Abstract
Crassostrea is the most farmed oyster genus worldwide and has significant economic and social impacts with environmental benefits. Hatchery oyster seed production is a highly costly phase, and a fluidized nursery system can help reduce this cost and reduce seed production time. The present study evaluated the survival and growth of two oyster species (Crassostrea gasar and Crassostrea gigas) in a fluidized bed bottle nursery system. With C. gasar, two experiments were performed; one tested three stocking densities and the other three bialgae diets. With C. gigas, one experiment with a bialgae and monoalgae in an initial bottle occupation of 8.8% produced more seeds per bottle, but an initial bottle occupation of 2.2% produced bigger seeds. Also, the experiment with C. gasar and with C. gigas tested diets did not affect seed survival, but the diets with bialgae I. galbana and N. oculate promoted more seed growth. The fluidized bed bottle nursery system developed for this study was adequate for the seeds of the oysters C. gasar and C. gigas in the nursery phase.
Key Contribution:
The fluidized bed bottle nursery system developed for this study was adequate for the oyster seeds of C. gasar and C. gigas in the nursery phase in the hatchery. All initial tested stocking densities can be used with the C. gasar and C. gigas seeds. The oyster seeds of C. gasar and C. gigas fed with a bialgae diet (I. galbana and N. oculate) showed high growth. The tested microalgae in monoalgae or bialgae diet affect more seed growth than survival. The fluidization velocity used in the present study for C. gasar and C. gigas showed a relationship with biomass in the bottle.
1. Introduction
The oyster genus Crassostrea (Sacco 1897) is the most cultured oyster group, with economic, social, and environmental importance. Around 26 species are in the genus Crassostrea [1] and according to FAO [2], Crassostrea gigas (Thunberg 1793) is the most cultured oyster species worldwide. In 2021, Crassostrea spp. production was 6.0 million tons, representing 33.1% of the world mollusk production in quantity [3] and 21.4% of the world mollusk production in value [4]. Seed oyster production in hatcheries has an important contribution to the large-scale culture of Crassostrea, especially of C. gigas, as in China [5] and Brazil [6]. In Brazil, besides Pacific oyster production, another oyster species farmed is the native oyster Crassostrea gasar (according to Ferreira et al. [7] the descriptor for C. gasar is Dillwyn 1817; syn. Crassostrea brasiliana and Crassostrea tulipa), commonly known as mangrove oyster, gasar oyster, and bottom oyster, among others.
Oyster hatchery production involves at least six key points: water supply, broodstock obtention and conditioning, larviculture, settlement and nursery, microalgae production for feed larvae, seed (spat), broodstock, and skilled staff. Settlement, and especially the nursery for early seeds, can be expensive. Depending on the culture system used, it requires a large quantity of microalgae, large areas, and a lot of human labor effort.
The hatcheries’ seed culture system (nursery) starts after larvae metamorphosis [7]. Larvae metamorphosis is generally induced with neurotransmitters, such as epinephrine, for single oyster hatchery production. When the metamorphosis is completed, the seeds are transferred to the nursery systems, which include the upwelling or downwelling systems [7]. Generally, the upweller and downweller use silos, which are containers with mesh in the bottom to hold the seeds and to permit water flow ascendant (upweller) or descendent (downweller), suspended in tanks. A combination of flow and seston concentration (i.e., microalgae) can affect the optimal stocking density used in the culture units [8,9], affecting oxygen and food availability and waste removal due to the water movement through the oyster bed. The upweller-using bottles are known as fluidized bed nurseries, generally used with oysters, and are capable of holding higher stocking densities than standard upwelling systems (i.e., silos). In this fluidized bed nursery system, oyster seeds are lifted by the flow [7] due to the fluidization of the bed. In high superficial flow velocity, the oyster’s seeds can be transported by the fluid and washed out from the culture unit. However, in intermediate velocities, each individual oyster is suspended in the fluid [10], inside of the culture unit. Many commercial hatcheries are using a fluidized bed system, but little has been published about the zootechnical performance of oyster seeds in this system. The fluidized bed nursery system was evaluated with the oyster Crassostrea virginica (Gmelin 1791) [10] and the clam Mercenaria mercenaria (Linnaeus 1758) [11].
Microalgae diets are another important aspect of the seed’s zootechnical performance during the nursery phase. The biochemical composition of microalgae can vary among species [12], where a combination of microalgae species in the diet needs to be considered to provide more nutrients for the oyster seeds. Monoalgae (single-species diets) and multialgae (mixed-species diets) diet have been studied for the oyster nursery phase, as for example monoalgae diets for C. gigas [13,14] and Ostrea edulis (Linnaeus 1758) [15] and bialgae diets for C. gigas [14], O. edulis [15,16], Crassostrea corteziensis (Hertlein 1951) [17], and Saccostrea commercialis (Iredale & Roughley 1933) [18].
In this sense, the zootechnical performance, measured by survival and growth, of oysters (C. gasar and C. gigas) in a fluidized bed bottle nursery system was evaluated. Two experiments with C. gasar were conducted: one testing stocking density and the other with bialgae diet; and one experiment with C. gigas testing bialgae and monoalgae diet in a fluidized bed bottle nursery system.
2. Materials and Methods
This study was performed at the Laboratory of Marine Mollusks of the Federal University of Santa Catarina (LMM-UFSC), located in Florianópolis, Brazil (27°35’06.35” S, 48°26’27.05” W). Single oyster seeds of the mangrove oyster C. gasar and the Pacific oyster C. gigas were obtained from the LMM-UFSC hatchery.
Three experiments were performed to evaluate oyster survival and growth (described below) in a fluidized bed bottle nursery system in a closed aquaculture system (FBBN-CAS). Two experiments were performed with the native mangrove oysters (C. gasar): the first (experiment I) tested stocking density and the second (experiment II) tested diet. A third experiment (experiment III) was performed with the Pacific oysters (C. gigas) to test the diet.
2.1. Fluidized Bed Bottle Nursery System in Closed Aquaculture System (FBBN-CAS)
Three identical fluidized bed bottle nursery (FBBN) systems in a closed aquaculture system (CAS) were developed for this study. Each FBBN system (Figure 1) was composed of cylinder-conical acrylic bottles (transparent; a total volume of 1.26 L with 0.9 L useful volume; experimental unit = EU), each EU with a shut-off valve (SOV; glass marble in the bottom of the bottle), two ball valve (BV) and one flowmeter (FW) coupled in the bottom, and a drain in the top of each bottle (BDR); a wood rack to fix the bottles and a PVC water drainer; a sump tank (SUMP; fiberglass; 664 L) with a magnetic pump (MP; Boyu, 5500 L.h−1); a distribution tank (Dtank; carboy; 20 L; steady head); and a feeding tank (Ftank; fiberglass; 250 L) with a peristaltic pump (PP; Seko Tekna EVO803; 20–54 L.h−1). The total volume of the system was 685 L. For the stocking density experiment (I), one FBBN-CAS system with three EUs (n = 3) for each treatment was used, totaling nine EUs randomly distributed in the FBBN-CAS system. For experiments with diet (II and III), three identical FBBN-CAS systems were used, each with four EUs (n = 4) randomly distributed in the system.
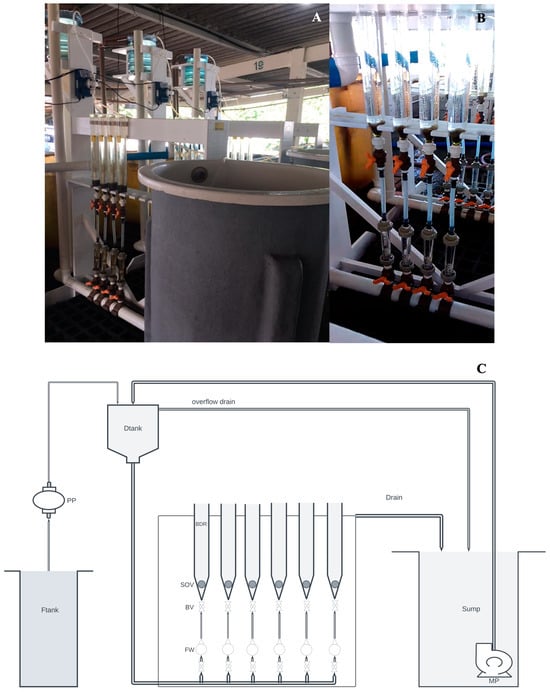
Figure 1.
Fluidized bed bottle nursery in closed aquaculture system (FBBN-CAS) system used in the experiments I, II, and III. (A), an overview of the FBBN; (B), a detail of the acrylic bottle and flow control system used; and (C), a schematic drawing showing water direction movement in the FBBN-CAS.
Seawater (filtered at 1 µm and sterilized with UV) was pumped from the sump tank to the distribution tank (highest level in the system) by gravity distribution through a pipe (lowest level in the system) to the bottom of each bottle, creating an ascendant flow, going from the bottom of the bottle, through the seeds, to the top drainers and returning to the sump tank by gravity. The distribution tank has an overflow. Drain microalgae were pumped (140 mL.min−1) from the feed tank by the peristaltic pump to the distribution tank. The flow was regulated through a flowmeter to maintain the seeds in suspension in the bottle. The flow of the inlet seawater in the bottles was fixed to be the same for all treatments in each experiment and regulated according to the seed’s growth (Table 1).

Table 1.
Mean and standard deviation of the temperature, salinity, pH, and residual algae; minimum and maximum temperature; and the experiment period in each experiment (I, II, and III), and the range of inlet seawater flow in the bottle in the sump of experiment I and in each treatment in experiments II and III, where Exp. = experiment; C = Crassostrea; D15 = 15 seeds.mL−1; D30 = 30 seeds.mL−1; D60 = 60 seeds.mL−1; IC = 50% of Isochrysis galbana and 50% of Chaetoceros muelleri; IN = 50% of I. galbana and 50% of Nannochloropsis oculata; RC = 50% of Rhodomonas salina and of 50% of C. muelleri; I = 100% of I. galbana; N = 100% of N. oculata; Min = minimum; and Max = maximum.
FBBS-CAS daily handling consisted of seawater (sump tank) and feed (feeding tank) exchange. For that, first, the shut-off valve of each bottle was closed to avoid seed escape, and then the pumps (magnetic and peristaltic) were turned off. The seawater from the sump tank and the feeding tank were drained, and both tanks were cleaned with fresh water before being refilled with seawater (sump tank) and microalgae (feeding tank). Before and after seawater exchange, the temperature (infrared sensor; Figure 2), salinity (refractometer: Kasvi), and pH (pHmeter; Alfakit AT-350) of the sump tank for each experiment were registered (Table 1).
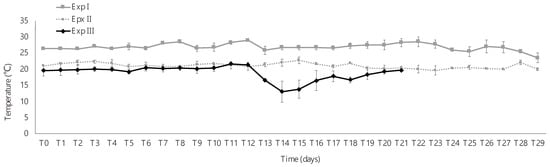
Figure 2.
Temperature (mean ± standard deviation) during the experimental period in experiments I, II, and III where Exp. = experiment and T = time.
2.2. Experiment I: Stocking Density (Crassostrea Gasar)
In experiment I, three initial stocking densities (D15, a stocking density of 15 seeds.mL−1 (20 mL of seeds; 2.2% of the total bottle volume occupation); D30, a stocking density of 30 seeds.mL−1 (40 mL of seeds; 4.4% of the total bottle volume occupation); and D60, a stocking density of 60 seeds.mL−1 (80 mL of seeds; 8.8% of the total bottle volume occupation)) of C. gasar seeds (1.98 ± 0.31 mm of shell height) in FBBS-CAS were tested. Each stocking density treatment was calculated as the percentage of the useful bottle volume filled with seeds. Experiment I lasted for 29 days, being T0 at the beginning of the experiment (planting day) and 29 days at the end (T29).
The seed diet during the experimental period was composed of two microalgae species, Isochrysis galbana (Parke 1949) (Iso) and Chaetocheros muelleri (Lemmermann 1898) (Cm), with a ratio of 30: 70 (Iso: Cm). The microalgae concentration started at 12 × 104 cells.mL−1 (1 to 5 days of the experiment), and increased to 15 × 104 cells.mL−1 (6 to 17 days), 20 × 104 cells.mL−1 (18 to 22 days), and 22 × 104 cells.mL−1 (23 to 30 days) according to the seed growth. The microalgae concentration was calculated using the total volume system (685 L). The microalgae concentration used in this experiment was chosen for the LMM protocol for C. gasar seeds maintenance. Daily, the residual microalgae concentration in the outlet seawater from the bottles was monitored (Table 1).
2.3. Experiment II: Diet (Crassostrea Gasar)
In experiment II, C. gasar seeds (1.77 ± 0.12 mm of shell height) were used to test three bialgae diets: IC, Iso, and Cm; IN, Iso, and Nannochloropsis oculata (Hibberd 1981) (N); and RC, Rhodomonas salina (Hill & Wetherbee 1989) (R), and Cm; with the proportion 1:1 of each microalgae species. The microalgae quantities in each treatment were calculated for 10% of the total seed whole weight (fresh weight) [16]. Weekly, the total microalgae quantities were adjusted to the total seed whole weight, maintaining the feed in 10% of the biomass. Experiment II lasted for 29 days. Each experimental unit was planted with 10 mL of seeds (7.5 seeds.mL−1). Daily, the residual microalgae concentration in the outlet seawater from the bottles was monitored (Table 1).
2.4. Experiment III: Diet (Crassostrea Gigas)
In experiment III, C. gigas seeds (1.68 ± 0.14 mm of shell height) were used to test three diets, two monoalgae diets (I: 100% of Iso; and N: 100% of N) and one bialgae diet (IN: 50% of Iso and 50% of N). The microalgae quantities in each treatment were calculated for 10% of the total whole weight of the seeds [16]. Weekly, the total microalgae quantities were adjusted to the total seed fresh weight, maintaining the feed at 10% of the total seed fresh weight. Experiment III lasted for 21 days. Each experimental unit was planted with 10 mL of seeds (7.5 seeds.mL−1). Daily, the residual microalgae concentration in the outlet seawater from the bottles was monitored (Table 1).
The dried microalgae weight for I. galbana and R. salina used in the present study is cited by Brown [14]; for N. oculata, we considered the dried weight of Nannochloropsis-like sp. CS-246 cited by Brown [14], and for C. muelleri, the dried weight is cited by Helm et al. [7].
2.5. Data Collection and Statistical Analysis
For data collection (Table 2), the seed number (total live seeds in each EU of each treatment) and survival (survival in each time in each EU of each treatment) in experiments I and II were measured after 14 and 29 days (T14 and T29, respectively) and in experiment III after 14 and 21 days (T14 and T21, respectively). For seed number quantification, the total volume of seeds from each EU for each treatment was measured, and after that, the seeds were sieved in four screen mesh (1, 2, 3, and 4 mm). Three samples (n = 3) from each screen (0.5 mL for the seeds retained in the screen 1 mm and below 1 mm, and 2.5 mL for the seeds retained in the screens 2, 3, and 4 mm) were taken. The sum of the number of live seeds from each screen was calculated to obtain the total number of seeds per EU. The seed survival (%) was quantified in each time sample (i.e., seed survival in T14 in relation to T0, seed survival in T29 in relation to T14 for experiments I and II, seed survival in T14 in relation to T0, and seed survival in T21 in relation to T14 for experiment III).

Table 2.
Analyzed parameters in each experiment (I, II, and III) in each time sampling (T), where C = Crassostrea; T = time; FV = fluidization velocity; TV = total volume of live seeds; IV = increase in volume of seeds; PSS = percentage of seeds by screen; TWW = total seed whole weight; and IWW = individual seed whole weight.
For seed growth analysis in the experiment with stocking densities (experiment I), the total volume (TV) of live seeds, the increase in the volume (IV) of seeds, and the percentage of seeds by screen (PSS) were quantified. IV was defined as the increase in the total volume of seeds in a container as a measurement of oyster growth. In the C. gasar diet experiment (experiment II), the seed growth per treatment was analyzed with TV, total seed whole weight (TWW) in each EU, and individual seed whole weight (IWW) at T14 and T29. For C. gigas diet experiment (experiment III), the seed growth per treatment was analyzed with TV, TWW, and IWW at T14 and T21, and shell height and shell length at T7, T14, and T21.
For TV per each EU in each treatment measurement graduated test tubes were used, and IV, the increase in the total volume (times more volume) of seeds compared to T0, was calculated according to Equation (1).
where
IV: increase in volume (times more volume of seeds than in T0)
TV: total volume of seeds in each EU at each time (T14 and T29 for experiments I and II and at T14 and T21 for experiment III; mL) for each treatment;
iV: initial volume of seeds planted at T0 (mL)
To analyze PSS, the following five size classes were used: (i) <#1: the seeds not retained in the screen of 1 mm (seeds < 1 mm); (ii) #1–2: the seeds retained the screens of 1 mm (seeds ≥ 1 mm and < 2 mm); (iii) #2–3: the seeds retained in between the screens of 2 mm (seeds ≥ 2 mm and < 3 mm); (iv) #3–4: the seeds retained in between the screens of 3 mm (seeds ≥ 3 mm and < 4 mm); and (v) ≥#4: the seeds retained in the screen of 4 mm (seeds ≥ 4 mm). These five size classes were used to calculate seed growth in total volume (TV). The percentage of seeds in each size class (<#1, #1–2, #2–3, #3–4, and ≥#4) was calculated with the total seed number in each size class and the total seed number per EU.
For TWW quantification, all animals from each EU were retained in a sieve (230 µm) and dried for 1 h over napkins (napkins were changed every 15 min to continue retaining water). They were then weighted (total whole weight) in an analytical balance (with a resolution of 0.001 g; Shimadzu, UX4200H). After weighing, seeds from each EU returned to their own bottle to continue the experiment.
For seed shell height and shell length, a sample (n = 30) from each EU for each treatment was tacked, and measurement was performed in microscopy (Leica; using software LAZ EZ 3.0.0) and stereomicroscope (Leica 4D) according to the size of the seeds.
Fluidization velocity (FV; cm·s−1), defined as the velocity that promotes oyster seed fluidization, was calculated for experiments II and III. According to Equation (2), the fluidization velocity was calculated for each flow in each treatment at T0, T14, and T29 (experiment II) and at T0, T14, and T21 (experiment III). The fluidization velocity was used for linear regression analyses.
where
FV: fluidization velocity (cm·s−1)
Q: flow (cm3.s−1) measured in the flowmeter
A: cross-sectional area of the bottle (cm2)
Seed specific weight (kg·m−3; Table 1) was calculated for each species using all data from each EU for experiment II (data from T0, T14, and T29; n = 28) and for experiment III (data from T0, T14, and T21; n = 36).
Seed number, survival, TV, IV, PSS, TWW, IWW, FV, shell height, and shell length data were tested for the basic assumptions for analysis of variance (ANOVA) using the Shapiro–Wilk test for the normality of residues and Levene’s test (for data with two factors) and Bartlett’s test (for data with one factor) for the homogeneity of variance. Pairwise comparisons of stocking density and sampling time means were carried out using Tukey’s test (p < 0.05) for parametrical data, the Wilcoxon test, and the Permutation with t-test for nonparametric data. The fluidization velocity was calculated and linear regression analysis was performed to evaluate the relationship between FV (y-axis) and TWW (x-axis) using all data at T0, T7, T14, T21, and T29 (experiment II) and at T0, T7, T14, and T21 (experiment III) for each flow. All statistical tests were performed in RStudio. The data of specific weight were not used for statistical analysis.
3. Results
3.1. Survival and Total Number of Seeds
The tested stocking density (D15, D30, and D60) did not significantly (p > 0.05) affect oyster survival after 14 and 29 days, with the final survivals (T29) of 95.3 ± 2.1%, 91.3 ± 3.4%, and 90.2 ± 6.5%, respectively, for the treatments D15, D30, and D60. The seed number in D60 was significantly (p < 0.05; Tukey’s test) higher than in D15 and D30 at T14 and T29 (Figure 2).
In experiment II, the diet did not significantly (p > 0.05) affect oyster survival and seed number after 14 and 29 days, with a final survival (T29) of 88.9 ± 2.8%, 94.2 ± 4.5%, and 89.6 ± 4.9%, respectively, for the treatments IC, IN, and RC. The seed numbers showed no differences between the treatments (Figure 3).
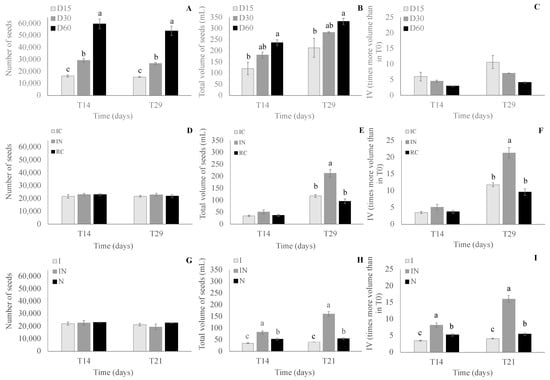
Figure 3.
Seed number, total volume, and times more volume than in T0 (increase in volume; IV) in experiment I (C. gasar; (A), (B), and (C), respectively) in each stocking density treatment (D15, D30, and D60) at T14 and T29, in experiment II (C. gasar; (D), (E), and (F), respectively) in each diet treatment (IC, IN, and RC, respectively) at T14 and T29, and in experiment III (C. gigas; (G), (H), and (I), respectively) in each diet treatment (I, IN, and N) at T14 and T21, where, D15: 15 seeds.mL−1; D30: 30 seeds.mL−1; D60: 60 seeds.mL−1; IC: 50% of Isochrysis galbana and 50% of Chaetoceros muelleri; IN: 50% of I. galbana and 50% of Nannochloropsis oculata (N); RC: 50% of Rhodomonas salina and of 50% of C. muelleri; I: 100% of I. galbana; N: 100% of N. oculata; and T = time. Different letters indicate statistical differences in each time sampling ((A, B, D, E, F, and G): Tukey’s test; (C, H, and I): Permutation t-test). The absence of letters represents that there were no significant differences between the treatments.
In experiment III, the diet showed a significant difference (p < 0.05; Tukey’s test) between survival at T14 and T21 independent of the treatment with higher survival at T14 (96.0 ± 5.7%) than at T21 (89.7 ± 7.9%). However, no differences were observed in the survival between the diet treatments (I, IN, and N) independent of the time sampling. No interaction was observed between the diets tested and time samplings in the survival. The final survival (T21) was 90.2 ± 4.4%, 82.7 ± 8.9%, and 96.2 ± 1.0%, respectively, for the treatments I, IN, and N. The seed numbers showed a significant difference (p < 0.05; Tukey’s test) between T14 and T21 independent of the treatment, with a higher number at T14 (22657 ± 1351 seeds) than at T21 (21169 ± 1874 seeds). However, no differences were observed in the seed numbers between the diet treatments (I, IN, and N) independent of the time sampling. No interaction was observed between the diets tested and time samplings in the seed numbers. The seed numbers at T14 and T21 showed no differences in all tested treatments (I, IN, and N; Figure 3).
3.2. Seed Growth
3.2.1. Experiment I
The total volume (TV) of the seeds increased significantly (p < 0.05; Tukey’s test) from T14 (179.2 ± 50.9 mL) to T29 (275.8 ± 54.8 mL), independent of the density, and the density, independent of the time sampling, also affect TV, being higher (p < 0.05; Tukey’s test) in D60 (283.7 ± 49.5 mL) than in D15 (167.2 ± 58.7 mL) and both not being different from D30 (231.7 ± 51.9 mL). No interaction was observed between the stocking density tested and time samplings in the TV. The seed TV in D60 was significantly (p < 0.05; Tukey’s test) higher than in D15, and both (D15 and D60), were not different from D30 (Figure 3) at T14 and T29.
The increase in volume (IV) was significant (p < 0.05; Tukey’s test) from T14 (4.5 ± 1.5) to T29 (7.3 ± 2.9) independent of the treatment. Also, the treatment affects IV independent of the sampling time in D15 (8.3 ± 2.9) and D30 (5.8 ± 1.3), with no differences between them, and both being higher (p < 0.05; Tukey’s test) than in D60 (3.5 ± 0.6). No interaction was observed between the stocking density tested and sampling times in the IV. At T14 and T29, the seed IV showed no difference between the treatments (Permutation t-test) (Figure 3).
Stocking density affected significantly (p < 0.05; Tukey’s test) the PSS in the size class <#1, #1–2, #2–3, and #3–4, independent of the sampling time. Analyzing sampling time, independent of the stoking density, a significant effect (p < 0.05; Tukey’s test) was observed in the size class <#1, #1–2, and #3–4. The PSS in the size class >#1 was higher (p < 0.05) in D30 compared to D15 and D60 at T14, and the PSS in D30 was higher (p < 0.05) than in D15 at T29. The PSS in the size class #1–2 was higher (p < 0.05) in D60 compared to D15, at T14 and T29, and compared to D30, at T29. The PSS in the size class #2–3 was higher (p < 0.05) in D15 and D30 compared to D60 at T14 and T29, and PSS in D30 was not different from in D60, at T14 and T29. The PSS in the size class #3–4 showed no differences between the tested stocking densities at T14 but was higher (p < 0.05) in D15 compared to D30 and D60 at T29. No seeds were observed in the size class ≥#4 at T14 and T29, and no differences were observed in the PSS between the tested stocking densities (Figure 4).

Figure 4.
Percentage (%) of seeds (C. gasar) by size class in each treatment (D15, D30, and D60) at T14 (A) and T29 (B) in experiment I, where D15: 15 seeds.mL−1; D30: 30 seeds.mL−1; D60: 60 seeds.mL−1. Different letters indicate statistical differences in each size class (A and B: Tukey’s test). The absence of letters represents that there were no significant differences between the treatments.
3.2.2. Experiment II
In experiment II, the total volume (TV) of the seeds increased significantly (p < 0.05; Tukey’s test) from T14 (41.9 ± 9.2 mL) to T29 (142.4 ± 52.1 mL), independent of the diet, and the diet treatment independent of the time sampling also affect TV, being higher (p < 0.05; Tukey’s test) in IN (132.1 ± 82.2 mL) than in IC (76.5 ± 41.7 mL) and RC (66.4 ± 30.4 mL). Interaction (p < 0.05) between the tested diets and time samplings in the TV was observed. At T14, no differences in TV were observed between the diets, but at T29, the seed TV in the IN diet was higher (p < 0.05; Tukey’s test) than in the IC and RC diets, and both (IC and RC) had no difference between them (Figure 3).
The increase in volume (IV) was significant (p < 0.05; Tukey’s test) from T14 (4.1 ± 0.9) to T29 (14.2 ± 5.2) independent of the treatment. Also, the treatment affected IV independently of the sampling time, with IN (13.2 ± 8.2) IV being significantly higher (p < 0.05; Tukey’s test) than in IC (7.6 ± 4.2) and RC (6.6 ± 3.0), both showing no difference between them. The interaction between the tested diets and time samplings in the IV was observed. At T14, no differences were observed between the treatments, but at T29, the IV of the diet treatment IN was higher (p < 0.05; Tukey’s test) than in IC and RC (Figure 3). The differences (p < 0.05; Tukey’s test) between time sampling (T14 and T29) in each treatment (IC, IN, and RC) were observed, and IV in each treatment at T29 was higher than at T14.
TWW was different (p < 0.05; Tukey’s test) from T14 (35.7 ± 6.4 g) to T29 (115.7 ± 36.1 g) independent of the treatment. Also, the diet treatment affected TWW independent of the sampling time, with it being higher (p < 0.05; Tukey’s test) in the IN diet (103.5 ± 61.5 g) than in IC (62.0 ± 31.5 g) and RC (61.4 ± 29.7 g). The interaction (p < 0.05) between the tested diets and the sampling times in the TWW was observed. At T14 and T29, the seed TWW in the diet IN were significantly (p < 0.05; Tukey’s test) higher than in IC and RC, and both (IC and RC) showed no difference between them (Figure 5).
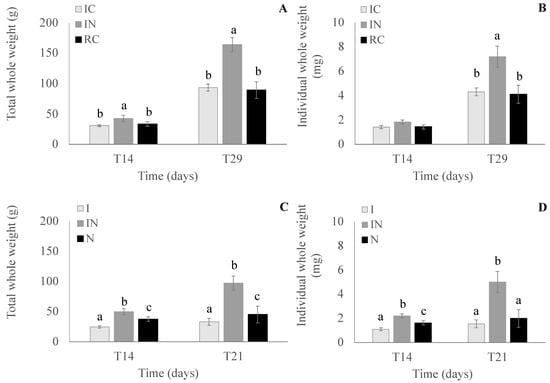
Figure 5.
Seed total whole weight and individual whole weight in experiment II (C. gasar; (A) and (B), respectively) in each diet treatment (IC, IN, and RC, respectively) at T14 and T29 and in experiment III (C. gigas; (C) and (D), respectively) in each diet treatment (I, IN, and N) at T14 and T21, where IC: 50% of Isochrysis galbana and 50% of Chaetoceros muelleri; IN: 50% of I. galbana and 50% of Nannochloropsis oculata (N); RC: 50% of Rhodomonas salina and of 50% of C. muelleri; I: 100% of I. galbana; N: 100% of N. oculate; and T = time. Different letters indicate statistical differences in each time sampling ((A,B,D): Tukey’s test; (C): Permutation t-test). The absence of letters represents that there were no significant differences between the treatments.
The IWW of the seeds increased significantly (p < 0.05; Tukey’s test) from T14 (1.6 ± 0.2 g) to T29 (5.2 ± 1.6 g), independent of the diet. The diet treatment, independent of the time sampling, affected IWW, with it being higher (p < 0.05; Tukey’s test) in IN (4.7 ± 2.7 mg) than in IC (2.9 ± 1.5 mg) and RC (2.8 ± 1.4 mg). The interaction (p < 0.05) between the tested diets and the sampling times in the IWW was observed. At T14, no differences in IWW were observed between the diets, but at T29, the seed IWW in the IN diet was higher (p < 0.05; Tukey’s test) than in the IC and RC diets and both (IC and RC) showed no difference between them (Figure 5).
3.2.3. Experiment III
In experiment III, total volume (TV) showed no significant difference between T14 (56.4 ± 19.8 mL) and T21 (85.6 ± 53.7 mL), independent of the diet. However, the diet treatment, independent of the time sampling, affected TV, with it being higher (p < 0.05; Wilcoxon test) in IN (121.19 ± 40.42 mL) than in I (38.1 ± 3.4 mL) and in N (53.7 ± 3.8 mL) diets and the TV of seeds in the diet treatment N being higher (p < 0.05; Wilcoxon test) than in I diet. The interaction (p < 0.05; Tukey’s test) between the tested diets and the time samplings in the TV was observed. The seed TVs at T14 and T21 were higher (p < 0.05; Permutation t-test) in the IN diet than in the I and N diets, and in the N diet were higher (p < 0.05; Permutation test) than in I diet (Figure 3).
The increase in volume (IV) showed no difference between time sampling T14 (5.6 ± 2.0) and T21 (8.5 ± 5.4) independent of the treatment. However, the treatment affected IV independent of the sampling time, with IV in IN (12.1 ± 4.0) being significantly higher (p < 0.05; Tukey’s test) than in I (3.8 ± 0.3) and N (5.4 ± 0.4) and IV in N being higher (p < 0.05; Tukey’s test) than in I. The interaction (p < 0.05; Tukey’s test) between the tested diets and sampling times in the IV was observed. At T14 and T21, the IV of the diet treatment IN was higher (p < 0.05; Permutation t-test) than in I and in N and IV in N was higher (p < 0.05; Permutation t-test) than in I (Figure 3). Differences (p < 0.05; Tukey’s test) between the time samplings (T14 and T21) in the diet treatments I and IN were observed, being at T21 higher (p < 0.05; Permutation t-test) than at T14 for both treatments, and for diet N, no differences in the IV between T14 and T21 were observed.
The seed TWWs showed no significant difference between T14 (37.3 ± 10.9 g) and T21 (58.5 ± 28.1 g), independent of the diet. However, the diet treatment, independent of the time sampling, affected TWW, being higher (p < 0.05; Wilcoxon test) in IN (73.6 ± 24.4 g) than in the I (28.8 ± 4.3 g) and in N (41.4 ± 4.6 g) diets and the TWW of seeds in the diet treatment N being higher (p < 0.05; Wilcoxon test) than in I. The interaction (p < 0.05) between the tested diets and time samplings in the TWW was observed. The seed TWWs at T14 and at T21 were higher (p < 0.05; Permutation test) in the IN diet than in the I and N diets, and in the N diet were higher (p < 0.05; Permutation test) than in the I diet (Figure 5).
The IWW of the seeds increased significantly (p < 0.05; Tukey’s test) from T14 (1.6 ± 0.5 mg) to T21 (2.8 ± 1.5 mg), independent of the diet. The diet treatment, independent of the sampling time, also affected IWW, being higher (p < 0.05; Tukey’s test) in IN (3.6 ± 1.4 mg) than in the I (1.3 ± 0.2 mg) and N (1.8 ± 0.2 mg) diets, and the IWW of the seeds in the diet treatment N being higher (p < 0.05; Tukey’s test) than in the I diet. The interaction (p < 0.05) between the tested diets and sampling times in the IWW was observed. The seed IWW at T14 was higher (p < 0.05; Tukey’s test) in the IN diet than in the I and N diets, and in the N diet, it was higher (p < 0.05; Tukey’s test) than in the I diet (Figure 5). At T21, the IWW of the seeds in the diet IN was higher (p < 0.05; Tukey’s test) than in the I and N diets, and in both (I and N) diets, IWW were not significantly different (Figure 5).
In the shell height and shell length analysis in experiment III, in the diet I, shell height showed significant (p < 0.05; Wilcoxon test) growth from T7 to T14 and no difference from T14 to T21, and shell length showed a significant (p < 0.05; Wilcoxon test) increase in shell length from T7 to T14 and decrease in shell length from T14 to T21. In the diet IN, the shell height and shell length showed significant (p < 0.05; Wilcoxon test) growth from T7 to T14 and from T14 to T21. In diet N, the shell height and shell length showed significant (p < 0.05; Wilcoxon test) growth from T7 to T14 and no difference from T14 to T21. Analyzing the shell height and shell length in each time sampling (T7, T14, and T21), high (p < 0.05; Tukey’s test) values were observed in the diet IN (Figure 6).
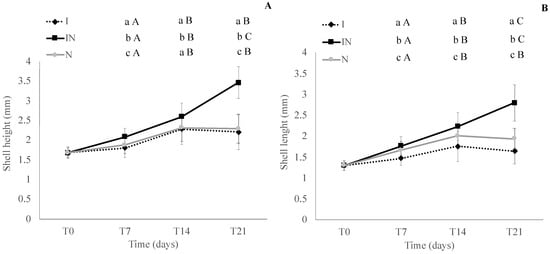
Figure 6.
Shell height (A) and shell length (B) (C. gigas) at T0 (beginning of the experiment) and after 7, 14, and 21 days (T7, T14, and T21, respectively) in each treatment (I, IN, and N) in experiment III, where I = diet with 100% of Isochrysis galbana; IN = diet with 50% of I. galbana and 50% of Nannochloropsis oculata; N = diet with 100% of N. oculate; and T = time. Different lower letters indicate statistical differences in each time sampling (T7, T14, and T21) comparing diets (I, IN, and N) (shell height at T7: Tukey’s test; shell height T14 and T21 and shell length at T7, T14, and T21: Wilcoxon test). Different capital letters indicate statistical differences in each diet treatment (I, IN, and N) comparing time sampling (T7, T14, and T21) (shell height in the diets I, IN, and N and shell length in the diet I and N: Wilcoxon test; shell length in the diet N: Tukey’s test). The absence of letters represents that there were no significant differences between the treatments.
3.3. Fluidization Velocity, Specific Weight, and Linear Regression of FV and TWW
The fluidization velocity (FV) varies from 0.7 to 2.5 cm·s−1 in experiment I, from 0.5 to 2.5 cm·s−1 in experiment II, and from 0.5 to 1.5 cm·s−1 in experiment III (Table 1). The general seed specific weight of C. gasar (experiment II) varies from 724.9 to 1004.2 kg·m3 with a mean of 860.3 ± 72.7 kg·m3 and of C. gigas (experiment III) from 571.4 to 841.6 kg·m3 with a mean of 706.5 ± 73.7 kg·m3.
The linear regression analysis of TWW and FV was significant (p < 0.05) in both experiments (II and III), with an R-squared of 0.8286 (adjusted R-squared of 0.8256) for experiment II with C. gasar and of 0.8644 (adjusted R-squared of 0.8414) for experiment III with C. gigas (Figure 7).
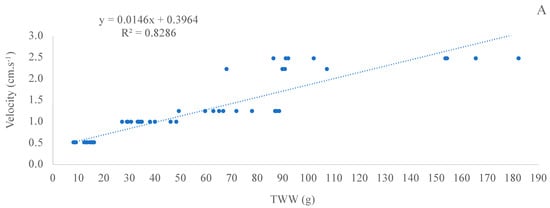
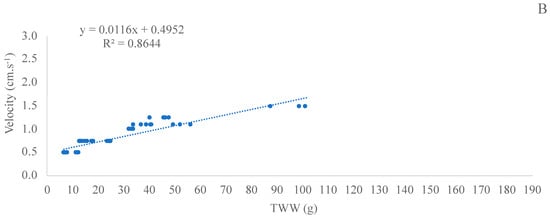
Figure 7.
Regression of fluidization velocity (cm·s−1) and total whole weight (TWW; g) in experiment II (C. gasar; A) and III (C. gigas; B) during the experiment period. Data of TWW and velocity used were obtained from T0, T7, T14, T21, and T29 (experiment II) and from T0, T7, T14, and T21 (experiment III) of all treatments and all experimental units.
4. Discussion
The seeds of oysters C. gasar and C. gigas showed high zootechnical performance in the fluidized bed bottle nursery in closed aquaculture system (FBBN-CAS) system used in the present study. The fluidized bed bottle system promotes faster oyster growth than the downwelling system [19]. The FBBN-CAS system designed for this study and flow used promote good conditions for seed development, where the seeds were constantly exposed to feed, observed by the microalgae concentration in the FBBN system before daily handling and by the constant biodeposits removed from the bottle observed in the outlet promoted by the seawater flow.
The fluidization velocity of the upward flow used in this study maintained the seeds fluidized in the bottle by visual analysis and demonstrated by the linear regression analysis was significant (with R-square values up to 80%). In the present study, the seeds of C. gasar with a shell height of 4.0 ± 1.7 mm (D60; experiment I) were fluidized with a velocity of 2.5 cm·s−1 and C. gigas with a shell height of 3.5 ± 0.4 mm (IN; experiment III) fluidized with a velocity of 1.5 cm·s−1, respectively. In a study developed by Ver and Wang [10] with C. virginica (6.5 mm of shell height), similar velocity (2 and 2.5 cm·s−1) was described as velocity at minimum fluidization maintained oyster seeds fluidized. The standardization of the FV by the seed TWWs in experiments II and III showed no variation between values, corroborating that the fluidization velocity used in the present study was adequate for the FBBN system tested.
The fluidization velocity of C. gasar and C. gigas in the FBBN system can be obtained from the linear regression equation presented in the present study with the TWW of seeds or by using specific weights to calculate TWW if the seed volume is known. The seeds of C. gasar showed a higher specific weight than C. gigas. More studies are suggested to evaluate the specific weight of this species in earlier seed stages and in different nursery culture systems.
For C. gasar, the initial bottle seed volumes with all tested stocking densities (D15, D30, and D60) can be feasible. The density of D60, which represents 8.8% of the total bottle volume, produces more seeds per bottle compared to D30 and D15, which represent 4.4% and 2.2% of the total bottle volume, respectively. Despite these higher seed numbers per bottle and consequently the higher total volume of seeds in the initial stocking density D60, oyster seeds in the density D15 showed more animals in the size class of #2–3 and #3–4 than in D60, suggesting that animals after 29 days grew more with the initial bottle area occupation of 2.2%. Both results are interesting for seed production, depending on the hatchery objective. If the aim is to produce a higher seed number or the hatchery has small infrastructures, higher initial stocking densities can be used. However, if the proposal is to produce a higher seed size, a low (2.2% of the bottle volume occupation) initial stocking density is recommended.
Higher growth in lower densities can be related to algae availability and feeding behavior in the bottle due to the relation between water flow and the number of seeds in each bottle. According to James [20], flow is more critical for oyster growth than population density. It could be possible that there was competition for food, even when residual algae was observed after each management. That flow in the high density promoted the fluidization of the seeds. Feeding rates and scope for growth analysis could help better understand seed growth dynamic in the different stocking densities tested.
Bialgae and monoalgae diets tested in the present study showed no effects on the seed’s survival and number, though these diets affected the seed’s growth. The bialgae diets with microalgae N. oculata improved the total volume of the seeds and increased the volume for both species (C. gasar and C. gigas). Seed growth of both oyster species with a bialgae diet of I. galbana and N. oculata was also observed by the high seed weight (total whole weight and individual whole weight) and shell height and shell length for both oyster species (C. gasar and C. gigas). The seed growth of both oyster species (C. gasar and C. gigas) observed in the bialgae diet of I. galbana with N. oculata can be related to the nutritional contribution of N. oculata. Analysis of fatty acid of N. oculata retorted that it is a microalgae rich in fatty acid, as in eicosatetraenoic acid (ETA 20:4n-3; [21]), eicosapentaenoic acid (20:5n-3; [22]) and palmitic acid (C16:0; [22]). Ohse et al. [23] observed that N. oculata has more total lipid content than C. muelleri, and Sühnel et al. [24] observed that I. galbana has more total lipid content than C. muelleri.
The bialgae diet, compared to the monoalgae diet, is preferred for better bivalve nutrition; however, even though feed was calculated by biomass as in experiment II, the appropriate selection of microalgae species for algae combination is important for better seed growth. Although Brown et al. [14] did not obtain good results using a monoalgae diet of Nannochloropsis-like sp. for C. gigas spat diet, in the present study, the monoalgae diet with the microalgae species N. oculate showed better zootechnical performance compared to the monoalgae diet with I. galbana for C. gigas.
The diet of I. galbana with C. muelleri did not provide the same nutritional value for the seeds to grow compared with N. oculata, as can be observed in seed weight growth with C. gasar. Lagreze et al. [25] observed that different combination of microalgae species in a bialgae diet promotes different survivals and shell growth of the clam Anomalocardia brasiliana (Gmelin 1791) larvae.
The combination of I. galbana and N. oculata was shown to be appropriate for both oyster species (C. gasar and C. gigas). Already for the clam A. brasiliana, Lagreze et al. [25] suggest a combination of N. oculata with C. muelleri, Pavlova lutheri (Green 1975; syn Diacronema lutheri) with C. calcitrans, and P. lutheri with C. muelleri. There is no one good microalgae combination for all bivalve species, but for each species and life cycle stage, this combination of two or more algae species in the diet needs to be evaluated. Diet of I. galbana with C. muelleri (IC) and R. salina with C. muelleri was unsuitable for C. gasar, with lower weight growth. However, if this microalgae species is the only species available, seed survival will not be affected by these diets (IC and RC) but will grow lower. For C. gigas, the monoalgae diet of I. galbana and N. oculata also promotes lower seed weight and shell growth than the bialgae diet.
Seed growth showed an increase in volume for the seeds fed with N. oculata compared to the seeds fed with I. galbana. However, the differences in seed volumes between both treatments are very low, as both monoalgae diets are not recommended if the hatchery objective is seed growth. However, if only one microalgae species is available, I. galbana and N. oculata can be used as monoalgae diets without affecting seed survival.
The reduction in seawater temperature, caused by cold weather (wintertime), observed from T14 to T21 in experiment III could explain the increasing residual algae in the treatments with N. oculata (IN and N). This fact can be related to the effect of temperature on seed clearance rate. Casas et al. [26] observed a lower clearance rate for C. virginica at 10 °C, compared to 20 °C and 30 °C.
The high survival rates observed in the present study for C. gasar (from 88 to 95%) and for C. gigas (from 82 to 96%) were also reported for other oyster species in the nursery phase (i.e., C. gigas, from 75 to 98%; and O. edulis, from 82 to 87%; [27]). The growth rate also demonstrated that the present configuration of the system is a good alternative for hatchery operation, reinforcing that FBBN is recommended to reduce hatchery space and optimize water use for oyster seed production. For C. gasar, the present study showed that the initial stocking density of 8.8% can produce high seed numbers, and the diet of I. galbana and N. oculate high seed growth for C. gasar and C. gigas in the nursery phase using the FBBN-CAS system.
5. Conclusions
In conclusion, the FBBN-CAS system developed for this study promotes a high zootechnical development for the seeds of C. gasar and C. gigas. For C. gasar, an initial stocking density of 8.8% of bottle occupation provides a higher seed number after 29 days of culture. Still, at 2.2% of the initial bottle occupation, bigger seeds can be achieved. All tested diets did not affect oyster seed survival, but the diet composition affects seed growth and the better seed growth of C. gasar and C. gigas can be achieved with a bialgae diet with I. galbana and N. oculata.
Author Contributions
Conceptualization, S.S., F.J.L.-S. and C.M.R.d.M.; methodology, S.S., F.J.L.-S. and C.M.R.d.M.; investigation, S.S., G.N.C., J.A., G.d.S., J.P.R.F., F.C.d.S. and C.H.A.d.M.G.; data curation, S.S., G.N.C. and G.d.S.; formal analysis, S.S., F.J.L.-S. and C.M.R.d.M.; writing—original draft preparation, S.S. and F.J.L.-S.; writing—review and editing, S.S., F.J.L.-S. and C.M.R.d.M.; supervision, F.J.L.-S. and C.M.R.d.M.; project administration, S.S. and C.M.R.d.M.; funding acquisition, S.S. and C.M.R.d.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Fisheries and Aquaculture grant number 003500063832013-82.
Institutional Review Board Statement
The study was bivalves, and the invertebrate animals do not need ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support this study are available from the corresponding author upon reasonable request.
Acknowledgments
We express out gratitude to the Ministry of Fisheries and Aquaculture (Process 003500063832013-82) for the post-doctoral scholarship for the first author (S. Sühnel) and project financial support. C.M.R. de Melo is a beneficiary of the “National Council for Scientific and Technological Development—Brazil (CNPq)” productivity fellowship (Process 305807/2022-6).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Guo, X.; Li, C.; Wang, H.; Xu, Z. Diversity and evolution of living oysters. J. Shellfish Res. 2018, 37, 755–771. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- FAO. Global Aquaculture Production Quantity (1950–2021). 2024. Available online: https://www.fao.org/fishery/statistics-query/en/aquaculture/aquaculture_quantity (accessed on 21 March 2024).
- FAO. Global Aquaculture Production Value (1950–2021). 2024. Available online: https://www.fao.org/fishery/statistics-query/en/aquaculture/aquaculture_value (accessed on 21 March 2024).
- Mao, Y.; Lin, F.; Fang, J.; Fang, J.; Li, J. Bivalve production in China. In Goods and Services of Marine Bivalves; Smaal, A.C., Ferreira, J.G., Grant, J., Petersen, J.K., Strand, Ø., Eds.; Springer Open: Cham, Switzerland, 2019; pp. 51–72. [Google Scholar] [CrossRef]
- Sühnel, S.; Picanço, T.; Medeiros, S.C.; Magalhães, A.R.M.; Melo, C.M.R. Effects of seeding data and seed size on Crassostrea gigas (Thunberg, 1793) culture in a subtropical climate. J. Shellfish Res. 2017, 36, 303–313. [Google Scholar] [CrossRef]
- Helm, M.M.; Bourne, N.; Lovatelli, A. Hatchery Culture of Bivalves. A Practical Manual; FAO Fisheries Technical Paper No. 471; FAO: Rome, Italy, 2004; pp. 5–166. [Google Scholar]
- Appleyard, C.L.; Dealteris, J.T. Growth of the northern quahog, Mercenaria mercenaria, in an experimental-scale upweller. J. Shellfish Res. 2002, 21, 3–12. [Google Scholar]
- Rodhouse, P.G.; O’Kelly, M. Flow requirements of the oysters Ostrea edulis L. and Crassostrea gigas Thunb. in an upwelling column system of culture. Aquaculture 1981, 22, 1–10. [Google Scholar] [CrossRef]
- Ver, L.M.B.; Wang, J.W. Design criteria of a fluidized bed oyster nursery. Aquacult. Eng. 1995, 44, 229–249. [Google Scholar] [CrossRef]
- Pfeiffer, T.J.; Rusch, K.A. An integrated system for microalgal and nursery seed clam culture. Aquacult. Eng. 2000, 24, 15–31. [Google Scholar] [CrossRef]
- Whyte, J.N.C. Biochemical composition and energy content of six species of phytoplankton used in mariculture of bivalves. Aquaculture 1987, 60, 231–241. [Google Scholar] [CrossRef]
- Langdon, C.J.; Waldock, M.J. The effect of algal and artificial diets on the growth and fatty acid composition of Crassostrea gigas spat. J. Mar. Biol. Assoc. 1981, 61, 431–448. [Google Scholar] [CrossRef]
- Brown, M.R.; McCausland, M.A.; Kowalski, K. The nutritional value of four Australian microalgal strains fed to Pacific oyster Crassostrea gigas spat. Aquaculture 1998, 165, 281–293. [Google Scholar] [CrossRef]
- Laing, I.; Millican, P.F. Relative growth and growth efficiency of Ostrea edulis L. spat fed various algal diets. Aquaculture 1986, 54, 245–262. [Google Scholar] [CrossRef]
- Ronquillo, J.D.; Fraser, J.; Mcconkey, A.J. Effect of mixed microalgal diets on growth and polyunsaturated fatty acid profile of European oyster (Ostrea edulis) juveniles. Aquaculture 2012, 360, 64–68. [Google Scholar] [CrossRef]
- Mazón-Suástegui, J.M.; Ruíz-Ruíz, K.M.; Parres-Haro, A.; Saucedo, P.E. Combined effects of diet and stocking density on growth and biochemical composition of spat of the Cortez oyster Crassostrea corteziensis at the hatchery. Aquaculture 2008, 284, 98–105. [Google Scholar] [CrossRef]
- O’Connor, W.A.; Nell, J.A.; Diemar, J.A. The evaluation of twelve algal species as food for juvenile Sydney rock oysters Saccostrea commercialis (Iredale & Roughley). Aquaculture 1992, 108, 277–283. [Google Scholar] [CrossRef]
- O’Connor, W.; Dove, M.; Finn, B.; O’Connor, S. Manual for Hatchery Production of Sydney Rock Oyster (Saccostrea glomerata); Nsw Department of Primary Industries: Cronulla, NSW, Australia, 2008; pp. 41–42. [Google Scholar]
- James, C. Soda Bottle Upweller System: Optimizing Production of Eastern Oyster Seed. 2022. Available online: https://projects.sare.org/wp-content/uploads/Coke-Bottle-Poster.pdf (accessed on 19 March 2024).
- Cheng, P.; Zhou, C.; Chu, R.; Chang, T.; Xu, J.; Ruan, R.; Chen, P. Effect of microalgae diet and culture system on the rearing of bivalve mollusks: Nutritional properties and potential cost improvements. Algal Res. 2020, 51, 102076. [Google Scholar] [CrossRef]
- Gu, N.; Lin, Q.; Li, G.; Tan, Y.; Huang, L.; Lin, J. Effect of salinity on growth, biochemical composition, and lipid production of Nannochloropsis oculata CS179. Eng. Life Sci. 2012, 12–16, 631–637. [Google Scholar] [CrossRef]
- Ohse, S.; Derner, R.B.; Ozório, R.A.; Correa, E.B.F.; Cunha, P.C.R. Lipid content and fatty acid profiles in ten species of microalgae. IDESIA 2015, 33, 93–101. [Google Scholar] [CrossRef]
- Sühnel, S.; Lagreze, F.; Pereira, A.; Da Silva, F.C.; Gurney-Smith, H.; Bercht, M.; Maraschin, M.; Magalhães, A.R.M.; Ferreira, J.F. Effects of astaxanthin on reproductive success in the tropical scallop Nodipecten nodosus (Linnaeus, 1758). J. Shellfish Res. 2014, 33, 89–98. [Google Scholar] [CrossRef]
- Lagreze, F.J.S.; Albuquerque, M.C.P.; Araujo, J.; Sühnel, S.; Melo, C.M.R. Survival and growth of the native clam Anomalocardia brasiliana (Gmelin, 1791) larvae in laboratory. Bol. Inst. Pesca SP 2015, 41, 133–143. [Google Scholar]
- Casas, S.M.; Figueira, R.; Lavaud, R.; Comeau, L.A.; LaPeyre, M.K.; Peyre, J.F. Combined effects of temperature and salinity on the physiology of two geographically-distant eastern oyster populations. J. Exp. Mar. Bio. Ecol. 2018, 506, 82–90. [Google Scholar] [CrossRef]
- Spencer, B.E.; Akester, M.J.; Mayer, I. Growth and survival of seed oysters in outdoor pumped upwelling systems supplied with fertilized sea water. Aquaculture 1986, 55, 173–189. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).