A Comparison of Polyvalent Passive Immunoprotection from Antibodies with Different Immunity Models of Live or Inactivated Vibrio fluvialis in Fish
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Animals
2.2. Preparation of the Mouse Antisera
2.3. Passive and Passive Cross-Protective Rates of the Mouse Antisera
2.4. Interaction between Mouse Antisera and Bacteria
2.5. Kidney Bacterial Content
2.6. White Blood Cell Phagocytosis Analysis
2.7. Organizational Pathological Analysis
2.8. Kidney Immunofluorescence Analysis
2.9. Antioxidant Factor Activity Analysis
2.10. mRNA Expression of Inflammatory Factors
2.11. Protein Chip Array to Assess the Outer Membrane Protein Antibodies in the Mouse Antisera
3. Results
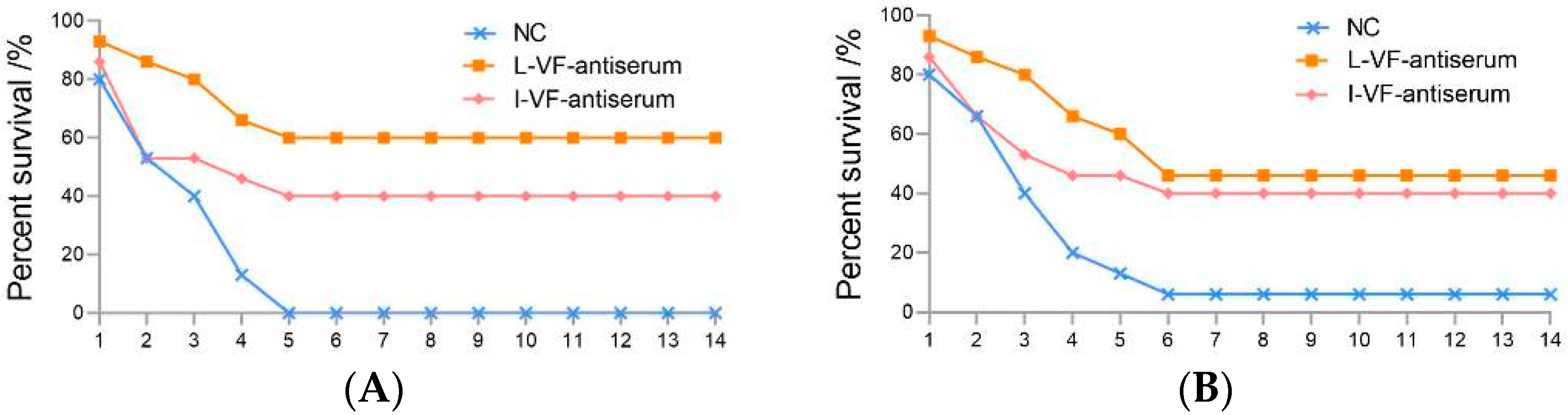
3.1. Passive and Passive Cross-Protective Rates of the Mouse Antisera in C. auratus
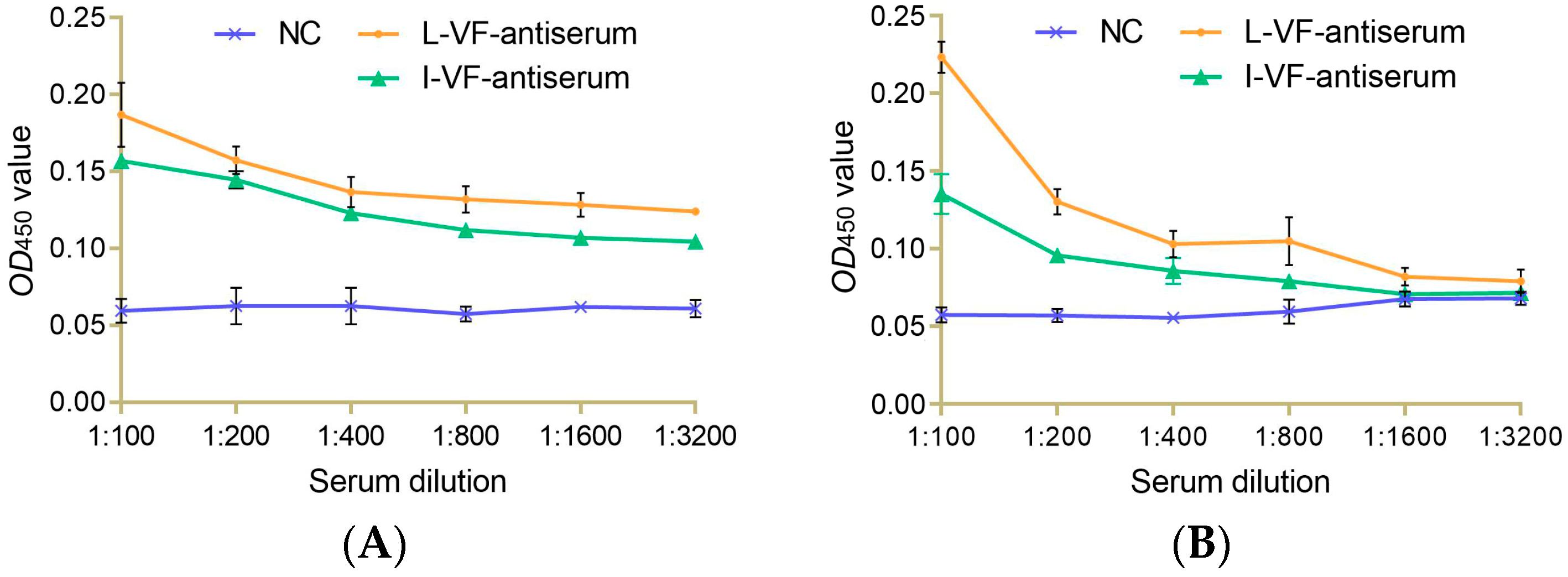
3.2. Immune Binding Capacity between the Mouse Antisera and Bacteria In Vitro
3.3. Bacterial Count in the Kidneys of the C. auratus
3.4. The Phagocytic Activity of C. auratus Leukocytes
3.5. Pathological Observation of the Visceral Tissue Morphology in C. auratus
3.6. Immunofluorescence of p53 and γH2A.X in the C. auratus’ Kidneys
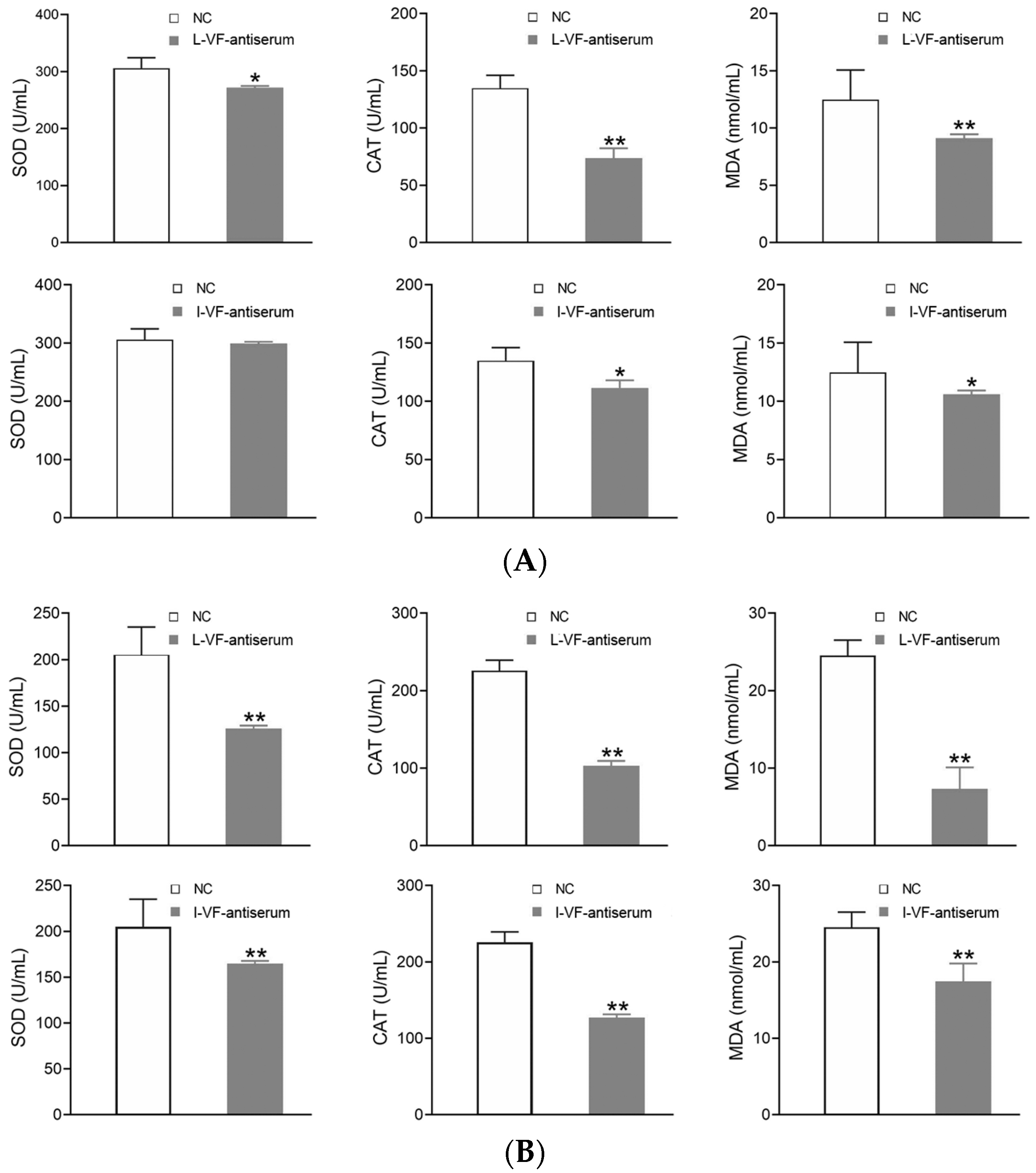
3.7. Detection of the Antioxidant Factors’ Activity in the C. auratus Serum
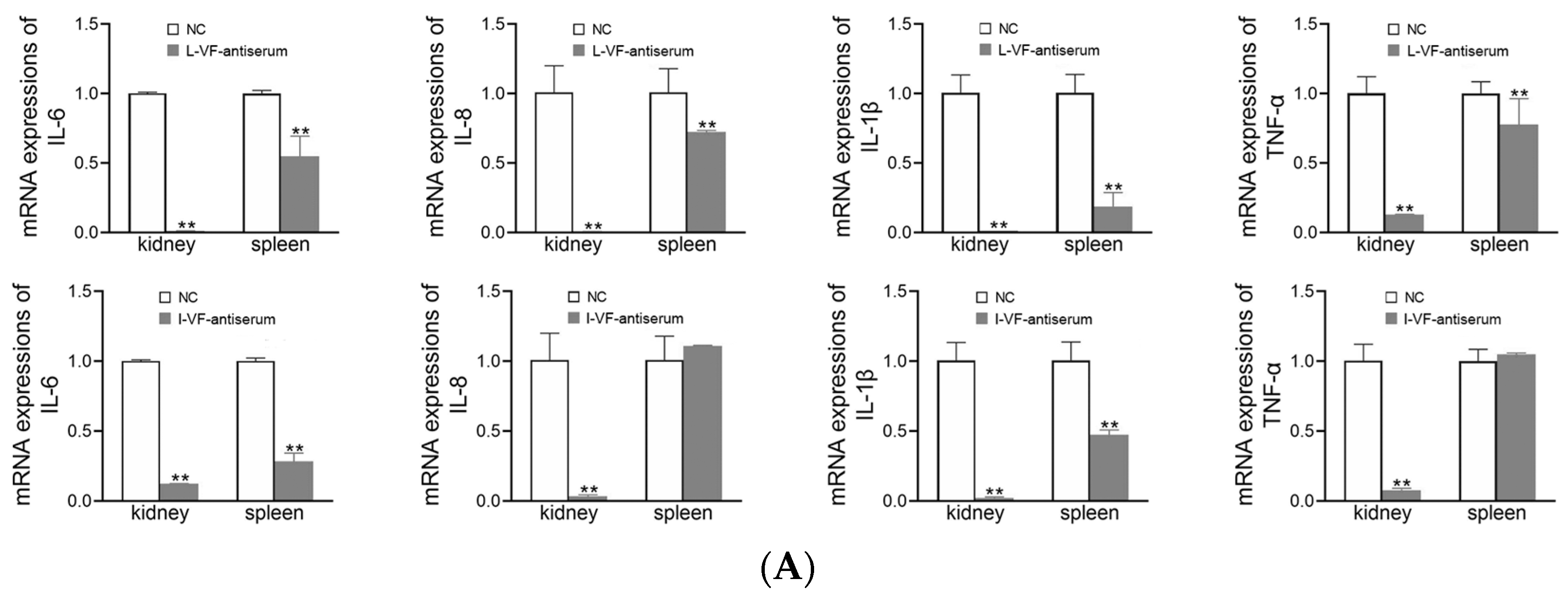
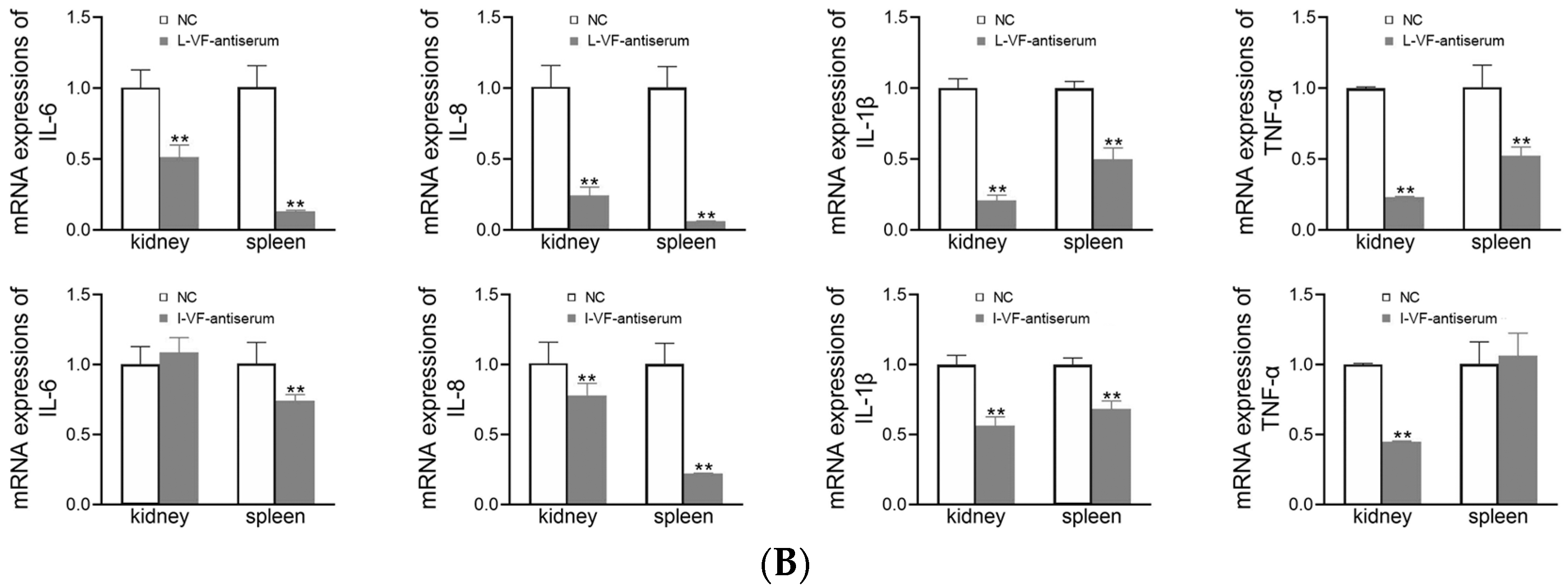
3.8. Inflammatory Gene Expression in C. auratus
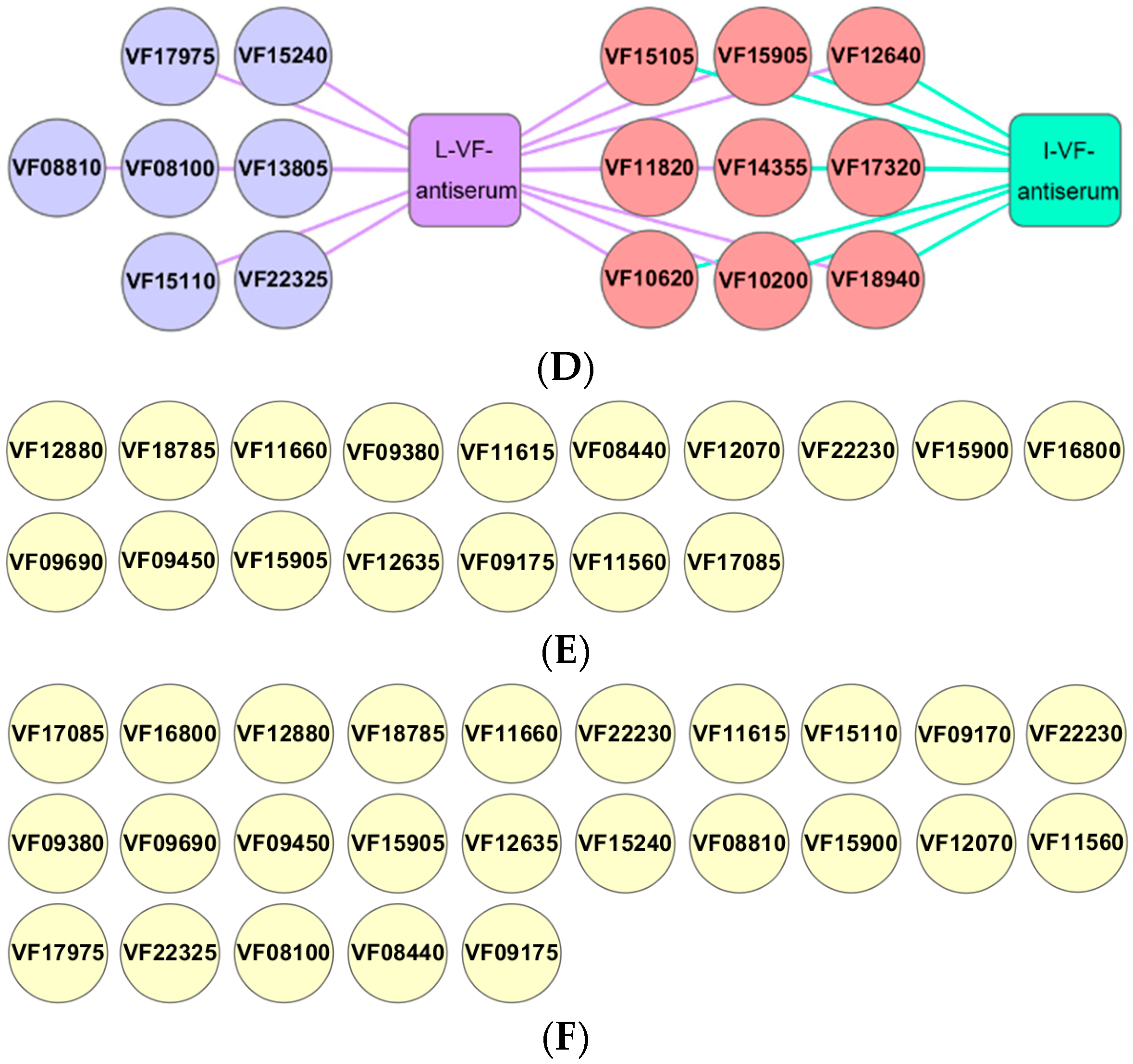
3.9. Differences in the Antibody Responses of the Outer Membrane Proteins Induced by Live and Inactivated V. fluvialis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Volpe, E.; Errani, F.; Zamparo, S.; Ciulli, S. Redspotted grouper nervous necrosis virus and the reassortant RGNNV/SJNNV in vitro susceptibility against a commercial peroxy-acid biocide under different conditions of use. Vet. Sci. 2023, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Abioye, O.E.; Osunla, C.A.; Nontongana, N.; Okoh, A.I. Occurrence of virulence determinants in Vibrio cholerae, Vibrio mimicus, Vibrio alginolyticus, and Vibrio parahaemolyticus isolates from important water resources of Eastern Cape, South Africa. BMC Microbiol. 2023, 23, 316. [Google Scholar] [CrossRef] [PubMed]
- De Silva, L.A.D.S.; Heo, G.J. Biofilm formation of pathogenic bacteria isolated from aquatic animals. Arch. Microbiol. 2022, 205, 36. [Google Scholar] [CrossRef] [PubMed]
- Ismail, E.T.; El-Son, M.A.M.; El-Gohary, F.A.; Zahran, E. Prevalence, genetic diversity, and antimicrobial susceptibility of Vibrio spp. infected gilthead sea breams from coastal farms at Damietta, Egypt. BMC Vet. Res. 2024, 20, 129. [Google Scholar] [CrossRef] [PubMed]
- Muzembo, B.A.; Kitahara, K.; Ohno, A.; Khatiwada, J.; Dutta, S.; Miyoshi, S.I. Vibriosis in South Asia: A systematic review and meta-analysis. Int. J. Infect. Dis. 2024, 141, 106955. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yu, L.; Liu, M.; Liang, W.; Li, Z.; Nan, Z.; Kan, B. Virulence, antibiotic resistance phenotypes and molecular characterisation of Vibrio furnissii isolates from patients with diarrhoea. BMC Infect. Dis. 2024, 24, 412. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.; Zheng, E.J.; Valeri, J.A.; Donghia, N.M.; Anahtar, M.N.; Omori, S.; Li, A.; Cubillos-Ruiz, A.; Krishnan, A.; Jin, W.; et al. Discovery of a structural class of antibiotics with explainable deep learning. Nature 2024, 626, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Barba, S.; Top, E.M.; Stalder, T. Plasmids, a molecular cornerstone of antimicrobial resistance in the One Health era. Nat. Rev. Microbiol. 2024, 22, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zeng, Z. Antibiotic residues, antimicrobial resistance and intervention strategies of foodborne pathogens. Antibiotics 2024, 13, 321. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Yang, Z.G.; Huang, Y.; Jian, J.C.; Tang, J.J. Effects of Chinese herbal medicines on growth performance, intestinal flora, immunity and serum metabolites of hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatu ♂). Fish Shellfish Immunol. 2023, 140, 108946. [Google Scholar] [CrossRef] [PubMed]
- Sihra, N.; Goodman, A.; Zakri, R.; Sahai, A.; Malde, S. Nonantibiotic prevention and management of recurrent urinary tract infection. Nat. Rev. Urol. 2018, 15, 750–776. [Google Scholar] [CrossRef]
- Swain, B.; Campodonico, V.A.; Curtiss, R. Recombinant attenuated Edwardsiella piscicida vaccine displaying regulated lysis to confer biological containment and protect catfish against Edwardsiellosis. Vaccines 2023, 11, 1470. [Google Scholar] [CrossRef] [PubMed]
- Jose Priya, T.A.; Kappalli, S. Modern biotechnological strategies for vaccine development in aquaculture—Prospects and challenges. Vaccine 2022, 40, 5873–5881. [Google Scholar] [CrossRef]
- Tharmalingam, T.; Han, X.; Wozniak, A.; Saward, L. Polyclonal hyper immunoglobulin: A proven treatment and prophylaxis platform for passive immunization to address existing and emerging diseases. Hum. Vaccin. Immunother. 2022, 18, 1886560. [Google Scholar] [CrossRef] [PubMed]
- Ying, B.; Darling, T.L.; Desai, P.; Liang, C.Y.; Dmitriev, I.P.; Soudani, N.; Bricker, T.; Kashentseva, E.A.; Harastani, H.; Raju, S.; et al. Mucosal vaccine-induced cross reactive CD8 (+) T cells protect against SARS-CoV-2 XBB.1.5 respiratory tract infection. Nat. Immunol. 2024, 25, 537–551. [Google Scholar] [CrossRef]
- Ratanabanangkoon, K.; Tan, K.Y.; Pruksaphon, K.; Klinpayom, C.; Gutiérrez, J.M.; Quraishi, N.H.; Tan, C.H. A pan-specific antiserum produced by a novel immunization strategy shows a high spectrum of neutralization against neurotoxic snake venoms. Sci. Rep. 2020, 10, 11261. [Google Scholar] [CrossRef]
- Mai, T.T.; Kayansamruaj, P.; Soontara, C.; Kerddee, P.; Nguyen, D.H.; Senapin, S.; Costa, J.Z.; Del-Pozo, J.; Thompson, K.D.; Rodkhum, C.; et al. Immunization of Nile Tilapia (Oreochromis niloticus) broodstock with Tilapia lake virus (TiLV) inactivated vaccines elicits protective antibody and passive maternal antibody transfer. Vaccines 2022, 10, 167. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, H.H.; Chao, J.; Jian, S.J.; Wu, X.Q.; Lu, J.; Wang, J.; Chen, C.L.; Liu, Y. Polyvalent passive vaccine candidates from egg yolk antibodies (IgY) of important outer membrane proteins (PF1380 and ExbB) of Pseudomonas fluorescens in fish. Fish Shellfish Immunol. 2023, 143, 109211. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Ye, J.Z.; Han, Y.; Zeng, L.; Zhang, J.Y.; Li, H. Identification of polyvalent protective immunogens from outer membrane proteins in Vibrio parahaemolyticus to protect fish against bacterial infection. Fish Shellfish Immunol. 2016, 54, 204–210. [Google Scholar] [CrossRef]
- Liu, X.; Rong, N.; Sun, W.; Jian, S.J.; Chao, J.; Chen, C.; Chen, R.; Ding, R.; Chen, C.; Liu, Y.; et al. The identification of polyvalent protective immunogens and immune abilities from the outer membrane proteins of Aeromonas hydrophila in fish. Fish Shellfish Immunol. 2022, 128, 101–112. [Google Scholar] [PubMed]
- Liu, X.; Chao, J.; Xiao, H.H.; Chen, J.; Cui, P.; Wu, X.Q.; Lu, J.; Wang, J.; Chen, C.L.; Zhang, X.Y.; et al. Identification of polyvalent passive vaccine candidates from egg yolk antibodies (IgY) of important outer membrane proteins of Aeromonas hydrophila in fish. Aquacult. Rep. 2024, 35, 102002. [Google Scholar] [CrossRef]
- Soto-Rodriguez, S.A.; Marrujo Lopez, F.I.; Aguilar-Rendon, K.G.; Guzmán, R.H. Pathogenic bacteria prevalence in cultured Nile tilapia in Southwest Mexico: A real-time PCR analysis. J. Fish Dis. 2024, 47, e13921. [Google Scholar] [CrossRef] [PubMed]
- Yaparatne, S.; Morón-López, J.; Bouchard, D.; Garcia-Segura, S.; Apul, O.G. Nanobubble applications in aquaculture industry for improving harvest yield, wastewater treatment, and disease control. Sci. Total Environ. 2024, 931, 172687. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ye, M.Z.; Peng, B.; Wu, H.K.; Xu, C.X.; Xiong, X.P.; Wang, C.; Wang, S.Y.; Peng, X.X. Immunoproteomic identification of polyvalent vaccine candidates from Vibrio parahaemolyticus outer membrane proteins. J. Proteome Res. 2010, 9, 2573–2583. [Google Scholar] [CrossRef] [PubMed]
- Bu, W.; Kumar, A.; Board, N.L.; Kim, J.; Dowdell, K.; Zhang, S.; Lei, Y.; Hostal, A.; Krogmann, T.; Wang, Y.; et al. Epstein-Barr virus gp42 antibodies reveal sites of vulnerability for receptor binding and fusion to B cells. Immunity 2024, 57, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, J.; Jin, L.; Li, X.; Zhen, Y.; Xue, H.; You, J.; Xu, Y. Passive protection of shrimp against white spot syndrome virus (WSSV) using specific antibody from egg yolk of chickens immunized with inactivated virus or a WSSV-DNA vaccine. Fish Shellfish Immunol. 2008, 25, 604–610. [Google Scholar] [CrossRef]
- Peng, B.; Lin, X.P.; Wang, S.N.; Yang, M.J.; Peng, X.X.; Li, H. Polyvalent protective immunogens identified from outer membrane proteins of Vibrio parahaemolyticus and their induced innate immune response. Fish Shellfish Immunol. 2018, 72, 104–110. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Hu, X.; Zhou, Y.; Yang, Z.; Hou, J.; Liu, F.; Liu, Q.; Mabrouk, I.; Yu, J.; et al. Dermal FOXO3 activity in response to Wnt/β-catenin signaling is required for feather follicle development of goose embryos (Anser cygnoides). Poult. Sci. 2024, 103, 103424. [Google Scholar] [CrossRef] [PubMed]
- Cen, X.; Chen, Y.; Xu, X.; Wu, R.; He, F.; Zhao, Q.; Sun, Q.; Yi, C.; Wu, J.; Najafov, A.; et al. Pharmacological targeting of MCL-1 promotes mitophagy and improves disease pathologies in an Alzheimer’s disease mouse model. Nat. Commun. 2020, 11, 5731. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Sun, H.; Sun, L.; Shi, K.; Chen, Y.; Ren, X.; Ge, Y.; Jiang, D.; Liu, X.; Knoll, W.; et al. Rapid, label-free histopathological diagnosis of liver cancer based on Raman spectroscopy and deep learning. Nat. Commun. 2023, 14, 48. [Google Scholar] [CrossRef]
- Liang, X.; Liang, J.; Cao, J.; Liu, S.; Wang, Q.; Ning, Y.; Liang, Z.; Zheng, J.; Zhang, Z.; Luo, J.; et al. Oral immunizations with Bacillus subtilis spores displaying VP19 protein provide protection against Singapore grouper iridovirus (SGIV) infection in grouper. Fish Shellfish Immunol. 2023, 138, 108860. [Google Scholar] [CrossRef] [PubMed]
- Parikh, P.; Britt, R.D.J.; Manlove, L.J.; Wicher, S.A.; Roesler, A.; Ravix, J.; Teske, J.; Thompson, M.A.; Sieck, G.C.; Kirkland, J.L.; et al. Hyperoxia-induced cellular senescence in fetal airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 2019, 61, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Reyes, A.; Ortiz, G.; Duarte, L.F.; Fernández, C.; Hernández-Armengol, R.; Palacios, P.A.; Prado, Y.; Andrade, C.A.; Rodriguez-Guilarte, L.; Kalergis, A.M.; et al. Contribution of viral and bacterial infections to senescence and immunosenescence. Front. Cell Infect. Microbiol. 2023, 13, 1229098. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Hassan, M.; Tacke, F.; Engelmann, C. Delineating the heterogeneity of senescence induced-functional alterations in hepatocytes. Cell Mol. Life Sci. 2024, 81, 200. [Google Scholar] [CrossRef] [PubMed]
- Suresh Babu, V.; Kizhakeyil, A.; Dudeja, G.; Chaurasia, S.S.; Barathi, V.A.; Heymans, S.; Verma, N.K.; Lakshminarayanan, R.; Ghosh, A. Selective induction of intrinsic apoptosis in retinoblastoma cells by novel cationic antimicrobial dodecapeptides. Pharmaceutics 2022, 14, 2507. [Google Scholar] [CrossRef] [PubMed]
- Joseph, T.T.; Schuch, V.; Hossack, D.J.; Chakraborty, R.; Johnson, E.L. Melatonin: The placental antioxidant and anti-inflammatory. Front. Immunol. 2024, 15, 1339304. [Google Scholar] [CrossRef] [PubMed]
- Biller, J.D.; Takahashi, L.S. Oxidative stress and fish immune system: Phagocytosis and leukocyte respiratory burst activity. An. Acad. Bras. Cienc. 2018, 90, 3403–3414. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.Y.; Liu, C.; Ding, J.; Gao, X.M.; Wang, J.Q.; Zhang, Y.B.; Du, C.; Hou, C.C.; Zhu, J.Q.; Lou, B.; et al. Scavenging reactive oxygen species is a potential strategy to protect Larimichthys crocea against environmental hypoxia by mitigating oxidative stress. Zool. Res. 2021, 42, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Winiarska-Mieczan, A.; Kwiecień, M.; Jachimowicz-Rogowska, K.; Donaldson, J.; Tomaszewska, E.; Baranowska-Wójcik, E. Anti-inflammatory, antioxidant, and neuroprotective effects of polyphenols-polyphenols as an element of diet therapy in depressive disorders. Int. J. Mol. Sci. 2023, 24, 2258. [Google Scholar] [CrossRef]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The role of oxidative stress and antioxidants in diabetic wound healing. Oxidative Med. Cell. Longev. 2021, 2021, 8852759. [Google Scholar]
- Dongjie, S.; Rajendran, R.S.; Xia, Q.; She, G.; Tu, P.; Zhang, Y.; Liu, K. Neuroprotective effects of Tongtian oral liquid, a traditional Chinese medicine in the Parkinson’s disease-induced zebrafish model. Biomed. Pharmacother. 2022, 148, 112706. [Google Scholar] [CrossRef] [PubMed]
- Naraki, K.; Rezaee, R.; Karimi, G. A review on the protective effects of naringenin against natural and chemical toxic agents. Phytother. Res. 2021, 35, 4075–4091. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xu, Y.P.; He, L.Y.; Zhang, M.X.; Wang, L.L.; Li, Z.; Li, X.Y. Immunomodulatory effects of chicken egg yolk antibodies (IgY) against experimental Shewanella marisflavi AP629 infections in sea cucumbers (Apostichopus japonicus). Fish Shellfish Immunol. 2019, 84, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.D.; Chen, K.C.; Li, S.S.; Zhang, Y.T.; Li, Z.M.; Liu, S.; Sun, Y.S. Panax quinquefolius polysaccharides ameliorate ulcerative colitis in mice induced by dextran sulfate sodium. Front. Immunol. 2023, 14, 1161625. [Google Scholar] [CrossRef] [PubMed]
- Janssens, A.; Nguyen, V.S.; Cecil, A.J.; Van der Verren, S.E.; Timmerman, E.; Deghelt, M.; Pak, A.J.; Collet, J.F.; Impens, F.; Remaut, H. SlyB encapsulates outer membrane proteins in stress-induced lipid nanodomains. Nature 2024, 626, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, I.C.; Rodrigues, S.C.; Duarte, F.V.; Costa, P.M.D.; Costa, P.M.D. The role of outer membrane proteins in UPEC antimicrobial resistance: A systematic review. Membranes 2022, 12, 981. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Salgado, L.; Souto, S.; Olveira, J.G.; Bandín, I. A potential Nervous Necrosis Virus (NNV) live vaccine for sole obtained by genomic modification. Animals 2024, 14, 983. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Meena, J.K.; Sharma, M.; Dixit, A. Recombinant outer membrane protein C of Aeromonas hydrophila elicits mixed immune response and generates agglutinating antibodies. Immunol. Res. 2016, 64, 1087–1099. [Google Scholar] [CrossRef]
- Tang, X.; Wang, H.; Liu, F.; Sheng, X.; Xing, J.; Zhan, W. Outer membrane protein A: An immunogenic protein induces highly protective efficacy against Vibrio ichthyoenteri. Microb. Pathog. 2017, 113, 152–159. [Google Scholar] [CrossRef]
- Zhang, B.Z.; Hu, D.; Dou, Y.; Xiong, L.; Wang, X.; Hu, J.; Xing, S.Z.; Li, W.; Cai, J.P.; Jin, M.; et al. Identification and evaluation of recombinant outer membrane proteins as vaccine candidates against Klebsiella pneumoniae. Front. Immunol. 2021, 12, 730116. [Google Scholar] [CrossRef] [PubMed]
- Olukitibi, T.A.; Ao, Z.; Azizi, H.; Ouyang, M.J.; Omole, T.; McKinnon, L.; Kobasa, D.; Coombs, K.; Kobinger, G.; Yao, X. UV-inactivated rVSV-M2e-based influenza vaccine protected against the H1N1 influenza challenge. Front. Biosci. 2024, 29, 195. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Duggan, C.; Texada, D.E.; Reden, T.B.; Kooragayala, L.M.; Langford, M.P. Immunogenicity of enterovirus 70 capsid protein VP1 and its non-overlapping N-and C-terminal fragments. Antiviral Res. 2005, 66, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Hankaniemi, M.M.; Stone, V.M.; Sioofy-Khojine, A.B.; Heinimäki, S.; Marjomäki, V.; Hyöty, H.; Blazevic, V.; Laitinen, O.H.; Flodström-Tullberg, M.; Hytönen, V.P. A comparative study of the effect of UV and formalin inactivation on the stability and immunogenicity of a Coxsackievirus B1 vaccine. Vaccine 2019, 37, 5962–5971. [Google Scholar] [CrossRef] [PubMed]




| Gene | NCBI Number | Forward Primer (5–3′) | Reverse Primer (5–3′) |
|---|---|---|---|
| IL-6 | XM_026289280.1 | TCTCCTCAGACCCTCAGACG | CGTTTGGTCCCGTGTTTGAC |
| IL-8 | XM_026267284.1 | GGAGTGCAGGCCACTGTTAG | ATCAGAAGCATGAAGGCGGA |
| IL-1β | AJ249136.1 | TTCAGGAAAGAGACGGGCAC | GTCAGTTGGCACCTGGATCA |
| TNF-α | EU069817.1 | GGGCCACATCGTGATTCGTA | GCCTCCAGTGTAGCATGTGT |
| GAPDH | XM_026284269.1 | GATTTCAACGGGGATGTGCG | TCACACACACGGTTGCTGTA |
| Bacterium | Antiserum | Total (No.) | Survived (No.) | Died (No.) | ADR (%) | RPS (%) |
|---|---|---|---|---|---|---|
| V. fluvialis | Control | 15 | 0 | 15 | 100 | -- |
| Live bacteria | 15 | 9 | 6 | 40 | 60 ** | |
| Inactivated bacteria | 15 | 6 | 9 | 60 | 40 ** | |
| A. hydrophila | Control | 15 | 1 | 14 | 93.33 | -- |
| Live bacteria | 15 | 7 | 8 | 53.33 | 42.86 * | |
| Inactivated bacteria | 15 | 6 | 9 | 60.00 | 35.71 * |
| Bacterium | Group | Phagocytic Percentage (PP%) | Phagocytic Index (PI%) |
|---|---|---|---|
| V. fluvialis | Control | 16.67 ± 1.53 a | 164.00 ± 3.46 a |
| Live bacteria | 32.67 ± 2.08 c | 236.73 ± 13.80 b | |
| Inactivated bacteria | 26.0 ± 1.00 b | 223.08 ± 3.85 b | |
| A. hydrophila | Control | 19.67 ± 1.53 a | 188.14 ± 13.45 a |
| Live bacteria | 40.33 ± 2.52 c | 202.48 ± 6.24 ab | |
| Inactivated bacteria | 29.33 ± 2.08 b | 226.14 ± 10.42 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, H.; Cui, P.; Chen, J.; Han, X.; Ma, Z.; Chen, C.; Liu, Y.; Liu, X. A Comparison of Polyvalent Passive Immunoprotection from Antibodies with Different Immunity Models of Live or Inactivated Vibrio fluvialis in Fish. Fishes 2024, 9, 302. https://doi.org/10.3390/fishes9080302
Xiao H, Cui P, Chen J, Han X, Ma Z, Chen C, Liu Y, Liu X. A Comparison of Polyvalent Passive Immunoprotection from Antibodies with Different Immunity Models of Live or Inactivated Vibrio fluvialis in Fish. Fishes. 2024; 9(8):302. https://doi.org/10.3390/fishes9080302
Chicago/Turabian StyleXiao, Huihui, Pan Cui, Jing Chen, Xiaohui Han, Ziye Ma, Chen Chen, Yong Liu, and Xiang Liu. 2024. "A Comparison of Polyvalent Passive Immunoprotection from Antibodies with Different Immunity Models of Live or Inactivated Vibrio fluvialis in Fish" Fishes 9, no. 8: 302. https://doi.org/10.3390/fishes9080302
APA StyleXiao, H., Cui, P., Chen, J., Han, X., Ma, Z., Chen, C., Liu, Y., & Liu, X. (2024). A Comparison of Polyvalent Passive Immunoprotection from Antibodies with Different Immunity Models of Live or Inactivated Vibrio fluvialis in Fish. Fishes, 9(8), 302. https://doi.org/10.3390/fishes9080302






