Abstract
This study evaluates the effects of dietary Clostridium butyricum on growth performance and intestinal health in triploid rainbow trout (Oncorhynchus mykiss). Administered in a 12-week trial, five isonitrogenous and isolipidic feeds contained different C. butyricum levels [G1 (0), G2 (1.6 × 106 CFU/g), G3 (1.2 × 107 CFU/g), G4 (1.1 × 108 CFU/g), and G5 (1.3 × 109 CFU/g)]. Significant enhancements in growth performance, including improved feed conversion ratios and specific growth rates in the G4 group, were indicative of enhanced nutrient utilization, corroborated by optimal digestive enzyme activity levels. Antioxidant capabilities were also enhanced in the G4 group, indicated by increased serum superoxide dismutase and catalase activities, along with a significant decrease in malondialdehyde levels. Gut microbiota analysis indicated a probiotic concentration-dependent modulation of microbial communities, with a marked enrichment in beneficial bacterial phyla like Firmicutes in the G4 group. This microbial shift correlated with significant downregulations in immune-related gene expressions, including interleukins (IL-1β, IL-8), NF-κB, MyD88, and TNF-α, highlighting an activated immune response. Correspondingly, serum lysozyme and immunoglobulin M contents were significantly elevated in the G4 group. Challenge tests with Aeromonas salmonicida exhibited the higher disease resistance of fish fed the G4 diet. In conclusion, the study shows the potential of dietary C. butyricum, especially at 1.1 × 108 CFU/g, in enhancing the growth, health, and disease resistance of triploid rainbow trout through modulating gut microflora and stimulating immune responses.
Keywords:
triploid rainbow trout; C. butyricum; low-fish-meal diet; growth performance; intestinal health Key Contribution:
Dietary supplementation with C. butyricum at 1.1 × 108 CFU/g significantly enhances growth performance; stimulates immune responses, and improves intestinal health in triploid rainbow trout, offering a promising strategy for aquaculture nutrition optimization.
1. Introduction
As global demand for aquacultured fish increases, the need for sustainable feed alternatives becomes critical [1,2]. With the continuous advancement of aquaculture technologies and economic benefits, the demand for fish meal, a high-quality protein source, has increasingly surged. Consequently, the search for suitable alternatives to fish meal in aquafeeds has become a topic of significant interest within the industry [3,4]. Soybean meal, as a plant protein source, offers advantages such as relatively high protein content, widespread availability, and lower cost, positioning it as an excellent alternative to fish meal [5]. However, the presence of antinutritional factors in soybean meal, including protease inhibitors, antivitamins, and allergens, can impair aquatic animals’ ability to absorb nutrients from feed, subsequently diminishing growth performance [6]. Research has shown that supplementing feed additives in diets where plant protein sources like soybean meal replace fish meal can effectively mitigate the adverse effects associated with plant protein substitution [7]. This approach enhances the utilization ratio of plant proteins in feeds, therefore contributing to more sustainable and cost-effective aquaculture practices.
Probiotics, as effective feed additives, have demonstrated advantages in promoting growth, enhancing feed utilization rates, and bolstering the immune system in fish. Despite these benefits, the literature reveals certain gaps, particularly in the specific applications of different probiotic strains and their optimal concentrations for different fish species. Many studies have generalized the effects of probiotics without delving into strain-specific responses or the ideal dosages required to elicit optimal health benefits in specific fish species [8]. Moreover, the mechanisms underlying the probiotic action, especially in relation to immune modulation and gut microbiota balance, are often not detailed, leaving a gap in the comprehensive understanding necessary for tailored probiotic applications in aquaculture.
Clostridium butyricum, capable of anaerobic growth and spore production, exhibits resilience to heat, acid, alkali, and high temperatures, categorizing it among the spore-forming probiotics [9]. C. butyricum has been identified as a superior probiotic in aquaculture due to its robust spore-forming ability that ensures survival under harsh conditions and its proven efficacy in enhancing growth, immune responses, and gut health, surpassing other commonly used probiotics [10,11]. Supplementing with appropriate amounts of C. butyricum has been shown to effectively maintain gut health in fish. On a cellular level, C. butyricum not only sustains cell viability but also increases mucin secretion; regarding the intestinal mucosa, it promotes repair and reduces permeability, aiding in the restoration of physiological functions [7]. This enhancement of mucosal barrier function by C. butyricum is crucial as it directly influences the composition and function of the gut microflora, establishing a more stable and beneficial microbial community that enhances overall gut health. The gut microflora’s dysbiosis can lead to various diseases, while C. butyricum fosters the proliferation of beneficial bacteria such as bifidobacteria, lactobacilli, and pseudobacilli and inhibits the growth of harmful bacteria like Staphylococcus, Candida, Klebsiella, Campylobacter, Pseudomonas, Escherichia coli, Shigella, Salmonella typhi, and putrefactive bacteria, therefore reducing the production of harmful substances within the organism [12]. Studies in hybrid tilapia (Oreochromis niloticus × O. aureus) [11] carp (Cyprinus carpio) [13] have revealed the significant role of C. butyricum in affecting aquatic animals’ growth performance, physiological and biochemical indicators, modulation of gut microflora, and enhancement of immunity. Current research on C. butyricum primarily focuses on fish meal-based feeds, with limited studies in plant protein substitutes. The potential of C. butyricum and its metabolites to repair intestinal damage caused by plant protein substitutes and to improve growth performance, antioxidative capacity, and immunity under such conditions is less reported.
Oncorhynchus mykiss, classified under the order Salmoniformes, family Salmonidae, and genus Oncorhynchus, is a prominent species among cold-water fishes cultivated in China. Notably, triploid rainbow trout obtains consumer preference for its rapid growth, efficient feed conversion, high muscle yield, lack of intramuscular bones, and palatable flesh compared to its diploid [14]. Despite the rapid advancement in aquaculture, the industry confronts challenges such as the limited availability of high-quality protein sources for feed, environmental contamination affecting water quality, and the widespread occurrence of diseases. By optimizing feed formulations and supplementing effective additives, it is feasible to sustain optimal nutrition and growth in rainbow trout, even with reduced fish meal content. Previous nutritional assessments in our laboratory have shown that triploid rainbow trout can maintain normal growth and development with a fish meal inclusion level of 15% [15,16]. However, research into the effects of C. butyricum supplementation in low-fish-meal diets, particularly its impact on the immune response, intestinal inflammation-related gene expression, and microflora in triploid rainbow trout, remains scarce. Therefore, this study investigates the effects of different levels of C. butyricum in low-fish-meal diets on the growth, digestive physiology, immune functions, and gut microflora of triploid rainbow trout. These findings aim to provide a basis for the dietary formulation of trout feeds.
2. Materials and Methods
2.1. Fish Management
The triploid rainbow trout (initial body weight: 259.73 ± 0.80 g) were sourced from the Benxi Aigemolin Rainbow Trout Farming Company in Liaoning Province (Benxi, China). At the start of the trial, trout were acclimatized to the experimental conditions for 15 days. Subsequently, the fish were randomly allocated into 15 tanks, each with a capacity of 500 L and 20 fish per tank. Water quality parameters were monitored once a day. During the 12-week trial, the tanks were maintained under a relatively constant dissolved oxygen level (>6.0 mg/L), temperature (18.0–20.0 °C), pH (6.5–6.8), ammoniacal nitrogen level (<0.02 mg/L), and photoperiod (12 h light: 12h dark). The fish were fed four times daily (6:00, 9:00, 14:00, 16:00) until apparent satiety.
2.2. Diets
Five isonitrogenous and isolipidic diets supplemented with different levels of C. butyricum [G1 (0), G2 (1 × 106 CFU/g), G3 (1 × 107 CFU/g), G4 (1 × 108 CFU/g), and G5 (1 × 109 CFU/g)] were formulated. The C. butyricum, provided by Hangzhou Huajia Biotechnology Co., Ltd. (Hangzhou, China), had a bacterial concentration of 1 × 109 CFU/g. This specific strain is referred to as C. butyricum CICC 24854. For feed preparation, the probiotic powder was first homogenized with a small quantity of the base feed to ensure even distribution. This premix was then thoroughly blended with the remaining feed components using a paddle mixer to maintain uniformity. The formulated diets utilized fish meal and soybean meal as the primary protein sources, supplemented with compound amino acids and brewer’s yeast. Fish oil and soybean oil were utilized as lipid sources, while wheat middings served as the carbohydrate sources (Table 1). The diets were passed through a 250-µm sieve. The dry, finely ground feed components were completely blended with lipids and then homogenized using a blender (GYJ-250B; Dashiqiao Bao’s Feed Machinery Factory; Yingkou, China). The dough was pelleted into 3.0-mm (diameter) granular feed using a lab pelletizer (SLP-45; Fishery Mechanical Facility Research Institute, Shanghai, China). The pellets were then air-dried to a moisture content of approximately 10% by weight, collected in vacuum-packed bags, and stored at −20 °C until use.

Table 1.
Composition and nutrient levels of the basal diet (air-dried basis, %).
2.3. Sample Collection
The fish were starved for 24 h before sampling. Anesthesia was administered using 40 mg/L of MS-222. After that, samples from the blood, whole body, liver, and mid-section of the intestine were collected. From each tank, four fish were chosen to analyze body composition and examine blood chemistry and liver antioxidant capacity. Three fish were selected from each tank for mid-intestine (the middle section of the intestine after the pyloric caecum) gene expression and digestive enzyme analysis. Blood was collected from each fish’s tail vein and then left to stand for 4 h at 4 °C. Then, the blood was centrifuged in a pre-cooled centrifuge for 15 min at 5000× g. The supernatant was transferred to a centrifuge tube and stored at −80 °C. Excess lipids and inclusions were removed from the mid-intestines of fish and placed in cryovials. The tubes were then immediately submerged in liquid nitrogen for RNA extraction and digestive enzyme analysis. Additionally, whole bodies from each treatment group were labeled and stored at −80 °C for the determination of body composition. Another two intestinal samples were collected under sterile conditions from two randomly selected fish per tank. The exterior of each fish was sanitized with 75% alcohol before the intestines were extracted using sterile scissors. Contents from the foregut to the hindgut were transferred into a sterile 2 mL centrifuge tube (RNase-Free, Axygen, Union City, CA, USA) using sterile tweezers and immediately frozen in liquid nitrogen for gut microflora high-throughput sequencing analysis.
2.4. Nutrient Content
The diets and whole fish were analyzed following the AOAC protocols (2016) [17]. The moisture content of the samples was analyzed using the drying method. The samples were put in an oven at 105 ± 0.5 °C for 3 h until they reached a constant weight. The protein content of the whole fish was determined using automatic rapid nitrogen-fixing (rapid N exceed, Elementar, Langenselbold, Germany). The ash content was determined using the cauterization method. First, 2.0 ± 0.5 g of the sample was weighed precisely and placed in the crucible, which was then heated in the muffle furnace. The temperature was set to 350 °C for half an hour, followed by an adjustment to 600 °C for 2 h. The crude lipid content was determined using the Soxhlet extraction method with a Soxhlet extraction system (Extraction system-811, BUCHI, Flawil, Switzerland) and petroleum ether as the extraction solvent. The extraction process lasted for a duration of 10 h to ensure complete extraction of the lipid content from the sample. The determination of crude fiber was conducted using the acid-alkali digestion method, which involves sequential digestion of the sample with sulfuric acid and sodium hydroxide, followed by combustion of the insoluble residue to quantify fiber content using a fiber analyzer (TH-SF22, Tianhong Co., Weifang, China). The gross energy content was determined through the utilization of a bomb calorimeter (LRY-600A, Chuangxin Ltd., Shijiazhuang, China).
2.5. Digestive Enzyme
Liver and intestinal samples were thawed at 4 °C. Add pre-cooled physiological saline solution with a concentration of 0.86% to the thawed sample at a ratio of 1:9 between the sample and physiological saline solution. The samples were homogenized by employing a high-speed dispersive tissue homogenizer (FJ-200, Shanghai Instrument Co., Shanghai, China). Following this, the samples were placed into a low-temperature centrifuge (H2050R, Shanghai Instrument Co., China) and centrifuged at a speed of 6000× g for 10 min to segregate the supernatant. Digestive enzyme analyses followed a protocol from the manufacturer, utilizing commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The lipase-specific substrate was decomposed, and lipase activity was determined by the rate of formation of red methyl halide. The product is measured at 580 nm (A054-2-1). α-Amylase (C016-1-1) hydrolyses starch to produce monosaccharides, which bind to unhydrolyzed starch by the addition of iodine solution to form a blue complex, the absorbance of which is measured at 660 nm. The intestinal protease activity was determined using the Folinphenol method. Casein (substrate) is broken down by protease to generate amino acids containing phenolic groups, which react with the Folinphenol reagent to produce a blue color. The absorbance of this color was measured at 680 nm to calculate protease activity.
2.6. Biochemical Analysis
Analyses of antioxidant and immune indicators were performed for superoxide dismutase (SOD), malondialdehyde (MDA), catalase (CAT), lysozyme (LZM), and immunoglobulin (IgM) following the manufacturer’s protocol, utilizing commercial kits from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China) (Table 2).

Table 2.
The chemical analysis kits were used in the experiment.
Biochemical parameters, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglycerides (TG), total protein (TP), glucose (GLU), and albumin (ALB), were quantitatively analyzed using an MNCHIP Celercare V5 automatic biochemical analyzer (MNCHIP, Tianjin, China). Serum samples, collected by centrifuging blood at 4000× g for 15 min, were processed according to the manufacturer’s guidelines to ensure accurate assessments of metabolic function indicators.
2.7. Real-Time Polymerase Chain Reaction (PCR) Analysis
The intestines were collected, an appropriate amount of liquid nitrogen was added, and ground in a cooled mortar until they became a fine powder. Total RNA was isolated from the powdered samples utilizing the TRIzol reagent method (Ambion, San Diego, CA, USA). To remove DNA, the sample was treated with Rnase-free DNase I (Thermo Scientific, Waltham, MA, USA). The mass of RNA was measured by agarose gel electrophoresis, for which a 1% agarose gel was prepared and run at 110 V for 30 min. The concentration and quality of the extracted RNA required for subsequent experiments were determined using an ultra-micro UV-visible spectrophotometer (Thermo Scientific NanoDrop 2000) featuring an A260 nm/A280 nm ratio ranging from 1.8 to 2.0. The conversion of RNA to cDNA was conducted with the PrimeScriptTM RT Reagent Kit (TaKaRa, Dalian, China) according to the kit instructions. The cDNA concentration was determined using an ultra-micro spectrophotometer, and samples with completed reverse transcription were diluted to 50 ng/μL for dispensing and storage.
Use the NCBI website to design primers, ensuring avoidance of repeated bases and adherence to a melting curve Tm value of 55–80 °C. It is typically recommended that primer length falls between 18 and 27 bp but should not exceed 38 bp, with a primer GC content of 30–80%. The length of the PCR amplification product should fall within the range of 80–250 bp, with a primer annealing temperature of around 60 °C (Table 3).

Table 3.
Primers used for determining gene expression of rainbow trout.
RT-PCR was performed using the TaKaRa TB Green® Premix Ex Taq™ II (Tli RNaseH Plus) Kit. The expression of β-actin was used as housekeeping genes. Real-time PCR assays were performed on an ABI 7500 Real-Time instrument (Applied Biosystems, Foster City, CA, USA). Three replicates of each amplification reaction were used for comparison (Table 4). Relative gene expression level was determined by the 2−∆∆CT method [18].

Table 4.
Real-time PCR amplification procedure.
2.8. Intestinal Microflora Analysis
The assessment of gut microflora composition utilized the approach reported by Poolsawat et al. (2020) [11]. These samples were analyzed using 16S rRNA gene sequencing. Total DNA was extracted from 0.2 g of intestinal feces using a DNA Extraction Kit (Beijing Tiangen Biochemical Technology Co. Ltd., Beijing, China), following the manufacturer’s protocol for total DNA extraction from intestinal microorganisms. DNA isolation from the extracted samples utilized the specific primers 341F (5′-CCTAYGGGRBGCASCAG-3′) and 806R (5′-GGACTACNNGGGTATCTAAT-3′), as described by Sun et al. (2020) [19]. PCR amplification was performed under optimized conditions: an initial denaturation at 95 °C for 5 min, followed by 27 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and elongation at 72 °C for 45 s, with a final extension at 72 °C for 10 min. The PCR amplicons were purified using a 0.7% agarose gel, quantified with a Qubit dsDNA Broad Range Assay Kit (Life Technologies, Carlsbad, CA, USA), and pooled at an equal concentration of 20 ng/μL. Sequencing was conducted on an Illumina MiSeq platform (300 bp paired-end reads) at Shanghai Meiji Biopharmaceutical Technology Co. Ltd., Shanghai, China. The raw sequencing data were deposited in the NCBI Sequence Read Archive (SRA) database under the accession number PRJNA1093281. An Operational Taxonomic Unit (OTU) was defined based on sequence samples demonstrating more than 97% similarity within their 16S rRNA gene sequences, analyzed using the QIIME platform managed by Shanghai Meiji Biopharmaceutical Technology Co. Ltd. (Shanghai, China).
2.9. Challenge with Aeromonas salmonicida
For the challenge experiment with A. salmonicida, the strain used was A. salmonicida subsp. salmonicida ATCC 33658, originally isolated from a diseased salmonid. Ten fish from each tank were selected after 12 weeks of cultivation. The A. salmonicida culture was grown in TSB medium at 28 °C for 24 h. The optical density (OD) of the culture was measured at 600 nm using a UV-vis spectrophotometer to calculate the concentration of the bacterial suspension. Following gradient dilution, a preliminary experiment was conducted to infect triploid rainbow trout and determine the 72-h median lethal dose (LD50) concentration of the bacterial strain. For the challenge, the bacterial culture was freshly agitated, and its concentration was adjusted to the LD50 value for infection. At the end of the cultivation period, each fish was intraperitoneally injected with 0.1 mL of A. salmonicida at an LD50 concentration of 6.8 × 108 CFU/mL. Subsequently, mortality rates were closely monitored for 72 h.
2.10. Calculations and Statistical Analysis
The assessment of growth performance in the study was carried out using the following parameters:
Survival rate (SR, %) = (total no. fish harvested/total no. fish cultured) × 100%;
Weight gain rate (WGR, %) = ((W1 − W2)/W2) × 100%;
Specific growth rate (SGR, %/d) = (ln(W1/W2)/D) × 100%;
Feed conversion ratio (FCR) = W3/total biomass gain (g);
Hepatosomatic index (HSI, %) = (W4/W1) × 100%;
Viscerasomatic index (VSI, %) = (W5/W1) × 100%;
Where W1, W2, and D mean the wet fish weight (g), initial fish weight (g), and feeding period (d), respectively. W3, W4, and W5 mean the dry feed intake (g), liver weight (g), and viscera weight (g), respectively.
Data were statistically analyzed using SPSS 22.0 (Chicago, IL, USA), and Levene’s test for equality of variances was applied to assess homogeneity of variance. The data were analyzed with one-way ANOVA, and mean differences were analyzed through Tukey’s multiple-range test. Images were drawn using GraphPad Prism 9.0 (Graph Pad Software, San Diego, CA, USA). Data are expressed as means ± S.E., and differences were considered statistically significant at p < 0.05.
3. Results
3.1. Growth Performance
The growth performance of triploid rainbow trout fed diets with different C. butyricum levels (Table 5). SR ranged from 89.67% to 94.33%. There were significant effects of dietary treatments on FBW, WGR, FCR, SGR, and HSI (p < 0.05). Notably, G4 demonstrated the highest growth performance, achieving the most efficient FCR of 1.08 ± 0.02 and the highest SGR of 1.61 ± 0.03. Furthermore, a significant difference was observed in HSI, with G2 exhibiting a larger liver size relative to body weight than the other groups (p < 0.05).

Table 5.
The growth performance of triploid rainbow trout fed diets containing different C. butyricum levels.
3.2. Body Composition
Dietary C. butyricum affected the whole-body composition of triploid rainbow trout, significantly altering moisture, ash, crude lipid, and crude protein content (p < 0.05) (Table 6). Notably, G5, which had the highest crude protein content at 17.32 ± 1.52%, was significantly different from G1, which contained the lowest protein content at 16.32 ± 2.28 (p < 0.05). There were no significant differences in moisture, ash, and crude lipid among the groups (p > 0.05).

Table 6.
Body composition of triploid rainbow trout fed diets containing different C. butyricum levels (%).
3.3. Digestive Enzyme
There were significant effects of dietary C. butyricum on lipase, protease, and amylase activities among the groups (p < 0.05) (Table 7). Lipase activity showed a rise, ranging from G1 at 42.79 ± 8.96 U/mgprot to peak in G4 at 126.55 ± 27.90 U/mgprot. Similarly, protease activity showed significant variations, with G1 showing minimal activity at 940.23 ± 151.04 U/mgprot, and G4 showing maximal at 1310.29 ± 233.30 U/mgprot. Conversely, amylase activity exhibited a decreasing trend, starting at 2.51 ± 0.05 U/mg protein in both G1 and G3 and reaching its lowest at 1.59 ± 0.03 U/mg protein in G5.

Table 7.
Effects of dietary C. butyricum supplementation on digestive enzyme activity of triploid rainbow trout.
3.4. Antioxidant Capacity and Immune Response
There were significant improvements in liver antioxidative capacity among the groups (Table 8) (p < 0.05). SOD activity increased significantly, peaking in G4 and G5.

Table 8.
The antioxidant capacity of triploid rainbow trout fed diets containing different C. butyricum levels.
There were significant differences in serum antioxidative and oxidative stress markers among the groups (p < 0.05). SOD activity exhibited an increase from G1 at 16.40 ± 2.23 U/mL to the highest in G4 at 41.48 ± 10.75 U/mL. MDA levels showed a decrease from G1 to the lowest in G5 at 8.48 ± 0.97 nmoL/mL. Additionally, CAT activity peaked in G4 at 24.46 ± 1.81 U/mL.
Moreover, different levels of C. butyricum in the diet significantly impacted the activities of LZM and IgM in serum (p < 0.05), with peak activities observed in G4 (Figure 1).
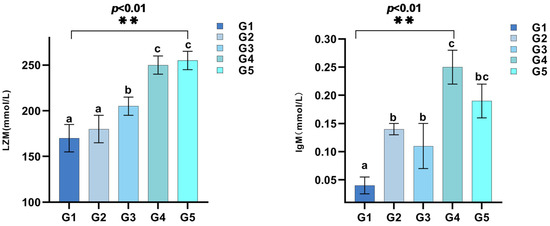
Figure 1.
Lysozyme (LZM) and immunoglobulin M (IgM) responses in triploid rainbow trout by dietary C. butyricum supplementation (n = 3). Values in the columns with different superscript letters are significantly different (p < 0.05). ** indicates p < 0.01, representing statistically significant differences.
3.5. Serum Metabolic Markers
There were significant differences in serum AST levels among the groups (p < 0.05) (Table 9), with G5 showing the lowest AST at 360.65 ± 70.15 U/L and G2 showing the highest at 400.18 ± 80.36 U/L. Despite the differences in GLU, TP, ALB, ALT, and TG, these variations did not exhibit significant changes among the groups (p > 0.05).

Table 9.
The serum indices of triploid rainbow trout fed diets containing different C. butyricum levels.
3.6. Relative Gene Expression in the Intestine
As shown in Figure 2 and Figure 3, supplementation with different levels of C. butyricum significantly affected the expression of intestinal immune-related genes in triploid rainbow trout, such as interleukins (IL-1β, IL-8, IL-10), NF-κB, and TNF-α (p < 0.05). Notably, IL-1β expression reached its peak in G3, with no significant differences between other groups and the control group (p > 0.05). The expression levels of IL-8, NF-κB, and MyD88 initially increased and then decreased as C. butyricum levels increased, with the peak expressions observed in G3. In contrast, IL-10 expression progressively rose with increasing C. butyricum level, significantly higher than in the control group (p < 0.05). TNF-α expression, however, exhibited a declining trend. Meanwhile, IL-2 expression remained consistent (p > 0.05), although a gradual decrease was observed.
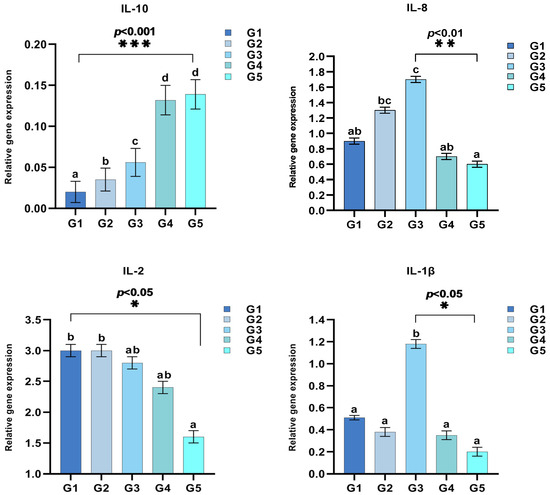
Figure 2.
Modulation of interleukin profiles in triploid rainbow trout by dietary C. butyricum supplementation (n = 3). Values in the columns with different superscript letters are significantly different (p < 0.05). * indicates p < 0.05, ** indicates p < 0.01, and *** indicates p < 0.001, representing statistically significant differences.
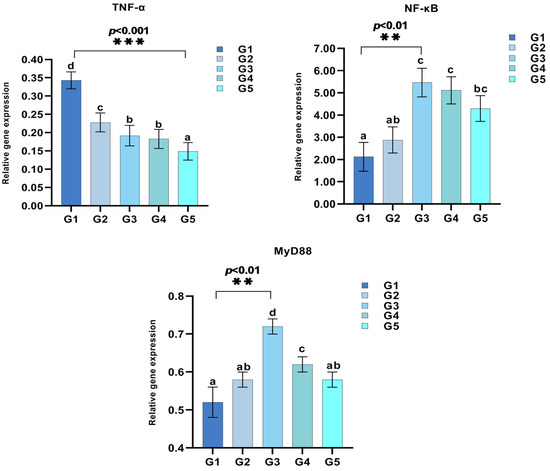
Figure 3.
Impact of C. butyricum supplementation on key inflammatory gene expression in triploid rainbow trout (n = 3). Values in the columns with different superscript letters are significantly different (p < 0.05). ** indicates p < 0.01, and *** indicates p < 0.001, representing statistically significant differences.
Intestinal occludin expression levels revealed no significant disparities between groups G1 through G4 and a minor enhancement in G5 (Figure 4). The subsequent graph demonstrates an increased trend in ZO-1 expression from G1 to G4 (p < 0.05).
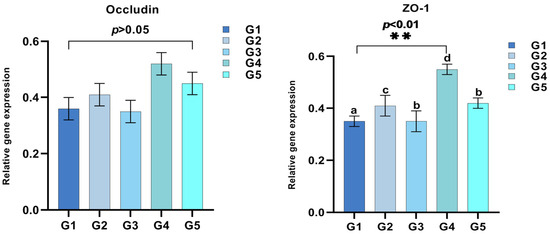
Figure 4.
Effects of different concentrations of C. butyricum on triploid rainbow trout intestinal occludin and ZO-1 expression (n = 3). ** indicates p < 0.01, representing statistically significant differences.
3.7. Intestinal Microflora
The addition of different levels of C. butyricum significantly impacted the gut microflora of triploid rainbow trout among the groups, as depicted in a Venn diagram (Figure 5). A core microbiome of 121 operational taxonomic units (OTUs) common to all groups suggests a stable microbial community across different supplementation levels. Despite different C. butyricum levels, there was no significant impact on the Chao1, ACE, Shannon, and Simpson diversity indices (Table 10).
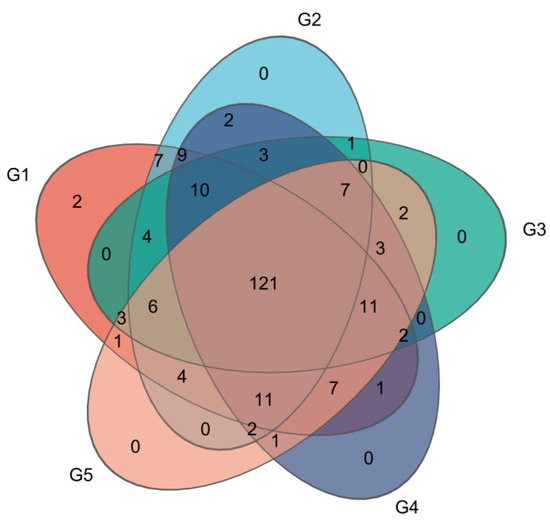
Figure 5.
Venn diagram of shared and unique operational taxonomic units (OTUs) among triploid rainbow trout gut microflora subjected to different levels of C. butyricum supplementation (n = 3).

Table 10.
The intestinal microflora diversity index of triploid rainbow trout fed diets containing different C. butyricum levels.
Barplot analyses showed the predominance of Proteobacteria and Firmicutes among all groups, with fluctuations in Firmicutes abundance (Figure 6). The presence of Actinobacteriota, Cyanobacteria, and other minor phyla contributed to microbial diversity. At the genus level, it illustrates a diverse bacterial community with genera like Burkholderia–Caballeronia–Paraburkholderia, Pseudomonas, and Clostridium present in different proportions across groups G1 to G5.
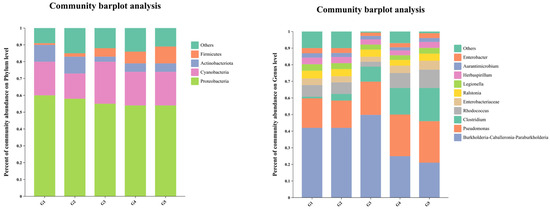
Figure 6.
Comparative analysis of microbial community functions on phylum and genus level in triploid rainbow trout: the role of C. butyricum in low-fish meal diets (n = 3).
Principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) showed distinct microbial profiles among the groups, with G1 showing a unique composition (Figure 7). This separation shows the potential of C. butyricum supplementation to modulate gut microflora at the genus level, with G4 exhibiting the greatest variance post-supplementation. Principal Coordinates Analysis (PCoA) and Non-metric Multidimensional Scaling (NMDS) revealed that C. butyricum supplementation subtly alters gut microflora without causing significant overall structural changes (PCoA: PC1, 21.09%, PC2, 11.23%, R = 0.0290, p = 0.327; NMDS stress: 0.188) (Figure 8). G1 presented a unique microbial profile, while G2, G3, and G4 showed overlapping clusters, and G5 showed greater variability.
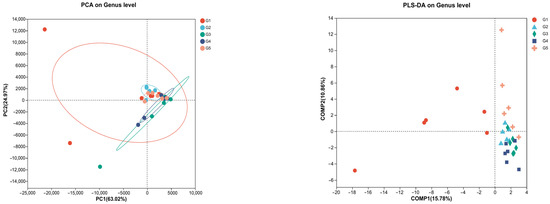
Figure 7.
Principal component analysis (PCA) and Partial least squares discriminant analysis (PLS-DA) on genus level of triploid rainbow trout gut microflora response to C. butyricum supplementation (n = 3).
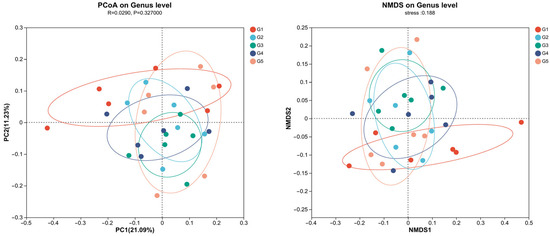
Figure 8.
Principal coordinates analysis (PCoA) and non-metric multidimensional scaling (NMDS) analysis: evaluating the C. butyricum impact on triploid rainbow trout microflora composition (n = 3).
The COG functional classification revealed that the supplementation levels might influence the gut microbiome’s functional potential, with categories related to carbohydrate transport and metabolism, translation, ribosomal structure and biogenesis, and amino acid transport and metabolism being significantly affected (Figure 9). This suggests that C. butyricum not only alters microbial composition but also affects the functional capabilities of the trout’s gut microflora.
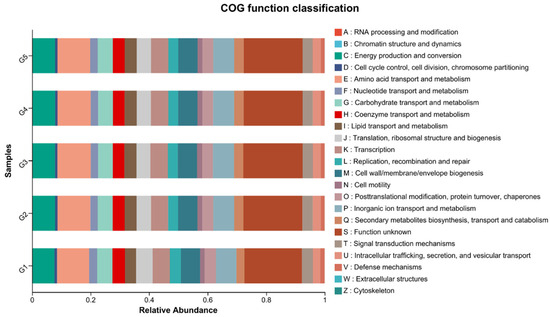
Figure 9.
Cluster of orthologous groups (COG) functional classification in triploid rainbow trout microflora influenced by C. butyricum supplementation (n = 3).
3.8. Challenge with A. salmonicida
The graph presents the SR of triploid rainbow trout over 72 h after being exposed to A. salmonicida, following 12 weeks of cultivation with different levels of C. butyricum supplementation (Figure 10). Initially, all groups exhibited an SR of 100%. However, with time, G3 and G1 showed earlier reductions in survival, with G3 experiencing a more apparent decline. By the conclusion of the 72-hour observation period, G4 recorded the highest SR (66.67%). Similarly, G5 and G3 showed higher SR, with G5 achieving 63.33% and G3 achieving 60.00%. G1 and G2 exhibited SR of 50% and 53.33%, respectively.
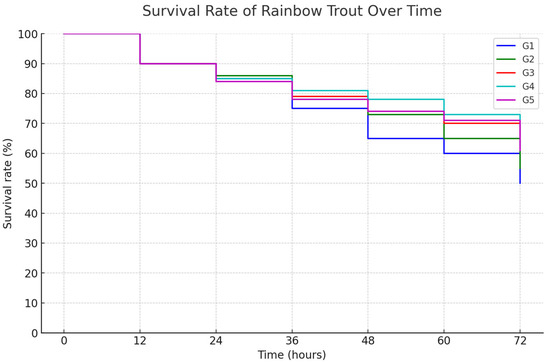
Figure 10.
Survival rate of triploid rainbow trout over time post-A. salmonicida infection with different levels of C. butyricum.
4. Discussion
4.1. Growth Performance
C. butyricum, as a feed additive, has been shown to significantly enhance growth performance, feed utilization rates and intestinal health in a variety of fish species (Table 11), including croaker (Miichthys miiuy) [20], tilapia [11], large yellow croaker (Larimichthys crocea) [21], pompano (Trachinotus ovatus) [12], and carp [13]. Similarly, findings by Lan (2019) indicated that C. butyricum, could improve weight gain in European eel (Anguilla anguilla), with insignificant effects on HSI and VSI [22]. These align with our results, showing that as dietary levels of C. butyricum increased, so did the WGR and SGR in triploid rainbow trout, with the lowest FCR observed at a supplementation concentration of 1 × 108 CFU/g. The presence of antinutritional factors in high soybean meal proportions could reduce nutrient absorption and utilization in triploid rainbow trout. However, our study found that adding C. butyricum to low-fish meal diets can counteract the growth-inhibitory effects of soybean meal and enhance feed efficiency to some extent. These findings suggest that C. butyricum facilitates better nutrient utilization and feed efficiency, potentially through the production of short-chain fatty acids (SCFAs) like butyrate, which enhance digestive processes and reduce gut inflammation. The production of SCFAs in the intestine is primarily by dietary C. butyricum supplementation, butyrate, and acetate. Butyrate serves as a fundamental energy source for epithelial cell growth and can release H+ into the neutral cytoplasm to reduce intracellular pH, potentially utilizing the remaining H+ in metabolism to lower cell mortality rates [23]. This process aids in maintaining epithelial tissue integrity, facilitating nutrient absorption. Additionally, butyrate may enhance feed utilization by balancing the gut’s microecological environment [24].

Table 11.
Comparative growth metrics of various fish species administered with C. butyricum as a feed additive.
4.2. Body Composition
In our study, the whole-body composition of triploid rainbow trout showed no significant differences in moisture, crude lipid, and ash content, with an increase observed in crude protein levels. This indicates that C. butyricum supplementation is beneficial for enhancing protein deposition in triploid rainbow trout. Research by Huang (2017) found that juvenile tiger grouper (Epinephelus fuscoguttatus × E. lanceolatus) fed diets containing C. butyricum exhibited increased crude protein content without affecting ash [25]. Within the intestines, C. butyricum engages in anaerobic fermentation, producing specific metabolites such as butyrate, which is known to enhance intestinal health by strengthening the gut barrier and modulating immune responses. Additionally, the enzymes and amino acids released by C. butyricum help in the efficient breakdown and assimilation of proteins, supporting muscle growth and protein deposition in fish. This direct interaction of butyrate and enzymatic activity not only corroborates the growth-promoting effects of C. butyricum but also shows its potential to improve overall fish health and feed efficiency.
4.3. Digestive Enzyme
In this study, as the level of C. butyricum increased, the activities of lipase and protease in the intestines of triploid rainbow trout significantly rose compared to the control group, reaching their peak at a concentration of 1.1 × 108 CFU/g, while the activity of α-amylase significantly decreased. Similar results were observed in studies on Cherax quadricarinatus, where the lipase and amylase activities in the C. butyricum treatment group significantly increased by 165.84% and 18.75%, respectively, compared to the control group [26]. Additionally, a feeding trial with juvenile hybrid grouper (Epinephelus fuscoguttatus × E. lanceolatus) also found that diets supplemented with C. butyricum not only enhanced gastric digestive enzyme activities but also increased intestinal trypsin, lipase, and amylase activities [27]. These findings suggest the need for detailed dose-response studies to identify the optimal concentrations of C. butyricum that maximize digestive efficiency without compromising the digestive processes. This could involve systematic variations of probiotic dosages in feeding trials to map out a full spectrum of enzymatic responses and establish a threshold beyond which no further benefits are observed, or adverse effects may begin to appear.
4.4. Antioxidant Capacity
The butyrate produced by C. butyricum plays a crucial role in maintaining the antioxidant capacity of the colonic epithelium. Dietary C. butyricum and Bacillus coagulans in rainbow trout diets have been shown to effectively increase serum total antioxidant capacity T-AOC and SOD activity while reducing MDA content, whether used singly or in combination [28]. In this study, increasing levels of C. butyricum enhanced the antioxidant capacity in the serum and liver of triploid rainbow trout. Studies on tilapia also found that dietary C. butyricum increased CAT, SOD, and hemoglobin activities, enhancing resistance to A. hydrophila infection [29]. Hasan et al. (2018) found elevated SOD activity in flounder (Paralichthys olivaceus) after probiotic supplementation [30]. Likewise, probiotics have been shown to boost SOD and CAT activities in various species, such as Japanese eel (Anguilla japonica) [31] and grouper (Epinephelus coioides) [32]. Thus, C. butyricum, as a probiotic, can enhance the antioxidant capacity of aquatic animals, therefore improving their disease resistance. Excessive oxidative stress and inflammatory responses can lead to the oxidation of lipids and proteins, with oxidized low-density lipoprotein (LDL) being internalized by macrophages to form foam cells, stimulating the production of pathogenic factors [33]. The antioxidant effects of C. butyricum may relate to the butyrate and NADH produced, potentially clearing reactive oxygen species (ROS) through NADPH generation, thus reducing oxidative stress [34].
4.5. Serum Metabolites
Feeding aquatic animals with diets substituting fish meal with plant proteins introduces antinutritional factors like protease inhibitors, phytic acid, and gossypol from plant proteins into the animal’s tissues via intestinal digestion and absorption, leading to stress responses that can alter serum biochemistry profiles. These changes include disruptions in protein digestion, mineral deficiencies, and increased oxidative stress markers, highlighting the critical need for optimized diet formulations to mitigate these adverse effects in aquaculture practices. Studies, such as those by Abdel-Tawwab et al. (2021), have shown no significant differences in AST and ALT activities when Macrobrachium rosenbergii was fed diets supplemented with C. butyricum [35]. In our study, an increase in the level of C. butyricum supplementation was associated with a gradual decrease in AST levels. Similarly, in carp [13], serum AST and ALT activity decreased, indicating an improvement in fish health with C. butyricum-enriched diets. The reduction in transaminase levels with higher C. butyricum supplementation suggests that adding C. butyricum to diets replacing fish meal with soybean meal can provide some protection against liver damage caused by plant proteins in triploid rainbow trout.
4.6. Immunity
C. butyricum, specifically, can regulate innate and adaptive immunity, enhancing the animal’s defense against microbes and maintaining immunological homeostasis [36]. Immunomodulation and suppression of excessive immune responses and inflammation are considered mechanisms through which probiotics exert therapeutic effects [37]. Experiments by Xiao et al. (2019) have shown that C. butyricum can lower IL-8 expression and increase IL-10 expression in yellow catfish (Pelteobagrus fulvidraco) suffering from acute pancreatitis [38]. In our study, the addition of C. butyricum significantly influenced the expression of intestinal genes IL-1β, IL-8, IL-10, MyD88, NF-κB, and TNF-α in triploid rainbow trout. This suggests that C. butyricum not only activates essential immune pathways but also regulates inflammatory responses, which is crucial for maintaining immune homeostasis and improving disease resistance in triploid rainbow trout. The immune system is activated upon exposure to adverse factors, with various cytokines being moderately expressed to protect the organism; however, excessive immune responses can lead to inflammation. The addition of C. butyricum in this study not only promoted the expression of pro-inflammatory cytokines but also anti-inflammatory cytokines, regulating pathways related to cytokine expression and modulating the immune response in triploid rainbow trout. Treatment with C. butyricum in mice with ulcerative colitis reduced the expression of cytokines such as IL-1β, TNF-α, and IL-6 [39]. Feeding carp with lactobacillus strains increased the expression of pro-inflammatory (TNF-α, IL-1β, IL-6, IL-12) and anti-inflammatory (IL-10) cytokine genes [40], enhancing IL-8 gene expression in grouper (Epinephelus coioides) intestines [41] and TNF-α expression in the small intestine of hybrid tilapia (Oreochromis niloticus × O. aureus) [42]. This suggests that probiotics can induce a sensitizing effect in the host by increasing pro-inflammatory cytokines (like IL-8, IL-6, and TNF-α) and provide beneficial effects through the synthesis of anti-inflammatory cytokines (like IL-10).
After being fed probiotics, fish showed changes in the secretion of IgM and lysozyme (LZM). Gong et al. (2019) found that Pediococcus pentosaceus, a type of lactic acid bacteria, could increase the expression of IgM and complement protein C3 genes in the liver and spleen of grass carp (Ctenopharyngodon idellus) [43]. In this study, as the level of C. butyricum in the diet increased, serum LZM activity gradually rose, with G4 and G5 groups significantly higher than the control group; serum IgM content also gradually increased, peaking in the G4 group. This aligns with the findings of Song et al. (2006), who observed increased serum LZM activity and IgM content in Lateolabrax japonicus fed with C. butyricum [20]. This may be due to C. butyricum or its products activating the immune system, producing various immune factors, and stimulating LZM and IgM [44], therefore enhancing the immune function of triploid rainbow trout, protecting body tissues from damage, and maintaining health.
This observation implies enhanced pathogen resistance, likely due to the positive impact of C. butyricum on gut health and the subsequent strengthening of the immune response [32]. G5 also showed a higher SR, suggesting advantageous effects at this specific level of supplementation. These findings emphasize the critical role of optimizing C. butyricum supplementation levels to improve resistance against infections in triploid rainbow trout, proposing that an appropriate supplementation approach could serve as an effective strategy to improve the health and survivability of triploid rainbow trout raised on diets low in fish meal.
4.7. Intestinal Barrier
As the level of C. butyricum increases, there is an upregulation in the expression of the occludin and ZO-1 genes. This protective role of C. butyricum in enhancing intestinal barrier function via the modulation of tight junction (TJ) proteins is proved by previous research. For example, Fu et al. (2023) have shown that butyrate, a short-chain fatty acid produced by C. butyricum, strengthens the intestinal barrier by elevating the expression of TJ proteins such as occludin and ZO-1, thus reducing inflammation and epithelial damage [45]. Similarly, Peng et al. (2009) found that butyrate improves intestinal barrier function through a mechanism that includes the upregulation of TJ proteins, highlighting the critical role of microbial metabolites in maintaining gut health [46]. Moreover, the activity of C. butyricum in boosting the expression of occludin and ZO-1 suggests a viable mechanism for its positive effects in preventing and alleviating gastrointestinal disorders, such as inflammatory bowel disease (IBD), where compromised barrier function is a key issue. Bertiaux-Vandaële et al. (2011) noted that disturbances in TJ protein expression are linked to increased intestinal permeability, a characteristic feature of IBD pathology, highlighting the therapeutic value of adjusting TJ protein expression via probiotic supplementation [47].
4.8. Intestinal Microflora
The resilience of C. butyricum to gastric acids and temperature variations enables it to reach the intestines unaffected by stomach acids and various digestive enzymes. This characteristic aids in understanding its role in modulating gut microflora abundance [48]. The alpha diversity indices, including Chao1 and ACE, indicate microbial abundance, while Shannon and Simpson indices represent community diversity, with higher Shannon values indicating greater diversity and higher Simpson values indicating lower diversity [49]. Our results suggest that Chao1 and ACE indices show no significant difference among the groups. Conversely, Shannon and Simpson indices suggest that high levels of C. butyricum may reduce microbial diversity. This could be due to C. butyricum’s inhibitory effect on harmful bacteria, reducing the diversity by lowering the survival chances of harmful microbes [50]. Additionally, the presence of categories related to inorganic ion transport and metabolism and coenzyme transport and metabolism further highlights the diverse metabolic capabilities of the trout microflora under different treatment conditions. Consistent with previous research, these findings support the concept that gut microflora plays a crucial role in influencing nutrient utilization and energy harvesting, significantly contributing to the improved growth performance and health status of fish under low-fish-meal dietary conditions [51].
5. Conclusions
This study demonstrates that supplementing a low fishmeal diet with C. butyricum (1.1 × 108 CFU/g) significantly increases growth and gut health in triploid rainbow trout. The probiotic C. butyricum boosts populations of beneficial bacteria such as Firmicutes, enhancing digestive enzyme activities and antioxidant defenses, resulting in improved nutrient utilization and reduced oxidative stress. Furthermore, this supplement strengthens immune defenses by regulating key immune genes and increasing serum immunoglobulin levels, therefore enhancing resistance to A. salmonicida. These benefits suggest that C. butyricum could be a valuable dietary supplement in aquaculture, promoting sustainable growth, improved gut health, and disease resistance. Integrating C. butyricum could also offer economic advantages by reducing the use of fishmeal, the most expensive component of fish feed, potentially leading to significant cost savings in commercial aquaculture. Future studies should explore the effects of this probiotic across different species and aquacultural environments, and an economic analysis might illuminate its financial viability.
Author Contributions
Conceptualization, C.W.; methodology, C.W.; resources, H.J. and S.H.; data curation, C.W.; writing—original draft preparation, F.L.; writing—review and editing, F.L., S.L. and C.W.; visualization, D.W., Z.L. and W.G.; supervision, C.W. and H.L.; project administration, C.W. and H.L.; funding acquisition, C.W. and H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Key Research and Development Program of China (2023YFD2400400-5), the China Agriculture Research System of MOF and MARA (CARS-46), the Key Scientific Research Project of Heilongjiang Province (JD22A017), the Natural Science fund of Heilongjiang Province (LH2023C057), the Central Public-interest Scientific Institution Basal Research Fund, CAFS (2023TD60; 2023TD96; HSY202202M), and the National Postdoctoral Fund (2022MD713817).
Institutional Review Board Statement
The animal study was reviewed and approved by the Committee for the Welfare and Ethics of the Laboratory Animals of Heilongjiang River Fisheries Research Institute, CAFS (20200615). Written informed consent was obtained from the owners for the participation of their animals in this study.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors thank the participants who gave their time to the trial.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mitra, A. Thought of alternate aquafeed: Conundrum in aquaculture sustainability? Proc. Zool. Soc. 2020, 74, 1–18. [Google Scholar] [CrossRef]
- Albrektsen, S.; Kortet, R.; Skov, P.V.; Ytteborg, E.; Gitlesen, S.; Kleinegris, D.; Mydland, L.; Hansen, J.Ø.; Lock, E.; Mørkøre, T.; et al. Future feed resources in sustainable salmonid production: A review. Rev. Aquac. 2022, 14, 1790–1812. [Google Scholar] [CrossRef]
- Linh, N.V.; Wannavijit, S.; Tayyamath, K.; Dinh-Hung, N.; Nititanarapee, T.; Sumon, M.A.A.; Srinual, O.; Permpoonpattana, P.; Doan, H.; Brown, C.L. Black soldier fly (Hermetia illucens) larvae meal: A sustainable alternative to fish meal proven to promote growth and immunity in koi carp (Cyprinus carpio var. Koi). Fishes 2024, 9, 53. [Google Scholar] [CrossRef]
- Linh, N.V.; Lubis, A.R.; Dinh-Hung, N.; Wannavijit, S.; Montha, N.; Fontana, C.M.; Lengkidworraphiphat, P.; Srinual, O.; Jung, W.-K.; Paolucci, M.; et al. Effects of shrimp shell-derived chitosan on growth, immunity, intestinal morphology, and gene expression of nile tilapia (Oreochromis niloticus) reared in a biofloc system. Mar. Drugs 2024, 22, 150. [Google Scholar] [CrossRef] [PubMed]
- Azarm, H.M.; Lee, S.M. Effects of partial substitution of dietary fish meal by fermented soybean meal on growth performance, amino acid and biochemical parameters of juvenile black sea bream Acanthopagrus schlegeli. Aquac. Res. 2014, 45, 994–1003. [Google Scholar] [CrossRef]
- Sookying, D.; Davis, D.A.; Soller Dias da Silva, F. A review of the development and application of soybean-based diets for Pacific white shrimp Litopenaeus vannamei. Aquac. Nutr. 2013, 19, 441–448. [Google Scholar] [CrossRef]
- Gemede, H.F.; Ratta, N. Antinutritional factors in plant foods: Potential health benefits and adverse effects. Int. J. Nutr. Food Sci. 2014, 3, 284–289. [Google Scholar] [CrossRef]
- Chen, X.; Xie, J.; Liu, Z.; Yin, P.; Chen, M.; Liu, Y.; Tian, L.; Niu, J. Modulation of growth performance, non-specific immunity, intestinal morphology, the response to hypoxia stress and resistance to aeromonas hydrophila of grass carp (Ctenopharyngodon idella) by dietary supplementation of a multi-strain probiotic. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 231, 108724. [Google Scholar] [CrossRef]
- Meng, X.; Cai, H.; Li, H.; You, F.; Jiang, A.; Hu, W.; Zhou, Z. Clostridium butyricum-fermented Chinese herbal medicine enhances the immunity by modulating the intestinal microflora of largemouth bass (Micropterus salmoides). Aquaculture 2023, 562, 738768. [Google Scholar] [CrossRef]
- Foysal, M.J.; Fotedar, R.; Tay, A.C.Y.; Gupta, S.K. Effects of long-term starvation on health indices, gut microbiota and innate immune response of freshwater crayfish, marron (Cherax cainii, Austin 2002). Aquaculture 2020, 514, 734444. [Google Scholar] [CrossRef]
- Poolsawat, L.; Li, X.; He, M.; Ji, D.; Leng, X. Clostridium butyricum as probiotic for promoting growth performance, feed utilization, gut health and microbiota community of tilapia (Oreochromis niloticus × O. aureus). Aquac. Nutr. 2020, 26, 657–670. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, Q.; Yu, W.; Xu, C.; Hong, M.; Jiang, K.; Mai, X.; Chen, H.; Lin, H.; Yang, K. Effects of dietary supplementation of Clostridium butyricum on growth performance, serum biochemical indexes, intestinal flora, and short-chain fatty acid contents of juvenile Trachinotus ovatus. Chin. J. Anim. Nutr. 2023, 35, 5904–5918. [Google Scholar] [CrossRef]
- Du, Y.; Cheng, L.; Zhao, J.; de Cruz, C.R.; Xu, H.; Wang, L.; Xu, Q. Effects of Clostridium butyricum and sodium butyrate on growth performance, immunity, and gut microbiota of mirror carp Cyprinus carpio fed with soybean meal based diet. Aquac. Rep. 2023, 29, 101501. [Google Scholar] [CrossRef]
- Bao, S.; Zhuo, L.; Qi, D.; Tian, H.; Wang, D.; Zhu, B.; Meng, Y.; Ma, R. Comparative study on the fillet nutritional quality of diploid and triploid rainbow trout (Oncorhynchus mykiss). Aquac. Rep. 2023, 28, 101431. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Liu, S.; Zhang, S.; Lu, S.; Liu, H.; Han, S.; Jiang, H.; Zhang, Y. Effects of dietary arginine on growth performance, digestion, absorption ability, antioxidant capability, gene expression of intestinal protein synthesis, and inflammation-related genes of triploid juvenile Oncorhynchus mykiss fed a low-fish meal diet. Aquac. Nutr. 2022, 2022, 3793727. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, C.; Liu, S.; Wang, Y.; Lu, S.; Han, S.; Jiang, H.; Liu, H.; Yang, Y. Effect of dietary phenylalanine on growth performance and intestinal health of triploid rainbow trout (Oncorhynchus mykiss) in low fish meal diets. Front. Nutr. 2023, 10, 1008822. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International; AOAC: Washington, DC, USA, 2016. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (−ΔΔC(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, M.; Xia, L.; Fang, Z.; Yu, S.; Gao, J.; Feng, Q.; Yang, P. Alteration of salivary microbiome in periodontitis with or without type-2 diabetes mellitus and metformin treatment. Sci. Rep. 2020, 10, 15363. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.F.; Wu, T.X.; Cai, L.S.; Zhang, L.J.; Zheng, X.D. Effects of dietary supplementation with Clostridium butyricum on the growth performance and humoral immune response in Miichthys miiuy. J. Zhejiang Univ. Sci. B 2006, 7, 596–602. [Google Scholar] [CrossRef]
- Yin, Z.; Liu, Q.; Liu, Y.; Gao, S.; He, Y.; Yao, C.; Huang, W.; Gong, Y.; Mai, K.; Ai, Q. Early life intervention using probiotic Clostridium butyricum improves intestinal development, immune response, and gut microbiota in large yellow croaker (Larimichthys crocea) larvae. Front. Immunol. 2021, 12, 640767. [Google Scholar] [CrossRef]
- Lan, F.F. The promotive Effect of Adding Clostridium butyricum to Feed on the Growth and Health of Eels. Ph.D. Thesis, Jimei University, Xiamen, China, 2019. [Google Scholar]
- Ichikawa, H.; Shineha, R.; Satomi, S.; Sakata, T. Gastric or rectal instillation of short-chain fatty acids stimulates epithelial cell proliferation of small and large intestine in rats. Dig. Dis. Sci. 2002, 47, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Piao, X.; Mahfuz, S.; Long, S.; Wang, J. The interaction among gut microbes, the intestinal barrier and short chain fatty acids. Anim. Nutr. 2022, 9, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Huang, L. Study on the Effects of Probiotics Supplementation in Feed on Growth, Non-Specific Immunity, and Disease Resistance of Juvenile Tiger Grouper (Epinephelus fuscoguttatus × E. lanceolatus). Ph.D. Thesis, Guangzhou University, Guangzhou, China, 2017. [Google Scholar]
- Fan, C.W. The Impact of Clostridium butyricum on the Intestinal Health of Cherax quadricarinatus. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2020. [Google Scholar]
- He, R.P.; Feng, J.; Tian, X.L.; Dong, S.L.; Li, H.D. Effects of butyric acid bacteria on growth, digestive enzymes, serum antioxidase, and lysozyme activity of pearl gentian grouper. J. Ocean Univ. China: Nat. Sci. Ed. 2017, 47, 15–23. [Google Scholar]
- Ye, H.B.; Fan, Y.; Yan, F. Effects of probiotic formulations on serum biochemical indices and immune functions in rainbow trout. J. South China Norm. Univ. 2018, 50, 65–71. [Google Scholar]
- Li, H.Q.; Zhou, Y.; Ling, H.Y.; Luo, L.; Qi, D.; Feng, L. The effect of dietary supplementation with Clostridium butyricum on the growth performance, immunity, intestinal microbiota and disease resistance of tilapia (Oreochromis niloticus). PLoS ONE 2019, 14, e0223428. [Google Scholar] [PubMed]
- Hasan, M.T.; Jang, W.J.; Tak, J.Y.; Lee, B.J.; Kim, K.W.; Hur, S.W.; Kong, I.S. Effects of Lactococcus lactis subsp. lactis I2 with β-glucooligosaccharides on growth, innate immunity and streptococcosis resistance in olive flounder (Paralichthys olivaceus). J. Microbiol. Biotechnol. 2018, 28, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Cheng, H.; Damte, D.; Lee, S.J.; Kim, J.C.; Rhee, M.H.; Park, S.C. Effects of dietary supplementation of Lactobacillus pentosus PL11 on the growth performance, immune and antioxidant systems of Japanese eel Anguilla japonica challenged with Edwardsiella tarda. Fish Shellfish Immun. 2013, 34, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Cheng, Y.; Chen, X.; Liu, Z.; Long, X. Effects of small peptides, probiotics, prebiotics, and synbiotics on growth performance, digestive enzymes, and oxidative stress in orange-spotted grouper, Epinephelus coioides, juveniles reared in artificial seawater. Chin. J. Oceanol. Limnol. 2016, 35, 89–97. [Google Scholar] [CrossRef]
- Kawasaki, S.; Nakagawa, T.; Nishiyama, Y.; Benno, Y.; Uchimura, T.; Komagata, K.; Niimura, Y. Effect of oxygen on the growth of Clostridium butyricum (type species of the genus Clostridium), and the distribution of enzymes for oxygen and for active oxygen species in Clostridia. J. Ferment. Bioeng. 1998, 86, 368–372. [Google Scholar] [CrossRef]
- Dan, J.; Fang, Z.; Chin, S.X.; Tian, X.F.; Su, T.C. Biohydrogen production from hydrolysates of selected tropical biomass wastes with Clostridium butyricum. Sci. Rep. 2016, 6, 27205. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Shukry, M.; Farrag, F.A.; El-Shafai, N.M.; Dawood, M.A.O.; AbdelLatif, H.M.R. Dietary sodium butyrate nanoparticles enhanced growth, digestive enzyme activities, intestinal histomorphometry, and transcription of growth-related genes in Nile tilapia juveniles. Aquaculture 2021, 536, 736467. [Google Scholar] [CrossRef]
- Haller, D. Non-pathogenic bacteria elicit a differential cytokine response by intestinal epithelial cell/leucocyte co-cultures. Gut 2000, 47, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, F. Probiotics in inflammatory bowel disease--therapeutic rationale and role. Adv. Drug Deliv. Rev. 2004, 56, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.P.; Yu, L.T.; Gui, G.H.; Gong, Y.; Wen, X.; Xia, W.; Zhang, L. Molecular cloning and expression analysis of interleukin-8 and -10 in yellow catfish and in response to bacterial pathogen infection. Biomed. Res. Int. 2019, 9617659. [Google Scholar] [CrossRef]
- Zuo, H.N.; Yang, W.F. The effect of combined use of Lactobacillus and Clostridium butyricum on acute ulcerative colitis in mice. Int. J. Lab. Med. 2009, 30, 33–35. [Google Scholar]
- Mohammadian, T.; Alishahi, M.; Mohammad, R.T.; Jangaran Nejad, A.; Karami, E.; Zarea, M. Effects of autochthonous probiotics, isolated from Tor grypus (Karaman, 1971) intestine and Lactobacillus casei (PTCC 1608) on expression of immune-related genes. Aquac. Int. 2019, 27, 239–260. [Google Scholar] [CrossRef]
- Sun, Y.Z.; Xia, H.Q.; Yang, H.L.; Wang, Y.L.; Zou, W.C. TLR2 signaling may play a key role in the probiotic modulation of intestinal microbiota in grouper Epinephelus coioides. Aquac. 2014, 430, 50–56. [Google Scholar] [CrossRef]
- He, S.; Zhang, Y.; Xu, L.; Yang, Y.; Marubashi, T.; Zhou, Z.; Yao, B. Effects of dietary Bacillus subtilis C-3102 on the production, intestinal cytokine expression and autochthonous bacteria of hybrid tilapia Oreochromis niloticus × Oreochromis aureus. Aquaculture 2013, 412, 125–130. [Google Scholar] [CrossRef]
- Gong, L.; He, H.; Li, D.; Cao, L.; Khan, T.A.; Li, Y.; Xia, L. A new isolate of Pediococcus pentosaceus (SL001) with antibacterial activity against fish pathogens and potency in facilitating the immunity and growth performance of grass carp. Front. Microbiol. 2019, 10, 1384. [Google Scholar] [CrossRef]
- Ringø, E. Probiotics in shellfish aquaculture. Aquac. Fish. 2020, 5, 1–27. [Google Scholar] [CrossRef]
- Fu, Y.; Lyu, J.; Wang, S. The role of intestinal microbes on intestinal barrier function and host immunity from a metabolite perspective. Front. Immunol. 2023, 14, 1277102. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzmanr, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Bertiaux-Vandaële, N.; Youmba, S.B.; Belmonte, L.; Lecleire, S.; Antonietti, M.; Gourcerol, G.; Coëffier, M. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am. Coll. Gastroenterol. | ACG 2011, 106, 2165–2173. [Google Scholar] [CrossRef] [PubMed]
- Schönherr-Hellec, S.; Klein, G.; Delannoy, J.; Ferraris, L.; Friedel, I.; Rozé, J.C.; Aires, J. Comparative phenotypic analysis of Clostridium neonatale and Clostridium butyricum isolates from neonates. Anaerobe 2017, 48, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Ma, M.C.; Yang, C.M.C.K. Stopping rules and estimation for recapture debugging with unequal failure rates. Biometrika 1993, 80, 193–201. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Plaza-Diaz, J.; Munoz-Quezada, S. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef]
- Ringø, E.; Zhou, Z.; Vecino, J.L.G.; Wadsworth, S.; Romero, J.; Krogdahl, Å.; Merrifield, D.L. Effect of dietary components on the gut microbiota of aquatic animals. A never-ending story? Aquac. Nutr. 2016, 22, 219–282. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).