Abstract
A four-week trial was conducted to compare the effects of a conventional flow-through system diet (FTS) and an experimental RAS diet (ERAS) on fish performance, water quality and general system implication in a replicated recirculation aquaculture system (RAS). Six identical RAS, each with a total system water volume of 1500 L and cylindrical rearing tanks of 1000 L were stocked with Atlantic salmon (Salmo salar) post-smolts with an average weight of 199.7 ± 28 g, to an average stocking density of 30 kg/m3 and reaching approximately 49 kg/m3 at the trial end. The ERAS diet were composed to inhabit typical RAS feed characteristics compared with the FTS diet, such as a higher fecal stability and reduced protein levels (−12%), but at the same time increased fat content (+8%) to secure similar gross energy levels (22–23 MJ kg−1) between the two diets. Water quality parameters were measured individually. The total accumulation of minerals and metals was analyzed in water from different parts of the system at the start and end of the trial period for both diets. No differences in growth, condition factor, feed conversion rate (FCR), or survival of fish fed the two dietary adaptations were observed. The system using the ERAS diet showed significantly higher pH (+1.2%) and alkalinity (+17%) and lower total ammonia nitrogen (TAN) (−18%) and NO2− (−46%) compared with the FTS diet. The count of drum filter activations was also significantly lower (−13%) with the ERAS diet. Compared with the FTS diet, the ERAS diet had a lower probability (−4%) of generating particles smaller than 50 μm, and that the RAS was also more effective in removing particles from the drum filter, prompting a lower daily activation of the filter of 22.1 ± 3.0 counts compared with 25.5 ± 3.5 for the FTS diet. Mineral analysis showed a significantly lower accumulation of total phosphorus (TP) (−90%) and dissolved phosphorus (DP) (−92%) in the RAS units using the ERAS diet compared with those using the FTS diet. Compared with a traditional flow-through diet, these results highlight the benefits of using an RAS-adapted diet that matches the energy requirement of flow-through diets regarding water quality, system performance, satisfactory growth, and condition.
Key Contribution:
This study highlights the benefits and disadvantages of using specifically adapted diets for fish production in recirculating aquaculture systems.
1. Introduction
Local pollution from Norwegian Atlantic salmon (Salmo salar) farming is an increasing challenge and a direct result of the rapid increase the salmon farming industry has experienced over the last years [1]. Mainly deriving from feed waste and feces, the effluents discharged from land-based flow-through systems (FTS) and open pens are rich in nutrients like nitrogen (N) and phosphorus (P), in addition to organic matter, and can thus pose an immediate threat to the local ecosystem, impacting the nutrient balance in water bodies and potentially causing zones of harmful algal blooms and oxygen depletion [2,3,4].
An established strategy to prevent local pollution has been to position farming locations in areas with strong enough water currents to avoid sedimentation of organic matter directly under an open pen or next to the effluent pipe for FTS [5]. With experience from the 1990s, aquaculture waste was commonly defined as a fertilizer [6]. The definition of pollution suggests that the negative impact of waste from salmon farming only occurred when a substance was added into an environment in such concentrations that damage to nature was a fact, suggested mainly as local pollution [5]. The modern-day release of N and P from Norwegian aquaculture is 24 and 45 times higher than the total from the rest of the Norwegian industry (Agriculture, municipal drainage, and industry) and 0.7 and 9 times that of natural drainage, respectively [1]. With the steadily increasing biomass production in Norway [7,8], the pressure on local ecology is growing along with the increasing aquaculture production.
Due to this, the demand for closed aquaculture systems (CAS), including recirculating aquaculture systems (RAS), is expected to increase in the future to secure an expansion of the aquaculture industry [9]. One benefit of the RAS technology is its ability to concentrate effluent discharge, allowing for end-of-pipe treatment [10,11]. In addition, the technology enables farmers to optimize the rearing environment, e.g., temperature control, and also reduces water consumption when compared with FTS through a combination of mechanical, biological, and chemical water treatment [10,11,12,13,14]. Combined with increased productivity, these capabilities enable RAS fish farms to improve the operations’ sustainability by reducing their environmental impact compared with FTS operations [15,16,17].
Exogenous feeding represents the primary nutrient input into any given system. Controlling the fish diet’s nutritional composition and altering feeding strategies will be critical for mitigating a farm’s emissions [18]. Based on the definitions of local pollution [5], it can be assumed that dietary solutions for open pen and FTS productions have historically been formulated to possess attributes preventing the local settling of fecal matter by breaking it up and spreading over a larger area. Compared with FTS diets, modern dietary solutions for RAS are an opposing concept, with attributes promoting the binding and settling of fecal particles for easier removal during mechanical water treatment [19,20]. These RAS diets are marketed with the promise of lowering the biofilter load, improving water quality, and reducing N discharge [19,20]. Salmon farmers can sometimes have the opportunity, permission, and tank capacity to increase their biological production, but they lack the biofilter capacity to match the intended increase and the investment cost of increasing capacity for an already established biofilter can be high. In addition, space can be a limiting factor. Upscaling a biological production without increasing the general capacity of a system, can also alter nutrient dynamics within the system as well as output from the system [21], potentially making nutritional waste harder to collect. Choosing an RAS diet can, therefore, provide a solution for reduction of N and P discharge from the facility facing such challenges.
Diet manipulation to strengthen fecal stability and increase particle size also binds more N and P to the feces, thus facilitating their removal from the system [22,23,24]. Nutritional digestibility or availability of ingredients in the diets is also a decisive factor for biological utilization and, thus, the fish’s nutritional excretion [6,25]. Lower N emission can be achieved by improving digestibility or reducing total protein within a diet [26,27,28]. This can reduce the excreted N and overall ammonia levels commonly accumulating in an RAS. The dietary levels of vegetable protein sources can also affect the discharged amounts because salmonids cannot synthesize the phytase [29,30] that cleaves phosphate bonds in the phytic acid molecule, thus releasing a proportion of P from its main storage form in vegetable ingredients [29,30]. Due to this, the availability of P from phytic acid is estimated to be 0% in vegetable ingredients fed to Atlantic salmon [30]. While the availability in some fishmeal can be as high as 87% [31], the phytic acid content in vegetable ingredients can range from 60–80% [29], leaving an available fraction of only 20–40% for salmonids species. The inorganic P sources used in diet formulations, e.g., monocalcium phosphate (MCP), dicalcium phosphate (DCP), monoammonium phosphate (MAP) or monosodium phosphate (MSP) also influence P excretion due to their various P availability [32].
The nutrients accumulated within an RAS are ultimately discharged into the environment [33,34,35,36], and can potentially result in ecological and regulatory challenges [1]. Though RAS diets have been developed specifically for RAS productions, some farmers may still rely on the traditional FTS diets in their productions.
The existing literature within the field has focused mainly on the distribution and removal of nitrogen-containing molecules and total accumulation of minerals [33,34,37,38]; however, less attention has been given to the direct system effect and fish performance as a result of different dietary adaptations. The current study, therefore, aimed to compare the system performance of an RAS- and FTS-adapted diet used in an RAS context, with a focus on the accumulation of particles, nitrogenous compounds, minerals, and metals throughout the different water treatment steps in the RAS. The goal was to highlight the possible environmental benefits and disadvantages of using diets with opposing attributes regarding RAS operations, while monitoring the biological performance of the Atlantic salmon post-smolts.
2. Materials and Methods
In order to determine a suitable location from which to measure the total accumulation of substances in the recirculation system, it was necessary to run a pre-trial to map the distributional pattern. Therefore, the results from the pre-trial are only described in Section 3.1. The pre-trial was carried out and analyzed using the same system and methods as the main trial described in Section 2.1 and from Section 2.3 and onward.
2.1. Experimental System
The pre-trial and the main trial were conducted in six independent RAS research units (nanoRAS, Alpha Aqua, Esbjerg, Denmark). Each system had a total water volume of 1500 L and consisted of a 1000 L cylindrical rearing tank, a mechanical drum filter (60 μm mesh), a three-step biofilter filled with approximately 0.23 m3 saddle-chips biomedia (Dania plast AS, Mariager, Denmark) in each step that functioned as a moving bed and a gas balancing filter (GB filter)/pump sump (Figure 1). The process water from the fish tank enters the drum filter through the filter screen and the water level rises until the ultrasound switch is flooded, which activates and rotates the filter. Simultaneously, the backwash pump starts and the spray bar cleans the rotating drum. When the water level drops beneath the ultrasound switch the drum stops rotating, and the solenoid valves close. To regulate pH, an external supply of bicarbonate is added through a solenoid valve after the drum filter (maintaining an alkalinity of >150 mg L−1) before the water flows over to the first biofilter chamber. Once this chamber is filled, the water rises in the baffle wall between the 1st and 2nd biofilter before entering the second biofilter. The process is repeated between the 2nd and 3rd biofilter, before the water continues to a perforated top plate in the GB filter. Each biofilter chamber is supplied with air from an external blower. In the GB filter, the water falls through the perforated top plate into the pump sump, where a ventilator removes the CO2 through dedicated pipes in the top plate. In the pump sump, water is treated with UV, and temperature is controlled when an actuator opens a cooling agent to transfer excess heat via a cooling coil. Water can also be heated by a ceramic heating element placed in the pump sump. For the current trial, setpoints were adjusted to achieve target temperatures of 13 °C. Pressurized process water is pumped through an oxygen cone before entering the fish tank. An emergency oxygen pipe leaks oxygen at the bottom of the fish tank when the oxygen content is too low or at power failure. The fish tank’s dissolved oxygen concentration was maintained at near 100% saturation for the duration of the trial. All tanks were equipped with a paddle flow meter at the vertical inlet pipe, a pressure transmitter, oxygen-, temperature- and salinity sensor in the tank, and lid lights. An oxygen-, temperature-, salinity- and pH sensor also continuously measured water quality in the sensor well. This sensor well was placed above the pump sump top plate and fed with water directly from the pump sump, reflecting the water quality parameters at this location.
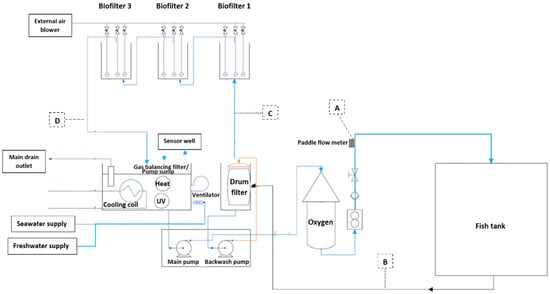
Figure 1.
Water flow and process design of a replicated Alpha Aqua RAS. Location for water sampling in the pre-trial is highlighted as follows. A: before the fish tank, B: after the fish tank, C: after the drum filter and D: after the biofilter.
2.2. Pre-Trial
The Atlantic salmon in the pre-trial was fed an experimental RAS diet (ERAS diet) for three days, followed by water sampling on the fourth day. This was determined as a sufficient time frame by which to map the distributional pattern of the different water treatment steps. For the pre-trial, 405 post-smolts with an average weight of 281 ± 46 g were distributed into three individual RAS units to achieve a fish density on 40 kg m−3 per tank. The fish were reared at average water temperature, salinity, and pH of 13 °C, 15 ppt, and 8.1, respectively. Each tank was fed 230 g feed daily, 690 g of feed in total pr. RAS unit over the period.
Water samples of 500 mL each were collected from the water stream as follows. A: before the fish tank, B: after the fish tank, C: after the drum filter, and D: after the biofilter (Figure 1).
This concluded the setup for the pre-trial, and from this point on, the material and methods discussed were exclusively connected to the main trial.
2.3. Culture Conditions
The fish performance and water quality were the focus of the study in the main trial. The start-up of the RAS began with the progressive stocking of 180 Atlantic salmon post-smolts between February and March 2023. Once the systems were stocked, they were acclimated for two weeks before the trial started to ensure satisfactory operating conditions, i.e., biofilter. In the start-up, acclimation, and trial phases, brackish water (15 ppt) was used.
All fish originated from fertilized eggs supplied by Aquagen (Atlantic QTL-innOva SHIELD, Vestseøra, Norway) and raised at Cargill Innovation Center (Dirdal Norway). The fish were pit-tagged, smoltified, and vaccinated before transfer into the RAS units. Following the acclimation period, the fish were weighed, measured, and pooled before, in total, 900 post-smolts, with an average weight of 199 ± 28 g, length of 25.7 ± 1.3 cm, and condition factor (K) of 1.16 ± 0.08 were distributed to 6 individual RAS units to achieve 30 kg m−3 per tank. Below are shown the equations for calculating K, SGR, and feed conversion rates (FCR).
K = 100 × (weight, g)/(total length, cm)3
SGR (% day−1) = 100 × (ln (final biomass, g) − ln (initial biomass, g))/(time, days)
FCR = Cumulative feed delivered to tank/fish biomass gain
Feed waste was not removed in an attempt to simulate commercial operating procedures, and FCR was therefore determined on the same foundation as in previous studies where feed waste was neither collected nor weighed [24,34,39].
2.4. Commercial Feed Formulations and Feeding
During the acclimation period, the fish were fed a commercial smolt feed (EWOS Micro, Cargill, Norway). During the 4-week experimental period, the fish were fed either an ERAS diet or an FTS diet. Both groups were fed the same amount of feed, weighed daily based on internal Cargill feeding tables, and delivered continuously by an automatic belt feeder. The fish were fed in excess and satiation was set when excess feed was visually observed at the bottom of the tank. Tanks were illuminated for 24 h. Diets were tested in triplicate and randomly allocated to each tank.
Trial feeds were formulated using a commercial formulation program and produced by extrusion at a Cargill feed factory (Florø, Norway). The formulation and chemical content of the experimental diets are described in Table 1, showing an ingredient and nutrient composition that meet the nutritional requirements of Atlantic salmon at this stage [25]. The ERAS diet was designed to have lower N content to reduce the biofilter pressure. To lower the N content, the protein levels were reduced (−12%) for the ERAS diet, but at the same time the fat content was increased (+8%) to secure similar gross energy levels (22–23 MJ kg−1) between the two diets. Reduction of protein was achieved by reducing the inclusion of plant protein while increasing the inclusion of a fish meal (FM) mix consisting of blue whiting (Micromesistius poutassou) and Atlantic herring (Clupea harengus), as a high-quality protein source. The primary plant protein sources for the FTS- and ERAS diet were soy protein concentrate (SPC) and sunflower, while wheat was used as a main source of starch. Both diets were optimized for essential amino acids according to the requirement [25]. The diets were formulated to contain 9 g available P per kg diet.

Table 1.
Formulation of the experimental recirculating aquaculture diet (ERAS diet) and flow-through system diet (FTS diet) used in the pre-trial and main trial.
2.5. Water Quality Sampling and Analysis
Environmental data and water quality were registered throughout the trial at different intervals in each RAS. From a partial flow after the drum filter (sensory pit), temperature, pH, salinity, and oxygen in each system were monitored five days a week (Monday to Friday) with a multi-parameter portable meter WTW multi 3620 IDS (Xylem Analytics, Mainz, Germany) and CO2 with a Franatech dissolved CO2 sensor HR (Franatech AS, Oslo, Norway). Total water consumption (L day−1) was constantly monitored with flow meters on the seawater and freshwater inlets for each tank. Total ammonia nitrogen (TAN), nitrite (NO2−), nitrate (NO3−), and alkalinity were measured three times a week (Monday, Wednesday, and Friday) from a water sample collected after the biofilter by chemical analysis. The TAN, NO2−, NO3− and alkalinity were prepared with Spectroquant cell test kits (Merck, Darmstadt, Germany) and analyzed photometrically using a Spectroquant Prove 300 (Merck, Darmstadt, Germany).
Turbidity was measured from the same sample using a turbidity meter WTW Turb750 IR (Xylem Analytics, Mainz, Germany). For the turbidity, 20 mL of water was analyzed five times, and then the average of the five analyses was deemed representative. Total suspended solids (TSS) were measured weekly (Monday) from the analysis of a sample collected after the biofilter of each RAS (n = 3) based on a standard method (NS-EN872:2005) [40]. Briefly, 300 mL of sampled water was filtered through a pre-weighed glass microfiber filter (1.5 μm) using a vacuum pump. The wet samples were then dried at 103 °C for 480 min before the dry samples were weighed again to determine TSS. Total gas pressure (TGP) was measured weekly (Monday) with an Oxyguard Handy Polaris TGP (Oxyguard, Denmark).
Percent daily recirculation and feed pr. liter of water was calculated as:
2.6. Minerals Sampling and Analysis
To measure mineral accumulation in the production systems, 500 mL of water sample was collected from the fish tank inlet (chosen based on results from the pre-trial) in each RAS unit at the start and end of the trial. Minerals and metals in the samples for the pre-trial and main trial were analyzed for total phosphorus (TP), dissolved phosphorus (DP), and phosphate (PO4-P) with a continuous flow analysis (CFA) (NS-EN ISO 15681-2:2018) [41]. The ISO standard defines TP as the total amount of P components within a sample. DP is the amount of P that passes through a filter with a pore size of 0.45 µm. This includes all inorganic and organic P that is left in the water after filtration and can include orthophosphate (PO4) and condensed phosphates. Orthophosphate, often referred to as “reactive phosphorus”, is a subset of DP but does not include other forms of P, e.g., organic or condensed P. This means that all PO4 are DP but not all DP is PO4.
Calcium (Ca), magnesium (Mg), iron (Fe), and zinc (Zn) were analyzed by inductively coupled plasma mass spectrometry (ICP-MS) before nitric acid digestion (SS-EN ISO 15587-2:2002 [42]/SS-EN ISO 17294-2:2016 [43]). Samples were filtered (0.45 µm) to a soluble fraction and digested to provide coverage of all components.
2.7. Fecal Stability and Drum Filter Activation
Approximately 0.7 g of feces were collected per tank after the rearing tank outlet (B) (Figure 1) using a Grunwerg fine-mesh stainless steel strainer (12.5 cm ø, Sheffield, UK) to evaluate fecal stability. Fecal stability was determined by density analysis where stability was expressed as the fraction of particles larger than 50 µm after undergoing ten consecutive runs (5 min stirring) in the Mastersizer 3000 instrument and where 50 µm was set as the lower limit of the mechanical filter to remove solid matter from the tank water. Each passage in the Mastersizer instrument will reduce the particle size of the fecal sample and a higher fraction of particles larger than 50 µm provides indication of a more stable fecal sample.
Additionally, the number of times the drum filter was activated during the trial to remove particles from the water stream was also registered (drum filter activation count). This would indicate how efficiently feces particles from different feed origins could be removed by mechanical filtration.
2.8. Statistical Analysis
The data are presented as average ± standard deviation (SD). For fecal stability analysis, the confidence interval (ci) was calculated by simulation and denotes the 95% ci for the mean of each sample. Fish performance and water quality data were tested with a one-way ANOVA (variable = diet), while mineral and metal accumulation was tested with a two-way ANOVA [44] (variable 1 = diet; variable 2 = sampling time, i.e., initial and final). The data from the pre-trial were tested with a one-way ANOVA (variable = sampling location). Effects were considered significant at a significance level of p < 0.05 and differences were tested using Tukey post hoc test. Mineral and metals were also structured with a principal component analysis (PCA), using a correlation matrix with two principal components to explain the variances. Minitab (20.4) [45] was used as statistical software.
3. Results
3.1. Pre-Trial
The distributional pattern for the minerals in the RAS showed no significant difference in accumulation between the different water treatment steps (Figure 2). Both filtered and digested Fe were below the detection limit and could, therefore, not be graphically presented.
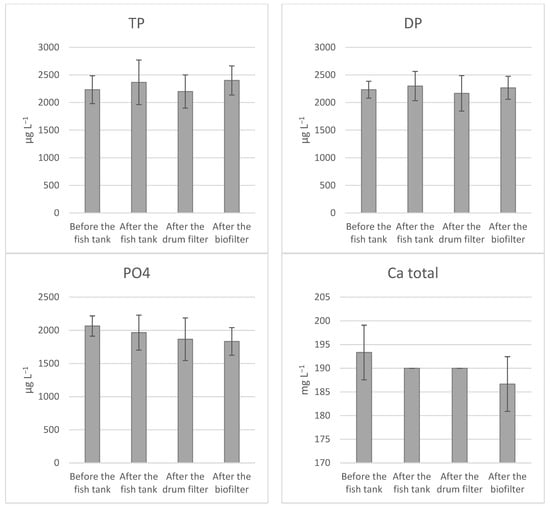
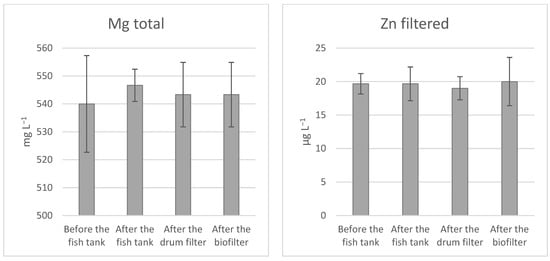
Figure 2.
The distributional pattern of total phosphorus (TP), dissolved phosphorus (DP), orthophosphate (PO4), zinc (ZN), calcium (Ca), and magnesium (Mg) in production water of RAS stocked with Atlantic salmon (Salmo salar) at 13 °C, fed an ERAS diet. Represented by sampling locations in water treatment before the fish tank, after the fish tank, after the drum filter, and after the biofilter. Data are expressed as average ± SD (n = 3).
3.2. Fish Performance
At the end of the 4-week trial, the fish had a weight gain of approximately 63%. No significant differences were observed between the two diets regarding final weight, length, condition factor, SGR, FCR, or survival (Table 2).

Table 2.
Growth performance indicators and survival in Atlantic salmon (Salmon salar) post-smolts reared during four weeks at 13 °C and fed either ERAS diet or FTS diet. Data are expressed as average ± SD (n = 3). Data were analyzed by two-way ANOVA (p < 0.05).
3.3. Water Quality and Fecal Stability
There was significantly higher water pH (<0.001) and alkalinity (<0.001) for the systems operating on the ERAS diet. The same systems also had a significantly lower accumulation of TAN (0.002) and NO2− (<0.001), in addition to a lower drum filter activation count compared with the system operating on the FTS diet. For the remaining water quality parameters, no significant difference was detected between the diets (Table 3).

Table 3.
Water quality and system performance of the RAS experimental units during the four-week trial. Data are expressed as daily average + SD (n = 3). Data were analyzed by one-way ANOVA (p < 0.05).
At the start of the trial, there was an immediate increase in the use of water in each of the RAS units and, subsequently, a reduction in the degree of recirculation (Figure 3). From 12 April, a manual cleaning step for the drum filter was implemented and an increase in the degree of recirculation could be observed in the period after. This means that none of the drum filters were self-cleaning, regardless of the diet. However, the results show no significant difference in the degree of recirculation between the two diets.
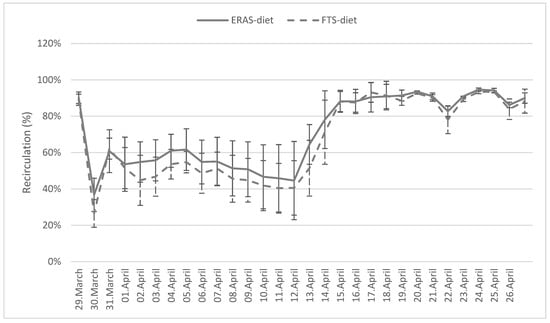
Figure 3.
Daily average degree of recirculation for RAS units with Atlantic salmon post-smolt fed an ERAS diet and an FTS diet in a period of four weeks. From 12 April, manual drum filter cleaning was initiated on a daily basis. Data are expressed as average ± SD (n = 3).
The fecal analysis showed that the ERAS diet had a significantly lower probability (approx. 89%) of generating feces particles smaller than 50 μm compared with the FTS diet (approx. 93%) (Figure 4). This translates to a significant higher fecal stability with the ERAS diet.
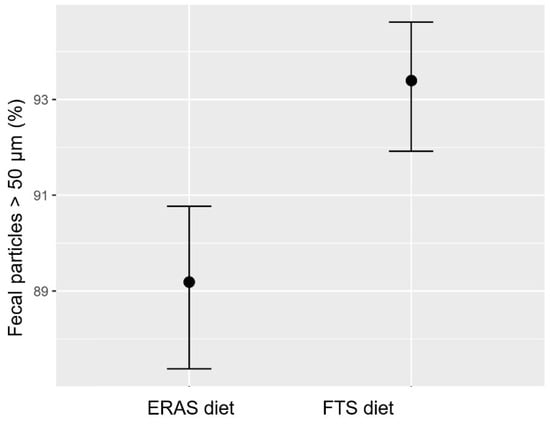
Figure 4.
Mastersizer comparison of fecal stability for the ERAS diet and FTS diet. Expressed as percentage probability of generating particles less than 50 μm when passing through a drum filter ± SD (n = 3). The ci are calculated by simulation and denote the 95% ci for the mean of each sample.
3.4. Total Mineral and Metal Accumulation
The mineral and metal analysis (Table 4), TP, DP, PO4, Ca, Mg, and total Zn, showed a significant increase in accumulation from the initial to the final sample for both diets. However, there was only a significantly lower accumulation of TP, DP and PO4 in the RAS units fed the ERAS diet. For total Fe, the initial sample was below the determination limit, so accumulation could not be determined.

Table 4.
Mineral and metal accumulation (µg L−1) in production water of Atlantic salmon (Salmo salar) post-smolts reared in RAS over four weeks at 13 °C and fed either ERAS diet or FTS diet. Data are expressed as average ± SD (n = 3). Data were analyzed by two-way ANOVA (p < 0.05) with diets as one variable and time as the other. The p values are presented between diet, between time (initial vs. final) and interaction (diet × time).
The first principal component accounts for 59.0% of the total variance (Figure 5). The second principal component accounts for 23.9% of the total variance. The PCA for the mineral and metal accumulation shows that three different groups were connected. The first group encompassed the dissolved Zn and Fe, and the second group encompassed the Ca, Mg, and total Zn. The third and last group encompassed the various P components.
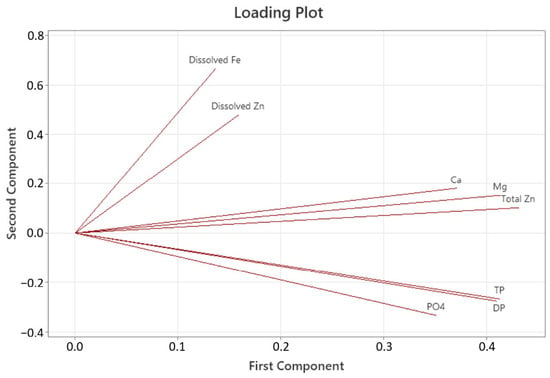
Figure 5.
The principal component analysis loading plot shows the connection for different minerals in the RAS units for the initial and final sampling.
4. Discussion
The pre-trial was used to screen the system for an optimal sampling location in the RAS. The results show low variation of mineral distribution among the different sampling locations. These results are not in line with previous findings as solid particles larger than 30 μm can be relatively easily removed with mechanical filters [46,47], while smaller particles under 30 μm have a residence time in the system which is inversely proportional to removal efficiency and water exchange rates [48,49,50]. It is also established that the size of particles can affect the amount of mineral bound within the particle itself [22], thus determining the effect of removal rate. Based on this effect, some sort of variation was expected between the different water treatment steps. Therefore, this low variation in mineral distribution within the system was unexpected. Nevertheless, the chosen sampling location for the current trial was also in line with previous studies investigating total mineral accumulation [33,34] and therefor strengthens the results found in the main trial.
The fish performance in the main trial showed no difference between the two dietary adaptations and is in line with internal expectations and studies showing equal performance with varying dietary ratios of energy and protein for salmonids [51,52]. Despite the short duration of the trial and 63% increase in body weight, the results indicate that the growth conditions and fish performance were as expected and are valuable contributions to the study.
An immediate and unexpected increase in water usage for each RAS unit was observed at the start of the trial, and subsequently, a reduction in degree of recirculation. This was due to a partial clogging of the drum filters caused by suboptimal automatic back washing of the microscreen in the drum filter, affecting each individual RAS unit regardless of diet. To increase the degree of recirculation and to maintain operating conditions, it was decided to implement one daily manual cleaning of the drum filters from 12 April (Figure 3). All RAS units were thereafter cleaned identically with a high-pressure washer on a daily basis. The new routine was successful and resulted in a relatively stable degree of recirculation (90%) from April 15th and to the end of the experiment. Total water consumption and feed pr. L showed a high standard deviation for both diets. This was most likely connected to the initial drop in the recirculation rate since some RAS units (independent of diet) had a higher drop than others. Though there was an unexpected issue with the drum filter that lasted for half of the study, it occurred similarly for both dietary adaptations. Therefore, the results are determined to be a representative comparison.
The significant differences observed in the accumulation of TAN and NO2− between the ERAS diet and the FTS diet (−18 and −42%, respectively) can partly be explained by the dietary composition. As ammonia is the end product of protein catabolism, lowering the dietary protein will have a significant effect on the emission of TAN if the nutritional digestibility is maintained [6,25,53,54,55]. Additionally, the nitrification efficiency for TAN and NO2− by the biofilter, in this experiment, could have been affected by pH, alkalinity and C/N ratios. The optimum pH for nitrification can range from 7.0 to 9.0, with different optimum pH levels for Nitrosomonas and Nitrobacter (7.2 to 8.8 and 7.2 to 9.0) [37]. The FTS diet resulted in a lower pH compared with the ERAS diet. The low pH favors the reduction of the toxicity of unionized ammonia, while a higher pH is suggestive of a more optimal biofilter efficiency, which favors the FTS diet [37]. The production water for the ERAS diet had higher alkalinity compared with the FTS diet. Nevertheless, both diets were well over the suggested alkalinity of 75 mg L−1 required to maintain the maximum nitrification rate in municipal wastewater treatments [56]. Alkalinity is, however, proposed to be better at higher levels of 200 mg L−1 for systems with minimal water exchange rates when considering possible stratification of alkalinity [37].
The C/N ratio in water is also linked to nitrification efficiency, where the TAN removal efficiency is lower at a higher C/N ratio [38]. The ERAS diet was formulated with a higher C/N ratio than the FTS diet (7% and 6%, respectively). However, the results show that the ERAS diet possessed increased fecal stability, prompting more efficient removal from the water treatment loop. This attribute is supported by the drum filter activation count, where the results indicate that feces from the ERAS diet were more efficiently removed from the drum filter. Thus, the filters did not operate as often as for the FTS diet. TOC is primarily present in organic waste, such as feces. Hence, removing more significant amounts of fecal particles will reduce the amount of C accumulating within a system. Though the ERAS diet had an initially higher C/N ratio, the attributes of the RAS feed could ensure lower accumulation of C in the production water and thus also a lower C/N ratio in the water, which is proven to be beneficial for the nitrification efficiency of the biological filter [38].
Accumulation of minerals and metals in RAS has now been documented in several studies [33,34,57,58,59] and it is known that the main reason for accumulation in RAS is linked to the recycling loop of water. However, the current study shows a significant difference in the accumulative tendencies depending on the dietary adaptation. Though the ERAS diet contained 1.3 g kg−1 more TP than the FTS diet, it resulted in approximately 90% less TP and DP accumulation in the water of the RAS units. The availability of P from the different raw material sources also determines total accumulation. The FTS diet had SPC as a primary plant ingredient, and this plant product is known to have a high phytic acid content, where the bound P molecules are estimated to have 0% availability [30,60]. This is in comparison with the sunflower, which is used as a primary plant ingredient for the ERAS diet, for which phytic acid content is generally low [61]. Different fish meals also differentiate in levels of available P (reviewed by: [62,63]), where fish meal from the Blue whiting is known to have a lower P availability compared with other species [64,65]. However, for the current trial, both diets were formulated with an LT fish meal, and there were no indications that the FM would affect the FTS diet negatively. The fish meal for the ERAS diet possessed a higher amount of Blue whiting, and it is therefore unlikely that P availability of the FM source was a main determining factor for the different accumulative levels. A bigger effect is more likely to have been caused by the inorganic P sources used in the different diet formulation. The MCP used for the ERAS diet has previously been shown to generate a higher amount of particle-bound P due to lower availability compared with MAP and MSP [32]. Though MCP will increase the total P of a diet, it will have a positive effect on the soluble and solid N discharge fractions compared with MAP where the fish is more likely to release a higher amount of undigested ammonium through feces [32]. The current study also supports the idea that an ERAS diet utilizing MCP will be able to increase the removal efficiency of P by the drum filter, thus also reducing the amount of P emitted from a facility, as long as water treatment by drum filter is applied. To further optimize an RAS-adapted diet and reduce the overall amount of total P within a diet, without potentially increasing N emissions, MSP may be a better alternative as an inorganic P source, when compared with MCP and MAP [32].
Interestingly, the total accumulation did not seem to differentiate between TP and DP for any of the RAS units given the ERAS diet or the FTS diet, which means that statistically, it is impossible to distinguish between TP and DP. Because the fecal stability and total accumulation were significantly different between the two diets, it is assumed that the ERAS diet was able to maintain a higher amount of particle-bound P in the feces due to the fecal binding effect of the raw material composition for this diet, thus prompting a significantly higher removal rate compared with the FTS diet [22,23]. This assumption is supported by previous findings, showing that P not accumulated within the fish are either found in the process water or in the sludge [66]. It is known that a highly intensive RAS that breaks down particles to 30 μm or less will render mechanical filtration less effective [67]. Because there was no difference between TP and DP, this indicates that the P left in the recirculation loop in the current trial was most likely 30 μm or less and is therefore impossible to remove by the drum filter and, due to this, accumulates over time. By not using diets specifically designed for production in RAS, the other reasonable ways to remove excess or unwanted phosphorus from the production water in commercial productions would be through precipitation or enhanced biological phosphorus removal (EBPR) [11,68]. However, this method will increase both investment, running and production costs for the production facility. Based on the current results, it can therefore be concluded that P precipitation is more likely to be required for systems fed a non-RAS diet, like the FTS diet used in this trial. For efficient removal of the P compounds excreted from fish, particle organic phosphorus (POP), dissolved organic phosphorus (DOP), and dissolved inorganic phosphorus (DIP) [69,70], a combination of mechanical, chemical and biological treatment is recommended [11]. Chemical separation of dissolved P involves precipitation and is a widespread method for wastewater treatment. Typically, aluminum, ferric chloride, or Ca ions are used [71], but adding lime in combination with high pH will also result in precipitation of dissolved P [11]. The added chemicals are rendered non-toxic to the fish due to the recirculation of water. P can also be removed by passing the water through a column that contains an absorption material, causing the dissolved P to be partly absorbed [11]. The microorganisms in the biofilter can also reduce the total accumulation of P in RAS. Some bacteria and algae are able to assimilate dissolved P and convert it for use in membranes, DNA, and ATP/ADP [72].
The biofilter bacteria Nitrosomonas and Nitrobacter need inorganic P to grow and develop [73]. The current trial seemed to indicate a trend in which the FTS diet accumulated higher levels of PO4 compared with the ERAS diet. The tanks fed the FTS diet showed an average accumulation of 1960 μm L−1. The observed high standard deviation (±950 μm L−1) may be caused by the differences in the average degree of recirculation, where two RAS units were able to recycle a respective 64% and 65%, while the last RAS unit recycled 71% of the total water. The ERAS diet on the other hand, showed a decumulation of −70 μm L−1 of PO4, meaning that the final samples showed a lower degree of PO4 in the water compared with the initial sample (in two of the three RAS units on the ERAS diet). The high standard deviation for the dietary adaptations may also indicate potential differences in the bacterial communities of the respective units. The current trial was conducted with brackish water, and research has shown differences between microbial communities in fresh, brackish, and seawater [74,75,76]. But this diversity amongst the microbial community seems to be higher in fresh water and in seawater, when compared with brackish water [75,76,77]. This indicates that the current trial setup should be the more optimal choice. The trial setup utilized three separate units for each dietary adaptation, and each biofilter represents a separate microbial community, meaning that slight differences between units are likely to occur, regardless of dietary adaptation.
By running a PCA it was possible to investigate which of the minerals and metals that followed similar trends and was grouped together (Figure 5). This PCA showed that TP, DP, and PO4 are highly correlated in their accumulative behavior, thus suggesting that that the accumulated PO4 would, similarly to TP and DP, be higher for the tanks receiving the FTS diet. The PCA additionally showed that the total level of Ca, Mg, and Zn share the same accumulative tendencies. This connection is believed to be connected to the inclusion of seawater. Seawater is rich in both Ca and Mg, and the results from the trial indicate no significant differences in the accumulation of Ca and Mg between diets, only from the initial to the final sampling. The ERAS diet contained more Ca and less Mg than the FTS diet, but the initial and final sampling showed almost an identical increase between the two diets. This strongly indicates that the gradual increase in water use as the fish grew mainly affected the Ca and Mg accumulation.
5. Conclusions
To our knowledge, this is the first study with tank replication that investigates the differences in accumulative tendencies between an RAS-adapted diet and an FTS diet in RAS. Though both diets will accumulate minerals and eventually lead to a saturation in the water, it is evident that a diet adapted for production in RAS will have a lower accumulation rate than a non-adapted diet in these systems. Therefore, we can conclude that an RAS-adapted diet that matches the energy requirement of today’s commercial flow-through diets will generate better water quality and increased system performance while still securing satisfactory growth and condition. The reduction of N and P emissions using an RAS-adapted diet can also significantly reduce the waste impact of the aquaculture industry, bringing the total emitted levels closer to the emission levels of the rest of the Norwegian industry. With this trial, it is evident that diet adaptation towards the production system and technology is one crucial factor in reducing local pollution and securing sustainable growth for the aquaculture industry in the future.
Author Contributions
V.Ø.F. designed and executed the study. He was also involved in the analysis and interpretation of the results, statistical analysis and writing of the manuscript. J.V. was involved in running the trial and data analysis. T.C.-R. was involved in design, interpretation of results, statistical analysis, and writing of manuscript. T.G. was involved in the statistical analysis of the study. J.Ø.H. was involved in interpretation of results and writing of manuscript. O.-I.L. was involved in interpretation of the results, writing of manuscript and supervision of the study. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Research Council of Norway, grant number 329419.
Institutional Review Board Statement
The experiment was performed according to the guidelines and protocols approved by the European Union (EU Council 86/609; D.L. 27 January 1992, no. 116) and by the National Guidelines for Animal Care and Welfare published by the Norwegian Ministry of Education and Research.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy restrictions.
Conflicts of Interest
Authors Vegard Øvstetun Flo, Thomas Cavrois-Rogacki, Jon Øvrum Hansen, Jannicke Vigen, Thomas Gitlesen were employed by the company Cargill. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Olaussen, J.O. Environmental problems and regulation in the aquaculture industry. Insights from Norway. Mar. Policy 2018, 98, 158–163. [Google Scholar] [CrossRef]
- Ackefors, H.; Enell, M. The release of nutrients and organic matter from aquaculture systems in Nordic countries. J. Appl. Ichthyol. 1994, 10, 225–241. [Google Scholar] [CrossRef]
- Ackefors, H.; Enell, M. Discharge of nutrients from Swedish fish farming to adjacent sea areas. Ambio 1990, 19, 28–35. [Google Scholar]
- Enell, M.; Löf, J. Environmental impact of aquaculture-sedimentation and nutrient loadings from fish cage culture farming. Vatten 1983, 39, 364–375. [Google Scholar]
- Røsvik, I.O. Biology for Aquaculture; Landbruksforlaget: Oslo, Norway, 1993; p. 183. [Google Scholar]
- Einen, O.; Mørkøre, T. Feeding Theory for Aquaculture; Landbruksforlaget: Oslo, Norway, 1996. [Google Scholar]
- Aas, T.S.; Åsgård, T.; Ytrestøyl, T. Utilization of feed resources in the production of Atlantic salmon (Salmo salar) in Norway: An update for 2020. Aquac. Rep. 2022, 26, 101316. [Google Scholar] [CrossRef]
- Aas, T.S.; Ytrestøyl, T.; Åsgård, T. Utilization of feed resources in the production of Atlantic salmon (Salmo salar) in Norway: An update for 2016. Aquac. Rep. 2019, 15, 100216. [Google Scholar] [CrossRef]
- Tande, T. Land i sikte. In Norsk Fiskerinæring, nr. 4; Norsk Fiskerinæring AS: Råholt, Norway, 2022; pp. 138–219. Available online: https://norskfisk.no/wp-content/uploads/2022/06/Land-i-sikte_SeafoodTalks2022.pdf (accessed on 4 March 2024).
- Losordo, T.M.; Hobbs, A.O.; DeLong, D.P. The design and operational characteristics of the CP&L/EPRI fish barn: A demonstration of recirculating aquaculture technology. Aquac. Eng. 2000, 22, 3–16. [Google Scholar] [CrossRef]
- Lekang, O.-I. Aquaculture Engineering, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Shnel, N.; Barak, Y.; Ezer, T.; Dafni, Z.; van Rijn, J. Design and performance of a zero-discharge tilapia recirculating system. Aquac. Eng. 2002, 26, 191–203. [Google Scholar] [CrossRef]
- Rakocy, J.E.; Masser, M.P.; Losordo, T.M. Recirculating Aquaculture Tank Production Systems: Aquaponics-Integrating Fish and Plant Culture; Oklahoma Cooperative Extension Service: Stillwater, OK, USA, 2016. [Google Scholar]
- Roque D’Orbcastel, E.; Blancheton, J.-P.; Belaud, A. Water quality and rainbow trout performance in a Danish Model Farm recirculating system: Comparison with a flow through system. Aquac. Eng. 2009, 40, 135–143. [Google Scholar] [CrossRef]
- True, B.; Johnson, W.; Chen, S. Reducing phosphorus discharge from flow-through aquaculture I: Facility and effluent characterization. Aquac. Eng. 2004, 32, 129–144. [Google Scholar] [CrossRef]
- Summerfelt, S.T.; Vinci, B.J. Better management practices for recirculating aquaculture systems. In Environmental Best Management Practices for Aquaculture, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 389–426. [Google Scholar] [CrossRef]
- Ahmed, N.; Turchini, G.M. Recirculating aquaculture systems (RAS): Environmental solution and climate change adaptation. J. Clean. Prod. 2021, 297, 126604. [Google Scholar] [CrossRef]
- Klinger, D.; Naylor, R. Searching for solutions in aquaculture: Charting a sustainable course. Annu. Rev. Environ. Resour. 2012, 37, 247–276. [Google Scholar] [CrossRef]
- Nutra RC for Atlantic Salmon 27. Available online: https://www.skretting.com/en/feed-for-aquaculture/nutra-rc-for-atlantic-salmon-27/ (accessed on 1 May 2024).
- Orbit. Available online: https://www.biomar.com/feed-and-services/feed-solutions/orbit (accessed on 1 May 2024).
- Palm, H.; Knaus, U.; Wasenitz, B.; Bischoff, A.; Strauch, S. Proportional up scaling of African catfish (Clarias gariepinus Burchell, 1822) commercial recirculating aquaculture systems disproportionally affects nutrient dynamics. Aquaculture 2018, 491, 155–168. [Google Scholar] [CrossRef]
- Brinker, A.; Koppe, W.; Rösch, R. Optimised effluent treatment by stabilised trout faeces. Aquaculture 2005, 249, 125–144. [Google Scholar] [CrossRef]
- Brinker, A. Guar gum in rainbow trout (Oncorhynchus mykiss) feed: The influence of quality and dose on stabilisation of faecal solids. Aquaculture 2007, 267, 315–327. [Google Scholar] [CrossRef]
- Davidson, J.; Good, C.; Barrows, F.T.; Welsh, C.; Kenney, P.B.; Summerfelt, S.T. Comparing the effects of feeding a grain-or a fish meal-based diet on water quality, waste production, and rainbow trout Oncorhynchus mykiss performance within low exchange water recirculating aquaculture systems. Aquac. Eng. 2013, 52, 45–57. [Google Scholar] [CrossRef]
- Hardy, R.W.; Kaushik, S.J. Fish Nutrition; Academic Press: New York, NY, USA, 2021. [Google Scholar]
- Cho, C.; Bureau, D. A review of diet formulation strategies and feeding systems to reduce excretory and feed wastes in aquaculture. Aquac. Res. 2001, 32, 349–360. [Google Scholar] [CrossRef]
- Kaushik, S.J.; de Oliva Teles, A. Effect of digestible energy on nitrogen and energy balance in rainbow trout. Aquaculture 1985, 50, 89–101. [Google Scholar] [CrossRef]
- Kaushik, S.; Cowey, C. Dietary factors affecting nitrogen excretion by fish. In 1. International Symposium; Fish Nutrition Research Laboratory: Guelph, ON, Canada, 1990; Available online: https://hal.inrae.fr/hal-02774770 (accessed on 1 May 2024).
- Skoglund, E.; Carlsson, N.-G.; Sandberg, A.-S. Phytate. In Analysis of Bioactive Components in Small Grain Cereals; Shewry, P.R., Ward, J.L., Eds.; AACC International Incorporated: St. Paul, MN, USA, 2009; pp. 129–139. [Google Scholar]
- Hua, K.; Bureau, D.P. Modelling digestible phosphorus content of salmonid fish feeds. Aquaculture 2006, 254, 455–465. [Google Scholar] [CrossRef]
- Lazzari, R.; Baldisserotto, B. Nitrogen and phosphorus waste in fish farming. Bol. Inst. Pesca 2008, 34, 591–600. [Google Scholar]
- Morales, G.A.; Azcuy, R.L.; Casaretto, M.E.; Márquez, L.; Hernández, A.J.; Gómez, F.; Koppe, W.; Mereu, A. Effect of different inorganic phosphorus sources on growth performance, digestibility, retention efficiency and discharge of nutrients in rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 495, 568–574. [Google Scholar] [CrossRef]
- Davidson, J.; Good, C.; Welsh, C.; Brazil, B.; Summerfelt, S. Heavy metal and waste metabolite accumulation and their potential effect on rainbow trout performance in a replicated water reuse system operated at low or high system flushing rates. Aquac. Eng. 2009, 41, 136–145. [Google Scholar] [CrossRef]
- Davidson, J.; Good, C.; Welsh, C.; Summerfelt, S. The effects of ozone and water exchange rates on water quality and rainbow trout Oncorhynchus mykiss performance in replicated water recirculating systems. Aquac. Eng. 2011, 44, 80–96. [Google Scholar] [CrossRef]
- Martins, C.I.; Eding, E.H.; Verreth, J.A. The effect of recirculating aquaculture systems on the concentrations of heavy metals in culture water and tissues of Nile tilapia Oreochromis niloticus. Food Chem. 2011, 126, 1001–1005. [Google Scholar] [CrossRef]
- van Bussel, C.G.; Schroeder, J.P.; Mahlmann, L.; Schulz, C. Aquatic accumulation of dietary metals (Fe, Zn, Cu, Co, Mn) in recirculating aquaculture systems (RAS) changes body composition but not performance and health of juvenile turbot (Psetta maxima). Aquac. Eng. 2014, 61, 35–42. [Google Scholar] [CrossRef]
- Chen, S.; Ling, J.; Blancheton, J.-P. Nitrification kinetics of biofilm as affected by water quality factors. Aquac. Eng. 2006, 34, 179–197. [Google Scholar] [CrossRef]
- Michaud, L.; Blancheton, J.-P.; Bruni, V.; Piedrahita, R. Effect of particulate organic carbon on heterotrophic bacterial populations and nitrification efficiency in biological filters. Aquac. Eng. 2006, 34, 224–233. [Google Scholar] [CrossRef]
- Wang, X.; Andresen, K.; Handå, A.; Jensen, B.; Reitan, K.I.; Olsen, Y. Chemical composition and release rate of waste discharge from an Atlantic salmon farm with an evaluation of IMTA feasibility. Aquac. Environ. Interact. 2013, 4, 147–162. [Google Scholar] [CrossRef]
- NS-EN 872:2005; Water quality—Determination of suspended solids—Method by filtration through glass fibre filters. ISO Standards: Geneva, Switzerland, 2005.
- NS-EN ISO 15681-2:2018; Water quality—Determination of orthophosphate and total phosphorus contents by flow analysis (FIA and CFA), Part 2: Method by continuous flow analysis (CFA). ISO Standards: Geneva, Switzerland, 2018.
- SS-EN ISO 15587-2:2002; Water quality—Digestion for the determination of selected elements in water, Part 2: Nitric acid digestion. ISO Standards: Geneva, Switzerland, 2002.
- SS-EN ISO 17294-2:2016; Water quality—Application of inductively coupled plasma mass spectrometry (ICP-MS), Part 2: Determination of selected elements including uranium isotopes. ISO Standards: Geneva, Switzerland, 2016.
- Zar, J.H. Biostatistical Analysis; Pearson Education India: Delhi, India, 1999. [Google Scholar]
- Minitab, LLC. 2021. Available online: https://www.minitab.com (accessed on 1 May 2024).
- Langer, J.; Efthimiou, S.; Rosenthal, H.; Bronzi, P. Drum filter performance in a recirculating eel culture unit. J. Appl. Ichthyol. 1996, 12, 61–65. [Google Scholar] [CrossRef]
- Davidson, J.; Summerfelt, S.T. Solids removal from a coldwater recirculating system—Comparison of a swirl separator and a radial-flow settler. Aquac. Eng. 2005, 33, 47–61. [Google Scholar] [CrossRef]
- Chen, S.; Timmons, M.B.; Aneshansley, D.J.; Bisogni Jr, J.J. Suspended solids characteristics from recirculating aquacultural systems and design implications. Aquaculture 1993, 112, 143–155. [Google Scholar] [CrossRef]
- Patterson, R.N.; Watts, K.C.; Timmons, M.B. The power law in particle size analysis for aquacultural facilities. Aquac. Eng. 1999, 19, 259–273. [Google Scholar] [CrossRef]
- Pfeiffer, T.J.; Osborn, A.; Davis, M. Particle sieve analysis for determining solids removal efficiency of water treatment components in a recirculating aquaculture system. Aquac. Eng. 2008, 39, 24–29. [Google Scholar] [CrossRef]
- Karalazos, V.; Bendiksen, E.; Dick, J.R.; Bell, J.G. Effects of dietary protein, and fat level and rapeseed oil on growth and tissue fatty acid composition and metabolism in Atlantic salmon (Salmo salar L.) reared at low water temperatures. Aquac. Nutr. 2007, 13, 256–265. [Google Scholar] [CrossRef]
- Hillestad, M.; Johnsen, F. High-energy/low-protein diets for Atlantic salmon: Effects on growth, nutrient retention and slaughter quality. Aquaculture 1994, 124, 109–116. [Google Scholar] [CrossRef]
- Randall, D.J.; Wright, P.A. Ammonia distribution and excretion in fish. Fish Physiol. Biochem. 1987, 3, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Rychly, J. Nitrogen balance in trout: II. Nitrogen excretion and retention after feeding diets with varying protein and carbohydrate levels. Aquaculture 1980, 20, 343–350. [Google Scholar] [CrossRef]
- Beamish, F.; Thomas, E. Effects of dietary protein and lipid on nitrogen losses in rainbow trout, Salmo gairdneri. Aquaculture 1984, 41, 359–371. [Google Scholar] [CrossRef]
- Gujer, W.; Boller, M. Design of a nitrifying tertiary trickling filter based on theoretical concepts. Water Res. 1986, 20, 1353–1362. [Google Scholar] [CrossRef]
- Ebeling, J.M.; Sibrell, P.L.; Ogden, S.R.; Summerfelt, S.T. Evaluation of chemical coagulation–flocculation aids for the removal of suspended solids and phosphorus from intensive recirculating aquaculture effluent discharge. Aquac. Eng. 2003, 29, 23–42. [Google Scholar] [CrossRef]
- Martins, C.I.; Ochola, D.; Ende, S.S.; Eding, E.H.; Verreth, J.A. Is growth retardation present in Nile tilapia Oreochromis niloticus cultured in low water exchange recirculating aquaculture systems? Aquaculture 2009, 298, 43–50. [Google Scholar] [CrossRef]
- Martins, C.I.; Pistrin, M.G.; Ende, S.S.; Eding, E.H.; Verreth, J.A. The accumulation of substances in Recirculating Aquaculture Systems (RAS) affects embryonic and larval development in common carp Cyprinus carpio. Aquaculture 2009, 291, 65–73. [Google Scholar] [CrossRef]
- Deak, N.A.; Johnson, L.A. Fate of phytic acid in producing soy protein ingredients. J. Am. Oil Chem. Soc. 2007, 84, 369–376. [Google Scholar] [CrossRef]
- Saeed, M.; Cheryan, M. Sunflower protein concentrates and isolates’ low in polyphenols and phytate. J. Food Sci. 1988, 53, 1127–1131. [Google Scholar] [CrossRef]
- Lall, S. Digestibility, Metabolism and Excretion of Dietary Phosphorus in Fish. In Proceedings of the First International Symposium on Nutritional Strategies in Management of Aquaculture Waste; University of Guelph: Guelph, ON, Canada, 1991. [Google Scholar]
- Riche, M.; Brown, P.B. Availability of phosphorus from feedstuffs fed to rainbow trout, Oncorhynchus mykiss. Aquaculture 1996, 142, 269–282. [Google Scholar] [CrossRef]
- Albrektsen, S.; Hope, B.; Aksnes, A. Phosphorous (P) deficiency due to low P availability in fishmeal produced from blue whiting (Micromesistius poutassou) in feed for under-yearling Atlantic salmon (Salmo salar) smolt. Aquaculture 2009, 296, 318–328. [Google Scholar] [CrossRef]
- Fjelldal, P.G.; Hansen, T.; Albrektsen, S. Inadequate phosphorus nutrition in juvenile Atlantic salmon has a negative effect on long-term bone health. Aquaculture 2012, 334, 117–123. [Google Scholar] [CrossRef]
- Strauch, S.M.; Wenzel, L.C.; Bischoff, A.; Dellwig, O.; Klein, J.; Schüch, A.; Wasenitz, B.; Palm, H.W. Commercial African catfish (Clarias gariepinus) recirculating aquaculture systems: Assessment of element and energy pathways with special focus on the phosphorus cycle. Sustainability 2018, 10, 1805. [Google Scholar] [CrossRef]
- Summerfelt, S. Solids Capture. In The Yellow Book of Recirculating Aquaculture, 5th ed.; Timmons, M.B., Vinci, B.J., Eds.; Ithaca Publishing Company LLC: Ithaca, NY, USA, 2022; pp. 147–200. [Google Scholar]
- Schleyken, J.; Gumpert, F.; Tränckner, S.; Palm, H.; Tränckner, J. Enhanced chemical recovery of phosphorus from residues of recirculating aquaculture systems (RAS). Int. J. Environ. Sci. Technol. 2023, 21, 3775–3778. [Google Scholar] [CrossRef]
- Uglem, I.; Järnegren, J.; Bloecher, N. Effekter av Organisk Utslipp fra Havbruk i Norge—En Kunnskapsoppsummering; Norsk Institutt for Naturforskning (NINA): Trondheim, Norway, 2020; Available online: https://hdl.handle.net/11250/2684245 (accessed on 7 February 2024).
- Wetzel, R.G. Limnology: Lake and River Ecosystems; Gulf Professional Publishing: Houston, TX, USA, 2001. [Google Scholar]
- Boyd, C.E. Practical aspects of chemistry in pond aquaculture. Prog. Fish-Cult. 1997, 59, 85–93. [Google Scholar] [CrossRef]
- Kroiss, H.; Rechberger, H.; Egle, L. Phosphorus in water quality and waste management. In Integrated Waste Management-Volume II; IntechOpen: London, UK, 2011; pp. 181–214. [Google Scholar]
- Meiklejohn, J. Minimum phosphate and magnesium requirements of nitrifying bacteria. Nature 1952, 170, 1131. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, T.C.; del Giorgio, P.A. Compositional changes in free-living bacterial communities along a salinity gradient in two temperate estuaries. Limnol. Oceanogr. 2002, 47, 453–470. [Google Scholar] [CrossRef]
- Herlemann, D.P.; Labrenz, M.; Jürgens, K.; Bertilsson, S.; Waniek, J.J.; Andersson, A.F. Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J. 2011, 5, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.J.; Kirchman, D.L. Bacterial diversity, community structure and potential growth rates along an estuarine salinity gradient. ISME J. 2013, 7, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Bakke, I.; Åm, A.L.; Kolarevic, J.; Ytrestøyl, T.; Vadstein, O.; Attramadal, K.J.K.; Terjesen, B.F. Microbial community dynamics in semi-commercial RAS for production of Atlantic salmon post-smolts at different salinities. Aquac. Eng. 2017, 78, 42–49. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).