similar to high-Tc cuprates since the Ti2Pn2O layer contains an anti-CuO2-type Ti2O square lattice. In addition to the structural similarity to the well-known high-Tc superconductors, BaTi2Pn2O shows unique physical properties: two superconducting domes appear in the electronic phase diagram for solid solutions of BaTi2(Sb1‒xBix)2O and a unique density-wave instability which coexists with superconductivity. In this short review, the early studies of titanium pnictide oxides, the discovery of novel superconductors BaTi2Pn2O, and recent progress are summarized.
1. Introduction
The discovery and the extensive studies of exotic superconductors have given rise to new classes of superconductors. The high-Tc superconductivity in cuprates has led to enormous interests in layered materials, and several classes of layered superconductors have been found mainly in light-element-based intermetallics (e.g., MgB2) and transition metal oxides (e.g., Sr2RuO4) [1,2,3,4,5]. The superconductivity in the Fe-based layered pnictides/chalcogenides is one of the intriguing discoveries in this trend, and it has triggered a surge of interest in materials containing relatively heavy elements like pnictogen [6]. Following the iron pnictides/chalcogenides, various kinds of superconductors have been discovered in pnictides and chalcogenides, such as BiS2-based superconductors [7,8,9,10]. The titanium pnictide oxide BaTi2Pn2O (Pn = Sb, Bi) is one of the new classes of these superconductors.
The first members of titanium pnictide oxides are Na2Ti2Pn2O (Pn = As, Sb), which were found in 1990 and show the anomaly in resistivity and magnetic susceptibility reminiscent of a charge-density-wave (CDW) or spin-density-wave (SDW) [11]. The unconventional superconductivity has often been found in the vicinity of other ordered phases such as CDW, SDW, and antiferromagnetic phase [6,12,13]. Hence, the titanium pnictide oxides have been considered as good candidates of the playground for exotic superconductivity due to their density-wave (DW) states. The titanium pnictide oxides have also attracted attention due to their similarity to the cuprates. Na2Ti2Pn2O crystallizes in a so-called anti-K2NiF4-type layered structure: it consists of an alternate stacking of double layers of Na and Ti2Pn2O layers. The Ti2Pn2O layer has an anti-CuO2-type Ti2O square lattice where Ti3+ (d1) is coordinated by two oxide anions and four pnictide anions. The square lattice can be regarded as an anti-configuration of the CuO2 (d9) square lattice.
The discovery of superconductivity in iron pnictides brought renewed attention in titanium pnictide oxides, and several novel compounds have been found in this family. In 2012, we discovered a novel layered superconductor BaTi2Sb2O (Tc = 1.2 K), and subsequently BaTi2Bi2O (Tc = 4.6 K) in 2013 [14,15]. These titanium pnictide oxides BaTi2Pn2O (Pn = Sb, Bi) consist of superconducting Ti2Pn2O layers and Ba blocking layers. At present, eight titanium pnictide oxides Na2Ti2Pn2O (Pn = As, Sb), (SrF)2Ti2Pn2O (Pn = As, Sb, Bi), and BaTi2Pn2O (Pn = As, Sb, Bi) have been synthesized, but only BaTi2Pn2O (Pn = Sb, Bi) shows superconductivity [11,14,15,16,17]. Though BaTi2Pn2O is not high-Tc superconductor, several unique and attracting physical properties have been found: two superconducting domes in the phase diagram of isovalent solid solutions of BaTi2(Sb1‒xBix)2O and unconventional DW instability [18,19]. In this short review, the superconductivity and DW instability in the titanium pnictide oxides will be discussed from discovery to recent progress.
2. Road to the Discovery of Superconductivity in Titanium Pnictide Oxides
We have been interested in the mixed anion compounds, such as oxyhydrides, oxyfluorides, and oxynitrides. The coexistence of multiple anions with different valences, ionic radii, electronegativities, and atomic polarizabilities in the structure provide great opportunities to realize unique electronic properties beyond the single anion coordination system, such as oxides. Hence, we initially focused on the titanium pnictide oxides in terms of their unique coordination environment discussed below. In this section, crystal structures and early studies of titanium pnictide oxides will be summarized as a background to the discovery of superconductivity in BaTi2Sb2O and BaTi2Bi2O.
2.1. Crystal Structure of ATi2Pn2O (A = Ba, Na2, (SrF)2; Pn = As, Sb)
The titanium pnictide oxides ATi2Pn2O crystallize in a tetragonal cell with stacked Ti2Pn2O layers and blocking layers A (A = Ba, Na2, (SrF)2). In Figure 1 the crystal structures of titanium pnictide oxides with three types of blocking layers are shown where the space group of these compounds are I4/mmm (A = Na2, (SrF)2) or P4/nmm (A = Ba). The structure is somewhat similar to cuprates, in particular La2CuO4 (K2NiF4 type structure): ATi2Pn2O has a Ti2O square lattice, which is an anti-configuration to the CuO2 square lattice in cuprates. The structure of Na2Ti2Pn2O can be viewed as an anti-K2NiF4 type structure where Na, Ti, Pn, and O, respectively, occupy apical F site, equatorial F site, K site, and Ni site. In Ti2O square lattice, the formal oxidation state of Ti is trivalent (d1), which is electron-hole symmetric to that of Cu (d9) in cuprates. After the discovery of high-Tc in cuprates, d1 analogues of cuprates, such as Sr2VO4, have been investigated to realize an electronic structure similar to that of cuprates [20]. In addition to the absence of superconductivity in these compounds, theoretical study suggested the splitting of the t2g orbital in these compounds is much smaller than that of the eg orbital in cuprates, resulting in these d1 analogues not showing a half-filled single band system in contrast to cuprates [21]. In titanium pnictide oxides, the Ti atoms are octahedrally coordinated by two oxide anions and four pnitide anions. Such a mixed anionic coordination provides a unique opportunity for the t2g orbitals to split to a greater extent than that of a single anionic coordination system, such as MO6 (M = transition metal) octahedra in oxides as discussed above. Thus, the titanium pnictide oxides might be a possible candidate for the d1 analogue of cuprates reflecting the mixed anionic coordination. Note that, as discussed later, theoretical studies revealed the crystal field of the TiO2Pn4 octahedron has indeed removed the degeneracy of t2g orbital, but three d orbitals still contribute significantly to the density of states (DOS) at the Fermi level.
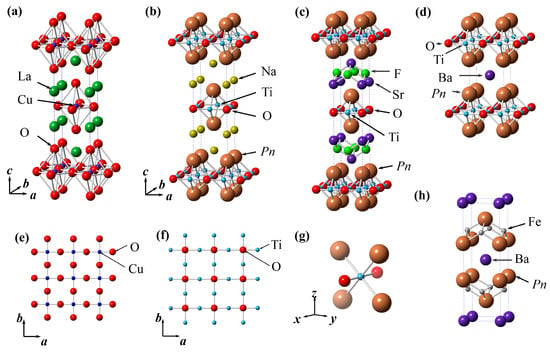
Figure 1.
Crystal structure of (a) La2CuO4; (b) Na2Ti2Pn2O (Pn = As, Sb); (c) (SrF)2Ti2Pn2O (Pn = As, Sb, Bi); (d) BaTi2Pn2O (Pn = As, Sb, Bi); (e) CuO2 square lattice in La2CuO4; (f) Ti2O square lattice in titanium pnictide oxides; (g) TiO2Pn4 octahedra in titanium pnictide oxides; (h) crystal structure of BaFe2Pn2 (Pn = P, As).
The discovery of superconductivity in iron pnictides and chalcogenides has triggered to a remarkable interest and extensive studies of exploring novel superconductors in layered pnictides and chalcogenides. In Fe-based superconductors, several structural types have been found such as REFeAsO (RE = rare earth) and AEFeAsF (AE = alkaline earth) with ZrCuSiAs-type structure, AEFe2As2 with ThCr2Si2-type structure, AFeAs (A = alkali) with PbClF/Cu2Sb-type structure, and FeCh (Ch = chalcogenides) with PbO-type structure. The difference of these structural types is mainly a blocking layer. The crystal structure of Na2Ti2Pn2O consists of alternate stacking of double Na layers and Ti2Pn2O layers, which is similar to that of AFeAs in that double A layers and the conducting layer (Ti2Pn2O or FeAs layer) are alternatively stacked. Hence, inspired by these structural types in Fe-based superconductors, new families of titanium pnictide oxides (SrF)2Ti2Pn2O and BaTi2As2O, where double Na layers have been replaced with (SrF)2 layers or Ba layers, were found in 2010 [14,17]. In analogy with Na2Ti2Pn2O, the structures of (SrF)2Ti2Pn2O and BaTi2As2O are respectively similar to that of AEFeAsF (ZrCuSiAs-type) and AEFe2As2 (ThCr2Si2-type) in that they have the same blocking layer (AEF2 or AE layer).
2.2. Physical Properties of ATi2Pn2O (A = Ba, Na2, (SrF)2; Pn = As, Sb)
Na2Ti2Pn2O (Pn = As, Sb) was first synthesized by Adam et al. in 1990 [11]. These compounds display anomalies in magnetic susceptibility and electrical resistivity at 320 K and 120 K for Pn = As and Sb, respectively. Namely, Na2Ti2Pn2O exhibits a sharp drop in magnetic susceptibility and jump in electrical resistivity as shown in Figure 2 [22]. Above the transition temperature, both compounds show metallic conductivity. The metallic conductivity is also observed below the transition temperature in Na2Ti2Sb2O, whereas Na2Ti2As2O shows semiconducting behavior. The Sommerfeld constant estimated from specific data is 4.1 mJ·mol−1·K−2 and 0 mJ·mol−1·K−2 for Na2Ti2Sb2O and Na2Ti2As2O, respectively, indicating that the densities of states remains finite below the transition temperature in Na2Ti2Sb2O while Na2Ti2As2O is fully gapped [23].
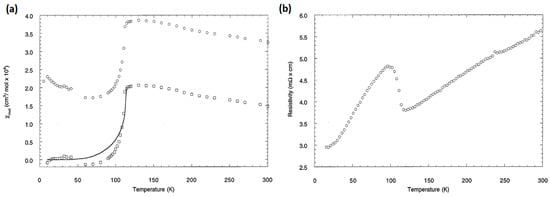
Figure 2.
Temperature dependence of (a) magnetic susceptibility under 1 T and (b) electrical resistivity for Na2Ti2Sb2O. Reprinted with permission from Axtell et al., J. Solid State Chem; published by Elsevier, 1997 [22].
The unique crystal structure and the anomalies in the magnetic susceptibility and electrical resistivity have attracted considerable attention, and Na2Ti2Pn2O have been extensively studied to clarify the origin of the anomaly. Theoretically, Picket suggested the origin of the anomaly is not ferromagnetic/antiferromagnetic ordering but the CDW/SDW instability [24]. Three Fermi surfaces have been found and one of them, a box-shaped Fermi surface around M and A points is almost dispersionless along the kz direction (perpendicular to the Ti2O square lattice), resulting in strong nesting of the surface. Biani et al. also found similar Fermi surface nesting and suggested CDW instability is the origin of the anomaly [25].
In the low-temperature powder neutron diffraction studies the structural distortion is observed at around 120 K: with a decrease in the temperature, the lattice is elongated along the a-axis but contracted along the c-axis around 120 K, which leads to distortion of TiO2Pn4 octrahedra, where the Ti–O bond is elongated and the Ti–Pn bond is shortened (Figure 3a) [26]. This indicates the anomaly observed in susceptibility and resistivity is accompanied with structural distortion. CDW/SDW compounds generally show a periodic structural modulation below the transition temperature. However, in Na2Ti2Sb2O, no peaks derived from magnetic ordering or the superlattice have been found in the low-temperature powder neutron diffraction data. Note that the neutron diffraction experiments do not exclude both CDW and SDW instability at low temperature because it is difficult to discern the modulation in powder neutron diffraction experiments when the modulation is quite small.
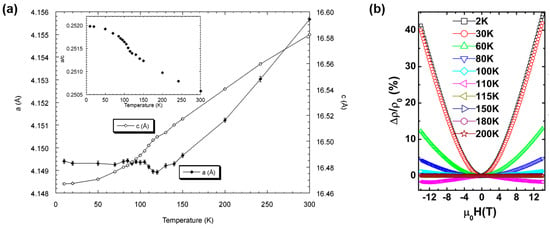
Figure 3.
(a) Temperature dependence of lattice parameters for Na2Ti2Sb2O. Reprinted with permission from Ozawa et al., J. Solid State Chem.; published by Elsevier, 2000 [26]. (b) Magnetic field dependence of magnetoresistance for Na2Ti2Sb2O. Reprinted with permission from Liu et al., Phys. Rev. B; published by APS, 2009 [27].
Na2Ti2Sb2O shows a negative Hall coefficient below 300 K and a steep rise in the Hall coefficient at around 120 K, indicating that the dominant carrier are electrons and the carrier density decreases below the transition temperature due to the formation of CDW/SDW [23]. A large magnetoresistance (MR), namely ~40% of MR at T = 2 K and H = 14 T, has been found in Na2Ti2Sb2O, which has been considered as a suppression of SDW instability by the magnetic field (Figure 3b). Thus, no experimental evidence for CDW/SDW instability has been found and the origin of the anomaly has not been unveiled for many years.
In 2004, Ozawa et al. reported single crystal growth of Na2Ti2Sb2O by using NaSb flux method [28]. Likewise, single crystal of Na2Ti2As2O was grown using NaAs flux by Shi et al. in 2013 [23]. Reflecting the layered structure, Na2Ti2Pn2O single crystals show anisotropy in electrical resistivity. The ratio of ρc/ρab (γρ) is ~140 and ~430 for Na2Ti2Sb2O and Na2Ti2As2O, whereas an anisotropy of less than a factor of 2 for Na2Ti2Sb2O is theoretically suggested [24]. These values of γρ are much larger than that of iron arsenide LiFeAs (γρ ~1.3–3.3) [29], which has an alikali double layer like Na2Ti2Pn2O. Note that γρ-values for iron arsenides with a different blocking layer have also been reported such as γρ ~1–6 for AEFe2As2 [30] and γρ ~20–200 for LaFeAsO [31]. From the structural point of view, Na2Ti2Sb2O is expected to have a more two-dimensional nature (stronger anisotropy) than Na2Ti2As2O, since the interlayer distance of Na2Ti2Sb2O is larger than that of Na2Ti2As2O (see Table 1). The weaker anisotropy in Na2Ti2Sb2O is possibly ascribed to the hybridization of the Pn-p orbital and the Ti-3d orbital, as theoretically suggested [32]. The replacement of As with Sb having a higher energy level than As leads to stronger hybridization with the Ti-3d orbital because the Pn-p orbital has a lower energy level than the Ti-3d orbital, resulting in the weakening of the anisotropy.

Table 1.
Cell parameters and transition temperature of DW (TDW) and superconductivity (Tc).
Similar anomalies in magnetic susceptibility and electrical resistivity have also been found in other members of titanium pnictide oxides, (SrF)2Ti2Pn2O and BaTi2As2O [14,17]. With decreasing temperature, a metal-to-semiconductor transition is clearly observed in the resistivity at around 200 K for (SrF)2Ti2Sb2O and 380 K for (SrF)2Ti2As2O, respectively, indicating these compounds are fully gapped below the transition temperature, as observed in Na2Ti2As2O (Figure 4a). The magnetic susceptibility shows a sharp drop at almost the same temperature as the resistive anomaly (Figure 4b). (SrF)2Ti2Sb2O shows a negative Hall coefficient above the density-wave transition temperature TDW and a steep rise of the Hall coefficient around the TDW, indicating the dominant carrier are electrons and the decrease in the carrier density at TDW is due to the opening gap. The Seebeck coefficient for (SrF)2Ti2Sb2O is negative above TDW and changes its sign from negative to positive below TDW with decreasing temperature, indicating multicarrier conduction in this compound. The temperature dependences of the lattice parameters a and c, obtained by XRD, exhibit a sharp drop in both a and c at the transition temperature, and behavior is different from that observed in Na2Ti2Sb2O (see above).
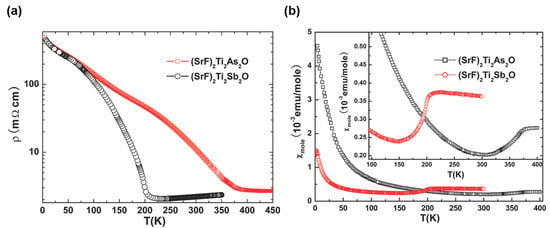
Figure 4.
Temperature dependence of (a) electrical resistivity and (b) magnetic susceptibility under 5 T for (SrF)2Ti2Pn2O. Reprinted with permission from Liu et al., Chem. Mater.; published by ACS, 2010 [17].
BaTi2As2O exhibits a sharp drop in magnetic susceptibility at around 200 K with decreasing temperature, as seen in other titanium pnictide oxides (Figure 5a). However, in contrast to the metal-to-semiconductor transition in Na2Ti2As2O and (SrF)2Ti2Pn2O, BaTi2As2O shows a metal-to-metal transition in electrical resistivity, indicating BaTi2As2O is not fully gapped below TDW, as observed in Na2Ti2Sb2O (Figure 5b). Below the transition temperature, a large MR is observed as seen in Na2Ti2Sb2O. The MR increases with decreasing temperature and reaches ~15% at 4 K and H = 14 T. The origin of the MR should be the same as that in Na2Ti2Sb2O, while the MR of BaTi2As2O is smaller than that of Na2Ti2Sb2O (~40%).
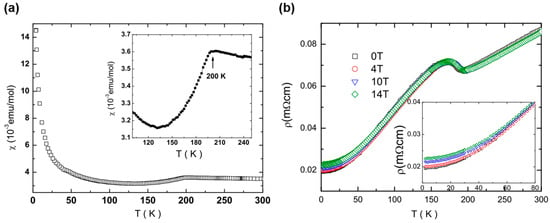
Figure 5.
Temperature dependence of (a) magnetic susceptibility under 1 T and (b) electrical resistivity for BaTi2As2O. Reprinted with permission from Wang et al., J. Phys. Condens. Matter.; published by IOP, 2010 [14].
The CDW and SDW instabilities are widely seen in layered compounds, such as transition metal dichalcogenides and iron arsenides. It is generally believed that such a DW state often competes with another electronic state, such as superconductivity, at low temperature. Hence, given the structural similarity to cuprates/iron pnictides and DW transition, titanium pnictide oxides are attractive candidates as novel superconducting materials. The suppression of the CDW/SDW transition has been examined in BaTi2As2O by an intercalation of Li+ into the interstitial site, namely LixBaTi2As2O [14]. The TDW decreases and the transition is broadened with increasing x, and finally the anomaly is smeared out. However, no superconductivity emerges. Note that carrier doping by replacing Ba with K, Na, La, Sm, Bi, and Al have been tried, but failed. Thus, none of the titanium pnictide oxides shown above exhibits superconductivity.
The lattice parameters and the interlayer distance in titanium pnictide oxides are summarized in Table 1, where the compounds are discussed in later section; namely BaTi2Sb2O, BaTi2Bi2O, and (SrF)2Ti2Bi2O are also listed. Reflecting the I-centered lattice, the interlayer distance of A = Na2 and (SrF)2 compounds is half of the c-axis length, while that of A = Ba (P-lattice) is the same as the c-axis length. In (SrF)2Ti2Pn2O, the (SrF)2 blocking layer is bulky, reflecting that the tetrahedral void within the double Sr layers are filled by additional F ions, which provides a much longer interlayer distance than that of Na2Ti2Pn2O (e.g., 8.281 Å for Na2Ti2Sb2O vs. 10.4429 Å for (SrF)2Ti2Sb2O). In contrast, BaTi2Pn2O has a single Ba layer between Ti2Pn2O layers, resulting in the smallest interlayer distance among the titanium pnictide oxides with the same Pn. Thus, the interlayer distance decreases in the order of (SrF)2Ti2Pn2O > Na2Ti2Pn2O > BaTi2Pn2O. From Table 1, we can find that TDW follows two kinds of trends, namely, A dependence and Pn dependence. First, given the same Pn, TDW decreases in the order of (SrF)2Ti2Pn2O > Na2Ti2Pn2O > BaTi2Pn2O, which is the same trend as that in interlayer distance, indicating these two parameters are closely related to each other. Second, given the same A, TDW decreases in the order of Pn = As > Pn = Sb > Pn = Bi. Before the discovery of the superconducting compound BaTi2Sb2O, only five compounds, Na2Ti2As2O, Na2Ti2Sb2O, (SrF)2Ti2As2O, (SrF)2Ti2Sb2O, and BaTi2As2O, have been known among titanium pnictide oxides. However, these five compounds are enough to find the trends mentioned above. We could expect that A = Ba and Pn = Sb, BaTi2Sb2O, should have lower TDW than the known titanium pnictide oxides, which may lead to superconductivity. Hence, we synthesized a new titanium pnictide oxides BaTi2Sb2O and found a DW transition at around 50 K and a superconducting transition at 1.2 K. Moreover, for further suppression of DW instability, we synthesized Pn = Bi compounds BaTi2Bi2O and (SrF)2Ti2Bi2O, which are the first examples of Pn = Bi in the titanium pnictide oxides family. Though DW instability disappeared in both compounds, only BaTi2Bi2O shows superconductivity at 4.6 K.
3. Superconductivity in BaTi2Pn2O (Pn = Sb, Bi)
3.1. Synthesis and Crystal Structure
Polycrystalline samples of BaTi2Sb2O and BaTi2Bi2O were synthesized by the conventional solid-state reaction [15,16]. Stoichiometric amounts of BaO, Ti, and Sb/Bi were mixed and pelletized. The pellet was wrapped with Ta foil and sealed in a quartz tube. The reaction temperature is 1000 °C for BaTi2Sb2O, and 850 °C for BaTi2Bi2O. Doan et al. also synthesized BaTi2Sb2O independently, where the precursors are sealed in Nb tubes under Ar gas and the reaction temperature is 900 °C [33]. The stability against ambient condition is lost when replacing As with Sb: BaTi2Sb2O is air- and moisture-sensitive while BaTi2As2O is not. Further replacement of Sb by Bi leads to increased sensitivity against air and moisture. The trend probably depends on the stability of Pn3− ion against the oxidation. BaTi2Bi2O is indeed readily decomposed by exposure to the air or solvent, and Bi-based phases, such as BaBi3 and Bi, are formed, where the Bi anion is oxidized from −3 to −0.66 and 0, respectively.
Crystal structure of BaTi2Sb2O determined by XRD and neutron diffraction data is the same as that of BaTi2As2O. The lattice constants are a = 4.11039(2) Å, c = 8.08640(4) Å determined by synchrotron XRD data, and a = 4.1055(2) Å, c = 8.0712(4) Å determined by neutron diffraction data. BaTi2Bi2O has also the same structure with lattice constants of a = 4.12316(4) Å, c = 8.3447(1) Å which is determined by synchrotron XRD [15]. Compared with BaTi2As2O (a = 4.046 Å, c = 7.272 Å), replacement of As with Sb/Bi leads to a distinct increase in the c-axis, whereas the increase in a-axis is small, as observed in other titanium pnictide oxides (see Table 1). Though the crystal structure of BaTi2Pn2O are similar to that of iron pnictides BaFe2Pn2 (ThCr2Si2-type structure), such a trend is not observed in BaFe2Pn2 [34]. Both the structure of BaTi2Pn2O and BaFe2Pn2 can be regarded as an alternate stacking of Ti2O or Fe square lattice and BaPn2 layer with CsCl-type structural arrangement (see Figure 1). As the structural difference between these compounds is the square lattice, the trend of the lattice constant is probably due to the rigid nature of the Ti2O square lattice.
It is well known that the formation of the interlayer X–X bond (the so-called lattice collapse) is often found in the AB2X2 compounds with the ThCr2Si2-type structure. As Hoffmann and Zheng pointed out, the formation of X–X bond is promoted when B is on the right-hand side of the periodic table and X is on the higher period of the periodic table [35]. In accordance with the trend in AB2X2 compounds, the replacement of As by Sb or Bi in BaTi2Pn2O may allow the formation of an interlayer Pn–Pn bond. However, the interatomic distance of Pn–Pn in BaTi2Pn2O is 3.725 Å for Pn = As, 4.079 Å for Pn = Sb, and 4.137 Å for Pn = Bi, respectively, indicating the absence of the Pn–Pn bond in all of the BaTi2Pn2O. As the formation of the X–X bond is often induced in AB2X2 compounds by applying physical pressure or chemical pressure, the lattice collapse transition might be induced when sufficient pressure is applied.
3.2. Superconductivity in BaTi2Pn2O and Its Superconducting Properties
BaTi2Sb2O shows an anomaly in magnetic susceptibility, electrical resistivity, and lattice constants at around 50 K in analogy with other titanium pnictide oxides. The resistivity shows a metal-to-metal transition at around 50 K as observed in BaTi2As2O (Figure 6b). With decreasing temperature, the magnetic susceptibility slightly increases and a small drop is observed at the same temperature (Figure 6c). An anomaly in the lattice constants a and c is highly similar to that observed in Na2Ti2Sb2O, as shown in Figure 6a. The transition temperature is much lower than that of BaTi2As2O (TDW = 200 K), as expected from the trends mentioned above.
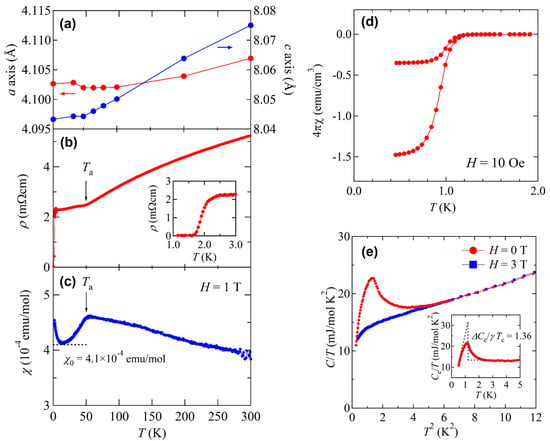
Figure 6.
Temperature dependence of (a) lattice constants a and c; (b) electrical resistivity; (c) magnetic susceptibility at 1 T for BaTi2Sb2O; (d) low-temperature magnetic susceptibility at 10 Oe for BaTi2Sb2O; (e) low-temperature specific heat for BaTi2Sb2O.
BaTi2Sb2O also shows superconducting transition at 1.2 K coexisting with the DW instability. As shown in Figure 6d, the magnetization shows a sharp drop at 1.2 K and shielding volume fraction is as large as 100% at 0.5 K. Electrical resistivity exhibits a sharp drop to zero at around 2 K as shown in the inset of Figure 6b. Specific heat of BaTi2Sb2O shows a distinct peak at around 1.2 K, which is firm evidence of bulk superconductivity in BaTi2Sb2O (Figure 6e). Note that the origin of the small fraction of superconductivity at around 2 K is not clear, but might be due to the sample quality. The Sommerfeld coefficient γ and the Debye temperature θD estimated from the specific data are 13.5 mJ·mol−1·K−2 and 239 K, respectively, which are similar to those of the As analogue (γ = 15.3 mJ·mol−1·K−2 and θD = 223 K). The value of ΔC(Tc)/γTc estimated from the specific heat jump is 1.36, which is almost consistent with the Bardeen–Cooper–Schrieffer (BCS) weak coupling limit of 1.43. Gooch et al. improved sample quality and reported the specific heat data where a sharper change at Tc was observed. The obtained γ, θD, and ΔC(Tc)/γTc are 10.9 mJ·mol−1·K−2, 230 K, and 0.9, respectively, which are smaller than those of our previous study [15,36]. The temperature dependence of electronic specific heat Cel/γT can be fitted by the so-called α model with α = 1.4, rather than BCS theory, suggesting a single-gap s-wave superconductivity with a gap of 2Δ = 0.3 meV.
The s-wave nature of the superconductivity is also suggested from the 121Sb-nuclear quadrupole resonance (NQR) measurements and the muon spin relaxation/rotation (μSR) measurements: the temperature dependence of 1/T1 in the 121Sb-NQR measurements shows a tiny coherence peak at around 1 K (Figure 7a), and temperature dependence of superconducting relaxation rate σSC obtained from μSR measurements can be fitted well by the BCS s-wave model in the weak coupling limit [37,38]. Note that the 121Sb-NQR measurements also revealed that the microscopic coexistence of superconductivity and DW instability in BaTi2Sb2O. Though a magnetic fluctuation has been observed at around TDW in 1/T1T, the origin of the fluctuation has not clarified yet (see Figure 7b).
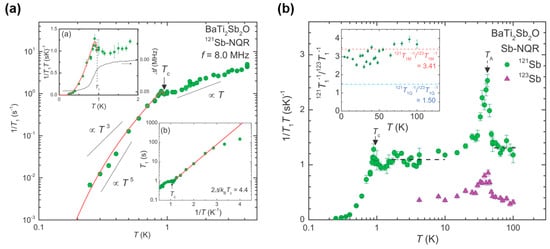
Figure 7.
(a) Temperature dependence of 1/T1 measured at 8.0 MHz; (b) Temperature dependence of 1/T1T measured with 121/123Sb-NQR. The inset shows temperature dependence of the 121/123Sb isotopic ratio 121T1−1/123T1−1. Reprinted with permission from Kitagawa et al., Phys. Rev. B; published by APS, 2013.
BaTi2Bi2O shows paramagnetic behavior in magnetic susceptibility and metallic temperature dependence in electrical resistivity, like BaTi2As2O and BaTi2Sb2O. However, no anomaly is observed in both data (Figure 8a), indicating the DW transition is further suppressed and disappears by replacing Sb with Bi, as expected from the trend discussed above. Reflecting the further suppression of DW instability, BaTi2Bi2O has an enhanced Tc of 4.6 K as seen in the magnetic susceptibility and electrical resistivity. Due to the extremely high air- and moisture-sensitivity, the specific heat of BaTi2Bi2O has not been measured. However, shielding volume fraction exceeds 60% at 1.85 K (Figure 8b), providing a firm evidence of bulk superconductivity in BaTi2Bi2O [16].
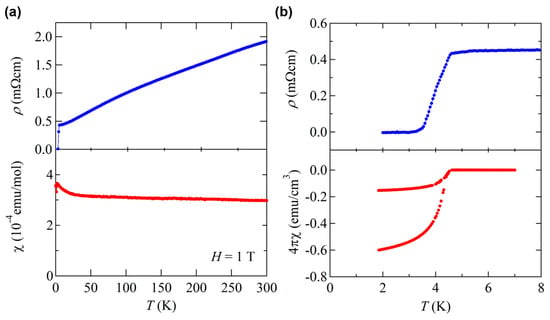
Figure 8.
(a) Temperature dependence of electrical resistivity and magnetic susceptibility under 1 T for BaTi2Bi2O. No DW transition is observed. (b) Low-temperature resistivity and magnetic susceptibility at 5 Oe for BaTi2Bi2O.
The magnetic field dependence of Tc for BaTi2Sb2O and BaTi2Bi2O are shown in Figure 9a,b where the Tc is determined from the midpoint of the resistive transition. As shown in Figure 9c, the temperature dependence of the upper critical field Hc2 increases almost linearly with decreasing temperature down to the lowest temperature measured. Hc2 at 0 K, Hc2(0), are 1.32 T for BaTi2Sb2O and 1.70 T for BaTi2Bi2O (Figure 9c), which is estimated from the linear extrapolation of the data. From the value of Hc2(0), the coherence length ξ0 is estimated to be 158 Å and 139 Å for BaTi2Sb2O and BaTi2Bi2O, respectively [39]. The superconducting parameters of BaTi2Sb2O determined by specific heat data is reported by Gooch et al. where Hc2(0) and ξ0 is estimated to be 0.08 T and 640 Å [36]. Though the origin of the discrepancy in the parameter of BaTi2Sb2O between [34] and [37] is not clear yet, it might come from the sample quality or the filamentary superconductivity which enhances the resistive Hc2 in BaTi2Sb2O. To clarify the origin, the measurements on single crystalline sample should be performed. The lower critical field Hc1 for BaTi2Bi2O is estimated from the magnetic field dependence of zero-field cooled magnetization at various temperatures (Figure 10a). The Hc1 as a function of (T/Tc)2 is shown in Figure 10b. The Hc1(0) is estimated to be 60 Oe from the linear extrapolation of the data (Figure 10b), which corresponds to the penetration depth of 2900 Å. Note that, Hc1(0) for BaTi2Sb2O has not been determined due to its low Tc.
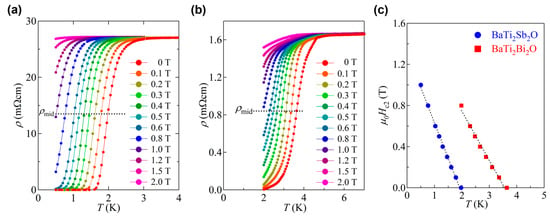
Figure 9.
Temperature dependence of the electrical resistivity for (a) BaTi2Sb2O and (b) BaTi2Bi2O under various magnetic fields; (c) temperature dependence of Hc2 for BaTi2Sb2O and BaTi2Bi2O.
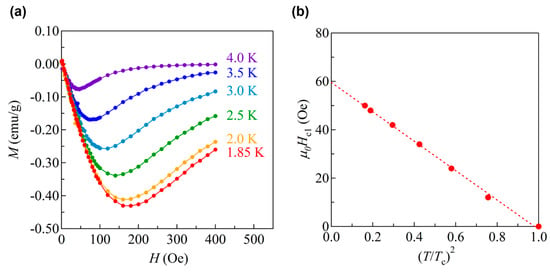
Figure 10.
(a) Magnetic field dependence of magnetization for BaTi2Bi2O at various temperature; (b) the temperature dependence of the lower critical field Hc1 for BaTi2Bi2O.
Though the specific heat data of BaTi2Bi2O is not available due to the extremely air- and moisture-sensitivity, D(EF) of BaTi2Bi2O can be estimated from the obtained superconducting parameters, the equation for weak-coupling limit, [μ0Hc(0)]2/8π = D(EF)Δ(0)2/2, and Δ(0) = 1.764kBTc, where Δ(0) is superconducting gap. The D(EF) of BaTi2Bi2O is estimated to be 4.79 states/eV/f.u. For BaTi2Sb2O, using γ obtained from specific heat data, D(EF) is estimated to be 5.7 states/eV/f.u. from the equation, γ = π2D(EF)kB2/3. Though Tc of BaTi2Bi2O is much higher than that of BaTi2Sb2O, the experimentally estimated D(EF) of BaTi2Bi2O is smaller than that of BaTi2Sb2O. Since BaTi2Bi2O is isostructural with BaTi2Sb2O, the slightly smaller Debye temperature θD is expected, reflecting the replacement of Sb by the heavier Bi. In the BCS weak-coupling limit, the decrease in both D(EF) and θD leads the decreased Tc. Thus, the enhanced Tc in BaTi2Bi2O cannot be explained by the BCS weak-coupling limit.
3.3. Electronic Structure of BaTi2Pn2O
The electronic structure is a key toward understanding of superconductivity and DW instability in BaTi2Pn2O. Several theoretical studies have addressed the electronic structure of BaTi2Sb2O. The electronic structure for BaTi2Sb2O based on the first principles calculations was first reported by Singh [40]. The calculation revealed three d orbitals, dxy, dx2‒y2, and dz2, contribute significantly at the Fermi level (EF), indicating the multiband nature of BaTi2Sb2O. The O 2p and Sb 5p bands are nominally full, while Sb 5p are hybridized with Ti 3d, and there are no Ba-derived occupied valence bands. The results indicate the formal valence is Ba2+Ti3+Sb3‒O2‒ as expected. The calculated Fermi surface (FS) is shown in Figure 11 where three sheets of FS, namely two electron sheets and one hole sheet, have been found. The square cylinder around the M and A points is an electron sheet with a two-dimensional character, which is the mixture of three d orbitals, dxy, dx2‒y2, and dz2. Another electron sheet is a three-dimensional complex-shaped section around the Γ and Z points, which has dz2 character. The three-dimensional hole sheet located around the R point is derived from the mixture of three d orbitals, where the sheet compensates other two electron sheets reflecting that an even electron count in BaTi2Sb2O. Consistent results for the FS of BaTi2Sb2O were also obtained in other theoretical studies [41,42,43]. The electronic structure for BaTi2Bi2O have been obtained by Suetin et al., [44]. As in the case with BaTi2Sb2O, at EF, three d orbitals contribute significantly and the topology of FS is highly similar to that in BaTi2Sb2O. These predicted FS of BaTi2Pn2O is highly similar to that theoretically predicted in Na2Ti2Pn2O [25,32,45]. Experimentally, Song et al. recently performed angle-resolved photoemission spectroscopy (ARPES) studies on Ba0.95Na0.05Ti2Sb2O single crystals and found the multiband electronic structure having electron and hole pockets, consistent with the theoretical studies [46].
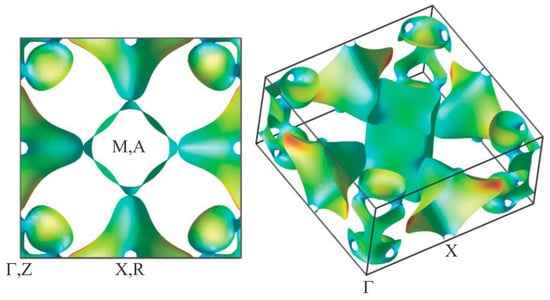
Figure 11.
Calculated Fermi surfaces of BaTi2Sb2O, where spin-orbit interaction are included. Square cylindrical section around M, A, and complex-shaped sections around Γ, Z are electron sections. The other section around R is a hole section. Reprinted with permission from Singh, New. J. Phys.; published by IOP, 2012 [40].
The D(EF) for BaTi2Sb2O is theoretically estimated to be 4.26 states/eV/f.u. by Singh, 3.88 states/eV/f.u. by Subedi, 3.599 states/eV/f.u. by Suetin et al. [32,41,47] where the calculations are based on the structure determined at room temperature (see Figure 1) and the structural distortion due to DW instability is not considered because the low temperature structure is not clarified yet. Subedi pointed out the possible lattice instability toward CDW with a √2 × √2 × 1 superlattice based on the calculation of phonon dispersions. When the suggested lattice distortion is taken into account, the D(EF) is found to be 2.83 state/eV/f.u., which suggests ~30% decrease through the DW transition. Note that the decrease in D(EF) below TDW is experimentally found to be 9% by NQR measurements (see Figure 7b) [38]. These D(EF) estimated from the theoretical studies are somewhat smaller than that estimated from the experimentally obtained γ (D(EF) = 5.7 states/eV/f.u.), implying that the γ is enhanced by electron-phonon coupling in BaTi2Sb2O [15]. For BaTi2Bi2O, the D(EF) of 3.42 states/eV/f.u. has been obtained by Suetin et al., which is also smaller than experimental D(EF) of 4.79 states/eV/f.u. [16,44].
The Tc of BaTi2Sb2O has been estimated by Subedi and Nakano et al. using the Allen-Dynes formula, and found to be 2.7 K and 2.30 K, respectively [43,47]. The estimated Tc is almost consistent with the experimental value (Tc = 1.2 K), suggesting BaTi2Sb2O is a conventional BCS-type superconductor. Nakano et al. also estimated the Tc of BaTi2Bi2O to be 2.45 K using the same method, which is relatively smaller than the experimental value (Tc = 4.6 K). The theoretically estimated Tc-values for BaTi2Sb2O and BaTi2Bi2O are similar to each other, where the experimental results, namely the enhancement of Tc by replacing Sb with Bi, could not be explained.
3.4. Isovalent Substitution Effect on BaTi2Pn2O
As discussed in above, the enhanced Tc of BaTi2Bi2O cannot be explained in the BCS weak-coupling limit. To find a clue, the effect of isovalent substitution, solid solutions of BaTi2(As1‒xSbx)2O and BaTi2(Sb1‒yBiy)2O, have been investigated [18]. The lattice constants and electronic phase diagram of the isovalent solid solutions are shown in Figure 12a. The lattice constants of these solid solutions follow Vegard’s law, indicating the solid solutions are formed and no structural transition is induced by isovalent substitution. With increasing x, TDW decreases and superconducting phase appears at around x = 0.9 (Tc = 0.5 K), where TDW and Tc are determined from the resistivity data. For the solid solution of BaTi2(Sb1‒yBiy)2O, Tc is determined from the magnetization data. With increasing y, Tc initially increases up to 3.5 K at y = 0.2, and then monotonically decreases down to 2.6 K at y = 0.3. For y = 0.4 and 0.5, bulk superconductivity is not observed down to 1.85 K, which is the instrumental limit. At y = 0.6, bulk superconductivity with Tc = 4.0 K appears again and Tc increases with increasing y up to 4.6 K at y = 1.0. The isovalent substitution effect for 0 ≤ y ≤ 0.4 has also been reported by Zhai et al., where the electronic phase diagram is almost the same as our results: Tc increases up to 3.7 K at y = 0.17, then gradually decreases above y = 0.2. Moreover, the TDW decreases with increasing y, and the DW transition could not be observed above y = 0.17 [48].
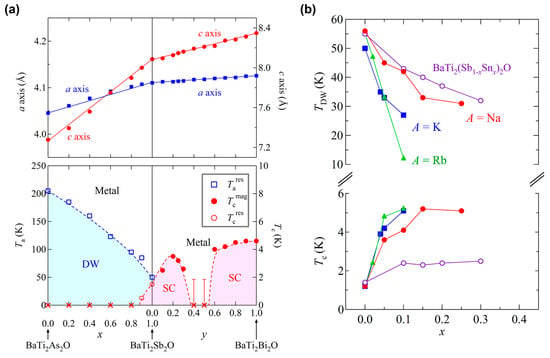
Figure 12.
(a) Lattice constants and electronic phase diagram of isovalent solid solutions BaTi2(Sb1‒xBix)2O; (b) TDW and Tc for aliovalent substitution compounds (Ba1‒xAx)Ti2Sb2O and BaTi2(Sb1‒xSnx)2O as a function of x.
From the overall electronic phase diagram shown in Figure 12a, we can find two-dome structure in Tc: the first dome ranges in 0.9 ≤ x ≤ 1 and 0 ≤ y ≤ 0.3, and the second dome ranges 0.6 ≤ y ≤ 1. The first superconducting phase seems to be induced by the suppression of DW phase, which is often seen in the CDW/SDW superconductors. However, the disappearance of superconductivity and a steep rise of Tc in the vicinity of the second superconducting phase implies the second superconducting phase may have a different mechanism behind superconductivity from the first superconducting phase. A two-dome structure in Tc has also seen in the iron pnictide superconductors such as LaFeAs(O1‒xHx) [49,50,51]. In the phase diagram of LaFeAs(O1‒xHx) (0 ≤ x ≤ 0.6), the first superconducting dome (0.05 ≤ x ≤ 0.20) with a maximum Tc of 29 K and the second dome (0.20 ≤ x ≤ 0.42) with a maximum Tc of 36 K appear with two antiferromagnetic phases (x < 0.05 and 0.4 < x) [49]. Though the two-dome structure in LaFeAs(O1‒xHx) emerges by electron doping, in contrast to the two-dome structure in BaTi2Pn2O emerging by isovalent substitution, both BaTi2Pn2O and iron based superconductors have a multiband nature. Hence, BaTi2Pn2O might offer an opportunity to understand the nature of high Tc superconductivity in iron pnictides.
3.5. Aliovalent Substitution Effect on BaTi2Pn2O
In BaTi2Sb2O, superconductivity emerges by the suppression of DW instability. However, DW instability is not fully suppressed and coexists with superconductivity, indicating that the further suppression of DW instability may enhance Tc. Several aliovalent substitutions, (Ba1‒xAx)Ti2Sb2O (A = alkali metal: Na, K, Rb) and BaTi2(Sb1‒xSnx)2O, have been reported, where all of them are hole-doped systems. The lattice constant a decreases with increasing x for all dopants, reflecting the size reduction of titanium ions upon hole doping. However, c of (Ba1‒xAx)Ti2Sb2O increases with increasing x, whereas that of BaTi2(Sb1‒xSnx)2O decreases. The increase of c for (Ba1‒xAx)Ti2Sb2O may come from the reduced Coulomb attraction between Ba/A and Sb due to the substitution of Ba for lower valence cation A.
On the basis of these substitution effects, TDW and Tc as a function of x is summarized in Figure 12b. The substitution effects are qualitatively the same: the DW phase is destabilized gradually and Tc is enhanced with increasing x. This indicates that the DW phase and superconducting phase compete with each other in BaTi2Sb2O, and hole doping is generally effective for the suppression of DW instability and the enhancement of Tc. In (Ba1‒xNax)Ti2Sb2O, Tc increases up to 5.5 K at x = 0.15 and show a plateau at higher x value [33]. Likewise, the maximum Tc of 6.1 K and 5.4 K are attained at x = 0.12 for (Ba1‒xKx)Ti2Sb2O [52] and x = 0.2 for (Ba1‒xRbx)Ti2Sb2O [53], respectively. In contrast to (Ba1‒xAx)Ti2Sb2O, BaTi2(Sb1‒xSnx)2O provides a quantitatively different phase diagram: the maximum Tc of 2.5 K is attained at x = 0.3, which is less than half of that for (Ba1‒xAx)Ti2Sb2O [54]. The quantitative difference between (Ba1‒xAx)Ti2Sb2O and BaTi2(Sb1‒xSnx)2O is probably due to disorder effects. The Sn substitution induces much greater disorder effects on the superconductivity than alkali substitutions since Sn substitutes the superconducting Ti2Sb2O layer while alkali substitutes the Ba blocking layer. Note that the similar behavior of the suppression of DW instability and enhancement of Tc, has been observed in a high-pressure study on BaTi2Sb2O where the electrical resistivity is measured under a pressure up to 16.12 kbar. By applying pressure, the TDW is monotonically suppressed down to ~40 K, whereas Tc increases up to 2.9 K [55].
The superconducting parameters for non-doped BaTi2Sb2O and hole-doped BaTi2Sb2O are summarized in Table 2, where two kinds of the parameters are listed for BaTi2Sb2O since that of BaTi2Sb2O are reported in different studies. In (Ba1‒xAx)Ti2Sb2O, the Sommerfeld constant γ is almost the same as BaTi2Sb2O, and the Debye temperature θD is slightly lower than that of BaTi2Sb2O. In contrast, in BaTi2(Sb1‒xSnx)2O, both γ and θD is higher than those of BaTi2Sb2O. Gooch et al. found the excellent agreement of the electronic specific heat data with the BCS theory in (Ba0.85Na0.15)Ti2Sb2O. Likewise, for all dopant, a ratio ΔC/γTc is close to the standard weak-coupling BCS value 1.43, suggesting conventional superconductivity in these compounds.

Table 2.
Superconducting parameters of non-doped BaTi2Sb2O and hole-doped BaTi2Sb2O.
In contrast to these hole-doped systems, electron-doped systems have not been found in BaTi2Sb2O, implying the difficulty of electron doping. Indeed, all of our attempts to synthesize electron-doped systems, such as (Ba1‒xLax)Ti2Sb2O and BaTi2(Sb1‒xTex)2O, have failed. In contrast, in BaTi2As2O, electron doping by Li+ intercalation has been reported as mentioned above. Recently, another electron doping system, Ba(Ti1‒xCrx)2As2O, has been synthesized where the TDW is gradually suppressed with increasing x and reaches 139 K at the solubility limit (x = 0.077) but no superconductivity is emerged [56]. These results may suggest electron-doping is not suitable for the superconductivity in titanium pnictide oxides. Though no superconductivity has been found in BaTi2As2O and its electron-doped systems, hole doping may be interesting since the hole-doped BaTi2Sb2O exhibits the suppression of DW instability and enhancement of Tc, as discussed above.
3.6. Substitution Effect on Other Titanium Pnictide Oxides
A Bi analogue of (SrF)2Ti2Sb2O and Na2Ti2Sb2O might further suppress the DW instability and lead to superconductivity, like BaTi2Sb2O and BaTi2Bi2O. Hence, we prepared a novel compound (SrF)2Ti2Bi2O by the conventional solid-state reaction. The structural refinement of synchrotron XRD and neutron diffraction data at room temperature revealed the structure is isostructual with As and Sb analogues. The lattice parameters of (SrF)2Ti2Bi2O are a = 4.11782(2) Å and c = 21.3703(2) Å from XRD, and a = 4.11720(9) Å and c = 21.3707(7) Å from neutron diffraction [16]. Compared with the Sb analogue (a = 4.1095(1) Å, c = 20.8858(5) Å), the c-axis is significantly elongated, whereas the a-axis is slightly elongated, whose trend is similar to those in BaTi2Pn2O. In analogy with the replacement of Sb by Bi in BaTi2Pn2O, the DW instability is much suppressed and disappears in (SrF)2Ti2Bi2O (Figure 13). Though superconductivity is expected, no superconducting transition has been found down to 0.5 K.
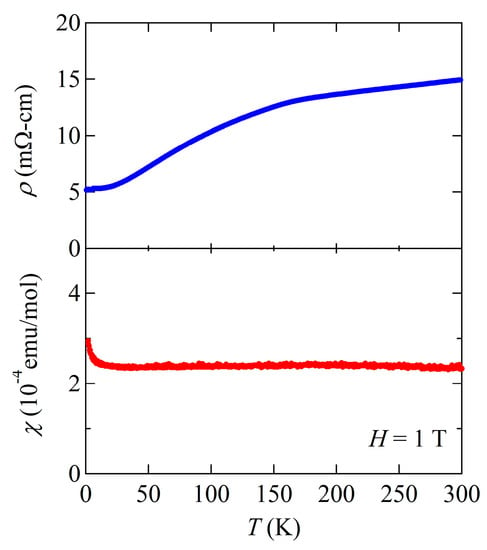
Figure 13.
Temperature dependence of electrical resistivity and magnetic susceptibility under 1 T for (SrF)2Ti2Bi2O. No anomaly is observed in both data, indicating complete suppression of DW instability.
In BaTi2Sb2O, hole doping suppresses DW instability and enhances Tc. Hence, the hole doping into Na2Ti2Pn2O and (SrF)2Ti2Pn2O might also induce superconductivity. Hole doping into Na2Ti2Sb2O has been reported by Ozawa et al. where Na2‒xTi2Sb2O has been synthesized by extraction of Na+ by polytetrafluoroethylene (PTFE) treatment. Though the maximum deintercalation amount x = 0.25 is achieved, no change in TDW and no superconductivity have been found [57].
4. The DW Instability in BaTi2Pn2O
In this section, experimental and theoretical studies on DW instability for BaTi2Pn2O will be discussed. The CDW/SDW has been widely observed in high-Tc cuprates and Fe-based superconductors and the relationship between DW instability and superconductivity is still under discussion. Likewise, since the DW instability competes with superconductivity in BaTi2Pn2O, it is important to clarify the origin of DW instability to understand whether the superconductivity in BaTi2Pn2O is mediated by spin fluctuation or electron-phonon interaction.
In theoretical studies, both SDW and CDW instability have been suggested: the SDW instability is predicted by Singh and Wang et al. while CDW instability is predicted by Subedi and Nakano et al. [40,41,43,47]. Singh found the Fermi surface nesting which lead to peaks in susceptibility, giving rise to SDW instability. Furthermore, based on the spin-fluctuation mediated scenario, possible unconventional superconductivity was also predicted. Wang et al. calculated bare susceptibility χ0(q) and found the peak of χ0(q) at the X point, which induces the SDW instability. Two degenerated antiferromagnetism, bi-collinear antiferromagnetism and blocked checkerboard antiferromagnetism, are suggested as the ground state. In contrast, Subedi calculated phonon dispersion and electron-phonon coupling and found a lattice instability which gives rise to a CDW instability. The √2 × √2 × 1 superstructure is predicted in the CDW phase. Nakano et al. also found that the phonon instability around the M and A points and predicted a √2 × √2 × 1 superstructure in both BaTi2Sb2O and BaTi2Bi2O (Figure 14). Note that they found phonon instabilities at the X and R points in BaTi2As2O, which is different from BaTi2Sb2O and BaTi2Bi2O.
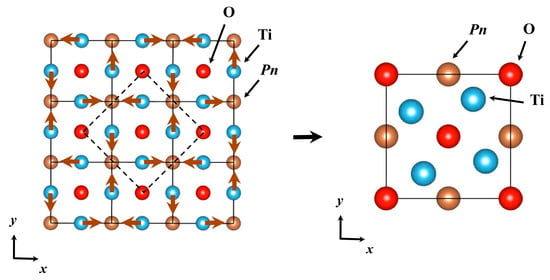
Figure 14.
Atomic displacements corresponding to the imaginary phonon mode at the M (1/2, 1/2, 0) point, leading to a √2 × √2 × 1 superlattice for BaTi2Pn2O (Pn = Sb and Bi). Dashed lines in the left panel indicate the unit cell of the superlattice, drawn in the right panel again with displaced atomic positions after the rotation by 45 degree, which is consistent with results predicted by Subedi. The symmetry of the superlattice is P4/mbm (No. 127). Reprinted with permission from Nakano et al., Sci. Rep.; published by NPG, 2016 [43].
Experimentally, the 121/123Sb-NQR/NMR spectra revealed the asymmetry parameter η becomes finite at 4.2 K without the internal field, indicating the breaking of the in-plane four-fold symmetry [37]. Furthermore, the spectra does not exhibit splitting and broadening, indicating that an incommensurate DW state is excluded. The μSR measurements exclude SDW instability. Figure 15 shows time dependence of asymmetry spectra for BaTi2As2O (TDW = 200 K) and BaTi2Sb2O (TDW = 50 K) [38]. The increased relaxation of the polarization could not be found below TDW, indicating the absence of magnetic order. Frandsen et al. performed low-temperature neutron diffraction measurements and found tiny lattice distortions from tetragonal to orthorhombic where the orthorhombicity parameter η = 2 × (a − b)/(a + b) reaches only 0.22% and 0.05% at 20 K for BaTi2As2O and BaTi2Sb2O, respectively [19]. The lattice distortion is much smaller than that observed in Jahn–Teller compounds and iron pnictides. Though a √2 × √2 × 1 superlattice was predicted in the theoretical study, no superlattice has been found in the diffraction studies. To account for the results, intra-unit-cell nematic charge order was proposed, such as that proposed in cuprates [58,59]. Recently, Song et al. reported direct observation of the CDW order with the wave vector of (π, π) by STM measurements on Ba0.95Na0.05Ti2Sb2O (Figure 16), which is consistent with that theoretically predicted by Subedi and Nakano et al. [46]. They also performed ARPES measurements and revealed the Fermi surface is well nested along the (π, π) direction, which is consistent with STM study, suggesting the electron-phonon mediated superconductivity in Ba0.95Na0.05Ti2Sb2O. The discrepancy between the absence of the superstructure in neutron scattering and the CDW order with the wave vector of (π, π) in STM and ARPES is not clear yet. Since both STM and ARPES is surface-sensitive technique, these results might not reflect bulk CDW instability due to the surface reconstruction. To unveil the nature of CDW instability in titanium pnictide oxides, the more extensive studies are required.
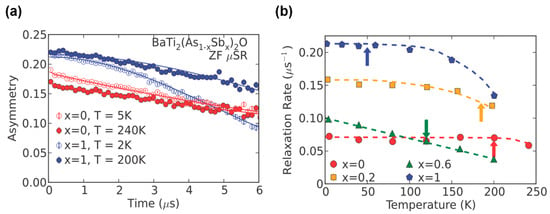
Figure 15.
Results of zero-field muon spin relaxation measurements on BaTi2(As1‒xSbx)2O. (a) Time dependence of asymmetry for BaTi2As2O and BaTi2Sb2O, indicating a slight increase in the relaxation rate at low temperatures; (b) refined relaxation rate for x = 0, 0.2, 0.6, 1, where the arrow indicates the TDW. Reprinted with permission from Nozaki et al., Phys. Rev. B; published by APS, 2013 [38].
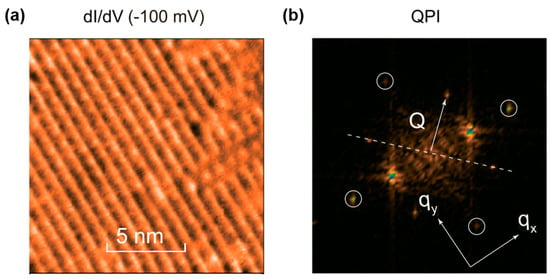
Figure 16.
(a) The differential conductance map taken with the bias voltage of −100 mV; (b) the corresponding Fourier transform of panel (a), giving a quasiparticle interference (QPI) plot. The four circled points are the lattice Bragg spots. Though the two bright points represent the 2 × 1 reconstruction of Ba atoms, additional weak peaks can be observed. Reprinted with permission from Song et al., Phys. Rev. B; published by APS, 2016 [46].
5. Conclusions
Since their discovery in 1990, titanium pnictide oxides, ATi2Pn2O, have attracted attention due to their structural similarity to high-Tc cuprates and Fe-based superconductors. Similar to these high-Tc superconductors, the DW instability has been found in Pn = As, Sb compounds. In 2012, we synthesized a new compound BaTi2Sb2O which exhibits superconducting transition at 1.2 K. Following BaTi2Sb2O, another superconductor BaTi2Bi2O (Tc = 4.6 K) has also been synthesized. To clarify the mechanism of superconductivity, DW instability has also been experimentally and theoretically studied. In early studies, possible SDW instability has been suggested. In contrast, recent results suggest that the DW instability is CDW. However, several phenomena have not been addressed: the mechanism of superconductivity in BaTi2Bi2O, large MR observed below TDW, and the enhancement of magnetic fluctuations at around TDW observed in 121/123Sb-NQR measurements. Nakaoka et al. recently predicted the formation of orbital ordering with q = (0, 0, 0), i.e., intra-unit-cell orbital order, which may account the magnetic fluctuation around TDW [43].
Recently, various ATi2Pn2O-related compounds, such as pnictide oxides and chalcogenide oxides, have been found [60,61,62,63,64]. For instance, Ba2Ti2Fe2As4O is an intergrowth of BaTi2As2O and BaFe2As2 where TDW of 120 K is observed, which is lower than that in BaTi2As2O due to the interlayer charge transfer [60]. Both CsV2S2O and Cs1‒xTi2Te2O have the same structure as BaTi2Pn2O, but they do not show any DW instability, while they show bad-metal and metallic conduction, respectively [61,62]. Though no superconductor has been found in ATi2Pn2O-related compounds yet, the discovery of superconductivity in titanium pnictide oxides might give rise to new classes of superconductors, just as cuprates and Fe-based superconductors have given rise.
Acknowledgments
The author gratefully acknowledge the support from JSPS KAKENHI Grant Number 15K17698.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bednorz, J.G.; Müller, K.A. Possible high Tc superconductivity in the Ba-La-Cu-O system. Z. Phys. B 1986, 64, 189–193. [Google Scholar] [CrossRef]
- Nagamatsu, J.; Nakagawa, N.; Muranaka, T.; Zenitani, Y.; Akimitsu, J. Superconductivity at 39 K in magnesium diboride. Nature 2001, 410, 63–64. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S.; Hotehama, K.I.; Kawaji, H. Superconductivity at 25.5 K in electron-doped layered hafnium nitride. Nature 1998, 392, 580–582. [Google Scholar] [CrossRef]
- Maeno, Y.; Hashimoto, H.; Yoshida, K.; Nishizaki, S.; Fujita, T.; Bednorz, J.G.; Lichtenberg, F. Superconductivity in a layered perovskite without copper. Nature 1994, 372, 532–534. [Google Scholar] [CrossRef]
- Takada, K.; Sakurai, H.; Takayama-Muromachi, E.; Izumi, F.; Dilanian, R.A.; Sasaki, T. Superconductivity in two-dimensional CoO2 layers. Nature 2003, 422, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Kamihara, Y.; Watanabe, T.; Hirano, M.; Hosono, H. Iron-Based Layered Superconductor La(O1‒xFx)FeAs (x = 0.05–0.12) with Tc = 26 K. J. Am. Chem. Soc. 2008, 130, 3296–3297. [Google Scholar] [CrossRef] [PubMed]
- Mizuguchi, Y.; Demura, S.; Deguchi, K.; Takano, Y.; Fujihisa, H.; Gotoh, Y.; Izawa, H.; Miura, O. Superconductivity in Novel BiS2-Based Layered Superconductor LaO1‒xFxBiS2. J. Phys. Soc. Jpn. 2012, 81, 114725. [Google Scholar] [CrossRef]
- Hor, Y.S.; Williams, A.J.; Checkelsky, J.G.; Roushan, P.; Seo, J.; Xu, Q.; Zandbergen, H.W.; Yazdani, A.; Ong, N.P.; Cava, R.J. Superconductivity in CuxBi2Se3 and its implications for pairing in the undoped topological insulator. Phys. Rev. Lett. 2010, 104, 057001. [Google Scholar] [CrossRef] [PubMed]
- Pyon, S.; Kudo, K.; Nohara, M. Superconductivity induced by bond breaking in the triangular lattice of IrTe2. J. Phys. Soc. Jpn. 2012, 81, 053701. [Google Scholar] [CrossRef]
- Kudo, K.; Ishii, H.; Takasuga, M.; Iba, K.; Nakano, S.; Kim, J.; Fujiwara, A.; Nohara, M. Superconductivity Induced by Breaking Te2 Dimers of AuTe2. J. Phys. Soc. Jpn. 2013, 82, 063704. [Google Scholar] [CrossRef]
- Adam, A.; Schuster, H.-U. Darstellung und Kristallstruktur der Pnictidoxide Na2Ti2As2O und Na2Ti2Sb2O. ZAAC 1990, 584, 150–158. (In German) [Google Scholar] [CrossRef]
- Morosan, E.; Zandbergen, H.W.; Dennis, B.S.; Bos, J.W.G.; Onose, Y.; Klimczuk, T.; Ramirez, A.P.; Ong, N.P.; Cava, R.J. Superconductivity in CuxTiSe2. Nat. Phys. 2006, 2, 544–550. [Google Scholar] [CrossRef]
- Wu, W.; Cheng, J.; Matsubayashi, K.; Kong, P.; Lin, F.; Jin, C.; Wang, N.; Uwatoko, Y.; Luo, J. Superconductivity in the vicinity of antiferromagnetic order in CrAs. Nat. Commun. 2014, 5, 5508. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Yan, Y.J.; Ying, J.J.; Li, Q.J.; Zhang, M.; Xu, N.; Chen, X.H. Structure and physical properties for a new layered pnictide-oxide: BaTi2As2O. J. Phys. Condens. Matter 2010, 22, 075702. [Google Scholar] [CrossRef]
- Yajima, T.; Nakano, K.; Takeiri, F.; Ono, T.; Hosokoshi, Y.; Matsushita, Y.; Hester, J.; Kageyama, H. Superconductivity in BaTi2Sb2O with a d1 square lattice. J. Phys. Soc. Jpn. 2012, 81, 103706. [Google Scholar] [CrossRef]
- Yajima, T.; Nakano, K.; Takeiri, F.; Hester, J.; Yamamoto, T.; Kobayashi, Y.; Tsuji, N.; Kim, J.; Fujiwara, A.; Kageyama, H. Synthesis and physical properties of the new oxybismuthides BaTi2Bi2O and (SrF)2Ti2Bi2O with a d1 square net. J. Phys. Soc. Jpn. 2012, 82, 013703. [Google Scholar] [CrossRef]
- Liu, R.H.; Song, Y.A.; Li, Q.J.; Ying, J.J.; Yan, Y.J.; He, Y.; Chen, X.H. Structure and Physical Properties of the Layered Pnictide-Oxides: (SrF)2Ti2Pn2O (Pn= As, Sb) and (SmO)2Ti2Sb2O. Chem. Mater. 2010, 22, 1503–1508. [Google Scholar] [CrossRef]
- Yajima, T.; Nakano, K.; Takeiri, F.; Nozaki, Y.; Kobayashi, Y.; Kageyama, H. Two Superconducting Phases in the Isovalent Solid Solutions BaTi2Pn2O (Pn = As, Sb, and Bi). J. Phys. Soc. Jpn. 2013, 82, 033705. [Google Scholar] [CrossRef]
- Frandsen, B.A.; Bozin, E.S.; Hu, H.; Zhu, Y.; Nozaki, Y.; Kageyama, H.; Uemura, Y.J.; Yinm, W.G.; Billinge, S.J. Intra-unit-cell nematic charge order in the titanium-oxypnictide family of superconductors. Nat. Commun. 2014, 5, 5761. [Google Scholar] [CrossRef] [PubMed]
- Deslandes, F.; Nazzal, A.I.; Torrance, J.B. Search for superconductivity in analogues of La2−xSrxCuO4: Sr2−xLnxVO4 (Ln = La, Ce, Pr, Nd, Eu). Physica C 1991, 179, 85–90. [Google Scholar] [CrossRef]
- Arita, R.; Yamasaki, A.; Held, K.; Matsuno, J.; Kuroki, K. Sr2VO4 and Ba2VO4 under pressure: An orbital switch and potential d1 superconductor. Phys. Rev. B 2007, 75, 174521. [Google Scholar] [CrossRef]
- Axtell, E.A.; Ozawa, T.; Kauzlarich, S.M.; Singh, R.R. Phase transition and spin-gap behavior in a layered tetragonal pnictide oxide. J. Solid State Chem. 1997, 134, 423–426. [Google Scholar] [CrossRef]
- Shi, Y.G.; Wang, H.P.; Zhang, X.; Wang, W.D.; Huang, Y.; Wang, N.L. Strong anisotropy in the electromagnetic properties of Na2Ti2X2O (X = As, Sb) crystals. Phys. Rev. B 2013, 88, 144513. [Google Scholar] [CrossRef]
- Pickett, W.E. Electronic instability in inverse-K2NiF4-structure Na2Sb2Ti2O. Phys. Rev. B 1998, 58, 4335–4340. [Google Scholar] [CrossRef]
- Fabrizi de Biani, F.; Alemany, P.; Canadell, E. Concerning the Resistivity Anomaly in the Layered Pnictide Oxide Na2Ti2Sb2O. Inorg. Chem. 1998, 37, 5807–5810. [Google Scholar] [CrossRef]
- Ozawa, T.C.; Pantoja, R.; Axtell, E.A.; Kauzlarich, S.M.; Greedan, J.E.; Bieringer, M.; Richardson, J.W. Powder Neutron Diffraction Studies of Na2Ti2Sb2O and Its Structure–Property Relationships. J. Solid State Chem. 2000, 153, 275–281. [Google Scholar] [CrossRef]
- Liu, R.H.; Tan, D.; Song, Y.A.; Li, Q.J.; Yan, Y.J.; Ying, J.J.; Xie, Y.L.; Wang, X.F.; Chen, X.H. Physical properties of the layered pnictide oxides Na2Ti2P2O (P = As, Sb). Phys. Rev. B 2009, 80, 144516. [Google Scholar] [CrossRef]
- Ozawa, T.C.; Kauzlarich, S.M. Single crystal growth and characterization of a layered transition metal pnictide oxide: Na2Ti2Sb2O. J. Cryst. Growth 2004, 265, 571–576. [Google Scholar] [CrossRef]
- Song, Y.J.; Ghim, J.S.; Min, B.H.; Kwon, Y.S.; Jung, M.H.; Rhyee, J.S. Synthesis, anisotropy, and superconducting properties of LiFeAs single crystal. Appl. Phys. Lett. 2010, 96, 212508. [Google Scholar] [CrossRef]
- Tanatar, M.A.; Ni, N.; Samolyuk, G.D.; Bud’ko, S.L.; Canfield, P.C.; Prozorov, R. Resistivity anisotropy of AFe2As2 (A = Ca, Sr, Ba): Direct versus Montgomery technique measurements. Phys. Rev. B 2009, 79, 134528. [Google Scholar] [CrossRef]
- Jesche, A.; Nitsche, F.; Probst, S.; Doert, Th.; Müller, P.; Ruck, M. Anisotropic electrical resistivity of LaFeAsO: Evidence for electronic nematicity. Phys. Rev. B 2012, 86, 134511. [Google Scholar] [CrossRef]
- Suetin, D.V.; Ivanovskii, A.L. Structural, electronic properties, and chemical bonding in quaternary layered titanium pnictide-oxides Na2Ti2Pn2O and BaTi2Pn2O (Pn = As, Sb) from FLAPW–GGA calculations. J. Alloy Compd. 2013, 564, 117–124. [Google Scholar] [CrossRef]
- Doan, P.; Gooch, M.; Tang, Z.; Lorenz, B.; Möller, A.; Tapp, J.; Chu, P.C.W.; Guloy, A.M. Ba1–xNaxTi2Sb2O (0.0 ≤ x ≤ 0.33): A Layered Titanium-Based Pnictide Oxide Superconductor. J. Am. Chem. Soc. 2012, 134, 16520–16523. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, S.; Shibauchi, T.; Hashimoto, K.; Ikada, K.; Tonegawa, S.; Okazaki, R.; Shishido, H.; Ikeda, H.; Takeya, H.; Hirata, K.; et al. Evolution from non-Fermi-to Fermi-liquid transport via isovalent doping in BaFe2(As1−xPx)2 superconductors. Phys. Rev. B 2010, 81, 184519. [Google Scholar] [CrossRef]
- Hoffmann, R.; Zheng, C. Making and Breaking Bonds in the Solid State: The ThCr2Si2 Structure. J. Phys. Chem. 1985, 89, 4175–4181. [Google Scholar] [CrossRef]
- Gooch, M.; Doan, P.; Tang, Z.; Lorenz, B.; Guloy, A.M.; Chu, P.C. Weak coupling BCS-like superconductivity in the pnictide oxide Ba1−xNaxTi2Sb2O (x = 0 and 0.15). Phys. Rev. B 2013, 88, 064510. [Google Scholar] [CrossRef]
- Kitagawa, S.; Ishida, K.; Nakano, K.; Yajima, T.; Kageyama, H. s-wave superconductivity in superconducting BaTi2Sb2O revealed by 121/123Sb-NMR/nuclear quadrupole resonance measurements. Phys. Rev. B 2013, 87, 060510. [Google Scholar] [CrossRef]
- Nozaki, Y.; Nakano, K.; Yajima, T.; Kageyama, H.; Frandsen, B.; Liu, L.; Cheung, S.; Goko, T.; Uemura, Y.J.; Munsie, T.S.J.; et al. Muon spin relaxation and electron/neutron diffraction studies of BaTi2(As1−xSbx)2O: Absence of static magnetism and superlattice reflections. Phys. Rev. B 2013, 88, 214506. [Google Scholar] [CrossRef]
- Yajima, T.; Nakano, K.; Nozaki, Y.; Kageyama, H. Superconducting properties of BaTi2Pn2O (Pn = Sb, Bi). Physica C 2014, 504, 36–38. [Google Scholar] [CrossRef]
- Singh, D.J. Electronic structure, disconnected Fermi surfaces and antiferromagnetism in the layered pnictide superconductor NaxBa1−xTi2Sb2O. New J. Phys. 2012, 14, 123003. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, H.; Zhang, L.; Liu, C. The electronic structure and magnetism of BaTi2Sb2O. J. Appl. Phys. 2013, 113, 243904. [Google Scholar] [CrossRef]
- Nakaoka, H.; Yamakawa, Y.; Kontani, H. Theoretical prediction of nematic orbital-ordered state in the Ti oxypnictide superconductor BaTi2(As,Sb)2O. Phys. Rev. B 2016, 93, 245122. [Google Scholar] [CrossRef]
- Nakano, K.; Hongo, K.; Maezono, R. Phonon dispersions and Fermi surfaces nesting explaining the variety of charge ordering in titanium-oxypnictides superconductors. Sci. Rep. 2016, 6, 29661. [Google Scholar] [CrossRef] [PubMed]
- Suetin, D.V.; Ivanovskii, A.L. Electronic properties and fermi surface for new Fe-free layered pnictide-oxide superconductor BaTi2Bi2O from first principles. JETP Lett. 2013, 97, 220–225. [Google Scholar] [CrossRef]
- Yan, X.W.; Lu, Z.Y. Layered pnictide-oxide Na2Ti2Pn2O (Pn= As, Sb): A candidate for spin density waves. J. Phys. Condens. Matter 2013, 25, 365501. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Yan, Y.J.; Ye, Z.R.; Ren, M.Q.; Xu, D.F.; Tan, S.Y.; Niu, X.H.; Xie, B.P.; Zhang, T.; Peng, R.; et al. Electronic structure of the titanium-based oxypnictide superconductor Ba0.95Na0.05Ti2Sb2O and direct observation of its charge density wave order. Phys. Rev. B 2016, 93, 024508. [Google Scholar] [CrossRef]
- Subedi, A. Electron-phonon superconductivity and charge density wave instability in the layered titanium-based pnictide BaTi2Sb2O. Phys. Rev. B 2013, 87, 054506. [Google Scholar] [CrossRef]
- Zhai, H.F.; Jiao, W.H.; Sun, Y.L.; Bao, J.K.; Jiang, H.; Yang, X.J.; Tang, Z.T.; Tao, Q.; Xu, X.F.; Li, Y.K.; et al. Superconductivity, charge-or spin-density wave, and metal-nonmetal transition in BaTi2(Sb1−xBix)2O. Phys. Rev. B 2013, 87, 100502. [Google Scholar] [CrossRef]
- Hiraishi, M.; Iimura, S.; Kojima, K.M.; Yamaura, J.; Hiraka, H.; Ikeda, K.; Miao, P.; Ishikawa, Y.; Torii, S.; Miyazaki, M.; et al. Bipartite magnetic parent phases in the iron oxypnictide superconductor. Nat. Phys. 2014, 10, 300–303. [Google Scholar] [CrossRef]
- Mukuda, H.; Engetsu, F.; Yamamoto, K.; Lai, K.T.; Yashima, M.; Kitaoka, Y.; Takemori, A.; Miyasaka, S.; Tajima, S. Enhancement of superconducting transition temperature due to antiferromagnetic spin fluctuations in iron pnictides LaFe(As1−xPx)(O1−yFy): 31P-NMR studies. Phys. Rev. B 2014, 89, 064511. [Google Scholar] [CrossRef]
- Matsuishi, S.; Maruyama, T.; Iimura, S.; Hosono, H. Controlling factors of Tc dome structure in 1111-type iron arsenide superconductors. Phys. Rev. B 2014, 89, 094510. [Google Scholar] [CrossRef]
- Pachmayr, U.; Johrendt, D. Superconductivity in Ba1−xKxTi2Sb2O (0 ≤ x ≤ 1) controlled by the layer charge. Solid State Sci. 2014, 28, 31–34. [Google Scholar] [CrossRef]
- Von Rohr, F.; Nesper, R.; Schilling, A. Superconductivity in rubidium-substituted Ba1−xRbxTi2Sb2O. Phys. Rev. B 2014, 89, 094505. [Google Scholar] [CrossRef]
- Nakano, K.; Yajima, T.; Takeiri, F.; Green, M.A.; Hester, J.; Kobayashi, Y.; Kageyama, H. Tc Enhancement by Aliovalent Anionic Substitution in Superconducting BaTi2(Sb1−xSnx)2O. J. Phys. Soc. Jpn. 2013, 82, 074707. [Google Scholar] [CrossRef]
- Gooch, M.; Doan, P.; Lorenz, B.; Tang, Z.J.; Guloy, A.M.; Chu, C.W. High pressure study of the normal and superconducting states of the layered pnictide oxide Ba1−xNaxTi2Sb2O with x = 0, 0.10, and 0.15. Supercond. Sci. Technol. 2013, 26, 125011. [Google Scholar] [CrossRef]
- Ji, Q.; Ma, Y.; Hu, K.; Gao, B.; Mu, G.; Li, W.; Hu, T.; Zhang, G.; Zhao, Q.; Zhang, H.; et al. Synthesis, Structural, and Transport Properties of Cr-Doped BaTi2As2O. Inorg. Chem. 2014, 53, 13089–13092. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T.C.; Naka, T.; Matsushita, A.; Kauzlarich, S.M.; Sasaki, T. Chemical composition and magnetic property modifications of Na2Ti2Sb2O using PTFE as an alkali–metal ion extraction reagent. J. Fluor. Chem. 2014, 168, 189–192. [Google Scholar] [CrossRef]
- Fujita, K.; Hamidian, M.H.; Edkins, S.D.; Kim, C.K.; Kohsaka, Y.; Azuma, M.; Takano, M.; Takagi, H.; Eisaki, H.; Uchida, S.; et al. Direct phase-sensitive identification of a d-form factor density wave in underdoped cuprates. Proc. Natl. Acad. Sci. USA 2014, 111, E3026–E3032. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, Y.; Kontani, H. Spin-Fluctuation-Driven Nematic Charge-Density Wave in Cuprate Superconductors: Impact of Aslamazov-Larkin Vertex Corrections. Phys. Rev. Lett. 2015, 114, 257001. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.L.; Jiang, H.; Zhai, H.F.; Bao, J.K.; Jiao, W.H.; Tao, Q.; Shen, C.Y.; Zeng, Y.W.; Xu, Z.A.; Cao, G.H. Ba2Ti2Fe2As4O: A new superconductor containing Fe2As2 layers and Ti2O sheets. J. Am. Chem. Soc. 2012, 134, 12893–12896. [Google Scholar] [CrossRef] [PubMed]
- Valldor, M.; Merz, P.; Prots, Y.; Schnelle, W. Bad-Metal-Layered Sulfide Oxide CsV2S2O. Eur. J. Inorg. Chem. 2016, 2016, 23–27. [Google Scholar] [CrossRef]
- Valldor, M.; Merz, P.; Prots, Y.; Watier, Y.; Schnelle, W. Synthesis and Characterization of Cs1–xTi2Te2O (x ≈ 0.2): Electron Doping by Te Resulting in a Layered Metal. Inorg. Chem. 2016, 55, 11337–11341. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, H.; Park, S.; Hiraka, H.; Ikeda, K.; Otomo, T.; Hosono, H. An Anti CuO2-type Metal Hydride Square Net Structure in Ln2M2As2Hx (Ln = La or Sm, M = Ti, V, Cr, or Mn). Angew. Chem. Int. Ed. 2015, 54, 2932–2935. [Google Scholar] [CrossRef] [PubMed]
- Takeiri, F.; Matsumoto, Y.; Yamamoto, T.; Hayashi, N.; Li, Z.; Tohyama, T.; Tassel, C.; Ritter, C.; Narumi, Y.; Hagiwara, M.; et al. High-pressure synthesis of the layered iron oxyselenide BaFe2Se2O with strong magnetic anisotropy. Phys. Rev. B 2016, 94, 184426. [Google Scholar] [CrossRef]
© 2017 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).