Abstract
As water temperatures rise in streams due to global temperature variations, dams, and increased water usage, native fish species face uncertain futures. Our study defines the thermal limits of flannelmouth sucker larvae. By raising sucker eggs at three acclimation temperatures (11 °C, 16 °C, and 22 °C), we defined ideal conditions for larval survival and the temperature tolerance range using critical thermal maximum (CTMax) and minimum (CTMin) trials. Larvae survived best at 16 °C. Within our three acclimation temperatures, our data suggest that larvae can survive static temperatures between 6.9 °C and 26.4 °C. Beyond an upper temperature of 34.8 °C and a lower temperature of 6.3 °C, these fish may fail to adapt. While flannelmouth suckers withstand high temperatures, even small temperature decreases prove detrimental. By defining the temperature limits of the flannelmouth sucker, we can make informed management decisions to preserve the populations of this desert fish.
Key Contribution:
Larval flannelmouth suckers have a narrow temperature tolerance range and a limited capacity to adapt to significant temperature shifts in warm and cold water. Their constrained cold tolerance and narrow 6.9 °C–26.42 °C thermal niche suggest that temperatures beyond this range could jeopardize their survival.
1. Introduction
Desert aquatic ecosystems are highly dependent on the availability of water. As water use increases, small streams become warmer, more fragmented, and less hospitable to native species. For desert fish, temperature is a critical factor influencing reproduction and survival [1]. Temperature tolerance ranges, which are shaped by evolutionary history and acclimatization capacity, determine how the fish cope with warming and cooling temperatures [2,3,4,5]. Species from warmer climes tolerate higher temperatures; those accustomed to cooler waters, lower [6,7]. Understanding these temperature tolerance relationships in desert ecosystems is key to developing effective management strategies to protect these populations.
The temperature tolerance of fish can be estimated in two ways: field observations and controlled laboratory studies. Critical thermal maxima and minima (CTMax and CTMin, respectively; CTMs collectively) are commonly used to develop temperature tolerance ranges for larval fish [7,8]. In the laboratory, CTM ranges are determined by gradually increasing (for CTMax) or decreasing (for CTMin) temperature from an initial point until fish experience loss of equilibrium (LOE), defined as when the fish can no longer maintain an upright dorsal–ventral orientation and would likely not survive in the wild [9]. It is also important to include multiple acclimation temperatures and high and low fish densities, and to measure water quality parameters when defining temperature ranges [7,9,10]. For instance, Riepe et al. [10] found the bluehead sucker Pantosteaus discobolus’s CTMax to be 6 °C lower when multiple fish were tested in the same tank compared to fish tested individually. Multiple stressors often have major effects on organisms and should be considered when developing temperature criteria. Additionally, CTMs provide information to infer upper and lower incipient lethal temperatures. For warm water species, the Colorado Water Quality Control Commission (WQCC) applies a correction factor of ±0.8 °C to define lethal temperature limits since lethal tests are long and are not typically conducted [11].
The flannelmouth sucker Catostomus latipinnis experienced substantial range declines due to habitat loss and hybridization with nonnative suckers [12], and presently occupies as little as 45% of its historic range [13]. Within Colorado, flannelmouth suckers are still present in the Colorado, Dolores, Green, Gunnison, San Juan, White, and Yampa rivers, and can be seasonally found in tributaries to these rivers where spawning and larval rearing occurs [14]. The spawning season is typically between March and June, depending on the region, and is heavily influenced by water temperature and discharge [15]. During spawning, flannelmouth suckers migrate from mainstem river habitats into perennial and intermittent tributaries to spawn. After spawning, adults migrate back to river habitats. Larval fish hatch within days to weeks of fertilization, and after emergence, they drift semi-passively downstream. During this drift phase, some larvae reach mainstem habitats, while others are retained in slower tributary waters, actively out-migrating at a later time or developing in perennial tributary habitats [16]. Temperature affects this reproductive strategy by impacting hatch timing and success, larval growth, and summer survival. While existing research shows that water temperature affects the timing of flannelmouth sucker spawning and hatching dates [1,17], no published studies define upper and lower tolerance ranges for flannelmouth sucker larvae once hatched. This study aimed to determine the critical thermal tolerances of larval flannelmouth suckers from a Gunnison River tributary in Colorado, USA, and compared temperatures for optimal hatch success and larval survival.
2. Materials and Methods
We collected adult flannelmouth suckers using backpack electroshocking units for streamside gamete collection from Cottonwood Creek (Delta, CO, USA). Cottonwood Creek is an intermittent tributary of Roubideau Creek, a perennial tributary to the Gunnison River. Most years, upwards of 3000 flannelmouth suckers migrate into Cottonwood Creek to spawn. Fish enter the creek in early April with the majority of the spawning occurring in May [18]. Daily average temperature data collected from Cottonwood Creek over seven seasons indicate that when eggs and larvae are present, daily average temperatures in the stream range from 11 °C to 22 °C. Our experiment reflects these temperatures with three acclimation temperatures of 11 °C, 16 °C, and 22 °C, described below.
2.1. Egg Collection
Gametes from twenty ripe, adult male (n = 10) and female (n = 10) flannelmouth suckers were stripped into dry containers. Adult fish were returned to the creek after gamete collection. Filtered creek water was added to each container and agitated for two minutes to induce fertilization. Eggs were poured into a fine-mesh aquarium net and transferred into a solution of filtered creek water and 400 ppm tannic acid for three minutes to prevent egg clumping. Eggs were then rinsed and transferred to a one-gallon jug with filtered creek water for transportation. All spawning and fertilization processes were conducted in the field. Within 24 h, all eggs were transported on ice maintaining a temperature of 15 °C to the Colorado Parks and Wildlife, Aquatic Toxicology Laboratory (Fort Collins, CO, USA).
2.2. Experimental Design
Three temperature-regulated water troughs (11 °C, 16 °C, and 22 °C) were set up in the laboratory with individual head tanks supplied with treated municipal water and regulated with chillers or heaters. Each water trough contained 15.3 L tanks (Figure 1) and received water from the head tanks at a flow rate of 0.11 L min−1. Tanks were fitted with one egg cup made of schedule 40 PVC pipes with a 0.5 mm screen floor. Egg cups were elevated on the sides of the tanks to ensure the eggs were at a depth of 30 mm inside each tank [10]. All tanks were supplied with atmospheric oxygen using airstones.

Figure 1.
Schematic diagram of the experimental setup. Fifteen 3 L tanks were placed in each of the three temperature treatment troughs (11 °C—blue; 16 °C—yellow; and 22 °C—red). Each tank was supplied with the designated water temperature, controlled in separate head tanks. Each tank contained 50 fertilized flannelmouth sucker eggs at the beginning of the experiment.
After the eggs arrived at the laboratory, they were treated with 1500 ppm formalin and rinsed with treated municipal water before 50 eggs were placed into each egg cup. We continued to treat the eggs and larvae with a 15 min static formalin treatment every three days through hatching and once per week until all fish reached the swim-up stage to prevent fungal growth. Eggs were checked daily to record mortality, hatching, and daily water temperatures. Egg mortality was determined when eggs appeared cloudy. Larvae were removed from the egg cups once hatched and placed into the coinciding tank. Larval mortality was counted daily, and dead larvae were removed to prevent fungus outbreaks in the tanks. Once 50% of the larvae reached swim-up, 2 mL of concentrated Artemia salina nauplii was added to each tank daily. A. salina was hatched with 25 ppt aerated salt water in a conical hatch tube for 24 h.
2.3. Critical Thermal Maxima and Minima
Critical thermal maxima (CTMax) and minima (CTMin) trials were conducted on the surviving 30-day post-swim-up (d psu) fish from the three temperature treatments. We followed the recommendations by Becker and Genoway [7] for thermal tests with the addition of high-fish-density trials, which have been shown to significantly reduce CTMax values [10]. Each CTMax and CTMin trial represented ten replicates of one fish per 2 L of water (low density) and ten replicates of 25 fish per 2 L of water (high density) for each of the three temperature treatments [10]. Unfortunately, we did not have enough 30 d psu larvae remaining in the 22 °C treatments to conduct ten trials of high fish density; thus, five trials were conducted for both CTMax and CTMin. Fish were netted and quickly placed into an individual, rectangular glass test tank with 2 L of water starting at the desired acclimation temperature (11 °C, 16 °C, or 22 °C) and desired fish density (1 fish or 25 fish). Fish were held in the glass tanks for five minutes before starting the experiment. 16B-series Love temperature controllers were programmed to change the water temperature ±3 °C per minute (18 °C per hour change; [9]). Water in individual test tanks was heated for CTMax trials with aquarium heaters connected to the temperature controllers or cooled with pumps connected to controllers that passed ice water through radiator pipes for CTMin. Pre- and post-trial temperatures from each test tank were recorded with NIST Traceable Lollipop thermometers that were calibrated before each test. Tanks were aerated with atmospheric air and stir bars were used to maintain a homogenous temperature throughout each of the test tanks. Dissolved oxygen was measured in each test tank before and after each trial and water was removed and replaced in each trial to start at the appropriate temperature. Each trial lasted until sustained LOE was observed (greater than 10 s; [19,20,21]). LOE was determined in high-density trials when half of the fish in a tank lost equilibrium during the trial [10]. After each trial, all fish recovered in a separate tank for up to 20 min [22], were euthanized with tricaine methanolsulfate (MS-222; Syndel, BC, Canada, were identified to larval stage, and were measured for standard length (SL; mm).
2.4. Larval Stage Identification
A subset of 30 d psu larvae from each temperature treatment (n = 150) were randomly selected from those preserved after CTM trials to identify the ontogenetic stage using an Olympic dissecting microscope. Staging followed Snyder and Muth [23] describing four identifiable stages: protolarvae (stage 1: no fin rays present); flexion mesolarvae (stage 2: few caudal fin rays present); post-flexion mesolarvae (stage 3: all caudal fin rays present); and metalarvae (stage 4: all caudal, dorsal, and anal fin rays present).
2.5. Partial Polygonal Temperature Profiles
A partial temperature polygon was created to represent the zone of temperatures that the larvae can survive in and how acclimation temperature affects that zone. To create the polygon, we combined data from the CTM experiments described above [19,24] and upper and lower lethal limit estimates using the Water Quality Control Commission (WQCC) correction factor of ±0.8 °C on the CTMin and CTMax temperatures at different acclimation temperatures for warm water species [11]. We connected the CTM LOE end temperatures and estimated lethal end temperatures for high- and low-density trials with separate regressions by upper and lower temperatures to produce a quadrilateral figure described with areal Celsius units per zone (°C2). The partial polygon was divided into three zones [24]: an optimal temperature tolerance zone independent of acclimation temperatures (Int: intrinsic temperature tolerance zone) and upper (Upt) and lower (LRt) acquired temperature zones (temperature tolerance gained by acclimation). The total area of the polygon was calculated by adding the areas (°C2) calculated for each of the three zones. The total area provides information to compare thermal profiles to other fish species.
Conte et al. [24] demonstrated at least three acclimation temperatures for both CTMin and CTMax values as well as upper and lower chronic thermal limits, which are required to create an accurate temperature tolerance polygon. Thus, data from temperature trials at all three acclimation temperatures (11 °C, 16 °C, and 22 °C) were used. We did not define the chronic high and low lethal temperature ranges of the larvae, so the full acclimation scope and thermal tolerance range of larval flannelmouth suckers were not identified, which are needed to create a full polygonal temperature profile; thus, a partial polygon was created.
2.6. Statistical Analysis
We calculated the probability of survival using a Kaplan–Meier survival model [25] starting from eggs to 30 d psu until the end of the experiment. We used a log-rank test to test for differences between the survival curves of each temperature treatment. This allowed us to understand overall survival during the hatch and larval rearing periods among the three temperatures. To determine if there were differences in hatch success and larval survival as a function of temperature treatment (11 °C, 16 °C, and 22 °C), we used an analysis of variance (ANOVA) with temperature treatment and assigned tanks as random variables. If a difference was detected, Tukey’s Honest Significance test was conducted. We also used a two-way ANOVA for an unbalanced design to determine if the density of fish and acclimation temperature interaction affected the upper (CTMax) and lower (CTMin) temperature limits. If there was evidence of a difference, a pairwise comparison using t-tests was used. Finally, ANOVA was used with larval stages and lengths of fish as functions of temperature treatments to determine if there were differences in stages or growth of the fish across the three temperatures. All analyses were performed in program R version 4.2.1 and significance was set at 0.05 .
3. Results
Acclimation temperatures in each water trough remained within the respective target temperatures. The 11 °C treatment held an average temperature of 11.1 ± 0.9 °C, the 16 °C treatment held an average 15.2 ± 2.6 °C, and the 22 °C treatment held an average temperature of 21.8 ± 1.0 °C.
3.1. Probability of Egg-to-Larval Survival
We used a Kaplan–Meier survival model to visualize an egg-to-larvae survival curve across temperature treatments from egg fertilization (day 1) through 30 d psu (Figure 2a). At time zero, the survival probability was 1.0 (i.e., 100% of the eggs are alive). The median survival time (50% mortality) in the 22 °C treatment was 30 days from egg to larvae. Fish in the 11 °C and 16 °C treatments never reached a point where 50% of the fish died. The log-rank test to determine a difference in survival gave a p-value of , indicating the temperature treatment groups differed significantly for egg-to-larval survival.
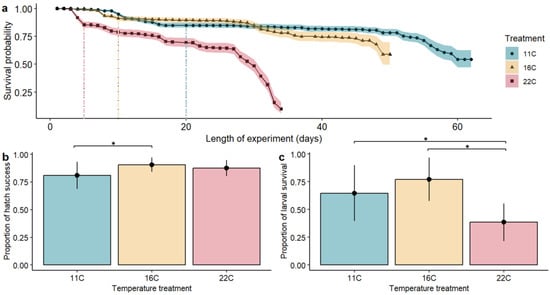
Figure 2.
(a). Kaplan–Meier survival plot of flannelmouth sucker eggs to larvae with three temperature treatments (11 °C—blue; 16 °C—yellow; and 22 °C—red), plotted daily over the 62-day experiment. Shaded areas represent 95% confidence intervals and dashed vertical lines represent when all eggs hatched for each treatment (11 °C—20 days; 16 °C—10 days; 22 °C—5 days). Survival post-hatch refers to the remaining larvae in each treatment. Hatch success (b) and larval survival (c) for the three temperature treatments. Vertical bars represent the standard deviation. Brackets with asterisks represent significant differences between treatments .
3.2. Hatch Success, Larval Survival, and Ontogenetic Stage
Time to peak hatch (50% of eggs hatching in a tank) from egg arrival doubled with decreasing temperatures (5 days to hatch at 22 °C; 10 days to hatch at 16 °C; 20 days to hatch at 11 °C; Figure 2a). Temperature treatment influenced the proportion of eggs that hatched (), whereas tank assignment did not (). Hatch success was greatest at 16 °C treatment but did not differ from 22 °C (11–16 °C ; 16–22 °C ; 11–22 °C ; Figure 2b). Larval survival was influenced by temperature treatment (), but not tank assignment (). Larval survival (30 d psu) was significantly greater at the 16 °C temperature treatment, with the lowest survival at the 22 °C treatment (11–16 °C ; 16–22 °C ; 11–22 °C ; Figure 2c).
We identified ontogenetic stages and standard lengths for 150 30 d psu larval fish from each temperature treatment. Larvae exhibited larger average lengths within the 11 °C treatment relative to the other treatments (11 °C: 15.2 mm, 16 °C: 15.0 mm, 22 °C: 14.5 mm: , ). Acclimation temperatures also affected ontogenetic stages. The 22 °C treatment contained more metalarvae (stage 4) than in the other treatments (p < 0.05, , whereas the 11 °C and 16 °C treatments contained more flexion mesolarvae (stage 2) and post-flexion mesolarvae (stage 3), respectively.
3.3. Critical Thermal Maximum and Minimum
Dissolved oxygen remained within 5% saturation during the CTM trials. Each CTM trial remained within the temperature ranges of the initial acclimation temperature targets before the CTM experiments started (10.9 ± 0.6 °C; 16.3 ± 1.1 °C; 21.9 ± 0.8 °C). Critical thermal maxima temperatures increased with acclimation temperature, ranging from 23.7 ± 2.4 °C to 34.0 ± 0.6 °C, as did CTMin temperatures, ranging from 6.3 ± 0.6 °C to 9.8 ± 0.4 °C (Table 1).

Table 1.
Critical thermal maxima (+0.3 °C min−1) and minima (−0.3 °C min−1) estimated from trials with flannelmouth sucker larvae for three acclimation temperatures (11 °C, 16 °C, and 22 °C) at two fish densities (low: 1 fish 2 L−1; high: 25 fish 2 L−1).
Differences in acclimation temperature and CTMax and CTMin trials indicate a difference in final LOE temperatures between all acclimation temperatures for CTMax (, Figure 3a) and CTMin (; Figure 3b). Fish density had an effect on CTMax at the 11 °C acclimation temperature (), indicating that fish at a higher density were less tolerant to warm temperatures than low-density fish. We did not detect a difference in the 16 °C and 22 °C acclimation temperatures (; Figure 3a). Similarly, fish density affected CTMin within the 16 °C () and 22 °C acclimation temperatures (), indicating that fish at a higher density were less tolerant of cold temperatures than low-density fish. We did not detect a difference in the 11 °C acclimation temperature (; Figure 3b). Different fish densities between acclimation temperatures of CTM trials also indicated a difference. Specifically, differences were detected between CTMax acclimation temperatures for both high () and low fish densities (), with post hoc analysis indicating a difference between 11 °C and 16 °C and between 11 °C and 22 °C among CTMax trials and between all acclimation temperatures for CTMin trials (Figure 3).
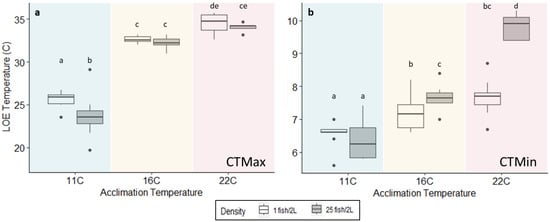
Figure 3.
Loss of equilibrium (LOE) of flannelmouth sucker larvae for (a) critical thermal maximum and (b) critical thermal minimum at three temperature treatments (11 °C, 16 °C, and 22 °C) and two fish densities (white = 1 fish 2 L−1; gray = 25 fish 2 L−1). Boxplot ranges represent the 25th and 75th percentiles; the band near the middle of the box represents the 50th percentile and the median value; whiskers range from the lowest to highest data ranges with points as outliers. Similar letters within the same figure (a,b) indicate no significance between average LOE temperatures, and different letters signify p < 0.05.
3.4. Polygonal Temperature Profile
Flannelmouth sucker larvae (30 d psu) exhibited a partial polygonal temperature profile of 251.7 °C2 (Figure 4) for the acclimation temperatures of 11 °C, 16 °C, and 22 °C. The area of the intrinsic tolerance zone was 195.5 °C2 (77.7% of the total area) and indicated an intrinsic tolerance zone (lnt) from 6.9 °C to 26.4 °C (dashed horizontal lines in Figure 4). The upper and lower tolerance zones were 49.5 °C2 and 6.7 °C2, respectively, making up 19.7% and 2.7% of the total area we examined. Upper and lower linear regressions indicate that as acclimation temperature increases by 1 °C, fish tolerate a temperature change of 0.86 °C, and as acclimation temperature decreases, fish tolerate a temperature change of 0.21 °C.
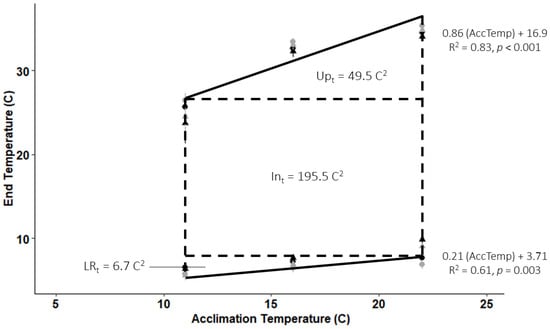
Figure 4.
A partial polygonal temperature profile generated from CTMax and CTMin (black data points) and upper and lower incipient lethal estimates for three acclimation temperatures using the WQCC conversion factor (gray data points) for flannelmouth sucker larvae. Points represent average end temperatures (°C; ±stdev) at each acclimation temperature from low-fish-density (●) and high-fish-density (▲) trials. End temperatures represent either the temperature for lethal estimates (gray data points) or loss of equilibrium temperature for CTM data points (black). The polygon includes three zones representing a partial temperature tolerance zone of 251.7 °C2 within the range of acclimation temperatures that we studied. Polygon zones include the optimal temperature zone independent of acclimation temperatures (Int: intrinsic temperature zone) and upper (Upt) and lower (LRt) temperature zones gained by acclimation (AccTemp)).
4. Discussion
The results of our study indicate that acclimation temperatures strongly influence the survival and upper and lower temperature ranges of larval flannelmouth suckers. On average, the CTMin and CTMax for high and low fish densities increased by 0.21 °C and 0.86 °C, respectively, for each 1 °C increase in acclimation temperature (Figure 4). The more proportional increase in CTMax than CTMin indicates that acclimation to cold temperatures is not as effective as acclimation to warm temperatures; this suggests larval flannelmouth suckers acclimate better to warmer temperatures than cold. This trend is expected, as tolerance ranges for desert fish tend to be narrow but more tolerant of high temperatures, given their warmer habitats [21].
Flannelmouth sucker larvae experience rapidly fluctuating temperatures that rise during the day, resulting in episodic stress. To effectively continue to conserve this species, management and policy decisions must be guided by data that define its thermal niche. Temperature trial data from experiments like CTMax and CTMin across a range of acclimation temperatures can describe the species’ polygonal temperature profile. Specifically, this polygon describes the optimal temperature range (intrinsic temperature range) and upper and lower acquired tolerance zones, which provide useful comparative indices of thermal tolerance ranges between species [9,26]. While the importance of acclimation temperature on upper and lower lethal temperatures is well established [26,27], polygonal temperature profiles further elucidate how acclimation temperature influences lethal limits [24]. Flannelmouth suckers showed smaller lower acquired tolerance zones versus upper acquired tolerance zones, suggesting temperature tolerance may confer short-term protection from extremely high temperatures. The larvae can survive static temperatures between 6.9 °C and 26.4 °C (optimal temperature zone) and acute exposures near 34.8 °C and 6.3 °C. Deacon et al. [28] reported higher CTMax values for juvenile flannelmouth suckers, ranging from 31.22 °C to 36.98 °C after acclimation to 10 °C and 25 °C, compared to the values we found at similar acclimation temperatures. This discrepancy may reflect potential regional differences in temperature tolerance, differences in larval and juvenile flannelmouth suckers, the exclusion of density as a factor in critical thermal methodology trials, or a combination of these factors.
Our acclimation temperature bounds that define the polygonal temperature profile do not define the full range of chronic lethal limits for flannelmouth sucker larvae, as including more extreme acclimation temperatures could reveal tolerance to additional temperatures extending the bounds of the polygon. However, the decreased survival observed at 22 °C and 11 °C suggests we are approaching those chronic limits, underscoring the importance of our data in identifying the potential optimal range for this species. Moreover, when comparing our findings to another recent study on the closely related bluehead sucker [10], the temperature ranges were similar. With an 18 °C acclimation temperature, bluehead suckers ranged from 7.2 ± 1.6 °C to 32.1 ± 2.4 °C, while our regressions for flannelmouth sucker larvae predicted limits ranging from 7.5 °C to 32.3 °C with the same 18 °C acclimation. Given the extensive overlap in spatial distribution and timing of reproduction, it is unsurprising that these two sucker species have comparable thermal tolerance ranges to larvae. This similarity further supports our findings as appropriate estimates of the optimal temperature ranges for flannelmouth sucker larvae.
Previous studies described potential temperature limits for flannelmouth suckers, but these were based on field observations measuring water temperature when larvae were present or absent rather than controlled laboratory experiments [29,30,31]. Reported egg and larval temperature ranges were 10–28 °C, with 20 °C deemed optimal for larvae [31]; however, these ranges were defined based on optimal growth, not survival. Our results showed lower hatch success at 11 °C, but all temperatures resulted in fairly high success. Larval survival was greatest at 16 °C. These optimal temperatures coincide with observations of high larval abundance in the field [1]. Our experiment was limited to only three acclimation temperatures; thus, testing a broader range of temperatures as well as defining chronic thermal limits may be needed to fully define the temperature limits for larval flannelmouth sucker survival.
Acclimation temperatures held an unanticipated effect on larval growth relative to the ontogeny stage. Our study found that larval flannelmouth suckers acclimated to cooler water temperatures developed more slowly but became larger than those acclimated to warmer waters. The differences in length and ontogenetic stages between acclimation temperatures were small but significant. Previous studies show that temperature can influence size at ontogenetic shifts, but the effects may be species-specific, with some species exhibiting larger sizes in warmer waters at more advanced ontogenetic stages [32], while others are smaller in warmer waters [33]. As early larval development is strongly affected by temperature through impacts on growth and survival [33], our laboratory results highlight the potential importance of water temperature in explaining variability in size and developmental stage during the larval phase.
We observed limited effects of fish density on CTM. Larval flannelmouth suckers in the 11 °C treatment had a significantly lower CTMax in high-density trials (25 fish/L) than when tested independently, and those in the 16 °C and 22 °C treatments had a significantly higher CTMin in the high-density trials than when tested independently. In a similar species, bluehead sucker, Riepe et al. [10] found that density effects on larval CTMs occurred at 18 °C acclimation temperature, and concluded that density was a stressor that should not be decoupled from thermal studies. Flannelmouth sucker larvae may experience physiological stress due to high cohort density at temperatures near the midpoint of their critical thermal range (16 °C). Both flannelmouth and bluehead sucker larvae hatch and develop in similar temporal and environmental conditions and use similar habitats, so biological explanations for the differences we observed are not apparent. For both species, however, the differences between low- and high-density trials observed consistently showed that the effect of density reduced the amount of warming or cooling tolerated. Therefore, we encourage continued evaluation of the effects of multiple stressors when defining thermal ranges for fishes, as many of these ranges may substantially differ when multiple stressors are present.
5. Conclusions
Our study found that the temperature tolerance range of larval flannelmouth suckers is narrow and their capacity to adapt to significant shifts in warm and cold water temperatures appears constrained. These desert fish seem predisposed to better withstand warming temperatures, rather than cooling, which is consistent with a common trait among species native to warmer environments. However, their limited cold tolerance and 6.9 °C–26.4 °C optimal temperature range suggest that exposures beyond this range could jeopardize their survival. While flannelmouth sucker larvae can withstand a wide spectrum of temperatures, their adaptability in rapidly changing environments should continue to be studied as stream temperatures continue to change.
Author Contributions
Conceptualization, T.B.R., Z.E.H.-U. and M.J.; methodology, T.B.R. and Z.E.H.-U.; formal analysis, T.B.R.; investigation, T.B.R., Z.E.H.-U. and M.J.; resources, T.B.R. and Z.E.H.-U.; data curation, T.B.R. and M.J.; writing—original draft preparation, T.B.R.; writing—review and editing, T.B.R., Z.E.H.-U. and M.J.; visualization, T.B.R.; supervision, T.B.R.; funding acquisition, T.B.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Colorado Parks and Wildlife, Species Conservation Trust Fund, grant number SCA23B.
Institutional Review Board Statement
Fish for this study were collected by Colorado Parks and Wildlife (CPW) Aquatic Research staff following the State of Colorado protocols for fish health inspections developed by and with the oversight of the CPW aquatic veterinarians and pathologists.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data available in this study are available upon request from the corresponding author. The data are not publicly available due to ethical reasons.
Acknowledgments
We are thankful to M. Sehlmeyer, J. Tauberman, C. Thorburn, C. Nicholson, B. Prior, and Z. Iverson for their valuable assistance in the field and R. Dils and R. Halford for fish care and larval fish staging. We are also thankful to T. Smith at the CPW Native Aquatic Species Restoration Facility for providing useful information on spawn take and egg care.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fraser, G.S.; Winkelman, D.L.; Bestgen, K.R.; Thompson, K.G. Temperature—Not flow—Predicts native fish reproduction with implications for climate change. Trans. Am. Fish. 2019, 148, 509–527. [Google Scholar] [CrossRef]
- Reynolds, W.W.; Casterlin, M.E. Behavioral thermoregulation and the “final preferendum” paradigm. Am. Zool. 1979, 19, 211–224. [Google Scholar] [CrossRef]
- Jobling, M. Temperature tolerance and the final preferendum—Rapid methods for the assessment of optimum growth Temperatures. J. Fish Biol. 1981, 19, 439–455. [Google Scholar] [CrossRef]
- Ho, P. Physiology and climate change. Science 2008, 322, 690–692. [Google Scholar]
- Moyano, M.; Candebat, C.; Ruhbaum, Y.; Alvarez-Fernandez, S.; Claireaux, G.; Zambonino-Infante, J.-L.; Peck, M.A. Effects of warming rate, acclimation temperature and ontogeny on the critical thermal maximum of temperate marine fish larvae. PLoS ONE 2017, 12, e0179928. [Google Scholar] [CrossRef]
- Fry, F.; Hart, J.; Walker, K. Lethal temperature relations for a sample of young speckled trout. Univ. Tor. Stud. Biol. Ser. 1946, 54, 9–35. [Google Scholar]
- Becker, C.D.; Genoway, R.G. Evaluation of the critical thermal maximum for determining thermal tolerance of freshwater fish. Environ. Biol. Fishes 1979, 4, 245–256. [Google Scholar] [CrossRef]
- Lutterschmidt, W.I.; Hutchison, V.H. The critical thermal maximum: History and critique. Can. J. Zool. 1997, 75, 1561–1574. [Google Scholar] [CrossRef]
- Beitinger, T.L.; Bennett, W.A.; McCauley, R.W. Temperature Tolerances of North American freshwater fishes exposed to dynamic changes in temperature. Environ. Biol. Fishes 2000, 58, 237–275. [Google Scholar] [CrossRef]
- Riepe, T.B.; Hooley-Underwood, Z.E.; McDevitt, R.E.; Sralik, A.; Cadmus, P. Increased density of bluehead sucker larvae decreases critical thermal maximum. N. Am. J. Fish. Manag. 2023, 43, 1135–1142. [Google Scholar] [CrossRef]
- Colorado Department of Public Health and Environment, Water Quality Control Commission. Temperature Criteria Methodology. (Policy Statement 06-1); Colorado Department of Public Health and Environment, Water Quality Control Commission: Denver, CO, USA, 2011.
- Rees, D.E.; Ptacek, J.A.; Carr, R.J.; Miller, W.J. Flannelmouth Sucker (Catostomus latipinnis): A Technical Conservation Assessment. USDA Forest Service, Rocky Mountain Region. Available online: http://www.fs.fed.us/r2/projects/scp/assessments/flannelmouthsucker.pdf (accessed on 6 May 2024).
- Bezzerides, N.; Bestgen, K. Status of Roundtail Chub Gila Robusta, Flannelmouth Sucker Catostomus Latipinnis, and Bluehead Sucker Catostomus Discobolus in the Colorado River Basin; Report to the US Bureau of Reclamation: Salt Lake City, UT, USA, 2002.
- McAda, C.W.; Wydoski, R.S. Growth and reproduction of the flannelmouth sucker, Catostomus Latipinnis, in the upper Colorado River basin, 1975–1976. Great Basin Nat. 1985, 45, 281–286. [Google Scholar]
- Bonjour, S.M.; Gido, K.B.; McKinstry, M.C.; Cathcart, C.N.; Bogaard, M.R.; Dzul, M.; Healy, B.D.; Hooley-Underwood, Z.E.; Rogowski, D.L.; Yackulic, C.B. Migration timing and tributary use of spawning flannelmouth sucker (Catostomus latipinnis). J Fish Biol. 2023, 103, 1144–1162. [Google Scholar] [CrossRef] [PubMed]
- Childs, M.R.; Clarkson, R.W.; Robinson, A.T. Resource use by larval and early juvenile native fishes in the Little Colorado River, Grand Canyon, Arizona. Trans. Am. Fish. 1998, 127, 620–629. [Google Scholar] [CrossRef]
- Fraser, G.S. Movement Patterns, Reproduction, and Potential Impacts of Climate Change on Three Native Fishes in the Upper White River Drainage, Colorado. Master’s Thesis, Colorado State University, Fort Collins, CO, USA, 2015. [Google Scholar]
- Hooley-Underwood, Z.E.; Stevens, S.B.; Salinas, N.R.; Thompson, K.G. An intermittent stream supports extensive spawning of large-river native fishes. Trans. Am. Fish. 2019, 148, 426–441. [Google Scholar] [CrossRef]
- Bennett, W.A.; Beitinger, T.L. Temperature tolerance of the sheepshead minnow, Cyprinodon variegatus. Copeia 1997, 1997, 77–87. [Google Scholar] [CrossRef]
- Selong, J.H.; McMahon, T.E.; Zale, A.V.; Barrows, F.T. Effect of temperature on growth and survival of bull trout, with application of an improved method for determining thermal tolerance in fishes. Trans. Am. Fish 2001, 130, 1026–1037. [Google Scholar] [CrossRef]
- Carveth, C.J.; Widmer, A.M.; Bonar, S.A. Comparison of upper thermal tolerances of native and nonnative fish species in Arizona. Trans. Am. Fish. 2006, 135, 1433–1440. [Google Scholar] [CrossRef]
- Cadmus, P.; Jefferson, A.L.; Townsend, A. Water Pollution Studies; Annual Progress Report; Colorado Parks and Wildlife: Denver, CO, USA, 2014. [Google Scholar]
- Snyder, D.E.; Muth, R.T. Catostomid Fish Larvae and Early Juveniles of the Upper Colorado River Basin—Morphological Descriptions, Comparisons, and Computer-Interactive Key; Technical Publication 42; Colorado Division of Wildlife: Fort Collins, CO, USA, 2004. [Google Scholar]
- Conte, M.; de Campos, D.F.; Eme, J. Effective practices for thermal tolerance polygon experiments using mottled catfish Corydoras paleatus. J. Therm. Biol. 2023, 115, 103616. [Google Scholar] [CrossRef]
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000. [Google Scholar]
- Rajaguru, S. Critical thermal maximum of seven estuarine fishes. J. Therm. Biol. 2002, 27, 125–128. [Google Scholar] [CrossRef]
- Beitinger, T.L.; Lutterschmidt, W.I. Encyclopedia of Fish Physiology—From Genome to Environment; Academic Press: Cambridge, MA, USA, 2011; pp. 1695–1702. [Google Scholar]
- Deacon, J.E.; Schumann, P.B.; Stuenkel, E.L. Thermal tolerances and preferences of fishes of the Virgin River system (Utah, Arizona, Nevada). Great Basin Nat. 1987, 47, 538–546. [Google Scholar]
- Bestgen, K.R.; Crist, L. Response of the Green River Fish Community to Construction and Re-Regulation of Flaming Gorge Dam, 1962–1996; Final Report of Colorado State University Larval Fish Laboratory to Upper Colorado River endangered fish recovery program; Colorado State University Larval Fish Laboratory: Denver, CO, USA, 2000. [Google Scholar]
- Clarkson, R.W.; Childs, M.R. Temperature effects of hypolimnial-release dams on early life stages of Colorado River basin Big-River fishes. Copeia 2000, 2000, 402–412. [Google Scholar] [CrossRef]
- Lamarra, V.A. San Juan River Fishes Response to Thermal Modification; A White Paper Investigation. Prepared for San Juan River Basin Recovery Implementation Program; US Fish and Wildlife Service: Albuquerque, NM, USA, 2007.
- Policansky, D. Influence of age, size, and temperature on metamorphosis in the starry flounder, Platichthys stellatus. Can. J. Fish. Aquat. Sci. 1982, 39, 514–517. [Google Scholar] [CrossRef]
- Fuiman, L.A.; Poling, K.R.; Higgs, D.M. Quantifying developmental progress for comparative studies of larval fishes. Copeia 1998, 3, 602–611. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).