Abstract
Siniperca chuatsi rhabdovirus (SCRV) is a major strain of viral fish virus resulting in multiple transmissions and devastating damage in aquaculture. Currently, there are no available approved therapeutics. In this study, we screened and identified a novel curcumin analog (EF-24) for evaluating its in vitro anti-SCRV properties and potential molecular mechanisms. Present results demonstrated that EF-24 could strongly delay the occurrence of cytopathic effects (CPEs) in epithelioma papulosum cyprinid cells (EPCs) and inhibit SCRV replication and viral nucleoprotein expression in the early stages of infection by the time-of-addition assay. Furthermore, flow cytometry analysis after Annexin V-FITC/PI double staining and immunofluorescence microscopy observation after JC-1 incubation showed that EF-24 downregulated cell mitochondrial apoptosis induced by SCRV. The enzymatic activities of caspase-3 and caspase-9 were also reduced after EF-24 treatment, indicating that EF-24 may protect cells from SCRV infection by decreasing mitochondrial intrinsic apoptosis in infected cells. Collectively, we demonstrated for the first time that the curcumin analog EF-24 possesses antiviral ability against SCRV, suggesting its potential for effective control of fish rhabdovirus spreading.
Key Contribution:
We demonstrated for the first time that curcumin analogue EF-24 possessed antiviral abilities against SCRV, suggesting its potential for effective control of fish rhabdovirus spreading.
1. Introduction
As highly invasive viral pathogens, fish rhabdoviruses can infect a wide range of host species, causing high economic losses, and representing a serious threat to aquaculture due to the lack of approved antiviral treatments [1,2]. Hence, there’s a strong demand to develop antiviral measures to prevent infection by different strains of fish rhabdoviruses. Until now, researchers have focused more on its isolation, identification, molecular sequencing, phylogenetic analysis of different strains, susceptibility, and pathology to fish hosts [3,4,5,6]. In addition, other basic theoretical research on their pathogenic process, including the immunological and cellular modulation of different fish rhabdoviruses, was also investigated [7,8,9,10]. These measures provide solid data for potential antiviral techniques or drugs in the future.
Presently, to prevent and restrict viral infection in actual production, several fish rhabdovirus DNA vaccines have been developed in the lab [11,12]. Wang et al. also applied live hirame novirhabdovirus (HIRRV) vaccines under temperature-controlled conditions, aiming to induce protective immunity in flounder [13]. In addition, subunit vaccines against different dominant epitopes of fish rhabdoviruses were also developed in the laboratory [14,15]. However, we should note that until now, no commercially used vaccine is available in aquaculture, considering real multiple-factor water environments, complicated rhabdoviruses–host relationships, and the high cost of vaccines. So, developing new antiviral techniques or molecules for potentially inhibiting infection of fish with rhabdoviruses is an urgent demand.
Siniperca chuatsi rhabdovirus (SCRV) was isolated and characterized from cultured turbot Scophthalmus maximus Linaeus, which is associated with lethal hemorrhagic disease [16]. Mechanically, we conducted pathogenic mechanism research and found that SCRV infection activated autophagy and apoptosis in one fish cell line and epithelioma papulosum cyprinid cells (EPC), whose interplay was also investigated [17]. Moreover, we also screened potential antiviral molecules and studied their anti-SCRV activities according to regular protocols [2]. Among them, one curcumin analog, 3,5-bis[(2-fluorophenyl)methylene]-4-piperidinone (EF-24, molecular formula C19H15F2NO) [18], exhibited its high ability to inhibit SCRV replication in EPC cells. In this study, we concluded that EF-24 could block the occurrence of cytopathic effects (CPEs) in SCRV-infected cells and reduce progeny virus titers in the early stage of infection. In addition, EF-24’s virucidal activity was confirmed by time-in-addition tests on EPC cells. Furthermore, E-24’s antiviral mechanism against SCRV replication was also explored. These studies clearly provided a potential chemical candidate for the characterization and future application of antiviral molecules to fish rhabdoviruses.
2. Materials and Methods
2.1. Chemicals and Cell Culture
Curcumin analog EF-24 was purchased from Sigma-Aldrich (Merck KGaA, Whitehouse Station, NJ, USA) and dissolved in dimethyl sulfoxide (DMSO; stock solution concentration 40 mM). EPC cells were cultured with M199 (Procell, Wuhan, China) medium containing 10% FBS in 25 cm2 culture flasks at 24 °C in an incubator. SCRV virus was stored in our laboratory in a −80 °C refrigerator.
2.2. Cell Viability Assay
EPC cells were inoculated in 6-well plates and grown overnight, then treated with DMSO and a series of EF-24 concentrations (1.25–20 µM) at different times, respectively. Changes in cellular morphology were detected by inverted microscopy (Canon, Tokyo, Japan). An MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, 5 mg/mL; Sorabio Technology Co., Ltd, Beijing, China) assay was applied to determine and analyze cell viability. Cells were inoculated at 1 × 104 cells/well into 96-well plates overnight, and treated with a range of EF-24 concentrations (1.25–20 µM) at different times. Then, 20 µL of MTT was added to each well and incubated for 4 h at 37 °C. After discarding the original medium, 100 µL of DMSO was added to each well. The plate was shaken at 110 rpm in a shaded shaker. Absorbances were measured using a microplate spectrometer (TECAN SPARK, Hombrechtikon, Switzerland) with a detection wavelength of 490 nm.
2.3. CPE Observation
EPC cells were first inoculated with 6 × 105 cells and grown overnight in 6-well plates. Then, EPC cells were treated with EF-24 and SCRV (300 TCID50) in three exposure manners for 12 h, 24 h, 36 h, and 48 h, respectively, including pre-exposure (the culture medium with EF-24 was added first and sucked out after 2 h; then, the culture medium with SCRV was added), co-exposure (add mixture solution to EF-24 and SCRV), and post-exposure (the culture medium with SCRV was added first and sucked out after 2 h; then, the culture medium with EF-24 was added). CPEs in the EPC monolayer were observed and photographed under a microscope (Canon, Tokyo, Japan).
2.4. Virus Titer Determination
EF-24 and SCRV viruses were treated with EPC cells at different exposure sequences for 24 h and 48 h, and the cell supernatants were collected to detect virus titers. Briefly, EPC cells were inoculated into 96-well plates and grown overnight. The supernatants of the infected cells were diluted into nine different viral dilutions from “10−1” to “10−9” with M199 culture medium containing 5% FBS and inoculated into each well (100 µL). Six replicate wells were set up for each group. The CPE numbers of the cell monolayers were counted by light microscopy 4–5 days after incubation. The viral TCID50 values were calculated according to the typical Reed–Muench method.
2.5. Reverse Transcription-Quantitative PCR (RT-qPCR)
EPC cells were inoculated into 25 cm2 cell culture flasks and cultured overnight. EPC cells were treated with EF-24 and SCRV viruses at different exposure sequences for 12 h and 24 h. Total RNA samples of the treated cells were extracted according to an experimental procedure recommended by the reagent kit manufacturer (Beijing Tsingke Biotech Co., Ltd., Beijing, China) and then reverse-transcribed into cDNAs. PCR experiments were performed using the following primer sequences: N-F. ATCCATCAGATCACAGAACGC, N-R: TCCCAGCCATTCTCCTCAGTCC, 18s-F: CATTCGTATTGTGCCGCTAGA, 18s-R: CAAATGCTTTCGCTTTGGTC [17]. The thermal cycling conditions were as follows: pre-denaturation (30 s at 95 °C), followed by 40 extension cycles (10 s at 95 °C, 15 s at 60 °C), and solubility curves (15 s at 95 °C, 60 s at 60 °C, 15 s at 95 °C). RT-qPCR analysis was performed using the SYBR Green real-time fluorescent quantitative PCR system. Finally, the relative mRNA values of each group were calculated using the 2−∆∆ Ct method.
2.6. Western Blot
After infecting EPC cells with EF-24 and SCRV of different chronologies, infected cells were collected and lysed in a lysis buffer containing a protease inhibitor cocktail (MedChemExpress, Monmouth Junction, NJ, USA) and phenylmethylsulfonyl fluoride. Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The target proteins in the gel were then transferred to nitrocellulose membranes (Solarbio, Beijing, China). The NC membrane was blocked in TBS containing 5% skim milk powder for 1 h, then incubated with the primary antibody against N and β-actin (as the internal reference antibody) at 4 °C for 18 h, respectively. After washing with TBST solution, the membranes were incubated with the corresponding secondary antibodies (LI-COR Biosciences, St. Lincoln, NE, USA) for 1 h at room temperature. Immunoblots were processed using the Odyssey CLX infrared imaging system and their fluorescence intensity was analyzed using the Odyssey application.
2.7. Hoechst 33342 Staining
EPC cells were inoculated into 24-well plates (2 × 105/well) and cultured overnight. Following different exposures to EF-24 and SCRV, the cells were incubated with Hoechst 33342 solution (600 μL) for 15 min and then washed twice with PBS. Cells were observed and photographed under a microscope with ultraviolet-stimulated light. CCCP was used as a positive control.
2.8. Flow Cytometry Analysis
After treatment with EF-24 (5 µM) and SCRV, EPC cells were washed with PBS solution and collected for flow cytometry analysis. Infected cells were resuspended in binding buffer and incubated with annexin V-FITC (5 μL) and PI (10 μL) for 20 min at room temperature in the dark. As a Ca2+-dependent phospholipid binding protein with a high affinity for phosphatidylserine, annexin V binds to the membrane of early apoptotic cells through exposure to phosphatidylserine outside the cell. The samples were then analyzed by flow cytometry (FACS Calibur, Becton Dickinson, NJ, USA). Data analysis was performed using Flowjo software, v10.
2.9. Caspase Activities Assay
Caspase-3, caspase-8, and caspase-9 activities in treated cells were determined according to the protocols of the Enzyme Activity Kit (Beyotime Institute of Biotechnology, Shanghai, China). After incubating EPC cells with SCRV for 24 h, the cell supernatants were collected and incubated overnight at 37 °C in the dark. The light absorbance values of the samples were analyzed at 405 nm using a microplate spectrometer (Bio-Tek Epoch, Beijing, China).
2.10. Mitochondrial Membrane Potential Detection
EPC cells were inoculated into 12-well plates overnight at a ratio of 1 × 104 cells/well. After being treated with SCRV and EF-24 (5 µM) for 24 h, respectively, cells were used to determine mitochondrial membrane potential (MMP) by JC-1 staining according to the manufacturer’s instructions. The mitochondrial probe JC-1, a fluorescence probe that can quickly and sensitively detect potential membrane changes in cells, tissues, or purified mitochondria, can be used for early apoptosis detection. Treated cells were stained with JC-1 (Beyotime, China) staining solution at 37 °C for 30 min, followed by fluorescence microscopy (Nikon Eclipse TS200-U, Tokyo, Japan). Carbonyl Cyanide3-ChloroPhenylhydrazone (CCCP, Beyotime, Shanghai, China) was used as a positive control.
2.11. Statistical Analysis
All experiments were repeated at least three times, and all data were analyzed using SPSS software (version 21, IBM, Armonk, NY, USA). Two sets of data were compared using unpaired two-tailed Student’s t-test, while multiple sets of data were compared using one-way ANOVA combined with Tukey’s multiple comparison tests. The data are expressed as mean ± standard error of the number of experiments specified. * p < 0.05; ** p < 0.01 and p < 0.001 (***) denote statistically significant differences.
3. Results
3.1. EF-24 at Low Concentrations Was Not Toxic to EPC Cells
EPC cells were exposed to different concentrations (1.25, 2.5, 5, 10, 15, and 20 µM) of EF-24 for 12, 24, 36, and 48 h. Microscopic detection showed that EF-24 at low concentrations (1.25 µM, 2.5 µM, and 5 µM) did not significantly impact morphological changes in EPC cells (Figure 1A). The cell monolayer was relatively complete 48 h after treatment. EF-24 at high concentrations (>10 µM) significantly damaged the cell monolayer, causing cells to become round and shed. At 48 h, there were almost no intact adherent cells in the cell monolayer treated with EF-24 (20 µM). To further examine the cytotoxicity effects of EF-24 on EPC, an MTT assay was used to estimate cellular survival rates after EF-24 treatment. Our results showed that cell viability in low-concentration treatment groups (1.25 µM, 2.5 µM, and 5 µM) was still maintained at higher levels (Figure 1B), exhibiting no significant cytotoxicity to EPC cells. These findings offer a solid basis for detecting EF-24’s antiviral activities in SCRV-infected EPC cells.
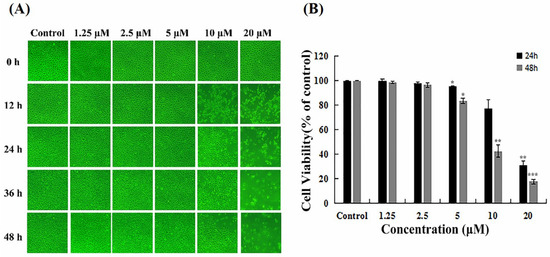
Figure 1.
EF-24 at low concentrations had no cytotoxicity to EPC cells. (A) Morphological changes in EPC cells after treatment with a series of EF-24 concentrations at different times. (original magnification: 100×). (B) Cell viability analysis of EPC cells treated with different EF-24 concentrations at 24 h and 48 h. (n = 3; * p < 0.05, ** p < 0.01 and *** p < 0.001).
3.2. Antiviral Activity of EF-24 against SCRV Replication in EPC Cells
To investigate the effects of EF-24 against SCRV progeny virus replication, we observed CPEs with different dosing temporalities in EPC cells. Here, a time-of-addition assay was conducted at different exposures. The results showed that compared to the other two groups, co-exposure of EF-24 and SCRV to EPC cells exhibited the best anti-viral effects on inhibiting replication, in which the cell monolayer was kept relatively complete at 24 h (Figure 2A). This finding indicated that EF-24 had direct virucidal activity on the SCRV virus and inhibited virus proliferation.
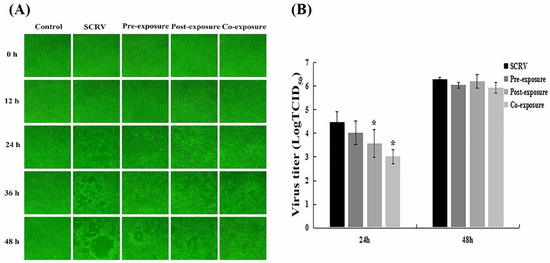
Figure 2.
EF-24 had direct virucidal activity on the SCRV virus in EPC cells. (A) Morphological changes in EPC cells from different exposure methods. (original magnification: 100×). (B) Virus titer analysis of EPC cell supernatants treated at different exposures for 24 h and 48 h. (n = 3; * p < 0.05). Pre-exposure, EF-24 was added first and sucked out after 2 h; then, the culture medium with SCRV was added. Post-exposure, SCRV was added first and sucked out after 2 h; then, the culture medium with EF-24 was added. Co-exposure involved adding the mixture solution to EF-24 and SCRV.
Furthermore, the cell supernatants were extracted from infected cells at 24 h and 48 h after the different exposure programs described above. Measurement of progeny virus titers by the Reed–Muench method showed that the co-exposure group also had a lower virus titer value (Figure 2B), especially at 24 h, which confirmed the antiviral effects of EF-24 and SCRV co-treatment.
3.3. EF-24 Inhibited SCRV Nucleoprotein Expression
The nucleoprotein (N) of rhabdoviruses plays a key role in viral replication. Hence, to identify the antiviral molecular mechanism of EF-24 against SCRV, we continued to explore transcriptional and expression changes in SCRV N in EPC cells at different exposure programs. Current RT-qPCR analysis showed that under all three treatment conditions (pre-exposure, post-exposure, and co-exposure), EF-24 effectively reduced N gene transcription (Figure 3A). Comparatively, in the co-exposure group, EF-24 was effective in decreasing N gene transcription at either 12 h or 24 h, with a statistically significant difference compared to the solely SCRV-infected group. Moreover, Western blot analysis demonstrated that the N protein also had a lower expression in EPC cells after co-exposure to EF-24 and SCRV (Figure 3B), which was consistent with the morphological observations and RT-qPCR results described above.
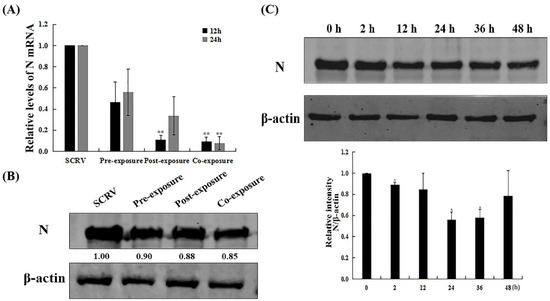
Figure 3.
EF-24 inhibited SCRV nucleoprotein expression in EPC cells. (A) RT-qPCR analysis of SCRV N mRNA at 12 h and 24 h with different exposure methods. (B) Western blot analysis of SCRV N protein at 24 h with different exposure methods. (C) Western blot analysis of SCRV N protein after co-exposure to SCRV and EF-24 at different time points. Bar graphs represent densitometric analysis of SCRV N protein to β-actin ratio. (n = 3; * p < 0.05, ** p < 0.01).
In addition, we detected expression changes in SCRV N protein after co-exposure to SCRV and EF-24 at different times (from 0 h to 48 h). The results showed that before 24 h, N expression presented a gradual downward, then upward trend at 36 h and 48 h (Figure 3C), which may be attributed to the release of more offspring viruses.
3.4. EF-24 Reduced Apoptosis Induced by SCRV Infection in EPC Cells
Cells were first analyzed by Hoechst 33342 staining. The results showed that typical apoptotic features, such as partial rupture and the crescent shape of the nucleus, reduced significantly in the co-exposure group with EF-24 and SCRV compared to the solely SCRV-infected group (Figure 4A, CCCP was used as a positive control), indicating that EF-24 could decrease apoptosis caused by SCRV.
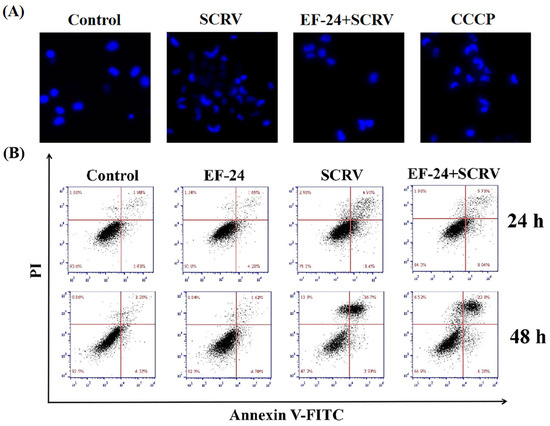
Figure 4.
EF-24 reduced SCRV-induced apoptosis in EPC cells. (A) Hoechst 33342 staining of cell nuclei after co-exposure to SCRV and EF-24. The number of cells with normal nuclear shape increased in the co-exposure group. CCCP treatment was used as a positive control (original magnification: 200×). (B) Flow cytometry analysis of EPC cells treated with different exposure methods. The number of early (AV staining) and late (PI staining) apoptotic cells significantly decreased in the co-exposure group at 24 h and 48 h, respectively.
In addition, cell apoptosis rates were counted by flow cytometry in the blank control group, EF-24-treated group, SCRV-infected group, and co-exposure to EF-24 and SCRV. The results showed that the apoptosis percentages in the solely SCRV-infected group were 18.5% and 39.4% (including early and late apoptotic cells) at 24 h and 48 h, respectively. EF-24 retarded cell apoptosis induced by SCRV to some extent, with cell apoptosis rates of 13.7% and 26.5% at 24 h and 48 h, respectively (Figure 4B). These findings also reaffirmed that EF-24 protects EPC cells from apoptotic cell death triggered by SCRV infection.
3.5. EF-24 Reduced Caspase-3 and Caspase-9 Activities in SCRV-Infected Cells
The cystatin family of proteases is closely associated with apoptosis. Changes in the enzyme activities of the main executive proteins of apoptosis (caspase-3, caspase-8, and caspase-9) after SCRV infection were examined in EPC cells. The results showed that effector caspase-3 activity increased 4.5-fold 24 h post-infection in the SCRV virus-infected group compared to the control group. By contrast, EF-24 significantly decreased caspase-3 activity in the co-exposure group compared to the only SCRV-infected group (Figure 5A).
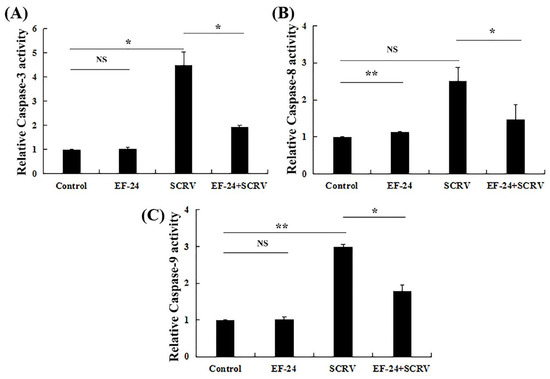
Figure 5.
EF-24 decreases caspase-3 and caspase-9 activities in SCRV-infected EPC cells. (A–C) show caspase-3, caspase-8, and caspase-9 activities and changes in cells treated with different exposure methods at 24 h, respectively. (n = 3; * p < 0.05, ** p < 0.01, NS, non-significant).
To explore potential apoptotic pathways, the activities of another two key caspases (caspase-8 and caspase-9) were also detected. Both enzymatic activities increased in the SCRV-infected group compared to the untreated group. However, there was no significant difference in caspase-8 activity compared to the control group (Figure 5B,C). Among them, caspase-9 activity increased 2.98-fold in the SCRV-infected group compared to the control group. Conversely, this value was significantly reduced in the co-exposure to SCRV and EF-24 compared to the SCRV-infected group (Figure 5C). Thus, we suggested that EF-24 might block the intrinsic mitochondrial apoptosis pathway activated by SCRV infection in EPC cells.
3.6. EF-24 Inhibited Mitochondrial Apoptosis in SCRV-Infected Cells
To further confirm EF-24’s inhibitory role in apoptosis induced by SCRV infection, we used a JC-1 probe to detect changes in EPC cells’ MMP. JC-1 produced red light in the matrix with high MMP, and green light when the potential was low. Current results demonstrated that green fluorescence signals were significantly enhanced in SCRV-infected cells, suggesting the activation of cellular mitochondrial intrinsic apoptosis. However, co-exposure to EF-24 and SCRV efficiently reduced green signal generation (Figure 6). These data indicated that EF-24 inhibited mitochondrial apoptosis in SCRV-infected EPC cells.
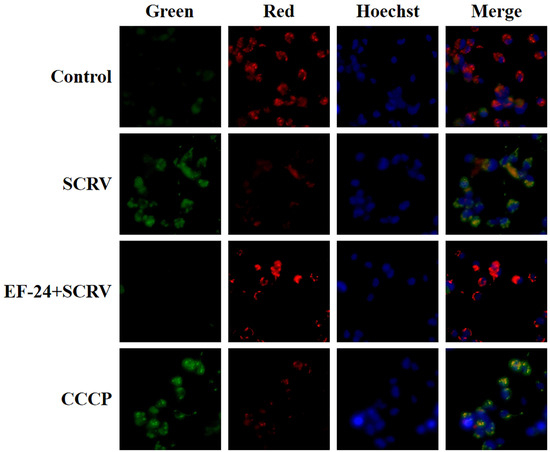
Figure 6.
Detection of MMP changes in EPC cells by different exposure methods. Co-exposure to EF-24 and SCRV effectively reduced green fluorescence signals in JC-1 staining cells. Green and red signals denote JC-1 monomers and JC-1 aggregates, respectively. The CCCP treatment group was used as a positive control, and Hoechst 33342 was used to stain the nuclei of treated cells. (original magnification: 200×).
4. Discussion
Over the past decades, several antiviral compounds or chemicals against fish rhabdoviruses were extracted or synthesized, including mixtures extracted from different organisms, proteins, nucleic acids, lipids and polysaccharides, and other chemical derivatives [19,20]. Recently, there have been reports on the screening and identification of new chemicals against fish rhabdoviruses, such as honokiol, bufalin, mangiferin, taurine, and so on [21,22,23]. But, apart from one commercially available protease, Neutrase®, which inactivates VHSV and koi herpesvirus (KHV) [24], most potential antiviral organic or inorganic substances have not been widely used in real aquaculture conditions due to time and space factors. Although significant progress has been made in identifying antiviral agents, new anti-fish rhabdovirus drugs or chemicals must be screened or characterized for their high mortality and transmission [20].
Curcumin has been intensively studied for its anti-tumor, antimicrobial, anti-inflammatory, and antioxidant pharmacological properties [25]. For its antiviral effects, some reports focused more on mediating curcumin’s antiviral activity through a variety of mechanisms. For example, curcumin inhibits the replication of Rift Valley fever virus by disrupting NF-κB signaling [26]. Curcumin also inhibits hepatitis C virus (HCV) replication by impairing viral binding and inhibiting virus particle entry [27]. In the field of aquatic virology [28], researchers found that curcumin could inhibit VHSV replication by suppressing viral entry via the rearrangement of the F-actin/G-actin ratio by downregulating the Hsc71 protein. However, low bioavailability restricts its utility, and novel different analogs with better pharmacokinetic and pharmacodynamic properties have been designed to overcome its limitations. As an important curcumin derivative, EF-24 has been characterized for its antiproliferative and anti-migration activities in different cancer cells by stimulating several cellular signaling pathways [29,30]. In this study, EF-24 was first applied to inhibit SCRV infection in fish cells, remarkably delaying the occurrence of CPEs in SCRV-infected cells. Further mechanistic studies indicated that EF-24 had virucidal activity against SCRV and demonstrated its most important inhibitory effects before viral entry. Considering the important role of nucleoproteins in rhabdoviruses’ replication cycles [31], their transcriptional and expression levels were also investigated in infected cells with EF-24 treatment. Our results suggested that the inhibition of transcription and translation of SCRV N could prevent progeny virus replication and decrease virus titers after EF-24 treatment. These findings are consistent with our results on SCRV N protein function by RNAi [31]. In fact, previous reviews of antiviral agents for fish rhabdoviruses suggested that several substances can suppress fish rhabdoviruses by directly inactivating viral particles, decreasing CPEs, inhibiting virus absorption (internalization), and blocking the expression of viral structural protein genes [20,32]. Our present study provides new experimental evidence for these theories.
Our previous report demonstrated that SCRV could induce mitochondrial apoptosis in EPC cells by increasing caspase-3 and caspase-9 activities, downregulating MMP, and increasing intracellular ROS [17]. Given its central role in cell apoptosis, MMP loss is a marker of mitochondrial apoptosis. In this study, our results demonstrated that EF-24 inhibited a SCRV-induced decrease in MMP through caspase-dependent mechanisms. In fact, many molecules that resist fish rhabdoviruses achieve their antiviral abilities by reducing cell apoptosis caused by virus infection [20]. For example, coumarin and arctigenin derivatives inhibit virus replication by inhibiting apoptosis in SVCV- or IHNV-infected cells [2,20]. Recent studies on honokiol’s antiviral mechanism against Micropterus salmoides rhabdovirus (MSRV) demonstrated that it significantly decreased virus titers and suppressed MSRV-induced apoptosis [21]. Hence, current research on EF-24’s antiviral effects against SCRV indicates a decrease in fish rhabdovirus-induced cell apoptosis for many chemical compounds and may be a common antiviral mechanism.
5. Conclusions
In conclusion, our study revealed that the curcumin analog EF-24 inhibited SCRV-induced mitochondrial apoptosis and blocked SCRV infection in the early stages of EPC cells. These findings provide a baseline for further studies on curcumin analogs’ anti-SCRV effects and anti-apoptosis mechanisms in experimental animal models infected with fish rhabdoviruses. Additionally, they might also be used as antivirus compounds in real aquaculture.
Author Contributions
Conceptualization, G.-Z.Z. and P.-M.J.; methodology, P.-M.J. and S.-W.M.; software, P.-M.J. and J.L.; validation, S.-F.Z. and Y.-Y.L.; formal analysis, P.-M.J.; investigation, P.-M.J., J.L. and S.-W.M.; resources, G.-Z.Z.; data curation, J.L.; writing—original draft preparation, P.-M.J. and S.-W.M.; writing—review and editing, J.L.; visualization, J.L.; supervision, G.-Z.Z.; project administration, G.-Z.Z.; funding acquisition, G.-Z.Z. and S.-W.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly supported by the Natural Science Foundation of Henan (232300420018), the Science and Technology Planning Project of Henan Province, China (222102110212), and the High-level Talents Fund from the Henan University of Technology (2023BS001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no financial/commercial conflicts of interest concerning this article.
References
- He, M.; Ding, N.Z.; He, C.Q. Novirhabdoviruses versus fish innate immunity. Virus Res. 2021, 304, 198525. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.C.; Li, B.Y.; Shen, Y.F.; Wang, T.; Wang, G.X. In vitro and in vivo inhibition of a novel arctigenin derivative on aquatic rhabdovirus. Virus Res. 2022, 316, 198798. [Google Scholar] [CrossRef]
- Huo, C.; Ma, Z.; Li, F.; Xu, F.; Li, T.; Zhang, Y.; Jiang, N.; Xing, W.; Xu, G.; Luo, L.; et al. First isolation and pathogenicity analysis of a genogroup U strain of infectious hematopoietic necrosis virus from rainbow trout in China. Transbound. Emerg. Dis. 2022, 69, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Perchun, A.V.; Pavlov, D.K.; Melnikov, V.P.; Antonychev, A.A.; Zinyakov, N.G.; Korennoy, F.I.; Moroz, N.V.; Chvala, I.A.; Metlin, A.E. Genetic characteristics of spring viraemia of carp virus strains Kirov/08 and Orenburg/14. Arch. Virol. 2022, 167, 681–685. [Google Scholar] [CrossRef]
- Sandlund, N.; Johansen, R.; Fiksdal, I.U.; Einen, A.C.B.; Modahl, I.; Gjerset, B.; Bergh, O. Susceptibility and Pathology in Juvenile Atlantic Cod Gadus morhua to a Marine Viral Haemorrhagic Septicaemia Virus Isolated from Diseased Rainbow Trout Oncorhynchus mykiss. Animals 2021, 11, 3523. [Google Scholar]
- Kim, S.S.; Kim, K.I.; Yoo, H.K.; Han, Y.S.; Jegal, M.E.; Byun, S.G.; Lim, H.J.; Park, J.S.; Kim, Y.J. Differential virulence of infectious hematopoietic necrosis virus (IHNV) isolated from salmonid fish in Gangwon Province, Korea. Fish Shellfish Immunol. 2021, 119, 490–498. [Google Scholar] [CrossRef]
- Li, C.; Shi, L.; Gao, Y.; Lu, Y.; Ye, J.; Liu, X. HSC70 inhibits spring viremia of carp virus replication by inducing MARCH8-mediated lysosomal degradation of G protein. Front. Immunol. 2021, 12, 724403. [Google Scholar] [CrossRef] [PubMed]
- Seong, M.S.; Jang, E.A.; Kim, J.; Kim, W.J.; Cheong, J. A single amino acid variation of NV protein of viral hemorrhagic septicemia virus increases protein stability and decreases immune gene expression. Fish Shellfish Immunol. 2021, 116, 84–90. [Google Scholar] [CrossRef]
- Liu, J.T.; Pham, P.H.; Wootton, S.K.; Bols, N.C.; Lumsden, J.S. VHSV IVb infection and autophagy modulation in the rainbow trout gill epithelial cell line RTgill-W1. J. Fish Dis. 2020, 43, 1237–1247. [Google Scholar] [CrossRef]
- Fu, X.; Ming, Y.; Li, C.; Niu, Y.; Lin, Q.; Liu, L.; Liang, H.; Huang, Z.; Li, N. Siniperca chuatsi rhabdovirus (SCRV) induces autophagy via PI3K/Akt-mTOR pathway in CPB cells. Fish Shellfish Immunol. 2020, 102, 381–388. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Lei, X.Y.; Zhang, Q.Y. The antiviral defense mechanisms in mandarin fish induced by DNA vaccination against a rhabdovirus. Vet. Microbiol. 2012, 157, 264–275. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Zhang, C.; Li, Y.; Zhang, P.Q.; Chen, G.; Wang, G.X.; Zhu, B. Immersion immunization of common carp with bacterial ghost-based DNA vaccine inducing prophylactic protective immunity against spring viraemia of carp virus. J. Fish Dis. 2021, 44, 2021–2029. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tang, X.; Xing, J.; Sheng, X.; Chi, H.; Zhan, W. Vaccination with live hirame novirhabdovirus (HIRRV) at temperature-controlled condition induced protective immunity in flounder (Paralichthys olivaceus). Microb. Pathog. 2021, 157, 104993. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.M.; Zhang, C.; Li, Y.; Chen, G.; Wang, G.X.; Zhu, B. Optimization of immunization procedure for SWCNTs-based subunit vaccine with mannose modification against spring viraemia of carp virus in common carp. J. Fish Dis. 2021, 44, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.X.; Zhao, Z.; Jin, Y.J.; Wang, Z.L.; Deng, J.F.; He, J.; Zhu, B. PEG-modified subunit vaccine encoding dominant epitope to enhance immune response against spring viraemia of carp virus. J. Fish Dis. 2021, 44, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.J.; Zhou, G.Z.; Gui, J.F.; Zhang, Q.Y. Genomic sequence of mandarin fish rhabdovirus with an unusual small non-transcriptional ORF. Virus Res. 2008, 132, 86–96. [Google Scholar] [CrossRef]
- Zhou, G.Z.; Li, J.; Sun, Y.H.; Zhang, Q.; Zhang, L.; Pei, C. Autophagy delays apoptotic cell death induced by Siniperca chuatsi rhabdovirus in epithelioma papulosum cyprinid cells. Viruses 2021, 13, 1554. [Google Scholar] [CrossRef]
- Monroe, J.D.; Millay, M.H.; Patty, B.G.; Smith, M.E. The curcuminoid, EF-24, reduces cisplatin-mediated reactive oxygen species in zebrafish inner ear auditory and vestibular tissues. J. Clin. Neurosci. 2018, 57, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Han, S.R.; Munangandu, H.M.; Yeo, I.K.; Kim, S.H. Bacillus subtilis inhibits viral hemorrhagic septicemia virus infection in olive flounder (Paralichthys olivaceus) intestinal epithelial cells. Viruses 2021, 13, 28. [Google Scholar] [CrossRef]
- Sun, S.S.; Ma, S.W.; Li, J.; Zhang, Q.; Zhou, G.Z. Review on the antiviral organic agents against fish rhabdoviruses. Fishes 2023, 8, 57. [Google Scholar] [CrossRef]
- Yang, F.; Yang, B.; Song, K.; Jin, Y.; Wang, G.; Li, P.; Yu, Q.; Ling, F. Natural product honokiol exhibits antiviral effects against Micropterus salmoides rhabdovirus (MSRV) both in vitro and in vivo. J. Fish Dis. 2024, 47, e12915. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, L.; Li, L.; Ren, G.; Shao, Y.; Liu, Q.; Lu, T. Traditional Chinese medicine bufalin inhibits infectious hematopoietic necrosis virus infection in vitro and in vivo. Microbiol. Spectr. 2024, 12, e0501622. [Google Scholar] [CrossRef]
- Dai, C.; Yu, L.; Wang, Z.; Deng, P.; Li, L.; Gu, Z.; He, X.; Wang, J.; Yuan, J. Mangiferin and taurine ameliorate MSRV infection by suppressing NF-κB signaling. Microbiol. Spectr. 2023, 11, e0514622. [Google Scholar] [CrossRef]
- Amtmann, A.; Ahmed, I.; Zahner-Rimmel, P.; Mletzko, A.; Jordan, L.K.; Oberle, M.; Wedekind, H.; Christian, J.; Bergmann, S.M.; Becker, A.M. Virucidal effects of various agents-including protease-against koi herpesvirus and viral haemorrhagic septicaemia virus. J. Fish Dis. 2020, 43, 185–195. [Google Scholar] [CrossRef]
- Lorenzo, R.D.; Forgione, F.; Bernardi, A.; Sacchi, A.; Laneri, S.; Greco, G. Clinical studies on topical curcumin. Skin Pharmacol. Physiol. 2023, 36, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, A.; Kehn-Hall, K.; Senina, S.; Lundberg, L.; Duyne, R.V.; Guendel, I.; Das, R.; Baer, A.; Bethel, L.; Turell, M.; et al. Curcumin inhibits Rift Valley fever virus replication in human cells. J. Biol. Chem. 2012, 287, 33198–33214. [Google Scholar] [CrossRef]
- Anggakusuma; Colpitts, C.C.; Schang, L.M.; Rachmawati, H.; Frentzen, A.; Pfaender, S.; Behrendt, P.; Brown, R.J.P.; Bankwitz, D.; Steinmann, J.; et al. Turmeric curcumin inhibits entry of all hepatitis C virus genotypes into human liver cells. Gut 2014, 63, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.H.; Vaidya, B.; Cho, S.Y.; Park, M.A.; Kaewintaju, K.K.; Kim, S.R.; Oh, M.J.; Choi, J.S.; Kwon, J.; Kim, D. Identification of regulators of the early stage of viral hemorrhagic septicemia virus infection during curcumin treatment. Fish Shellfish Immunol. 2015, 45, 184–193. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.H.; Liu, Y.T.; Zhang, Q.; Zhou, G.Z. Inhibition of autophagic flux by the curcumin analog EF-24 and its antiproliferative effect on MCF-7 cancer cells. J. Biochem. Mol. Toxicol. 2023, 37, e23307. [Google Scholar] [CrossRef] [PubMed]
- Su, S.C.; Hsin, C.H.; Lu, Y.T.; Chuang, C.Y.; Ho, Y.T.; Yeh, F.L.; Yang, S.F.; Lin, C.W. EF-24, a curcumin analog, inhibits cancer cell invasion in human nasopharyngeal carcinoma through transcriptional suppression of matrix metalloproteinase-9 gene expression. Cancers 2023, 15, 1552. [Google Scholar] [CrossRef]
- Zhou, G.Z.; Zhu, R.; Gui, J.F.; Zhang, Q.Y. Inhibition of Siniperca chuatsi rhabdovirus by RNA interference in a fish cell line. Fish Pathol. 2012, 47, 30–32. [Google Scholar] [CrossRef][Green Version]
- Liu, G.; Zhu, L.; Wu, Y.; Wang, C.; Wang, Y.; Zheng, Q.; Tian, M.; Wang, H.; Chen, Y. Herbal active small molecule as an immunomodulator for potential application on resistance of common carp against SVCV infection. Fish Shellfish Immunol. 2023, 137, 108782. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).