Variation in Isotopic Trophic Niche of Sablefish (Anoplopoma fimbria) and Shortraker Rockfish (Sebastes borealis) in the Northeast Pacific
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Areas
2.2. Sampling
2.3. Stable Isotope Analysis Preparation
2.4. Statistical Analysis
3. Results
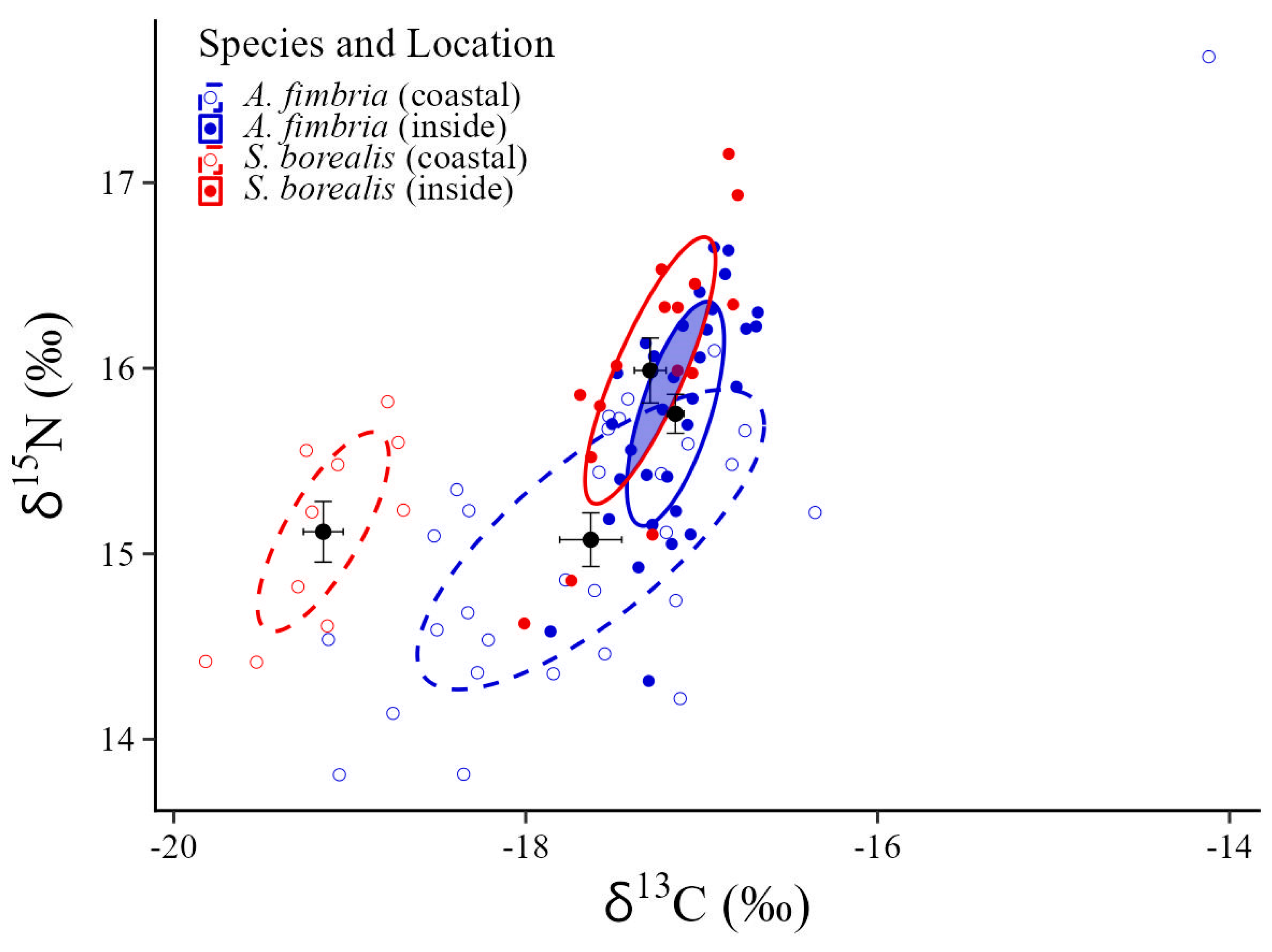
3.1. Isotopic Trophic Niche Position
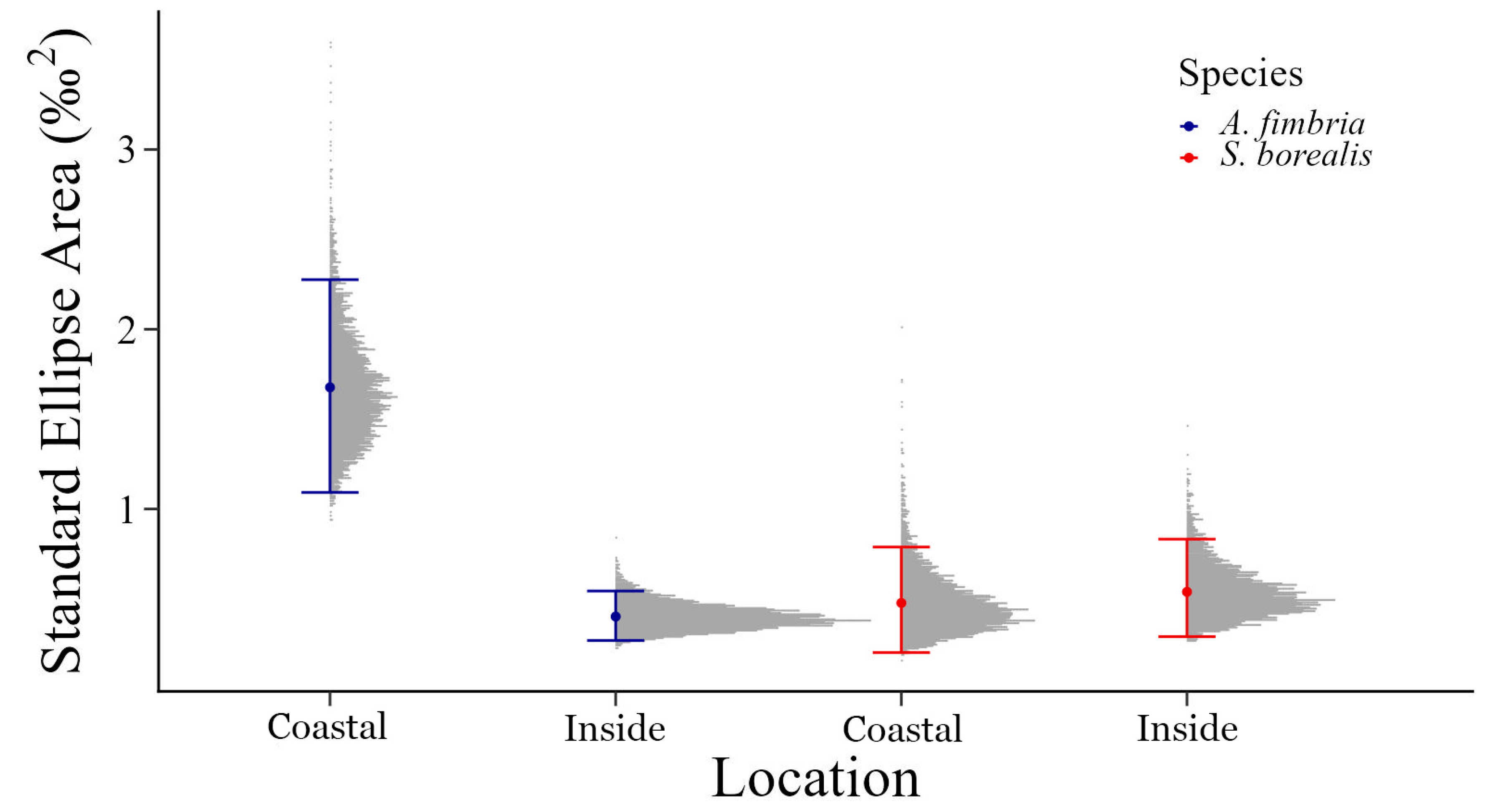
3.2. Isotopic Trophic Niche Breadth
3.3. Isotopic Trophic Niche Overlap
3.4. Isotopic Trophic Niche and Total Length
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clark, M. Are deepwater fisheries sustainable?—The example of orange roughy (Hoplostethus atlanticus) in New Zealand. Fish. Res. 2001, 51, 123–135. [Google Scholar] [CrossRef]
- Clark, M.R.; Althaus, F.; Schlacher, T.A.; Williams, A.; Bowden, D.A.; Rowden, A.A. The impacts of deep-sea fisheries on benthic communities: A review. ICES J. Mar. Sci. 2016, 73, 51–69. [Google Scholar] [CrossRef]
- Koslow, J.A.; Boehlert, G.W.; Gordon, J.D.M.; Haedrich, R.L.; Lorance, P.; Parin, N. Continental slope and deep-sea fisheries: Implications for a fragile ecosystem. ICES J. Mar. Sci. 2000, 57, 548–557. [Google Scholar] [CrossRef]
- Ramirez-Llodra, E.; Brandt, A.; Danovaro, R.; De Mol, B.; Escobar, E.; German, C.R.; Levin, L.A.; Arbizu, P.M.; Menot, L.; Buhl-Mortensen, P.; et al. Deep, diverse and definitely different: Unique attributes of the world’s largest ecosystem. Biogeosciences 2010, 7, 2851–2899. [Google Scholar] [CrossRef]
- Eschmeyer, W.N.; Herald, E.S.; Hammann, H. A Field Guide to Pacific Coast Fishes of North America; Houghton Mifflin Company: Boston, MA, USA, 1983. [Google Scholar]
- Love, M.S.; Yoklavich, M.; Thorsteinson, L.K. The Rockfishes of the Northeast Pacific; University of California Press: Berkeley, CA, USA, 2002; ISBN 978-0-520-23438-3. [Google Scholar]
- Echave, K.B.; Hulson, P.J.F. Assessment of the shortraker rockfish stock in the Gulf of Alaska. In Stock Assessment and Fishery Evaluation Report for the Groundfish Fisheries of the Gulf of Alaska; North Pacific Fishery Management Council: Anchorage, AK, USA, December 2019. [Google Scholar]
- Sigler, M.F.; Zenger, H.H. Assessment of Gulf of Alaska Sablefish and Other Groundfish Based on the Domestic Longline Survey, 1987; NOAA Technical Memorandum NMFS F/NWC; National Oceanic and Atmospheric Administration: Washington, DC, USA, 1989; Volume 169. [Google Scholar]
- Pirtle, J.L.; Shotwell, S.K.; Zimmermann, M.; Reid, J.A.; Golden, N. Habitat suitability models for groundfish in the Gulf of Alaska. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2019, 165, 303–321. [Google Scholar] [CrossRef]
- Shotwell, S.K.; Sullivan, J.; Echave, K.; Markowitz, E.; Ortiz, I.; Siddon, E.; Spencer, P.; Spies, I. Assessment of the shortraker rockfish stock in the Bering Sea and Aleutian Islands. In Stock Assessment and Fishery Evaluation Report for the Groundfish Fisheries of the Bering Sea and Aleutian Islands; North Pacific Fishery Management Council: Anchorage, AK, USA, December 2022. [Google Scholar]
- Krieger, K.J.; Ito, D.H. Distribution and abundance of shortraker rougheye, Sebastes borealis, and rougheye rockfish, S. aleutianus, determined from a manned submersible. Fish. Bull. 1999, 97, 264–272. [Google Scholar]
- Kimura, D.K.; Shimada, A.M.; Shaw, F.R. Stock structure and movement of tagged sablefish, Anoplopoma fimbria, in offshore northeast Pacific waters and the effects of El Nino Southern Oscillation on migration and growth. Fish. Bull. 1998, 96, 462–481. [Google Scholar]
- Sablefish (Anoplopoma fimbria) Species Profile by Alaska Department of Fish and Game. Available online: https://www.adfg.alaska.gov/index.cfm?adfg=sablefish.main (accessed on 10 October 2023).
- NOAA Fisheries Commercial Fishing Landings Database. Available online: https://www.fisheries.noaa.gov/foss/f?p=215:3:0 (accessed on 20 July 2024).
- Goethel, D.R.; Cheng, M.L.H.; Echave, K.B.; Marsh, C.; Rodgveller, C.J.; Shotwell, K.; Siwicke, K. Assessment of the Sablefish Stock in Alaska; North Pacific Fishery Management Council: Anchorage, AK, USA, 2023. [Google Scholar]
- Echave, K.B.; Siwicke, K.A.; Sullivan, J.; Ferriss, B. Assessment of the Shortraker Rockfish stock in the Gulf of Alaska; North Pacific Fishery Management Council: Anchorage, AK, USA, 2023. [Google Scholar]
- Krigbaum, M.J.; Anderson, C.M. Increasing value through gear flexibility: A case study of U.S. West Coast Sablefish. Can. J. Fish. Aquat. Sci. 2021, 78, 1130–1145. [Google Scholar] [CrossRef]
- Xiao, H.; Dee, L.E.; Chadès, I.; Peyrard, N.; Sabbadin, R.; Stringer, M.; McDonald-Madden, E. Win-wins for biodiversity and ecosystem service conservation depend on the trophic levels of the species providing services. J. Appl. Ecol. 2018, 55, 2160–2170. [Google Scholar] [CrossRef]
- Newsome, S.D.; Martinez del Rio, C.; Bearhop, S.; Phillips, D.L. A niche for isotopic ecology. Front. Ecol. Environ. 2007, 5, 429–436. [Google Scholar] [CrossRef]
- Perkins, M.J.; McDonald, R.A.; Frank van Veen, F.J.; Kelly, S.D.; Bearhop, S. Application of nitrogen and carbon stable isotopes (δ15N and δ13C) to quantify food chain length and trophic structure. PLoS ONE 2014, 9, e93281. [Google Scholar] [CrossRef] [PubMed]
- Fry, B. Stable Isotope Ecology; Springer: New York, NY, USA, 2006. [Google Scholar]
- Callahan, M.W.; Beaudreau, A.H.; Heintz, R.A.; Mueter, F.J.; Rogers, M.C. Temporal and age-based variation in juvenile sablefish diet composition and quality: Inferences from stomach contents and stable isotopes. Mar. Coast. Fish. 2021, 13, 396–412. [Google Scholar] [CrossRef]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- McCutchan, J.H.; Lewis, W.M.; Kendall, C.; McGrath, C.C. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 2003, 102, 378–390. [Google Scholar] [CrossRef]
- Davenport, S.; Bax, N. A trophic study of a marine ecosystem of Southeastern Australia using stable isotopes of carbon and nitrogen. Can. J. Fish. Aquat. Sci. 2011, 59, 514–530. [Google Scholar] [CrossRef]
- Lindeman, R.L. The trophic-dynamic aspect of ecology. Ecology 1942, 23, 399–417. [Google Scholar] [CrossRef]
- O’Gorman, E.J.; Emmerson, M.C. Perturbations to trophic interactions and the stability of complex food webs. Proc. Natl. Acad. Sci. USA 2009, 106, 13393–13398. [Google Scholar] [CrossRef] [PubMed]
- McDonald-Madden, E.; Sabbadin, R.; Game, E.T.; Baxter, P.W.J.; Chadés, I.; Possingham, H.P. Using food-web theory to conserve ecosystems. Nat. Commun. 2016, 7, 10245. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.K.; Worm, B. Cascading top-down effects of changing oceanic predator abundances. J. Anim. Ecol. 2009, 78, 699–714. [Google Scholar] [CrossRef]
- Lynam, C.P.; Llope, M.; Möllmann, C.; Helaouët, P.; Bayliss-Brown, G.A.; Stenseth, N.C. Interaction between top-down and bottom-up control in marine food webs. Proc. Natl. Acad. Sci. USA 2017, 114, 1952–1957. [Google Scholar] [CrossRef]
- Buckley, T.W.; Tyler, G.E.; Smith, D.M.; Livingston, P.A. Food Habits of Some Commercially Important Groundfish Off the Coasts of California, Oregon, Washington, and British Columbia; NOAA Technical Memorandum NMFS-AFSC-102; U.S. Department of Commerce: Washington, DC, USA, 1999; p. 173. [Google Scholar]
- Yang, M.-S.; Nelson, M.W. Food Habits of the Commercially Important Groundfishes in the Gulf of Alaska in 1990, 1993, and 1996; NOAA Technical Memorandum NMFS-AFSC-22; U.S. Department of Commerce: Washington, DC, USA, 2000; pp. 107–123. [Google Scholar]
- Laidig, T.E.; Adams, P.B. Feeding habits of sablefish, Anoplopoma fimbria, off the coast of Oregon and California. In Proceedings of the International Symposium on the Biology and Management of Sablefish, Seattle, WA, USA, 13–15 April 1993; NOAA Technical Report NMFS 130; U.S. Department of Commerce: Washington, DC, USA, 1997; pp. 65–80. [Google Scholar]
- Yang, M.-S.; Dodd, K.; Hibpshman, R.; Whitehouse, A. Food Habits of Groundfishes in the Gulf of Alaska in 1999 and 2001; NOAA Technical Memorandum NMFS-AFSC-164; U.S. Department of Commerce: Washington, DC, USA, 2006. [Google Scholar]
- Coleman, D.C.; Fry, B. Carbon Isotope Techniques, 1st ed.; Academic Press: Cambridge, MA, USA, 1991. [Google Scholar]
- Sweeting, C.J.; Polunin, N.V.C.; Jennings, S. Effects of chemical lipid extraction and arithmetic lipid correction on stable isotope ratios of fish tissues. Rapid Commun. Mass Spectrom. 2006, 20, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Post, D.M.; Layman, C.A.; Arrington, D.A.; Takimoto, G.; Quattrochi, J.; Montaña, C.G. Getting to the fat of the matter: Models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 2007, 152, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.C.; Sutton, T.T. Lipid correction for carbon stable isotope analysis of deep-sea fishes. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2010, 57, 956–964. [Google Scholar] [CrossRef]
- R Core Team, R. A Language and Environment for Statistical Computing; The R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org (accessed on 10 November 2023).
- Jackson, A.L.; Inger, R.; Parnell, A.C.; Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER—Stable isotope bayesian ellipses in R: Bayesian isotopic niche metrics. J. Anim. Ecol. 2011, 80, 595–602. [Google Scholar] [CrossRef] [PubMed]
- McCrum-Gardner, E. Which is the correct statistical test to use? Br. J. Oral Maxillofac. Surg. 2008, 46, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Suchomel, A.D.; Belk, M.C. Comparison of trophic niche position, size, and overlaps in an assemblage of pacific rockfish (genus Sebastes) for testing community composition models. Diversity 2022, 14, 689. [Google Scholar] [CrossRef]
- Stabeno, P.J.; Bond, N.A.; Hermann, A.J.; Kachel, N.B.; Mordy, C.W.; Overland, J.E. Meteorology and oceanography of the Northern Gulf of Alaska. Cont. Shelf Res. 2004, 24, 859–897. [Google Scholar] [CrossRef]
- Mclain, D.R. Heat and Water Balance of Lynn Canal, Alaska (Order No. 7014595); University of Michigan ProQuest Dissertations Publishing: Ann Arbor, MI, USA, 1969. [Google Scholar]
- Fransson, A.; Chierici, M.; Nojiri, Y. Increased net CO2 outgassing in the upwelling region of the southern Bering Sea in a period of variable marine climate between 1995 and 2001. J. Geophys. Res. Oceans 2006, 111, C08008. [Google Scholar] [CrossRef]
- Wesner, J.S.; Belk, M.C. Variation in the trophic position of common stream fishes and its relationship to the presence of a rare fish, northern leatherside chub (Lepidomeda copei). Ecol. Freshw. Fish 2014, 24, 234–241. [Google Scholar] [CrossRef]
- Lorrain, A.; Graham, B.S.; Popp, B.N.; Allain, V.; Olson, R.J.; Hunt, B.P.V.; Potier, M.; Fry, B.; Galván-Magaña, F.; Menkes, C.E.R.; et al. Nitrogen isotopic baselines and implications for estimating foraging habitat and trophic position of yellowfin tuna in the Indian and Pacific Oceans. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2015, 113, 188–198. [Google Scholar] [CrossRef]
- Vizzini, S.; Savona, B.; Chi, T.D.; Mazzola, A. Spatial variability of stable carbon and nitrogen isotope ratios in a Mediterranean coastal lagoon. Hydrobiologia 2005, 550, 73–82. [Google Scholar] [CrossRef]
- Dalponti, G.; Guariento, R.D.; Caliman, A. Hunting high or low: Body size drives trophic position among and within marine predators. Mar. Ecol. Prog. Ser. 2018, 597, 39–46. [Google Scholar] [CrossRef]
- Jennings, S.; Pinnegar, J.; Polunin, N.; Randall, K. Linking size-based and trophic analyses of benthic community structure. Mar. Ecol. Prog. Ser. 2002, 226, 77–85. [Google Scholar] [CrossRef]
- Shipley, O.N.; Matich, P. Studying animal niches using bulk stable isotope ratios: An updated synthesis. Oecologia 2020, 193, 27–51. [Google Scholar] [CrossRef] [PubMed]
- Hette-Tronquart, N. Isotopic niche is not equal to trophic niche. Ecol. Lett. 2019, 22, 1987–1989. [Google Scholar] [CrossRef] [PubMed]
- Cicala, D.; Sbrana, A.; Valente, T.; Berto, D.; Rampazzo, F.; Gravina, M.F.; Maiello, G.; Russo, T. Trophic niche overlap of deep-sea fish species revealed by the combined approach of stomach contents and stable isotopes analysis in the Central Tyrrhenian Sea. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2024, 206, 104281. [Google Scholar] [CrossRef]
- Potapov, A.M.; Pollierer, M.M.; Salmon, S.; Šustr, V.; Chen, T.-W. Multidimensional trophic niche revealed by complementary approaches: Gut content, digestive enzymes, fatty acids and stable isotopes in Collembola. J. Anim. Ecol. 2021, 90, 1919–1933. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Wei, W.; Pu, D.; Qubi, S.; Zhou, H.; Hong, M.; Tang, J.; Han, H. Comparative analysis of trophic niche using stable isotopes provides insight into resource use of giant pandas. Integr. Zool. 2023, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Balčiauskas, L.; Skipitytė, R.; Garbaras, A.; Stirkė, V.; Balčiauskienė, L.; Remeikis, V. Stable isotopes reveal the dominant species to have the widest trophic niche of three syntopic microtus voles. Animals 2021, 11, 1814. [Google Scholar] [CrossRef]
- Bearhop, S.; Adams, C.E.; Waldron, S.; Fuller, R.A.; Macleod, H. Determining trophic niche width: A novel approach using stable isotope analysis. J. Anim. Ecol. 2004, 73, 1007–1012. [Google Scholar] [CrossRef]
- Gong, Y.; Li, Y.; Chen, X.; Yu, W. Trophic niche and diversity of a pelagic squid (Dosidicus gigas): A comparative study using stable isotope, fatty acid, and feeding apparatuses morphology. Front. Mar. Sci. 2020, 7, 642. [Google Scholar] [CrossRef]
- D’Iglio, C.; Famulari, S.; Albano, M.; Giordano, D.; Rinelli, P.; Capillo, G.; Spanò, N.; Savoca, S. Time-scale analysis of prey preferences and ontogenetic shift in the diet of European hake Merluccius merluccius (Linnaeus, 1758) in southern and central Tyrrhenian Sea. Fishes 2022, 7, 167. [Google Scholar] [CrossRef]
- Orlov, A.M.; Abramov, A.A. Age, rate of sexual maturation, and feeding of the shortraker rockfish, Sebastes borealis (Scorpaenidae) in the Northwestern Pacific Ocean. J. Ichthyol. 2001, 41, 279–288. [Google Scholar]
- McClenachan, L.; Ferretti, F.; Baum, J.K. From archives to conservation: Why historical data are needed to set baselines for marine animals and ecosystems. Conserv. Lett. 2012, 5, 349–359. [Google Scholar] [CrossRef]
- Cheeseman, T.; Barlow, J.; Acebes, J.M.; Audley, K.; Bejder, L.; Birdsall, C.; Bracamontes, O.S.; Bradford, A.L.; Byington, J.; Calambokidis, J.; et al. Bellwethers of change: Population modelling of North Pacific humpback whales from 2002 through 2021 reveals shift from recovery to climate response. R. Soc. Open. Sci. 2024, 11, 231462. [Google Scholar] [CrossRef] [PubMed]
- Muhlfeld, C.C.; Thorrold, S.R.; McMahon, T.E.; Marotz, B. Estimating westslope cutthroat trout (Oncorhynchus clarkia lewisi) movements in a river network using strontium isoscapes. Can. J. Fish. Aquat. Sci. 2012, 69, 906–915. [Google Scholar] [CrossRef]
- Brennan, S.R.; Zimmerman, C.E.; Fernandez, D.P.; Cerling, T.E.; McPhee, M.V.; Wooller, M.J. Strontium isotopes delineate fine-scale natal origins and migration histories of Pacific salmon. Sci. Adv. 2015, 1, e1400124. [Google Scholar] [CrossRef]
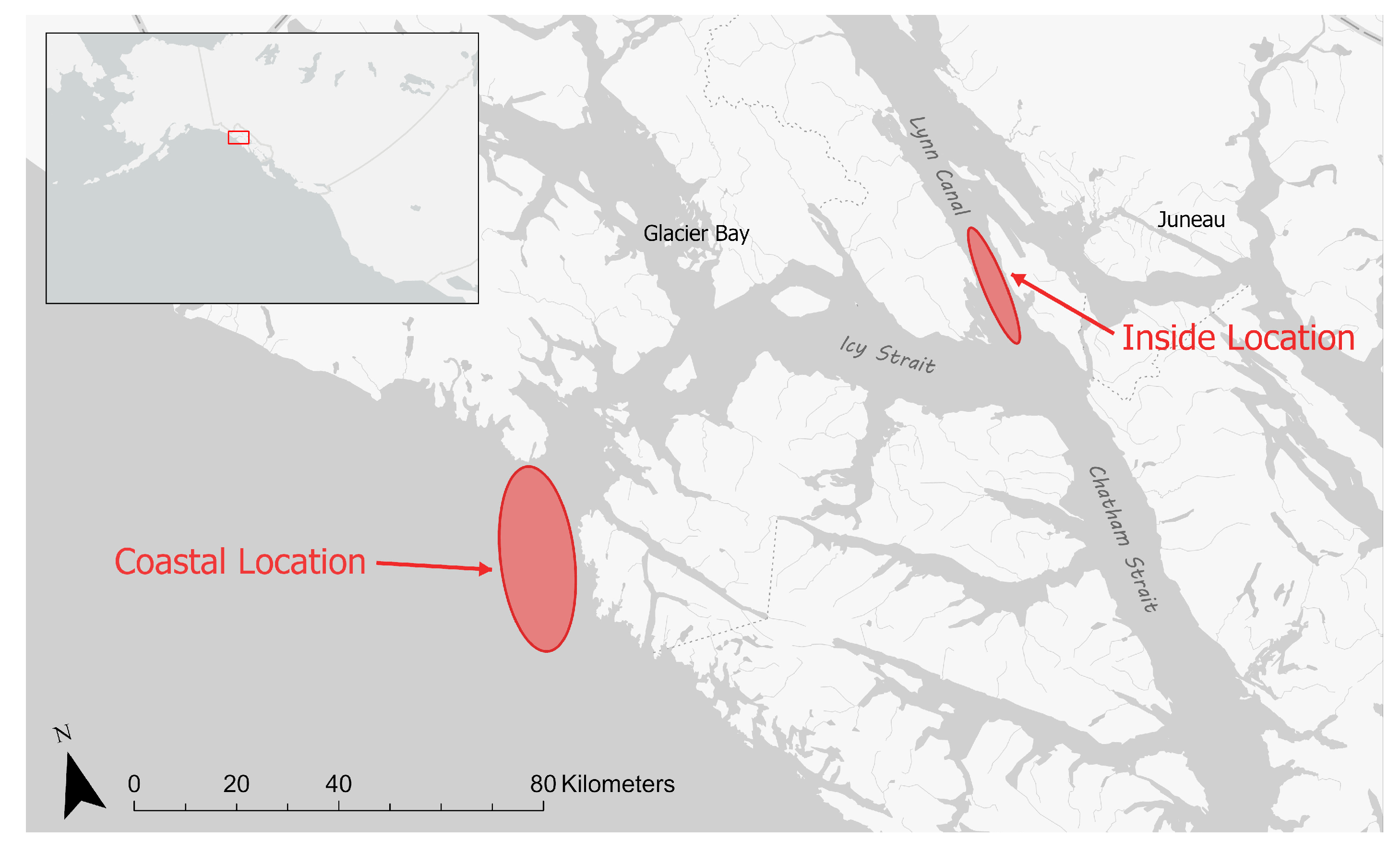
| Species | Location | Sample Size (N) | Mean TL (95% CI) (Range) |
|---|---|---|---|
| Anoplopoma fimbria | Inside | 32 | 723 (707–739) (584–902) |
| Coastal | 30 | 711 (705–717) (622–766) | |
| Sebastes borealis | Inside | 16 | 669 (647–692) (394–800) |
| Coastal | 10 | 575 (551–599) (443–652) |
| Species | Location | Mean δ15N (‰) | Mean δ13C (‰) |
|---|---|---|---|
| Anoplopoma fimbria | Inside Area | 15.75 (15.65–15.86) | −17.15 (−17.20–17.10) |
| Coastal Area | 15.08 (14.93–15.22) | −17.63 (−17.81–17.45) | |
| Sebastes borealis | Inside Area | 15.99 (15.81–16.16) | −17.29 (−17.38–17.20) |
| Coastal Area | 15.12 (14.96–15.28) | −19.15 (−19.26–19.04) |
| Variable | Degrees of Freedom | F-Value | p-Value |
|---|---|---|---|
| Species | 1/84 | 2.050 | 0.156 |
| Location | 1/84 | 25.136 | 2.93 × 10−6 |
| Species:Location | 1/84 | 0.347 | 0.558 |
| Variable | Degrees of Freedom | F-Value | p-Value |
|---|---|---|---|
| Species | 1/84 | 18.51 | 4.54 × 10−5 |
| Location | 1/84 | 42.64 | 4.75 × 10−9 |
| Species:Location | 1/84 | 21.61 | 1.23 × 10−5 |
| Species | Location | SEA (‰2) | SEAc (‰2) | SEAB (‰2) |
|---|---|---|---|---|
| Anoplopoma fimbria | Inside Area | 0.38 | 0.39 | 0.40 |
| Coastal Area | 1.55 | 1.60 | 1.67 | |
| Sebastes borealis | Inside Area | 0.45 | 0.48 | 0.54 |
| Coastal Area | 0.38 | 0.43 | 0.47 |
| Species | Location | Overlap (%) |
|---|---|---|
| Anoplopoma fimbria | Inside Area | 58.56 |
| Coastal Area | 14.34 | |
| Sebastes borealis | Inside Area | 0 |
| Coastal Area | 0 | |
| Between species | Inside Area | 28.22–34.48 |
| Coastal Area | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson, R.; Hatcher, T.J.; Suchomel, A.D.; Belk, M.C. Variation in Isotopic Trophic Niche of Sablefish (Anoplopoma fimbria) and Shortraker Rockfish (Sebastes borealis) in the Northeast Pacific. Fishes 2024, 9, 299. https://doi.org/10.3390/fishes9080299
Wilson R, Hatcher TJ, Suchomel AD, Belk MC. Variation in Isotopic Trophic Niche of Sablefish (Anoplopoma fimbria) and Shortraker Rockfish (Sebastes borealis) in the Northeast Pacific. Fishes. 2024; 9(8):299. https://doi.org/10.3390/fishes9080299
Chicago/Turabian StyleWilson, Raquel, Tessa J. Hatcher, Andrew D. Suchomel, and Mark C. Belk. 2024. "Variation in Isotopic Trophic Niche of Sablefish (Anoplopoma fimbria) and Shortraker Rockfish (Sebastes borealis) in the Northeast Pacific" Fishes 9, no. 8: 299. https://doi.org/10.3390/fishes9080299
APA StyleWilson, R., Hatcher, T. J., Suchomel, A. D., & Belk, M. C. (2024). Variation in Isotopic Trophic Niche of Sablefish (Anoplopoma fimbria) and Shortraker Rockfish (Sebastes borealis) in the Northeast Pacific. Fishes, 9(8), 299. https://doi.org/10.3390/fishes9080299








