Abstract
The genus Dichichthys was resurrected for five species previously allocated to the genus Parmaturus in the family Pentanchidae. Supraorbital crests on the chondrocranium distinguish Dichichthys from Parmaturus and other members of the family Pentanchidae. A new family, Dichichthyidae, has been proposed to contain Dichichthys. The sequence of the NADH2 mitochondrial gene confirms the placement of Dichichthys outside of the Pentanchidae family, as well as separate from the Atelomycteridae and Scyliorhinidae families. Dichichthys albimarginatus was described using a holotype collected off the coast of New Caledonia. A second juvenile specimen collected off the coast of Papua New Guinea was tentatively assigned as D. cf. albimarginatus. Dichichthys bigus is known from the holotype collected in the Coral Sea off the coast of Queensland, Australia. A new, parasite-afflicted underwater observation was reported further north of Queensland. The type species Dichichthys melanobranchus, previously only known from juvenile specimens, was redescribed based on adult specimens. Dichichthys nigripalatum is known from the holotype collected off Sumbawa, Indonesia, and a tentatively identified photo record from West Java. Dichichthys satoi n. sp. is described from the West Norfolk Ridge and off the North Island of New Zealand. Members of the genus Dichichthys have unique curved egg cases which have pliable ridges made up of numerous fibres and long coiled tendrils on the posterior end.
Key Contribution:
A new family of sharks, the bristle sharks Dichichthyidae, is defined. The new family contains five species previously assigned to the genus Parmaturus, one of which is formally named and described.
1. Introduction
Catsharks were originally considered to belong to the highly diverse and speciose family Scyliorhinidae Gill in 1862 [1] (e.g., [2]). As a family, they were global in the marine environment, occurring in cool temperate to tropical seas; they were small (generally <100 cm TL) and benthic [2]. In Compagno’s detailed revision of Carcharhiniformes [3], he divided the Scyliorhinidae into four subfamilies: Atelomycterinae White (1936) [4], Pentanchinae Smith (1912) [5], Schroederichthyinae Compagno (1988) [3], and Scyliorhininae Gill (1862) [1]. These subfamilies can be readily separated from each other based on a combination of morphological and skeletal features such as relative dorsal fin heights, cranial morphology, clasper morphology, intestinal spiral valve counts, labial furrow lengths, nasal flaps, and vertebral counts [3]. A single catshark family was retained until [6] identified several paraphylies during their study on Carcharhiniformes, including within the catsharks. They elevated the subfamily Pentanchinae to family level, i.e., Pentanchidae, for catsharks which lack a supraorbital crest on the chondrocranium. This character is an important first step in the dichotomous keys of catsharks starting from the work of [2]. The other three subfamilies were retained under the family Scyliorhinidae for those catsharks which have a supraorbital crest on the chondrocranium. Despite the important work of Iglésias et al. [6], a single family of catsharks (Scyliorhinidae) was retained by many subsequent authors (e.g., [7,8]).
A recent phylogenetic study by Soares and Mathubara [9] examined a large morphometric dataset integrated with molecular data where available. The results of this study support Pentanchidae as a valid family and recognise a third catshark family, Atelomycteridae, comprising 13 species amongst three genera: Atelomycterus, Aulohalaelurus, and Schroederichthys. This further restricts the family Scyliorhinidae to only 36 species amongst three genera: Cephaloscyllium, Poroderma, and Scyliorhinus.
The Pentanchidae (115 species) currently comprises 11 genera which are defined as lacking supraorbital crests on the chondrocranium, i.e., Apristurus Garman (1913) [10] (41 spp), Asymbolus Whitley (1939) [11] (9 spp), Bythaelurus Compagno (1988) [3] (14 spp), Cephalurus Bigelow and Schroeder (1941) [12] (1 spp), Figaro Whitley (1928) [13] (2 spp), Galeus Rafinesque 1810 [14] (19 spp); Halaelurus Gill 1862 [1] (7 spp); Haploblepharus Garman (1913) [10] (4 spp), Holohalaelurus Fowler (1934) [15] (5 spp), Parmaturus Garman (1906) [16] (11 spp), and Pentanchus Smith and Radcliffe (1912) [5] (1 spp). The genus Akheilos was allocated to the Pentanchidae by [9] despite [17] tentatively placing it in the subfamily Schroederichthyinae.
Evidence that the genus Parmaturus was not monophyletic was first documented by [18]. Specimens of Parmaturus melanobranchus (Chan, 1966) [19] were found to possess supraorbital crests on the chondrocranium in contrast to Parmaturus pilosus Garman (1906) [16], which lacked them. It was suggested by [18] that the genus Dichichthys Chan (1966) [19] should be resurrected for P. melanobranchus. The characters he provided for distinguishing Dichichthys from Parmaturus were multiple oviparity (vs. single), egg cases ridged with a fibrous covering (vs. smooth), supraorbital crests present on chondrocranium (vs. crests absent), and a more posteriorly located first dorsal fin.
Support for the genus Parmaturus being paraphyletic was also found by [20] based on mitochondrial DNA sequences (NADH2 gene). In that study, Parmaturus xaniurus (Gilbert, 1892) [21] was grouped close to Galeus sauteri (Jordan and Richardson, 1909) [22] in the Pentanchidae, while Parmaturus sp., an undescribed species from New Zealand [23], was grouped closest to members of the subfamilies Atelomycterinae and Schroederichthyinae. However, these were the only two Parmaturus species available for analysis.
The collection of specimens of Parmaturus from Japan (East China Sea), Australia, Papua New Guinea, and New Zealand has provided the genetic samples and specimens required to revise this group of deepwater catsharks. This paper’s main aim is to formally resurrect the genus Dichichthys as a valid genus and define a new family, Dichichthyidae, for this genus, including formal naming and description of a new species from New Zealand.
2. Materials and Methods
2.1. Morphology
Egg cases and postnatal specimens of Dichichthys species (previously placed in the genus Parmaturus) in various ichthyological collections were examined. A total of 68 morphometric characters were measured following [24] on four whole adult specimens of D. melanobranchus from the Ryukyu Islands and from the holotype and 13 paratypes of the new species from New Zealand (Table S1). The morphometric data generated are presented in Table 1. For comparison, measurements of D. albimarginatus, D. bigus, and D. nigripalatum were taken from the original descriptions, and the same methodology was used for morphometric measurements [25,26]. Measurements are presented as a percentage of total length (TL).
Egg case morphological measurements followed those proposed in [27] with the addition of egg case body length and maximum egg case width following [28] for skates. Two additional measurements specific to this genus of sharks were also taken: the depth of the single lateral keel on the left side of the egg case and the depth of the lower (broader) lateral keel on the right side of the egg case. The two D. bigus egg cases (CSIRO H 947-10) and seven of the D. satoi n. sp. egg cases (NMNZ P.017655 [×2], NMNZ P.035371 [×2] and NMNZ P.042524 [×3]) were measured and measurements expressed as a percentage of egg case length.
2.2. Meristics
Vertebral counts were obtained from radiographs of five adult specimens of D. melanobranchus and the holotype and six paratypes of the new species from New Zealand specimens (Table S1). Counts were obtained separately for trunk (monospondylous precaudal centra and diplospondylous precaudal centra (to the origin of the ventral caudal fin lobe)), precaudal (monospondylous precaudal centra + diplospondylous precaudal centra), and diplospondylous caudal centra (centra of the caudal fin) vertebrae following the methods used by [3] for carcharhiniform sharks.
The enlarged denticle crest on the dorsal and ventral caudal margin, which extends onto the midline of the caudal peduncle, prevents accurate identification of the upper and lower caudal fin origins. Thus, morphometrics which rely on this feature, e.g., dorsal–caudal space, may vary between researchers and studies and should be treated cautiously. For vertebral counts, the origin of the ventral–caudal lobe was more reliably determined from a radiograph rather than from the specimen itself. However, differences between radiographs, orientation of the specimen, and reader methodology lead to subjectivity in the placement of the ventral–caudal origin for vertebral counts. Thus, although provided in the descriptions herein, the precaudal vs. caudal diplospondylous counts should be treated with caution, and it is considered more accurate to use total diplospondylous counts only.
Tooth file counts were taken directly from specimens. Intestinal spiral valve counts and egg cases were taken from a subset of excised specimens.
2.3. Skeletal Features
Skeletal characteristics follow those mentioned in [3] for carcharhiniform sharks and are based on micro-CT scans of two specimens, the D. bigus holotype (CSIRO H 947-10) and an adult specimen of D. melanobranchus (OCF-P10795). Micro-CT scanning and segmentation of these two specimens was undertaken at the University of Florida. Due to the poor calcification of some parts of the cranium, some skeletal characters could not be adequately assessed, e.g., subnasal plates, suborbital shelves, and sphenopterotic ridges.
2.4. Denticle Morphology
Denticle descriptions for the genus are based on scanning electron micrographs (SEMs) of skin patches from the holotype NMNZ P.042517 of D. satoi n. sp. and from one adult specimen of Dichichthys melanobranchus (CSIRO H 9464-01) from two positions, i.e., dorsolateral and ventrolateral trunk just anterior to first dorsal fin on right side.
2.5. Molecular Analyses
Tissue samples were only available for a subset of the specimens examined (Table S1). Specimens were sampled for liver or muscle tissue and temporarily stored in 95% alcohol. DNA was extracted using phenol-chloroform extraction [29] or using the High Pure PCR Template Preparation Kit by Roche Diagnostics (Indianapolis, IN, USA). Extracted total DNA was stored at −20 °C until used for amplification via the polymerase chain reaction (PCR). Samples were amplified using Takara ExTaq (Clonetech, Mountain View, CA, USA) with primers designed to target the complete coding sequence for NADH dehydrogenase subunit 2 (NADH2). A single set of new universal primers (ILEM_LY: 5′-AAG GAY CAC TTT GAT AGA GT-3′; ASNM_LY: 5′-AAC RCT TAG CTG TTA AYT AAG AT-3′) designed to bind to the ASN and ILE tRNA regions of the mitochondrial genome was used to amplify the target fragment. PCR reactions were generally carried out in 25 µL tubes by adding 14.775 µL of PCR grade water, 2.5 µL of PCR buffer, 2.0 µL of MgCL2 (25 mM), 2.0 µL of dNTP mix (2.5 mM each), 0.8 µL of each primer (10 µM), 0.125 µL of Takara ExTaq (5 U/µL), and 2 µL of DNA template. The reaction cocktail was de-natured at 94° C for 3 min, after which it was subjected to 35 cycles of denaturation at 94° C for 30 s, annealing at 54° C for 30 s, and extension at 72° C for 90 s. PCR products were sent off to commercial sequencing centres for purification and Sanger sequencing (Retrogen Inc., San Diego, CA, USA). Sequence trace files were evaluated for quality, translated to amino acids, and aligned using the software package Geneious 11.0.5 (https://www.geneious.com, accessed on 5 October 2023). The aligned amino acid sequences were translated back, in frame, to their original nucleotide sequences to yield a nucleotide alignment that was 1044 base pairs long.
A maximum likelihood analysis framework was used to analyze the aligned NADH2 data set. Each site in the alignment was assumed to be independent of the next and identically distributed (IID assumption). We used a general time reversible (GTR) model with 6 rate categories of change (A< >C; A< >G; A< >T; T< >G; C< >T; C< >G) for the analysis. The reversibility of the model has the appealing quality that it generates the same topology and set of branch lengths independent of the choice of root (the root can be assigned after the analysis is run). We included an among-site rate variation (ASRV) parameter in the model to accommodate the fact that some sites, particularly in protein-coding genes, are more fast-evolving than others as a consequence of their functional constraints. The distribution of rates was estimated as a gamma distribution approximated with 4 rate categories. We also included a parameter to accommodate the fact that some sites are so highly conserved that they are effectively invariant. We used the average empirical nucleotide frequencies taken directly from the aligned data set for our inference model.
We then simultaneously estimated the parameter values for the parameterization described above (transition frequencies among the 6 categories of change, the shape of the gamma distribution and the proportion of invariant sites, and the best fitting topology and branch length combination). This yielded the ML tree presented and the following parameter values:
State frequencies A = 0.307875; C = 0.277879; G = 0.102944; T = 0.311303,
Exchangeabilities = AC = 3.70545; AG = 14.1668; AT = 2.0172; CG = 0.110565; CT = 20.0864; GT = 1.0,
Proportion of Invariable Sites = 0.467111,
Gamma Shape parameter = 2.21894.
The number of distinct character patterns under this model was 370.
Starting branch lengths used to initiate the search were obtained using the Rogers Swofford approximation method. Maximum likelihood (ML) analyses were conducted for the NADH2 dataset using PAUP*4.0 [30] using a GTR + I + G model. Bootstrap analyses (1000 replicates) were also conducted using RAxML [31,32] with the same partitioning strategy and nucleotide substitution model as above. PAUP 4.0.b10 [30] was then employed to obtain the 50% majority rule consensus tree and bootstrap values (BP). Sequences have been uploaded to GenBank (accession numbers: JQ519147, PP329559– PP329574, PPPP481950, and PPPP481951).
2.6. Distribution
The distribution map was generated in QGIS 3.24.2 (QGIS Development Team, 2022) using Google Earth base layers.
2.7. Institutional Acronyms
Museum acronyms used for the specimens examined: AIM, Auckland War Memorial Museum, New Zealand; AMS, Australian Museum, Sydney, Australia; ASIZP, Academia Sinica, Biodiversity Research Center, Taipei, Taiwan; BMNH, Natural History Museum, London, UK; CSIRO, Australian National Fish Collection, Hobart, Australia; HUMZ, The Hokkaido University Museum, Hakodate, Hokkaido, Japan; OCF, Okinawa Churashima Foundation, Okinawa, Japan; MNHN, Muséum National d’Histoire Naturelle, Paris, France; NCIP, Indonesian Institute of Sciences (LIPI), Jakarta, Java, Indonesia; NMNZ, Museum of New Zealand Te Papa Tongarewa, Wellington, New Zealand (see [33]).
3. Results
3.1. Family Dichichthyidae n. fam.
urn:lsid:zoobank.org:act:E108EF25-4A0D-475D-9637-26B40244F756
Type genus. Dichichthys Chan (1966) [19]: 223 (South China Sea).
3.1.1. Definition
Catsharks with eyes that are lenticular and slit-like, with subocular ridges broad below the eyes. Their anterior nasal flaps are short, narrowly triangular, and do not reach the upper lip; posterior nasal flaps are present but low. Labial furrows are moderately long, and uppers are continuous with lowers. Uppers are shorter than the nostril width and fall well behind symphyses; lowers are subequal to or slightly longer than uppers, much shorter than the space between anterior ends. Nasoral and postoral grooves are absent.
The trunk is relatively long, tapering gradually to the tail, with pectoral–pelvic space 18.4–26.1% TL. The tail is moderately long, with pelvic-fin insertion to ventral caudal-fin origin 22.2–29.4% TL, compressed, tapering gradually to the caudal fin. The precaudal tail is 0.4–0.6 times the snout–vent length. The caudal peduncle is short and compressed, with dorsal–caudal space 0.2–0.8 times the size of the second dorsal fin base. The prominent crest of enlarged denticles on the dorsal and ventral midline of the caudal peduncle extends to varying degrees onto the upper and lower caudal fin lobes, respectively (Figure 1).
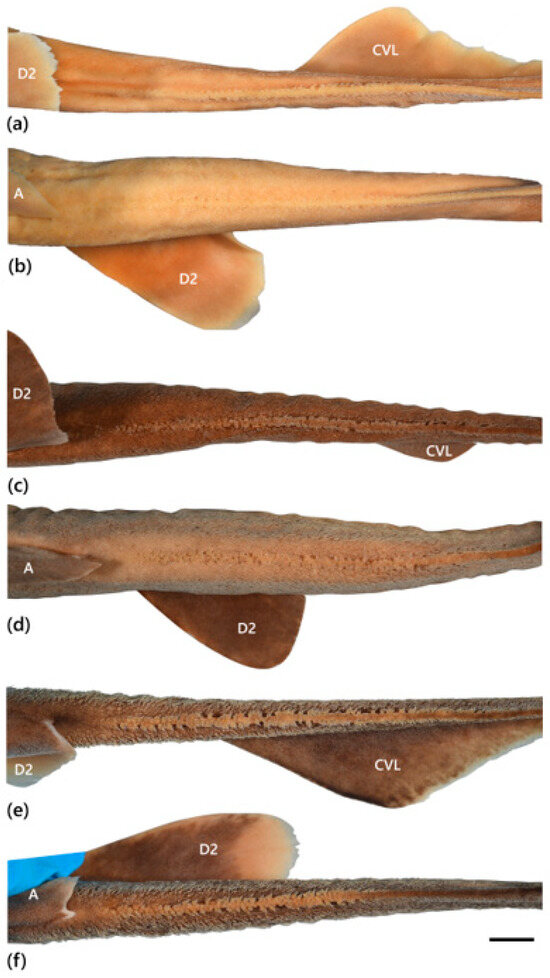
Figure 1.
Crests of enlarged denticles on dorsal (a,c,e) and ventral (b,d,f) midlines of caudal peduncle and anterior portions of the upper and lower caudal fin lobes in the family Dichichthyidae: (a,b) Dichichthys bigus (holotype, CSIRO H 947-10); (c,d) Dichichthys melanobranchus (CSIRO H 9464-01); (e,f) Dichichthys satoi n. sp. (paratype, NMNZ P.25376). Scale bar is 10 mm. Acronyms: A, anal fin; CVL, ventral caudal fin lobe; D2, second dorsal fin. Note blue colour in (f) is part of a latex glove.
Pectoral fins with mesopterygium larger than the propterygium. Pelvic fins are smaller in size than anal fin; the inner margins are not fused, forming a pelvic apron. Claspers are short and moderately robust; a rhipidion is present; an envelope is present; a distinct, short pseudosiphon is present; an exorhipidion is present without clasper hooks and is more posteriorly located than the cover rhipidion; the cover rhipidion is moderately large, without a free anterior fold.
The first dorsal-fin origin just forward of or behind pelvicfin insertions. The second dorsal fin is considerably larger than the first; it is subequal in size to the anal fin, 1.0–1.4 in the anal fin base, with origin over the midbase of the anal fin.
Postero-dorsal labial cartilage is present, with ventral labial cartilage large (Figure 2a). The quadratomandibular joint of Meckel’s cartilage with condyles is merged into one unit (Figure 2b). Palatoquadrate without labial ridges; orbital processes are present but reduced, not invading orbit, situated about half the length of each antimere (Figure 2c). The branchial skeleton without fourth ventral extrabranchials evident on CT scans (Figure 2d). The pectoral girdle has small, barely evident lateral processes; there is no medial projection of the coracoid bar (Figure 2e).
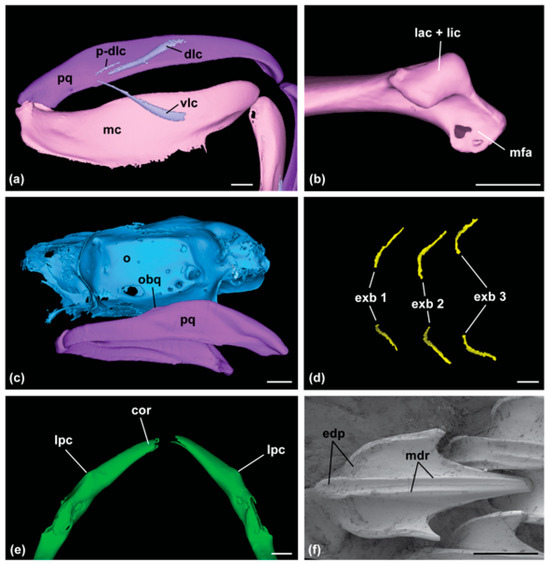
Figure 2.
Key characteristics of the family Dichichthyidae from micro-CT (a–e) and scanning electron microscope (f) images: (a) labial cartilages (dlc, dorsal labial cartilage; mc, Meckel’s cartilage; p-dlc, postero-dorsal labial cartilage; pq, palatoquadrate; vlc, ventral labial cartilage); (b) articular region of the quadratomandibular joint of Meckel’s cartilage (lac + lic, merged labial and lingual condyles; mfa, mandibular facet); (c) lateral palatoquadrate and chondrocranium (o, orbit on chondrocranium; obp, orbital process of the palatoquadrate; pq, palatoquadrate); (d) extrabranchial cartilage of the branchial region (exb 1, first extrabranchials; exb 2, second extrabranchials; exb 3, third extrabranchials); (e) pectoral girdle (cor, coracoid bar; lpc, lateral process); (f) dermal denticle from lateral trunk (edp, ectodermal pits; mdr, median ridges). Scale bar for (a–e) equals 5 mm, and for (f), it equals 100 µm.
Dermal denticles with ectodermal pits are absent or restricted to the anterior portion of the crown only; two median ridges were observed on crowns in larger specimens (Figure 2f).
The cranium has tips of rostral cartilage forming a distinct rostral node, not with free ends. The nasal capsules are anteriorly convex and spherical in shape; nasal apertures are not restricted anteriorly. Ectethmoid chambers at the posterior edges of nasal fontanelles are visible in ventral view (Figure 3a). The anterior fontanelle is broadly subquadrate, with a length of about 1.2–1.3 times the width; an epiphyseal notch is present on the posterior border. Internal carotid foramina are almost touching the midline of the basal plate, well separated from laterally placed stapedial foramina (Figure 3b). Orbits with prominent supraorbital crests (Figure 3c). Postorbital processes are low and bluntly pointed. The postorbital groove is narrow and shallow, less than a third of the height of the hyomandibular facet. The parietal fossa is almost parallel with the anterior fontanelle in lateral view. Otic capsules without prominent pterotic processes on the sphenopterotic ridges. Occiput with low pointed occipital condyles (Figure 3d).
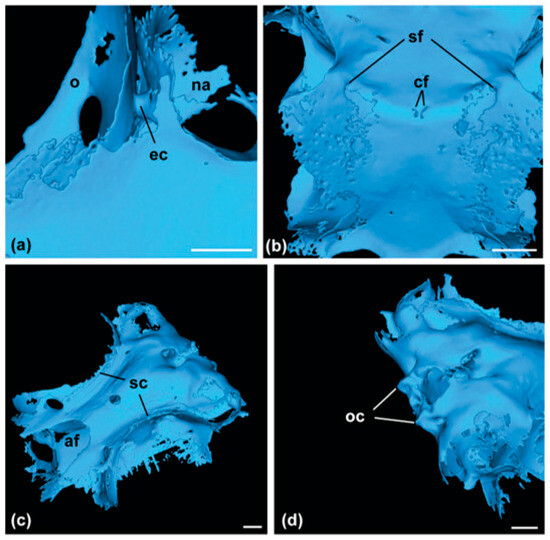
Figure 3.
Chondrocranium characteristics of the family Dichichthyidae from micro-CT scans: (a) ectethmoid chamber (ec) between posterior nasal aperture (na) and orbit (o); (b) internal carotid foramina (cf) and stapedial foramina (sf); (c) supraorbital crests (sc) and anterior fontanelle (af); (d) occipital condyles (oc). Scale bar equals 5 mm.
The total vertebral centra is 130–145; the precaudal centra (to lower caudal fin origin) is 90–102.
Intestinal valve with 9–14 turns.
The colour pattern is uniformly pale to dark brown (one species sometimes with white spots over the dorsal surface); fins are sometimes with white or dark margins.
The species are oviparous. Egg cases are asymmetrical, with one lateral edge straight or slightly concave and the other lateral edge moderately convex, giving the egg case a curved (banana-like) appearance. Dorsal and ventral surfaces are present with 10–16 longitudinal, pliable ridges; long attachment fibres are present on the anterior end; long coiled tendrils are present on the posterior end.
3.1.2. Genera
Monotypic: Dichichthys Chan (1966) [19].
3.1.3. Distribution
Only known from the eastern Indian and Western Pacific Oceans (Figure 4).
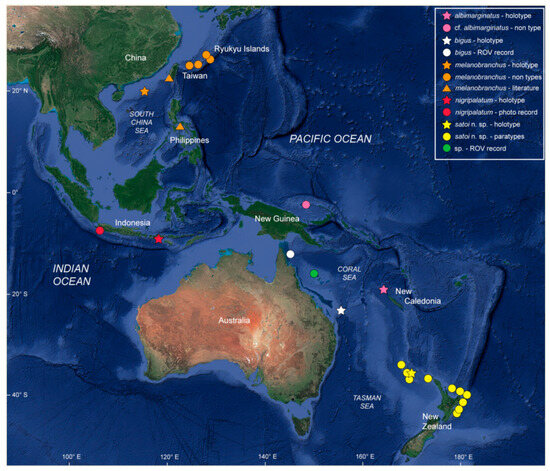
Figure 4.
Known occurrences of Dichichthys species in the Indo–West Pacific. Pink star denotes D. albimarginatus holotype, and pink circle denotes the Papua New Guinea specimen assigned to D. cf. albimarginatus; white star denotes the holotype, and white circle a live individual of D. bigus; orange star denotes the holotype; orange circles for other specimens examined and orange triangle other records not examined of D. melanobranchus; red star denotes holotype and red circle photographic evidence of D. nigripalatum; yellow star denotes the holotype and yellow circles paratypes of D. satoi n. sp.; green circle denotes live individuals of D. sp. (Image © Google 2023, SIO, NOAA, U.S. Navy, NGA, GEBCO).
3.1.4. Etymology
Chan [19] stated the name Dichichthys is a combination of the Greek dicho- meaning “to branch in two” and the Greek -ichthys for “fish”. This name was in allusion to the author, considering this genus intermediate between the Galeus-Parmaturus complex and Apristurus.
Vernacular name. Since catsharks have recently been separated into separate families, i.e., Atelomycteridae, Pentanchidae, Scyliorhinidae, and now Dichichthyidae, the authors have ascribed a new familial name for this new family, the bristle sharks. Chan [19] referred to the denticles of the juvenile D. melanobranchus holotype as bristle-like. Indeed, the most obvious characteristic when examining specimens of this species is the large denticles that appear bristle-like in texture macroscopically.
3.1.5. Molecular Analysis
Results of the maximum likelihood analysis of the NADH2 sequences are shown in Figure 5. The two representatives of the Dichichthyidae from which DNA sequence data were available are clearly separated from representatives of Atelomycterus, Aulohalaelurus, and Schroederichthys.
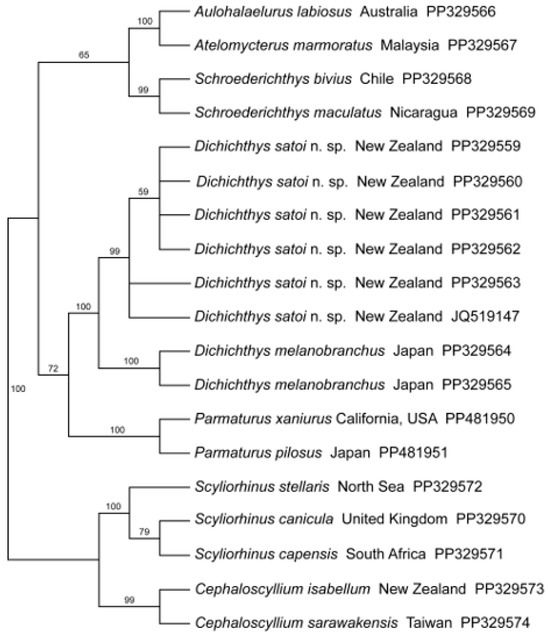
Figure 5.
Maximum likelihood tree estimated using a general time reversible model (GTR) with model terms to accommodate both invariant site (I) and gamma-distributed rates (G). Bootstrap support values are shown from a separate ML bootstrap analysis. GenBank accession numbers are provided.
3.1.6. Remarks
The family Dichichthyidae n. fam. differs from the family Pentanchidae, in which it was previously included, in possessing supraorbital crests (vs. no supraorbital crests). The family Dichichthyidae n. fam. differs from the family Scyliorhinidae in the following characteristics: clasper envelope present (vs. absent); second dorsal fin larger than first (vs. first much larger than second); second dorsal fin subequal in area to anal fin (vs. second dorsal fin much smaller than anal fin); tips of rostral cartilage fused into a rostral node (vs. tapering to abrupt free ends, not fused into a lobe); pseudosiphon distinct on claspers (vs. absent or rudimentary); ventral labial cartilage large (vs. small); postero-dorsal labial cartilage present (vs. absent). The family Dichichthyidae n. fam. differs from the genera Atelomycterus and Aulohalaelurus (family Atelomycteridae sensu [9]) in the following characteristics: subocular ridges below eyes broad (vs. narrow); clasper pseudosiphon short but distinct (vs. greatly elongate, slit-like); second dorsal fin larger than first (vs. dorsal fins subequal or second only slightly larger than first); posterior nasal flaps present (vs. absent). The family Dichichthyidae n. fam. differs from the genus Schroederichthys (family Atelomycteridae sensu [9]) in the following characteristics: second dorsal fin larger than first (vs. dorsal fins subequal or second only slightly larger than first); higher intestinal valve turns (10–14 vs. 6); two upper labial cartilages (vs. a single upper labial cartilage). While the analysis of the molecular data shows that the family Dichichthyidae is unambiguously distinct from Schroederichthys, Atelomycterus, and Aulohalaelurus, the exact relationship among these genera changes according to the taxon sampling scheme and the method of analysis used. We present the maximum likelihood tree that results from the taxon sampling scheme employed but note that the inferred topology presented changes when different taxon sampling schemes are used. Further analysis using a more extensive suite of markers is beyond the scope of the current contribution but will be pursued in the future.
3.2. Genus Dichichthys (Chan, 1966 [19])
Dichichthys (Chan, 1966 [19]): 223, figs 2, 3—type Dichichthys melanobranchus (Chan, 1966 [19]) by original designation.
Definition. Monogeneric; see family definition.
Species. Dichichthys albimarginatus (Séret and Last, 2007 [25]); Dichichthys bigus (Séret and Last, 2007 [25]); Dichichthys melanobranchus (Chan, 1966 [19]); Dichichthys nigripalatum (Fahmi and Ebert, 2018 [26]); Dichichthys satoi n. sp.
3.3. Dichichthys albimarginatus (Séret and Last, 2007 [25])
Whitetip Bristle Shark.
3.3.1. Synonymy
Parmaturus sp.—[34]: 35, 42 (New Caledonia).
Parmaturus albimarginatus Séret and Last, 2007 [25]: 25, figs 2, 3 (New Caledonia)—[8]: 362, figs (New Caledonia); [35]: 41 (Southwest Pacific); [36]: 400, figs (New Caledonia)
Parmaturus sp. 1 (tentatively)—[37]: 28 (west of Manus Island, Papua New Guinea); [38]: 114, figs (west of Manus Island, Papua New Guinea).
3.3.2. Specimens Examined
Holotype: MNHN 1997-3584, adult male 577 mm TL, Grand Passage, New Caledonia, 18°54′ S, 163°05′ E, 688–732 m depth, 21 November 1994 (Figure 6a and Figure 7a).

Figure 6.
Lateral view of (a) Dichichthys albimarginatus, adult male 577 mm TL (holotype MNHN 1997–3584, preserved); (b) Dichichthys cf. albimarginatus, juvenile male 250 mm TL (ASIZ P0080722, preserved).

Figure 7.
Ventral anterior view of (a) Dichichthys albimarginatus, adult male 577 mm TL (holotype MNHN 1997–3584, preserved); (b) Dichichthys cf. albimarginatus, juvenile male 250 mm TL (ASIZ P0080722, preserved).
Other material: Dichichthys cf. albimarginatus—ASIZ P0080722, putative juvenile male 250 mm TL, west of Manus Island, Papua New Guinea, 2°14.127′ S, 147°25.045′ E, 980–985 m depth, 29 September 2010 (Figure 6b, Figure 7b and Figure 8).
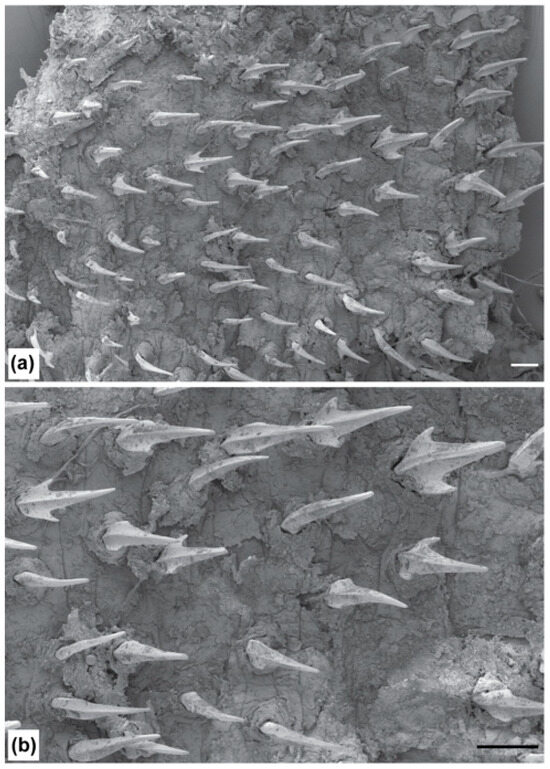
Figure 8.
Scanning electron micrographs of lateral trunk denticles of Dichichthys cf. albimarginatus (ASIZ P.80722, juvenile): (a) dorsolateral trunk (×30 magnification); (b) dorsolateral trunk (×60 magnification). Scale bar denotes 200 µm.
3.3.3. Diagnosis
A small bristle shark with the following combination of adult characteristics (Figure 6 and Figure 7): preanal length, 59.1% TL; pre-first dorsal length, 51.1% TL; short prenarial length, 3.7% TL; head depressed, its height 0.5 times its width; mouth moderately wide, its width 9.1% TL; lower labial furrows longer than uppers (upper furrows 1.7% TL, lower furrows 2.0% TL); anal fin moderately large, its base is 10.7% TL; posterior margin, 4.8% TL; pectoral–pelvic space 24.8% TL; body light to medium brown above, paler below, with the demarcation between darker dorsal and paler ventral colouration on ventrolateral surfaces relatively distinct; fins with distinct, broad white margins; teeth in 92 files in both upper and lower jaws; monospondylous centra, 43–46; precaudal centra, 92–95.
3.3.4. Description
See [25]. Proportional measurements of the holotype and putative PNG juvenile are provided in Table 1.

Table 1.
Morphometric measurements and meristics of Dichichthys species. Morphological measurements expressed as a percentage of total length. Measurements provided for the holotype of D. albimarginatus (MNHN 1987-3584), D. cf. albimarginatus (ASIZ P.80722), D. bigus (CSIRO H 947-10), D. melanobranchus (BMNH 1965.8.11.6), D. nigripalatum (NCIP 6567), and D. satoi n. sp. (NMNZ P.42517). Ranges provided for the 5 adult specimens of D. melanobranchus and the 13 paratypes of D. satoi n. sp.
3.3.5. Size
The adult male holotype is 577 mm TL; the juvenile male from Papua New Guinea is 250 mm TL.
3.3.6. Distribution
The holotype was collected northwest of New Caledonia at a depth of 688–732 m. The Papua New Guinea specimen tentatively assigned to this species was collected west of Manus Island in northern Papua New Guinea at 980–985 m depth (Figure 4).
3.3.7. Remarks
The allocation of this species to the genus Dichichthys is based on the presence of a supraorbital crest on the holotype and its similar morphology to the other species assigned to this genus. In particular, the second dorsal fin is distinctly larger than the first and smaller pectoral fins. No genetic data are available for this species to confirm its allocation.
The Papua New Guinea juvenile male has distinct, white-edged dorsal, anal, and caudal fins similar to those of D. albimarginatus and D. satoi n. sp. This specimen was first thought to represent an undescribed species based on differences with its closest congener, D. albimarginatus [38]. The juvenile differs from the holotype of D. albimarginatus via the following characteristics: dorsal fins more anteriorly placed (pre-first dorsal length 46.2 vs. 51.1% TL, pre-second dorsal length 62.0 vs. 66.9% TL), pectoral and pelvic fins closer together (pectoral–pelvic space 18.4 vs. 24.8% TL, 0.93 vs. 1.29 times head length), more anteriorly located cloaca (snout–vent length 44.8 vs. 49.7% TL) and anal fin (preanal length 55.2 vs. 59.1% TL), larger eyes (eye length 5.6 vs. 3.9% TL), larger spiracle (length 0.7 vs. 0.2% TL), longer snout (prenarial length 5.2 vs. 3.7% TL, preoral length 7.0 vs. 6.3% TL), longer first dorsal fin inner margin (its length 3.1 vs. 1.6% TL), and larger second dorsal fin (anterior margin 13.0 vs. 10.4% TL, base length 10.7 vs. 8.0% TL). The juvenile also differs from the holotype in having a naked area on the ventral head, extending down the midline (vs. entirely denticulated), less enlarged denticles on the upper caudal fin crest (up to twice as large as adjacent denticles vs. greatly enlarged, more than three times adjacent denticles), a dark grey to blackish incomplete collar-like marking on the head at the level of gills, extending both dorsally and ventrally (vs. no dark markings on the head), and a dark greyish triangular blotch on sides broadest at anterior origin above pectoral fin rear tips (vs. no black blotch on sides). The juvenile specimen has small, bristle-like, unicuspid to tricuspid denticles (Figure 8).
The juvenile male differs from the three smaller (<700 mm TL) specimens of D. satoi n. sp. (see later) via the following characteristics: dorsal fins more anteriorly placed (pre-first dorsal length 46.2 vs. 50.5–51.4% TL, pre-second dorsal length 62.0 vs. 65.2–66.6% TL), more anteriorly placed anal fin (preanal length 55.2 vs. 58.1–59.3% TL) and cloaca (snout–vent length 44.8 vs. 47.8–48.9% TL), pectoral and pelvic fins closer together (pectoral–pelvic space 18.4 vs. 22.4–23.6% TL), larger eyes (eye length 5.6 vs. 3.8–4.6% TL), broader head (interorbital space 8.6 vs. 6.5–7.4% TL), longer first dorsal fin inner margin (its length 3.1 vs. 1.7–1.9% TL), and longer pectoral fin anterior margin (10.6 vs. 8.5–8.8% TL).
Despite the large number of differences listed above for the juvenile Papua New Guinea male vs. both D. albimarginatus and D. satoi n. sp., intraspecific variation is poorly known in this genus due to the small number of specimens examined. No other small juveniles for any Dichichthys species were available for comparison. Without additional specimens for comparison or genetic samples to compare sequences of similar genes, the juvenile Papua New Guinea specimen is tentatively assigned to D. cf. albimarginatus rather than D. satoi n. sp. given the tropical distribution of the former species compared to the temperate distribution of the latter species. The tentative New Caledonia to northern Papua New Guinea distribution for D. albimarginatus resembles that found for Apristurus nakayai at similar depths [39]. Catsharks from the mid to lower slope are poorly known throughout much of the tropical Indo-Pacific; thus, D. albimarginatus could be more widespread than currently known.
3.4. Dichichthys bigus (Séret and Last, 2007 [25])
Beige Bristle Shark.
3.4.1. Synonymy
Parmaturus sp. A—[40]: 204, figs, pl. 20 (Queensland, Australia).
Parmaturus bigus Séret and Last, 2007 [25]: 32, figs 6, 7 (Queensland, Australia)—[7]: 221, figs, pl. 29 (Queensland, Australia); [8]: 364, figs (Queensland, Australia); [41]: 206 (Coral Sea); [35]: 41 (Southwest Pacific); [36]: 402, figs (Queensland, Australia).
3.4.2. Material Examined
Holotype: CSIRO H 947-10, gravid female 710 mm TL (two egg cases removed and registered as CSIRO H 947-34), south of Saumarez Reef, Marion Plateau, Queensland, 22°56′ S, 154°21′ E, 590–606 m depth, 17 November 1985.
3.4.3. Other Records
An underwater sighting recorded during an ROV SuBastian dive onboard the RV Falkor in the Coral Sea in 2020 is tentatively assigned to this species: adult male ~610 mm TL, Southern Small Detached Reef, 12°31.87′ S, 143°51.563′ E, 838–881 m depth, 18 October 2020.
3.4.4. Diagnosis
A medium-sized bristle shark with the following combination of adult characteristics (Figure 9 and Figure 10): preanal length, 62.4% TL; pre-first dorsal length, 54.4% TL; short prenarial length, 4.2% TL; head subcylindrical, its height subequal to its width; mouth relatively narrow, its width 7.7% TL; labial furrows subequal in length, 1.6% TL; anal fin moderately large, its base 9.8% TL, posterior margin 5.8% TL; pectoral–pelvic space, 25.0% TL; body pale brown above, whitish below, demarcation between darker dorsal and paler ventral coloration on ventrolateral surfaces distinct; fins with narrow white margins; dorsal fins with dusky subterminal blotches when live; teeth in 120 files in upper jaw; intestinal valve with 9 turns; monospondylous centra, 47; precaudal centra, 102; total centra, 144.

Figure 9.
Lateral view of holotype of Dichichthys bigus (CSIRO H 947-10, gravid female 710 mm TL, fresh).
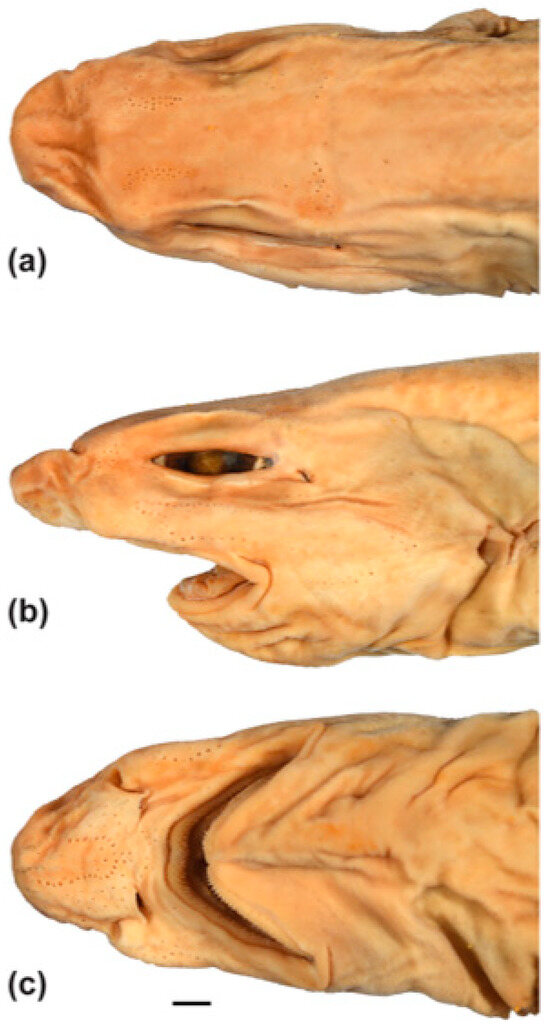
Figure 10.
Head of holotype of Dichichthys bigus (CSIRO H 947-10, gravid female 710 mm TL): (a) dorsal view; (b) lateral view; (c) ventral view. Scale bar denotes 10 mm.
3.4.5. Description
See [25].
3.4.6. Egg Case
Based on CSIRO H 947-34 (Figure 11a; Table 2): egg case elongate, its total length 89.41 mm and maximum width 27.89 mm, slightly dorsoventrally depressed; 12 longitudinal, pliable ridges extend the entire length of the egg case on both dorsal and ventral surfaces; the egg case is asymmetrical in shape, one lateral edge almost straight, the other lateral edge strongly convex, more so posteriorly, with posterior end tapering distinctly towards the straight edge. The anterior margin truncate; projections at each corner are absent; long thread-like attachment fibres extend from the anterior margin. The posterior margin is closed (no posterior border) and the posterior horns are curved strongly and medially, forming tightly coiled tendrils. The anterior waist is weak and the posterior waist is absent. A single, low, broad keel present on the straight lateral edge, most prominent at the anterior and posterior ends; a convex lateral edge with two prominent keels, similar in size, present alongthe entire length of the egg case, separated by a narrow groove; keels on each lateral edge at the posterior end folding on opposing dorsal and ventral surfaces, enveloping outermost pliable ridges. They are uniformly brown in colour.

Figure 11.
Egg case (preserved) of (a) Dichichthys bigus (CSIRO H 947-34, preserved); (b) Dichichthys melanobranchus (HUMZ 101534); (c) Dichichthys satoi n. sp. (from CSIRO H 9287-01). Scale bar denotes 10 mm.

Table 2.
Egg case morphometrics, expressed as a percentage of egg case length, for the two Dichichthys bigus egg cases and ranges for the seven D. satoi n. sp. egg cases measured.
3.4.7. Size
The only specimen in collections is the adult female holotype of 710 mm TL. One of the adult males seen during ROV footage (Figure 12) was estimated to be ~610 mm TL (estimated using parallel laser beams).
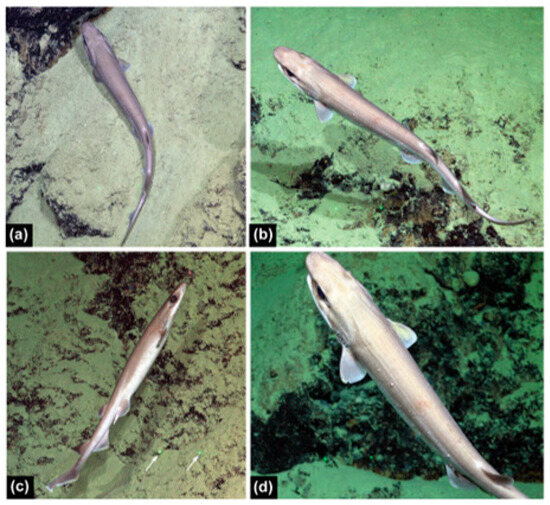
Figure 12.
Underwater images of Dichichthys bigus (adult male ~610 mm TL) from the ROV SuBastian during Coral Sea surveys on the RV Falkor at Southern Small Detached Reef, Queensland, at 838–881 m depth: (a) resting on sandy ledge on steep rock wall; (b) left dorsolateral view while swimming; (c) right lateral view while swimming (white arrows denote the parallel laser beams used for size estimation); (d) close-up of lesions and isopods on left side. Photo copyright Permission was granted by copyright holder Schmidt Ocean Institute.
3.4.8. Distribution
Type locality south of the Saumarez Reef on the Marion Plateau of southern Queensland (22°56′ S) at a depth of 590–606 m. Underwater records from the Southern Small Detached Reef (~12°32′ S) at depths of 838–881 m (Figure 4).
3.4.9. Remarks
The allocation of this species into the genus Dichichthys is confirmed based on the presence of supraorbital crests on the chondrocranium, as well as egg case morphology.
The morphometrics taken from the holotype in this study largely agreed with those provided in [25], except for the following measurements. The preanal length was reported as being 51.6% TL, but this is clearly an error as it is only slightly larger than the snout–vent length (50.8% TL). A preanal length of 62.4% TL is provided in this study. Pectoral-fin inner margin and posterior margin lengths also differed, but this is likely due to the interpretation of the apex of the free rear tip, which is broadly rounded. Pelvic-fin base length and length were much lower in [25] than recorded in this study, i.e., 4.6 vs. 7.5% TL and 7.3 vs. 10.2% TL, respectively. These measurements were consistent on both fins. The anal-fin length appears to be incorrect in [25] as it is the same percentage of length as its base length, i.e., 9.7% TL, despite having a free rear tip. An anal-fin length of 11.2% TL was recorded in this study. The dorsal–caudal margin measurement was much smaller in this study than in [25], i.e., 14.3 vs. 20.2% TL. It is likely that the measurement in [25] was taken from the origin of the dorsal–caudal crest, which commences anterior to the origin of the upper caudal lobe. However, the origin is difficult to determine as it is largely concealed by the crest.
Dichichthys bigus differs from the adult holotype of D. albimarginatus via the following characteristics: head barely depressed in D. bigus vs. distinctly depressed in D. albimarginatus (head height 8.6 vs. 6.9% TL, head width 1.1 vs. 1.9 times head width); head narrow (its width 9.6 vs. 12.9% TL, interorbital space 5.4 vs. 7.7% TL); first dorsal fin shorter (base length 5.1 vs. 6.8% TL, anterior margin 5.8 vs. 8.2% TL); larger gill slits (height of first 2.3 vs. 1.7% TL, the height of fifth 1.5 vs. 0.8% TL); a narrower mouth (its width 7.7 vs. 9.1% TL). Dichichthys bigus also has slightly more vertebrae than D. albimarginatus and D. cf. albimarginatus (144 vs. 133–136), though based on only the holotype.
The underwater images of the adult male specimen observed during an ROV SuBastian dive (Figure 12) in the Coral Sea are tentatively assigned to this species. The larger second dorsal fin and overall morphology agree well with D. bigus, and the narrow white posterior edges on the dorsal and caudal fins agree with the fresh D. bigus holotype (Figure 9). Several parasites are clearly visible on this live specimen. Three isopods from the family Aegidae, possibly Aega (A. Hosie, Western Australian Museum, pers. comm.), are attached to the gill slits on either side and one above the anterior pelvic-fin base (Figure 12d). Two other smaller parasites are visible on the lateral surface anterior to the left pelvic fin and one on the dorsal midline anterior to the first dorsal fin. These are possibly larval gnathiid isopods (A. Hosie, Western Australian Museum, pers. comm.). This shark also had many variable-sized raised nodular or cyst-like lesions, mostly on the left side (Figure 12b,d).
Another similar adult male catshark was observed on a different ROV SuBastian dive on 21 May 2020 at Moore Reef on the Queensland Plateau (15°52.64′ S, 149°08.54′ E) at depths of 847–849 m (Figure 13). This specimen lacks white-edged fins, with the fins being much darker. Additionally, the body is more robust, with a visibly shorter snout, trunk, and interdorsal space. It agrees with other Dichichthys species but cannot be reliably assigned to any species treated in this paper. This is the only record of this taxon from the Queensland Plateau and is referred to herein as Dichichthys sp. with specimens required to resolve its taxonomic position.
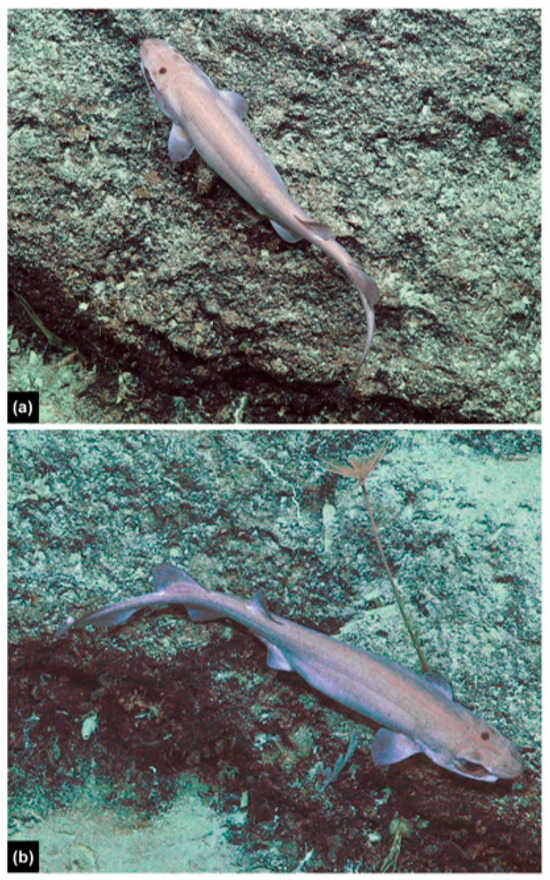
Figure 13.
Underwater images of Dichichthys sp. (adult male) from the ROV SuBastian during Coral Sea surveys on the RV Falkor at Moore Reef, Queensland, at 847–849 m depth: (a) dorsal view while swimming; (b) right dorsolateral view while swimming. Photos copyright: Permission was granted by copyright holder Schmidt Ocean Institute.
3.5. Dichichthys melanobranchus (Chan, 1966 [19])
Blackgill Bristle Shark.
3.5.1. Synonymy
Dichichthys melanobranchus (Chan, 1966 [19]): 223, figs 2, 3; holotype BMNH 1965.8.11.6 (South China Sea; see Figure 13a).
Parmaturus melanobranchus—[2]: 101 (South China Sea); [25]: 23 (China and Japan); [42]: 320 (Taiwan); [35]: 41 (Northwest Pacific); [36]: 404, figs (Northwest Pacific).
Figaro piceus Chu, Meng, and Liu, 1983 [43]: 104, figs 1, 2 (South China Sea).
Parmaturus melanobranchius—[44]: 341, figs (South China Sea); [45]: 1291, figs (South China Sea); [46]: 517; [47]: 355, fig 3A (Yonaguni Island, Ryukyu Archipelago, Japan); [48]: 179; [49]: 386, figs 2, 3 (Taiwan); [8]: 366, figs (Northwest Pacific).
Parmaturus sp.—[50]: 54 (Okinawa Islands).
3.5.2. Material Examined
Holotype: BMNH 1965.8.11.6, juvenile female 235 mm TL, about 135 miles south-southeast of Hong Kong, South China Sea, 20°05′ N, 115°03′ E, depth 549 m, 22 August 1964 (Figure 14a).

Figure 14.
Lateral view of Dichichthys melanobranchus: (a) preserved holotype (BMNH 1965.8.11.6, juvenile female 235 mm TL), photo copyright: Natural History Museum, London; (b) live specimen (OCF-P20110802, adult female 742 mm TL) taken in a pressurised chamber at the Okinawa Churaumi Aquarium; photo copyright: Okinawa Churaumi Aquarium; (c) fresh specimen (OCF-P20061022-3, female 600 mm TL).
Other material (n = 6): HUMZ 101534, egg case removed from HUMZ 95194 (gravid female 619 mm TL), Ryukyu Islands, East China Sea, Japan; OCF-P20110802 (GenBank accession PP329565), adult female 742 mm TL, off Ie-jima Island, Okinawa, East China Sea, Japan, 26°58.7′ N, 127°26.1′ E, 760 m depth, 1 June 2011; CSIRO H 9464-01, adult male 762 mm TL, CSIRO H 9464-02, adult female 678 mm TL, OCF-P10795, adult male 718 mm TL, OCF-P10796, adult male 650 mm TL, East China Sea, off Okinawa, Ryukyu Islands, Japan, 540–835 m depth.
3.5.3. Diagnosis
A medium-sized bristle shark with the following combination of adult characteristics: preanal length, 60.6–62.3% TL; pre-first dorsal length, 52.1–53.9% TL; moderate prenarial length, 4.8–5.4% TL; head moderately depressed, its height 0.5–0.9 times its width; mouth moderately wide, its width 9.2–9.9% TL; labial furrows subequal in length or lowers slightly longer than uppers (upper furrows 1.6–1.9% TL, lower furrows 1.8–2.0% TL); anal fin moderately large, its base 11.2–11.9% TL, posterior margin, 5.6–7.0% TL; pectoral–pelvic space, 24.0–26.0% TL; body brownish above, paler below, demarcation between darker dorsal and paler ventral colouration on ventrolateral surfaces distinct; fins uniform in colour, without markings; teeth in about 86–102 files in upper jaw and about 83–97 lower jaw; monospondylous centra, 45–46; precaudal centra, 96–101; total centra, 133–145.
3.5.4. Description
Based on five adult specimens from Ryukyu Island: body relatively firm, trunk slightly depressed, and tail compressed and slightly tapering to caudal fin (Figure 14b,c); abdomen much longer than head; pectoral–pelvic space, 24.0–26.0% TL; pectoral–pelvic space 1.19–1.32 times head length; pelvic–anal space 1.41–2.12 in anal-fin base; caudal peduncle short, compressed; anal–caudal space 1.41–1.57 in anal-fin base; caudal peduncle width 1.36–1.81 in height; prominent crest of enlarged denticles on the dorsal and ventral midline of caudal peduncle; dorsal crest originating over or just posterior to second dorsal fin free-rear tip and extending to the midpoint of the dorsal–caudal margin; ventral crest originating just posterior to the anal-fin free-rear tip and extending slightly onto ventral–caudal margin.
Head relatively short, depressed, width 10.5–13.0% TL (Figure 15); dorsal profile mostly straight anteriorly becoming slightly convex behind eyes in lateral view; prominent subocular ridges, originating at anterior nostrils extending almost to first gill slit, broadest below eyes, becoming less prominent posteriorly. Snout moderate, narrowly parabolic in dorsoventral view (Figure 15a,c), strongly indented at the level of anterior nostrils, tip narrowly rounded in lateral view (Figure 15b); preoral length 6.4–7.1% TL, 0.67–0.76 times mouth width; preorbital length 1.32–1.58 times eye length. Eyes large, narrow, length 4.5–4.8% TL, 4.45–5.59 times eye height, 4.22–4.63 in head length; eyes situated mostly laterally on the head (lower margin of eyes only slightly visible in dorsal view). Spiracles are small, located just behind the eye at about level with the lower margin of the eye. Mouth large, long, parabolic (arched in adult males, Figure 16b); mouth corners level with posterior margin of eye; symphysis of lower jaw about level with anterior margin of eye. Labial furrows are moderately long, well-defined; lowers are slightly longer than uppers. Nostrils large with tube-like incurrent apertures, nostril length 0.55–0.66 times eye length, nostrils well separated, internarial space 2.1–2.6% TL, 0.70–1.02 times nostril width; well separated from mouth; anterior nasal flaps moderate-sized, not overlapping posterior nasal flap, posterior tip forming a pronounced triangular lobe; posterior nasal flaps present, low, its width about a third of nostril width. Gills small, only slightly taller than eye height, fourth largest and above pectoral-fin origin, fifth smallest and over anterior pectoral-fin base; upper margins of gill slits well below the lower level of the eye.
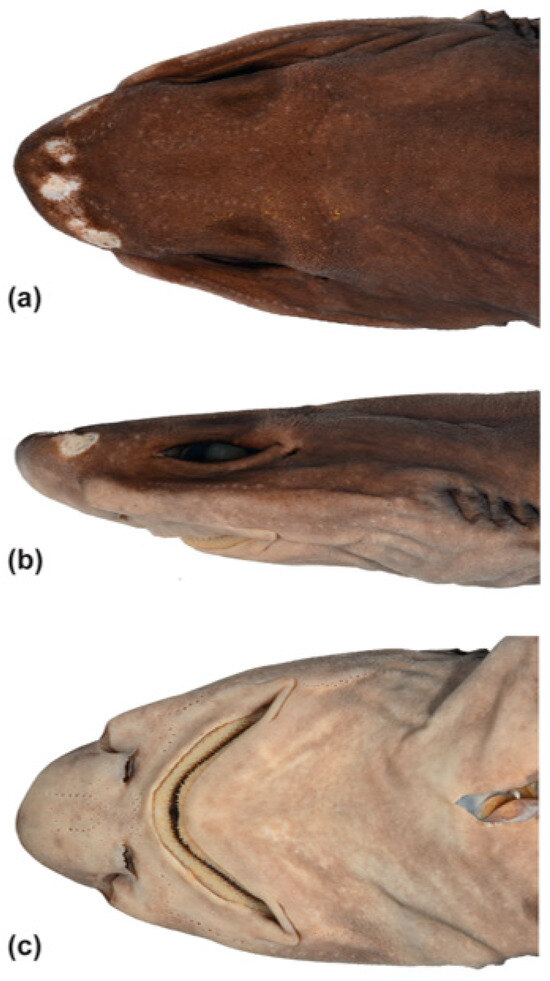
Figure 15.
Head of Dichichthys melanobranchus (OCF-P20110802, adult female 742 mm TL): (a) dorsal view; (b) lateral view; (c) ventral view.
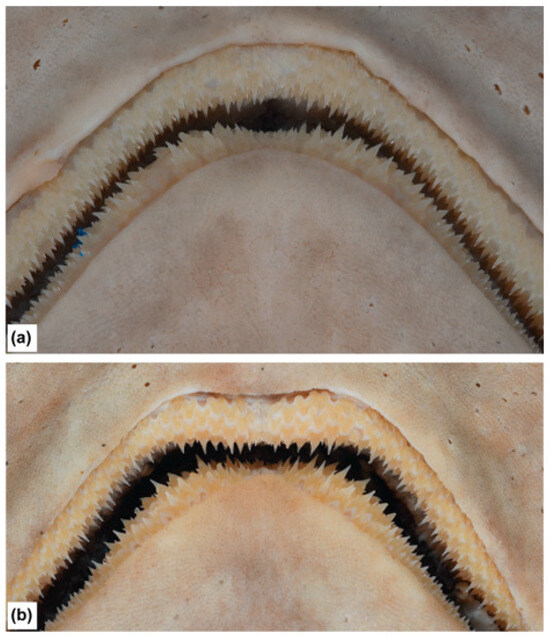
Figure 16.
Mouth of Dichichthys melanobranchus: (a) adult female (OCF-P20110802, 742 mm TL); (b) adult male (CSIRO H 9464-01, 762 mm TL).
Pores distinct on ventral head (Figure 15a,b); enlarged pores in two parallel longitudinal lines extending from snout tip, which converge rapidly level of nostrils, and near lateral extremity anterior to upper labial furrow towards nostrils; numerous smaller pores on preoral snout; hyomandibular pores distinct, 8–20 (mean 15.1) on each side; mandibular pores less distinct, often obscured by denticles, 3–7 (mean 4.3) on each side. Enlarged pores on the dorsal head extending from near the snout tip and curving posterolaterally, roughly following the contour of the rostral cartilage, and on subocular ridges below the eyes (Figure 15a); slightly smaller pores on either side of the head following the contour of orbits and on posterior head lateral line pores, becoming distinct anterior to gill openings.
First dorsal fin much smaller than second dorsal. First dorsal-fin height 0.70–0.75 times second dorsal-fin height; first dorsal-fin base 0.68–0.75 times second dorsal-fin base; originating over rear quarter of pelvic-fin base; anterior margin nearly straight to slightly convex, apex narrowly to moderately rounded, posterior margin slightly convex to nearly straight (slightly concave in OCF-P20110802, possibly damaged), inner margin short, rear tip rounded. Second dorsal fin originating over anal-fin midbase; anterior margin nearly straight to slightly convex, apex rounded, posterior margin nearly straight; inner margin short, free tip rounded to subangular.
Pectoral fins small, somewhat paddle-like, anterior margin 7.7–9.1% TL; anterior margins slightly to moderately convex, apex moderately rounded, posterior margin moderately convex, free-rear tip broadly rounded. Pelvic fins small, subtriangular, length 9.1–10.7% TL, anterior margin slightly convex, apex broadly rounded, posterior margin slightly to moderately convex, free-rear tip rounded.
Claspers of adult males short, relatively broad, tapering rapidly distally to a narrowly pointed tip (Figure 17a); clasper glans more than 90% of clasper outer length; fleshy envelope present, covering clasper groove; rhipidion well developed with a prominent posterior margin (Figure 17b); short but distinct pseudosiphon present; cover rhipidion large, without free anterior tab or fold; exorhipidion present, extending more posteriorly than cover rhipidion; dorsal surface of clasper naked except for exorhipidion, which is covered in small denticles.
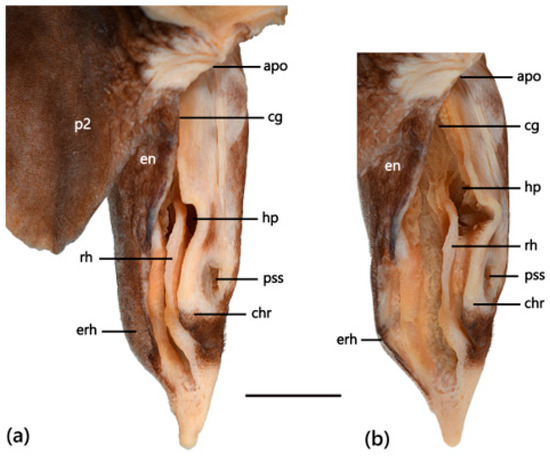
Figure 17.
Clasper of adult male Dichichthys melanobranchus (CSIRO H 9464-01): (a) glans not dilated; (b) glans dilated. Abbreviations: apo, apopyle; cg, clasper groove; chr, cover rhipidion; en, envelope; erh, exorhipidion; hp, hypopyle; p2, pelvic fin; pss, pseudosiphon; rh, rhipidion. Scale bar denotes 10 mm.
Anal fin relatively low, triangular, subequal in area to second dorsal fin; base longer than second dorsal fin base, base 11.2–11.9% TL, 1.28–1.45 times second dorsal-fin base, 1.12–1.42 times interdorsal space; origin level with or just posterior to first dorsal fin free rear tip, anal-fin height 2.60–2.91 in base length. Caudal fin short, dorsal–caudal margin length 15.6–17.8% TL; origins of upper and lower lobes obscured by crest of enlarged denticles; lower lobe weakly developed distally; terminal lobe prominent, terminal margin slightly convex to nearly straight.
The teeth of both jaws are exposed when the mouth is closed. The teeth are visibly larger in adult males than in females (Figure 16); anterior teeth of adult females have 4–6 cusps, those of adult males have mostly 3 cusps (Figure 18); the central cusp is longest and upright, flanked by smaller but distinct lateral cusps, much larger in adult males; the central cusp becomes progressively shorter posteriorly and more oblique; lateral cusps remain the same size; posteriormost teeth with three central cusps are almost equal in size; teeth are arranged in ~86–102 files in the upper jaw, and ~83–97 files in the lower jaw.

Figure 18.
Scanning electron micrographs of anterolateral teeth of Dichichthys melanobranchus (CSIRO H 9464-01): (a) upper tooth; (b) lower tooth (×35 magnification). Scale bar denotes 1 mm.
Dorsolateral trunk dermal denticles sometimes overlap and are slightly elevated; they have moderately long, relatively slender, tricuspidate crowns with a long pointed median cusp and short but distinct lateral cusps; there are two strong median ridges along the entire length of the crown; the crown surface is smooth, with ectodermal pits restricted to the anteriormost portion (Figure 19a,b). Ventrolateral trunk dermal denticles are smaller, with slightly shorter median cusps (Figure 19c,d).
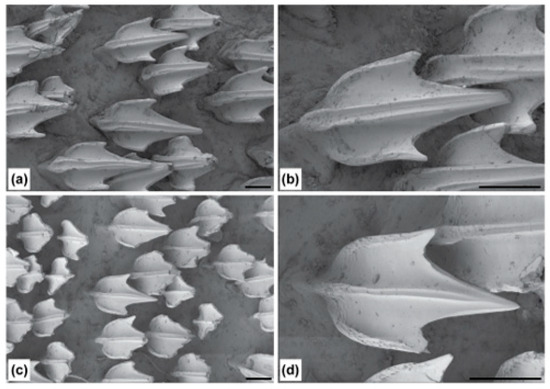
Figure 19.
Scanning electron micrographs of lateral trunk denticles of Dichichthys melanobranchus (CSIRO H 9464-01): (a) dorsolateral trunk (×60 magnification); (b) dorsolateral trunk (×150 magnification); (c) ventrolateral trunk (×60 magnification); (d) ventrolateral trunk (×180 magnification). Scale bar denotes 200 µm.
A strong caudal crest of enlarged denticles can be found on the dorsal midline of the caudal peduncle and the basal half of the upper caudal margin, its origin below the second dorsal-fin inner margin (Figure 1c,d). Denticles of the caudal crest are enlarged laterally: they are strongly tricuspidate, about twice the length of denticles below the crest; lateral cusps are long but distinctly shorter than the median cusp, directed slightly posterolaterally, with three or four rows of smaller denticles between the enlarged lateral denticles; denticles are largest over the origin of the dorsal caudal-fin lobe, decreasing in size posteriorly on the crest to merge with denticles of the tail. The crest is elevated, with a distinct naked area of skin separating crest denticles from those on the side of the tail (most obvious at its midlength). A similarly well-developed, low crest can be found on the caudal peduncle ventrally, extending from below the anal-fin free rear tip onto the basal part of the lower caudal lobe. Lateral denticles of the crest are the largest, with 2–4 (mostly 3) rows of smaller denticles between them. A narrow naked area is evident only at around its midlength, separating enlarged crest denticles from those on the side of the tail.
Monospondylous centra, 45–46; diplospondylous precaudal centra, 51–53; diplospondylous caudal centra, 37–46; total precaudal centra, 96–99 (juvenile holotype = 102); total centra, 133–145.
Intestinal valve with 10 turns (n = 1).
3.5.5. Egg Case
Based on an image of egg case HUMZ 101534 (Figure 11b): the egg case is moderately elongated, its total length ~90 mm and maximum width ~28 mm; ~16 longitudinal ridges extend the entire length of egg case on the visible dorsoventral surface; the egg case asymmetrical, one lateral edge is almost straight, and the other lateral edge is strongly convex, with posterior end tapering distinctly towards the straight edge. The anterior margin is truncate; long thread-like attachment fibres extend from the anterior margin; no posterior border is apparent, and tightly coiled tendrils are present at the posterior end. Anterior and posterior waists are present but barely distinguishable. They are golden brown in colour.
3.5.6. Colour
When fresh, dorsal and lateral surfaces are uniform brownish with lateral line distinctly pale from above the pectoral fin midbase extending posteriorly to the terminal caudal lobe; ventral surfaces are much paler (Figure 14b,c). Demarcation of pale ventral and brownish lateral surfaces (waterline) is distinct in lateral view; waterline on the head extending from the anterior nostrils, below the eyes, to the lower margin of gill slits and merging with the pectoral-fin origin; waterline on the ventrolateral trunk extending from the pectoral-fin insertion to pelvic-fin origin where it forms a distinct pale blotch; the waterline is less evident on the tail. The insides of the gill openings are brownish black; the external gill slits are dusky. The dorsal, caudal, anal, and dorsal surfaces of paired fins are brownish without distinct markings; the second dorsal fin and caudal fin are somewhat darker brown distally; the anterior inner margin of dorsal, anal, and paired fins is distinctly paler. The floor and roof of the mouth are medium greyish brown; the anterior pores on the roof of the mouth are dark-edged.
3.5.7. Size
The specimens examined in this study were 650–762 mm TL. Two females of 678 and 742 mm TL, and the three males (650–762 mm TL) were mature. The egg case (HUMZ 101534) was removed from a gravid female of 619 mm TL. Yano [47] examined five males (639–781 mm TL) and two females (692 and 730 mm TL) from the Ryukyu Islands in southern Japan. A female of 850 mm TL was reported in [36]. A 148 mm TL free-swimming juvenile was reported by [49].
3.5.8. Distribution
Previously known from specimens from the South China Sea off Hong Kong in China (20°05′ N, 115°03′ E), Taiwan Strait (22°19.8′ N, 120° E), the Sibuyan Sea in the Philippines (13°10.02″ N; 122°21′ E), and off Hateruma, Yonaguni, and Minna Islands in the Ryukyu Archipelago in southern Japan at depths of 448–1110 m [19,47,49,50] (Figure 4).
3.5.9. Remarks
The allocation of this species into the genus Dichichthys is confirmed based on the presence of supraorbital crests on the chondrocranium, its egg case morphology, and molecular data.
Dichichthys melanobranchus differs from D. albimarginatus in lacking distinct white margins on the fins. It is closest to D. bigus in general morphology but can be distinguished based on the following characteristics: narrower head (head width 9.6 vs. 10.5–13.0% TL, head width 1.1 vs. 1.3–2.0 times its height); caudal fin slightly smaller (dorsal caudal margin 14.3 vs. 15.6–17.8% TL, preventral caudal margin 6.8 vs. 8.2–10.1% TL), shorter anal fin (its base 9.8 vs. 11.2–11.9% TL); first dorsal fin shorter (anterior margin 5.8 vs. 7.2–8.0% TL, base 5.1 vs. 6.0–6.7% TL); mouth narrower (its width 7.7 vs. 9.2–9.9% TL); snout slightly shorter (prenarial length 4.2 vs. 4.8–5.4% TL); eyes slightly smaller (its length 4.1 vs. 4.5–4.8% TL).
3.6. Dichichthys nigripalatum (Fahmi and Ebert, 2018 [26])
Indonesian Bristle Shark.
3.6.1. Synonymy
Parmaturus nigripalatum Fahmi and Ebert, 2018 [26]: 531, figs 2–6 (Sumbawa, Indonesia)—[36]: 405, figs (Sumbawa, Indonesia).
3.6.2. Type Material
Holotype: NCIP 6567, adult male 548 mm TL, south of Sumbawa, Indonesia, 9.04° S, 117.92° E, 170–190 m depth, 16 April 2016.
3.6.3. Diagnosis
A small bristle shark with the following combination of adult characteristics (Figure 20): preanal length, 62.2% TL; pre-first dorsal length, 53.8% TL; prenarial length moderately long, 4.7% TL; head moderately depressed, its height 0.7 times its width; mouth moderately wide, its width 9.0% TL; labial furrows subequal in length, 1.9% TL; anal fin moderately large, its base 10.7% TL; posterior margin 5.4% TL; pectoral–pelvic space, 27.2% TL; body medium brown above, paler below; demarcation between darker dorsal and paler ventral colouration on ventrolateral surfaces diffuse and barely distinct on body; fins with dark posterior margins, most evident on ventral surface of paired fins; teeth in 52 files in upper jaw and 48 lower jaw; monospondylous centra, 42; precaudal centra, 90; total centra, 130.
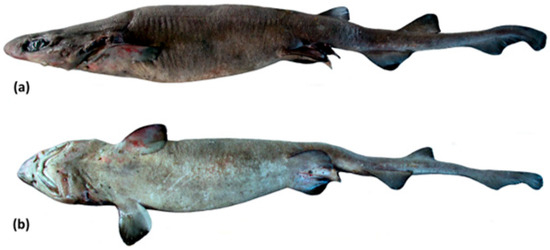
Figure 20.
Dichichthys nigripalatum, adult male 548 mm TL (holotype NCIP 6567, fresh): (a) lateral view; (b) ventral view. Photo copyright: Galih Rakasiwi (Tanjung Luar fisheries officer).
3.6.4. Description
See [26].
3.6.5. Size
Only known from the adult male holotype of 548 mm TL. An adult male specimen photographed by the late J. Widodo from Pelabuhanratu, West Java, in the 1990′s may also be this species (Figure 21), and based on the pencil in the image as a rough scale, it appears to be an adult male between 600 and 700 mm TL.
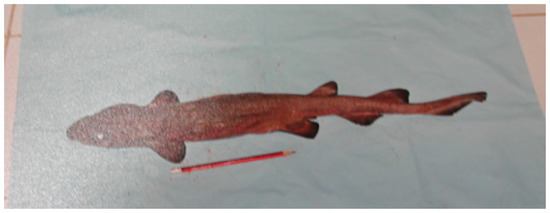
Figure 21.
Poor quality image of a photograph shown to one of the authors (WW) by the late Johannes Widodo taken from the Pelabuhanratu fish landing site (prior to 2001) tentatively assigned to Dichichthys nigripalatum.
3.6.6. Distribution
Only known from the type locality south of Sumbawa, Indonesia. The specimen photographed off Pelabuhanratu, West Java, is possibly this species (Figure 4).
3.6.7. Remarks
The allocation of this species to Dichichthys is based on similar morphology to the other species confidently assigned to this genus, i.e., the second dorsal fin is distinctly larger than the first and smaller pectoral fins. No genetic data are available for this species to confirm its allocation, and CT scans are also not available to confirm that supraorbital crests are present.
Dichichthys nigripalatum was described from a single specimen from off the coast of Sumbawa in Indonesia [26]. The shallow depth of this specimen seems questionable. The specimen was collected by a fisheries officer at the Tanjung Luar landing site in Lombok, so the depth information would have likely been anecdotal from the fishers involved. This shallow depth for this species should be treated with caution and requires validation. The holotype of D. nigripalatum differs from D. albimarginatus, D. bigus, and D. satoi n. sp. in that the fins are darker distally and lack white edges on the fins (vs. at least some fins with distinct white edges).
In their comparison with D. melanobranchus, [26] state that D. nigripalatum differs in having similar-sized dorsal fins vs. the first dorsal fin noticeably smaller than the second dorsal fin. However, the lateral image of the holotype and measurements of the dorsal fins clearly show that the second dorsal fin is distinctly larger than the first, as in D. melanobranchus. Another difference they used is the pre-first dorsal length being larger in D. nigripalatum than in D. melanobranchus, i.e., 53.8 vs. 45.9% TL. This latter measurement is based on Chan’s [19] description of D. melanobranchus, which was based on the unique holotype of a juvenile female 235 mm TL (BMNH 1965.8.11.6). The pre-first dorsal length of the larger specimens (650–762 mm TL) of D. melanobranchus examined in this study was similar to D. nigripalatum, i.e., 52.5–53.9% TL. Thus, this difference is likely due to ontogenetic variation, which is poorly understood in this genus and in many deepwater catsharks. Similarly, [26] reported that D. nigripalatum had near equal length labial furrows vs. much longer lower labial furrows in D. melanobranchus. However, several of the larger D. melanobranchus examined in this study had subequal-length labial furrows.
Another characteristic [26] used was vertebral counts, which were lower in D. nigripalatum than in D. melanobranchus, i.e., precaudal count 90 vs. 102 and total count 130 vs. 140. Vertebral counts of the four specimens examined in this study were 96–101 precaudal centra and 133–145 total centra. While these counts remain greater than those for D. nigripalatum, the difference in vertebral counts is much less. Tooth counts were also considered to be vastly different in D. nigripalatum than in other members of the genus Parmaturus to which it was originally allocated. Their counts of 52 upper and 48 lower tooth files are far lower than for other Dichichthys species (mostly >90 in upper teeth in adults). However, the ventral head view image (see Figure 3 in [26]) shows teeth similar in size to adult males examined in this study, and more than 52 upper tooth files can be counted in the image, which only shows part of the upper jaw. A recount of the number of upper and lower tooth files in the holotype of D. nigripalatum is needed.
Comparison of D. nigripalatum with the four larger specimens of D. melanobranchus examined in this study revealed the former species differs in having the following characteristics: slightly shorter but deeper eyes (eye length 4.2 vs. 4.5–4.8% TL; eye height 2.2 vs. 0.8–1.1% TL); a shorter pre-oral snout (5.0 vs. 6.4–7.1% TL); slightly larger first dorsal fin (anterior margin 8.6 vs. 7.2–8.0% TL); longer pectoral–pelvic space (27.2 vs. 24.0–26.0% TL); longer adult claspers in males (clasper outer length 5.4 vs. 4.3–4.9% TL, inner length 8.8 vs. 6.0–6.1% TL).
Despite the image of a specimen from Pelabuhanratu in West Java being poor quality (Figure 21), it clearly depicts a member of this genus with the first dorsal fin origin over pelvic-fin insertions and the second dorsal fin distinctly larger than the first. The dark fin margins of this specimen match closest to D. nigripalatum; thus, it is tentatively assigned to this species pending the collection of additional specimens as confirmation.
3.7. Dichichthys satoi n. sp.
Roughback Bristle Shark.
urn:lsid:zoobank.org:act:8559A429-367D-4FE3-8C1F-795BA5699D80.
3.7.1. Synonymy
3.7.2. Type Material
Holotype: NMNZ P.042517 (GenBank accession PP329559), adult male 954 mm TL, Tony B Seamount, West Norfolk Ridge, 34°01.7′ S, 168°09.50′ E, 870–1044 m depth, October 2005.
Paratypes: CSIRO H 9287-01, female 1038 mm TL, outer northern Bay of Plenty, New Zealand, 36°30.42′ S, 168°55.65′ E, 915–1028 m depth, 18 July 1998; AIM MA73694 (GenBank accession PP329561), gravid female 984 mm TL, West Norfolk Ridge, 34°25′ S, 168°0.3′ E, 100–800 m depth, 6 March 2008; NMNZ P.017655, gravid female 1012 mm TL, East Cape Ridge, New Zealand, 38°27.25′ S, 178°48.40′ E, 666–714 m depth, 18 July 1985; NMNZ P.020141, adult male 856 mm TL, Wanganella Bank, south-western Norfolk Ridge, 32°23.65′ S, 166°21.15′ E, 1059–1070 m depth, 7 June 1986; NMNZ P.025376, female 637 mm TL, Hikurangi Trough, New Zealand, 39°41.30′ S, 177°57.95′ E, 1013–1094 m depth, 28 September 1989; NMNZ P.042524 (GenBank accession PP329562), gravid female 971 mm TL, West Norfolk Ridge, 33°39.50′ S, 167°51′ E, 622–1051 m depth, 7 July 2006; NMNZ P.044582 (GenBank accession JQ519147), female 995 mm TL, NMNZ P.044583 (GenBank accession PP329560), female 832 mm TL, West Norfolk Ridge, 34°25′ S, 168°0.3′ E, 100–800 m depth, 6 March 2008; NMNZ P.045528 (GenBank accession PP329563), female 951 mm TL, Raukumara Plain, east of East Cape, New Zealand, 37°12.6′ S, 178°22.2′ E, December 2008; NMNZ P.047247, juvenile male 632 mm TL, Omakere Ridge, southeast of Cape Kidnappers, New Zealand, 39°59.29′ S, 177°53.18′ E, 1073–1140 m depth, 18 March 2003; NMNZ P.057059, juvenile male 642 mm TL, ~97 km southwest of Cape Reinga, New Zealand, 34°44.4′ S, 171°40.8′ E, 1000–1175 m depth, September 2014; NMNZ P.062238, adult male 973 mm TL, north of Tunanui Bank, off East Cape, 37°38.4′ S, 179°26.4′ E, 1009–1498 m depth, April 2019; NMNZ P.062313, gravid female 1046 mm TL and three excised egg cases, off eastern North Island.
3.7.3. Diagnosis
A large bristle shark with the following combination of characteristics: preanal length, 58.1–62.1% TL; pre-first dorsal length, 50.5–53.4% TL; prenarial length moderately long, 4.0–5.2% TL; head depressed, its height 0.6–1.1 times its width; mouth moderately wide, its width 9.0–11.4% TL; lower labial furrows distinctly longer than uppers (uppers 1.4–2.0% TL, lowers 2.0–2.8% TL); anal-fin moderately large, its base 11.0–12.0% TL, posterior margin, 5.4–7.4% TL; pectoral–pelvic space, 21.2–26.1% TL; body uniformly medium brown to greyish brown; fins with white margins, variable but mostly broad; teeth in 102–106 files in upper jaw and ~94–101 lower jaw; monospondylous centra, 45–47; precaudal centra, 95–101; total centra, 133–143.
3.7.4. Description
Body relatively firm, trunk slightly depressed, tail compressed and slightly tapering to caudal fin; abdomen longer than head; pectoral–pelvic space 24.6 (21.2–26.1% TL); pectoral–pelvic space 1.27 (1.02–1.31) times head length; pelvic–anal space 1.61 (1.57–2.19) in anal-fin base; caudal peduncle short, compressed; anal–caudal space 1.53 (1.23–1.74) in anal-fin base, caudal peduncle width 2.34 (1.57–2.25) in height (Figure 22). Prominent crest of enlarged denticles on dorsal and ventral midline of caudal peduncle; dorsal crest originating over or just posterior to second dorsal fin free-rear tip and extending two-thirds of the length of dorsal–caudal margin; ventral crest of enlarged denticles originating below midlength of anal-fin inner margin and extending slightly onto ventral caudal margin.

Figure 22.
Lateral view of Dichichthys satoi n. sp. (fresh): (a) holotype, NMNZ P.042517, adult male 954 mm TL; (b) paratype, NMNZ P.042524, gravid female 971 mm TL; (c) paratype, NMNZ P.045528, female 951 mm TL. Photos copyright Carl Struthers, Museum of New Zealand Te Papa Tongarewa.
Head relatively short, width 10.8 (11.3–13.9)% TL; dorsal profile mostly straight anteriorly becoming slightly convex behind eyes in lateral view (Figure 23b); prominent subocular ridges, originating at anterior nostrils extending to anterior of first gill slit, broadest below eyes, becoming less prominent posteriorly. Snout moderate, parabolic in dorsoventral view, slightly indented at level of anterior nostrils, tip bluntly pointed in lateral view; preoral length 5.4 (5.3–6.9% TL, 0.58 (0.51–0.69) times mouth width; preorbital length 1.36 (1.26–2.00) times eye length. Eyes large, narrow, length 4.2 (3.8–4.6)% TL, 4.25 (3.44–6.38) times eye height, 4.58 (4.43–5.16) in head length; eyes situated mostly laterally on the head (lower margin of eyes only slightly visible in dorsal view). Spiracles small, located just behind eyes at about level with lower half of the eye. Mouth large, long, broadly rounded in females, strongly arched in adult males (Figure 24); mouth corners level with posterior margin of eye; symphysis of lower jaw about level with anterior margin of eye. Labial furrows moderately long, well defined, lowers much longer than uppers: uppers 1.4 (1.4–2.0)% TL; lowers 2.5 (2.0–2.8)% TL. Nostrils large with tube-like incurrent apertures, nostril length 0.61 (0.55–0.84) times eye length; anterior nasal flaps moderate-sized, overlapping posterior nasal flap, posterior tip forming a blunt-tipped triangular lobe, posterior nasal flaps present, moderately large, its width slightly more than a third of nostril width; nostrils well separated, internarial space 2.2 (2.0–2.7)% TL, 0.86 (0.75–0.92) times nostril width; well separated from mouth. Gills small, about 1.5 times eye height, first four subequal in size, fifth smallest; fourth gill slit above pectoral-fin origin, fifth over anterior pectoral-fin base; upper margins of gill slits slightly below lower level of eyes.
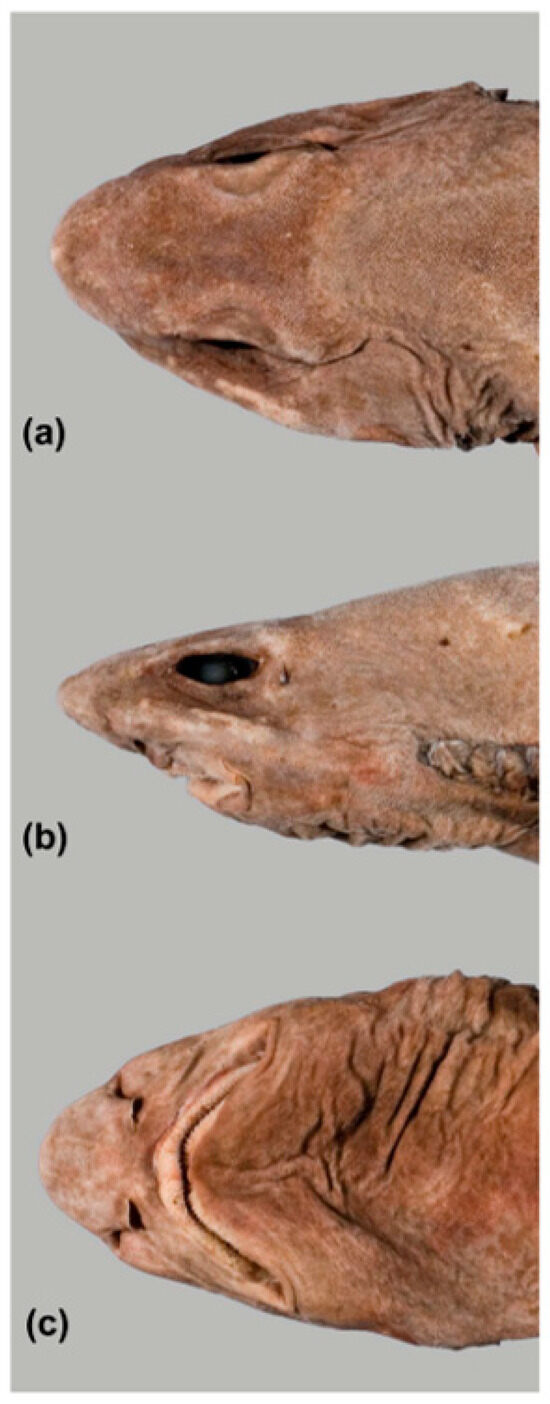
Figure 23.
Head of holotype of Dichichthys satoi n. sp. (NMNZ P.042517, adult male 954 mm TL, fresh): (a) dorsal view; (b) lateral view; (c) ventral view. Photos copyright: Carl Struthers, Museum of New Zealand Te Papa Tongarewa.
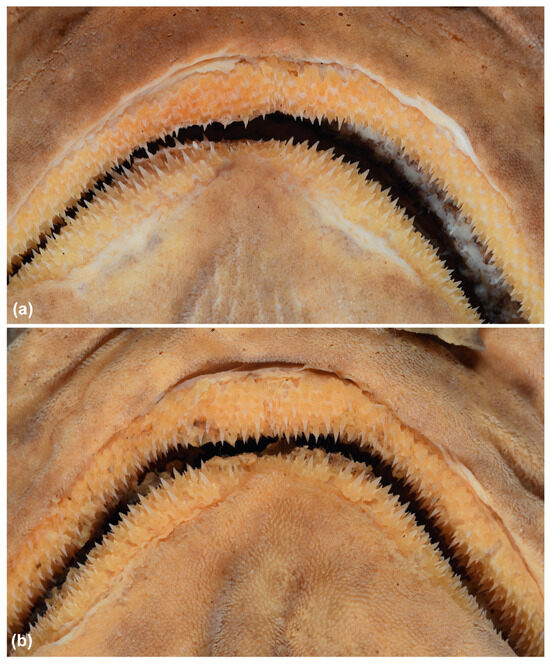
Figure 24.
Mouth of Dichichthys satoi n. sp.: (a) adult female (NMNZ P.042524, gravid female 971 mm TL, paratype); (b) adult male (NMNZ P.042517, adult male 954 mm TL, holotype).
Pores distinct on ventral head (Figure 23c); enlarged pores in two parallel longitudinal lines extending from snout tip, which converge rapidly to the level of anterior nostrils, and near lateral extremity extending posteriorly from behind nostrils almost to first gill slit; numerous smaller pores on preoral snout; hyomandibular pores distinct, ~10–20 (mean 15.9) on each side; mandibular pores less distinct, often obscured by denticles, 2–9 (mean 3.8) on each side. Enlarged pores on dorsal head extending from near snout tip and curving posterolaterally, roughly following the contour of the rostral cartilage, and on subocular ridges below eyes; pores less evident elsewhere on the head, often concealed by denticles (Figure 23a).
First dorsal fin much smaller than second dorsal. First dorsal-fin height 0.78 (0.65–0.77) times second dorsal fin height; first dorsal fin base 0.69 (0.60–0.82) times second dorsal fin base, originating over rear quarter of pelvic-fin base; anterior margin nearly straight to slightly convex, apex moderately to broadly rounded, posterior margin slightly convex to nearly straight; inner margin short, directed posterodorsally (strongly in smallest specimens), rear tip rounded. Second dorsal fin originating just posterior to anal-fin midbase; anterior margin nearly straight to slightly convex, apex moderately rounded, posterior margin nearly straight to slightly convex; inner margin short, directed slightly posterodorsally (strongly in smallest specimens), free tip rounded to subangular.
Pectoral fins small, somewhat paddle-like, anterior margin 7.7 (8.3–9.6)% TL; anterior margins slightly to moderately convex, apex bluntly subangular, posterior margin slightly convex, free-rear tip broadly rounded. Pelvic fins small, subtriangular, length 9.8 (9.2–12.2)% TL, anterior margin nearly straight to slightly convex, apex broadly rounded, posterior margin slightly convex, free-rear tip rounded. Claspers of adult males short, relatively broad, tapering rapidly distally to a narrowly pointed tip (Figure 25a); clasper glans more than 90% of clasper outer length; fleshy envelope present, covering clasper groove (Figure 25b); rhipidion well developed with a prominent posterior margin; short but distinct pseudosiphon present; cover rhipidion large, without free anterior tab or fold; exorhipidion present, extending more posteriorly than cover rhipidion; dorsal surface of clasper naked except for exorhipidion, which is covered in small denticles.
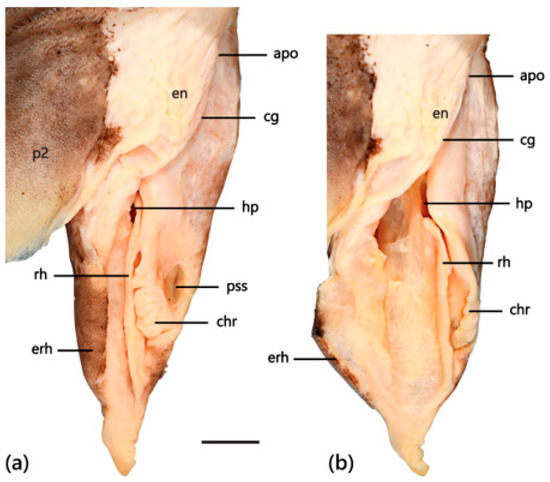
Figure 25.
Clasper of adult male Dichichthys satoi n. sp. (holotype, NMNZ P.042517): (a) glans not dilated; (b) glans dilated. Abbreviations: apo, apopyle; cg, clasper groove; chr, cover rhipidion; en, envelope; erh, exorhipidion; hp, hypopyle; p2, pelvic fin; pss, pseudosiphon; rh, rhipidion. Scale bar denotes 10 mm.
Anal fin relatively low, triangular, larger than second dorsal fin; base longer than second dorsal fin base, base 11.5 (11.0–12.0)% TL, 1.02–1.45 times second dorsal fin base, 1.44 (1.16–1.50) times interdorsal space; origin just anterior to or posterior to first dorsal fin free rear tip, anal-fin height 2.23 (2.05–2.75) in base length. Caudal fin short, dorsal–caudal margin length 18.1 (16.3–19.7)% TL, origins of upper and lower lobes obscured by the crest of enlarged denticles; lower lobe weakly developed distally; terminal lobe prominent, terminal margin slightly to moderately convex.
Teeth of both jaws exposed when mouth closed, visibly larger in adult males than females (Figure 24); anterior teeth of adult females with mostly 5 cusps, juvenile (NMNZ P.025376) with mostly 6 cusps; central cusp longest and upright, flanked by smaller but distinct lateral cusps, much larger in adult males (Figure 26); central cusp becoming slightly shorter posteriorly and becoming slightly oblique, lateral cusps remaining the same size, posteriormost teeth with shorter central cusp, but still larger than lateral cusps (much larger in adult males); teeth in 102–106 files in upper jaw, ~94–101 files in lower jaw.

Figure 26.
Scanning electron micrographs of anterolateral teeth of the holotype of Dichichthys satoi n. sp. (NMNZ P.042517, adult male 954 mm TL): (a) upper tooth; (b) lower tooth (×35 magnification). Scale bar denotes 1 mm.
Denticles large; denticles on dorsolateral surfaces above lateral line distinctly larger than ventrolateral denticles below lateral line; some specimens with distinctly larger dorsolateral denticles than other specimens (e.g., NMNZ P.017655 with dorsolateral denticles up to 1.1 mm long, vs. 0.8 mm in NMNZ P.042517). Dorsolateral trunk dermal denticles mostly not imbricate, somewhat erect, variable in size; long, slender, tricuspidate crowns with a very long pointed median cusp and short but distinct lateral cusps; two strong median ridges along entire length of crown; crown surface smooth, without ectodermal pits (Figure 27a,b). Ventrolateral trunk dermal denticles weakly imbricate, somewhat erect, relatively consistent in size; long, slender, tricuspidate crowns with a very long pointed median cusp and short but distinct lateral cusps; two median ridges extending along entire length of crown; crown surface smooth, without ectodermal pits (Figure 27c,d).
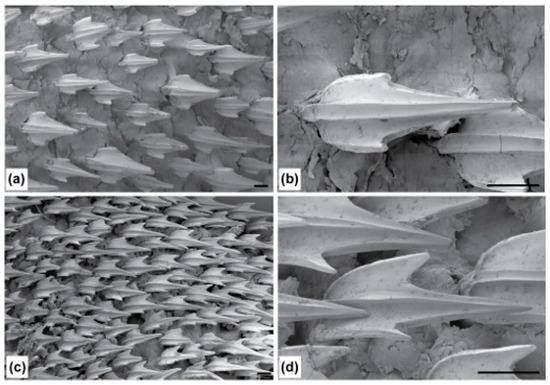
Figure 27.
Scanning electron micrographs of lateral trunk denticles of Dichichthys satoi n. sp. (holotype, NMNZ P.042517, adult male 954 mm TL): (a) dorsolateral trunk (×30 magnification); (b) dorsolateral trunk (×120 magnification); (c) ventrolateral trunk (×30 magnification); (d) ventrolateral trunk (×150 magnification). Scale bar denotes 200 µm.
A strong caudal crest of enlarged denticles was observed on the dorsal midline of the caudal peduncle and onto the basal half of the upper caudal margin, its origin almost at second dorsal fin insertion. Denticles of the caudal crest enlarged laterally: they are strongly tricuspidate, about twice the length of the denticles below the crest; lateral cusps are long but distinctly shorter than median cusp; they are directed slightly posterolaterally, with 2–4 rows of smaller denticles between the enlarged lateral denticles; denticles are largest anteriorly, decreasing in size posteriorly on the crest to merge with denticles of the tail; the crest is well elevated, a distinct naked area of skin separating crest denticles from those on the side of the tail (most obvious at its midlength). A slightly less-developed, low crest on the caudal peduncle was observed ventrally, extending from just posterior to anal-fin insertion onto the basal part of the lower caudal lobe; the lateral denticles of the crest were largest with 2–3 rows of smaller denticles between them; there was a distinct but less obvious naked area between enlarged crest denticles and those on the side of the tail (only obvious at its midlength) (Figure 1e,f).
Monospondylous centra, 47 (45–47; n = 5); diplospondylous precaudal centra, 49 (48–54; n = 5); diplospondylous caudal centra, 44 (36–48; n = 5); total precaudal centra, 96 (95–101; n = 5); total centra, 140 (133–143; n = 5).
Intestinal valve with 13–14 turns (n = 2).
3.7.5. Egg Case
Based on seven egg cases (CSIRO H 9287-01, NMNZ P.017655 and NMNZ P.042524) (Figure 11c, Figure 28 and Figure 29b): the egg case is moderately elongated, its total length 92.0–112.3 mm and maximum width 30.3–34.2 mm, slightly dorsoventrally depressed; the egg case is asymmetrical, with one lateral edge slightly to moderately concave, and the other lateral edge strongly convex, with the entire egg case appearing banana shaped; a thick mat of attachment fibres (fibrous mat) cover most of the egg case; longitudinal, pliable ridges extend the entire egg case length and are visible beneath the fibrous mat. Ridges numbered 10–15 (mean 12) on the dorsal surface and 12–16 (mean 14) on the ventral surface. The anterior margin is truncate; projections at each corner are absent; long thread-like attachment fibres extend from the anterior margin. The posterior margin is closed (no posterior border), and posterior horns curve strongly and medially, forming tightly coiled tendrils. Anterior and posterior waists are present but barely distinguishable. A single low, broad keel on the straight lateral edge was observed, most prominent at the anterior and posterior ends; two prominent keels were observed on the convex lateral edge, one larger in size, separated by a wide groove running the entire egg case length; keels on each lateral edge at the posterior end folding on opposing dorsal and ventral surfaces, enveloping outermost ridges. The eggs are brown to golden brown in colour.
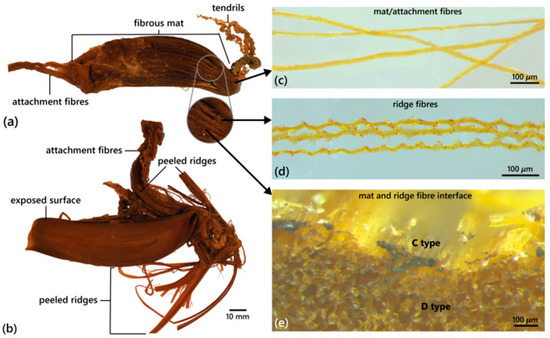
Figure 28.
Egg case structure of Dichichthys satoi n. sp. (NMNZ P.042524): (a) dorsal view of egg case with fibrous mat and ridges intact; (b) ventral view of egg case with attachment fibres, fibrous mat and ridges peeled back revealing inner (exposed) surface; (c) straight mat/attachment fibres; (d) zigzag-like fibres from ridges; (e) cross section of ridge showing straight mat/attachment fibres (C type) and zigzag-like ridge fibres (D type).
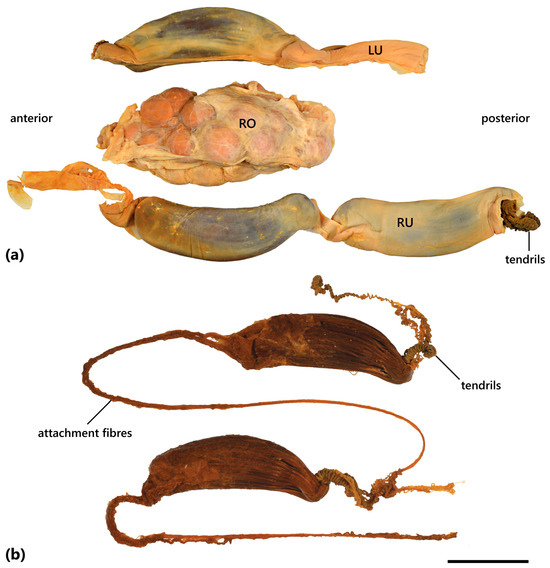
Figure 29.
Reproductive tract of a gravid female Dichichthys satoi n. sp. (CSIRO H 9287-01, 1038 mm TL): (a) left (LU) and right (RU) uteri containing egg cases and right ovary with large oocytes; (b) two egg cases removed from right uterus.
When the outer fibrous mat was removed, it became apparent that the fibrous mat transitioned into the ridge fibres, and continued removal of the fibrous mat resulted in peeling the ridges away. Peeling away the pliable ridges exposes a smooth, thin, delicate egg case layer was exposed, more closely resembling a scyliorhinid egg case (Figure 28b).
Microscopic examination of attachment fibres and ridge fibres revealed different structures: fibrous mat fibres are straighter and thread-like (Figure 28c), while ridge fibres are zigzag-like (Figure 28d). Microscopic examination of a cross-section of one of the ridges shows the interface between the outer fibrous mat and ridge fibres; all fibres within the ridge are densely packed (Figure 28e).
3.7.6. Colour
When fresh, the head and body are uniformly medium brown to greyish brown, and the lateral line is slightly paler. Gill margins are brownish black. Dorsal, anal, pelvic, and pectoral fins and the terminal lobe of the caudal fin have broad white posterior margins, with the remainder of the fin similar in colour to the body; white margins are somewhat variable: sometimes less evident (Figure 22b), sometimes very distinct (Figure 22c). The floor and roof of the mouth are brownish (much paler in smallest specimens), and the pores on the roof of the mouth are not black-edged. NMNZ P.025376 has scattered large white spots over the head and body, more concentrated on the dorsal area.
3.7.7. Size
The females examined ranged from 637 to 1046 mm TL; the smallest mature female was 971 mm TL. The males examined ranged from 632 to 973 mm TL; the smallest mature male was 856 mm TL; two males of 632 and 642 mm TL possessed immature claspers with no evidence of maturation beginning.
3.7.8. Biology
The reproductive mode is multiple oviparous, with several females containing three egg cases (two in one uterus, one in the other). One gravid female paratype (CSIRO H 9287-01) had its reproductive tract removed (Figure 29). The right uterus contained two egg cases, with the attachment fibres at the anterior end of the posteriormost egg case entwined with the tendrils at the posterior end of the anteriormost egg case. The left uterus contained a single egg positioned just posterior to the oviducal gland. The functional (right) ovary contained 18 large oocytes.
The stomach of the gravid female paratype (CSIRO H 9287-01), from which the reproductive tract was removed, contained one 400–450 mm long whiptail, Coelorinchus trachycarus (identified by P. McMillan, Museum of New Zealand Te Papa Tongarewa) and beaks of two octopus, Muusoctopus clyderoperi (identified by T.J. Verhoeff, Tasmanian Museum and Art Gallery).
3.7.9. Distribution
This species is known to occur on the West Norfolk Ridge–Wanganella Bank and off the North Island of New Zealand between latitudes 32°24′ and 39°59′ S. off the coast of North Island, recorded southwest of Cape Reinga, and on the eastern side from northern Bay of Plenty to the Ritchie Ridge off Hawke Bay. Depths for the type of specimen range from 100 to 1498 m, but the shallowest comes from three specimens taken over a depth range of 100–800 m by a commercial vessel, so this was probably generalised data. A depth range of 666–1140 m for this species (as Parmaturus sp.) was provided by [23]. The upper depth range of 666 m should be considered the shallowest for this species. Two specimens caught since [23] were taken at depth ranges of 1000–1175 and 1009–1498 m. The broad depth range for the latter specimen should not be considered an accurate depth distribution for this species, especially as all other specimens were caught in less than 1175 m depth. Thus, a tentative depth range of 666–1175 m is provided for this species based on current data.
3.7.10. Etymology
The species is named after Dr Keiichi Sato (Okinawa Churaumi Aquarium), whose extensive work on the taxonomy of deepwater catsharks has been crucial for the field; in particular, Dr Sato was the first to determine that Parmaturus melanobranchus was not congeneric with other Parmaturus species.
3.7.11. Remarks
The smallest specimen of this species collected is 632 mm TL. As such, juvenile characters are not known. It is likely that small juveniles differ in some characteristics from adults, as in D. melanobranchus. Therefore, small juveniles could be easily misidentified, so future research will hopefully be able to provide a description of juveniles of this species if collected.
Dichichthys satoi n. sp. can be readily distinguished from D. bigus, D. melanobranchus, and D. nigripalatum in having distinct white margins on the fins (vs. no distinct white markings on fins). While superficially similar to D. albimarginatus in having white fin margins, D. satoi n. sp. is a far larger species, attaining up to 1046 mm TL compared to the 577 mm TL adult male holotype of D. albimarginatus. The smallest adult male D. satoi n. sp. was 856 mm TL, while two males of 632 and 642 mm TL were immature with no evidence of any clasper calcification having occurred. Dichichthys satoi n. sp. further differs from D. albimarginatus in the following characteristics: interorbital space slightly narrower (5.8–7.4 vs. 7.7% TL); snout slightly shorter (4.0–5.2 vs. 3.7% TL); slightly longer based pectoral fins (5.0–5.8 vs. 4.4% TL); anal-fin taller (its height 4.3–5.7 vs. 3.8% TL); more teeth (upper jaw with 102–106 vs. 92 files).
Dichichthys satoi n. sp. differs from D. bigus in the following characteristics: more intestinal valve turns (13–14 vs. 9); head and body uniform in colour vs. ventral surface distinctly paler than dorsal and lateral surfaces; head broader (its width 10.8–13.9 vs. 9.6% TL, interorbital width 5.8–7.4 vs. 5.4% TL); mouth broader (its width 9.0–11.4 vs. 7.7% TL); lower labial furrows distinctly longer than uppers vs. labial furrows equal in length; lower labial furrows longer (2.0–2.8 vs. 1.6% TL); first dorsal fin shorter (anterior margin 7.9–9.1 vs. 5.8% TL, base 6.1–7.3 vs. 5.1% TL); anal-fin longer (its length 12.0–13.4 vs. 11.2% TL, base 11.0–12.0 vs. 9.8% TL); caudal fin longer (dorsal–caudal margin 14.16.3–19.7 vs. 14.3% TL, preventral caudal margin 7.7–10.8 vs. 6.8% TL). The egg cases of D. satoi n. sp. differ from those of D. bigus in being larger (egg case length 92.0–112.3 vs. 85.4–89.4 mm) and having a much broader lower keel on the right lateral edge (4.7–6.2 vs. 2.7% ECL) (Table 2).
Dichichthys satoi n. sp. attains a larger size than D. melanobranchus, i.e., the smallest adult male is 856 vs. 650 mm TL. It further differs from D. melanobranchus in the following characteristics: more intestinal valve turns (13–14 vs. 10); head and body uniform in colour vs. ventral surface distinctly paler than dorsal and lateral surfaces; lower labial furrows distinctly longer than uppers vs. labial furrows subequal in length; anal-fin slightly taller (its height 4.3–5.7 vs. 3.9–4.3% TL).
Dichichthys satoi n. sp. is a far larger species than D. nigripalatum, with the smallest adult male D. satoi n. sp. being 856 mm TL, while two males of 632 and 642 mm TL were immature. In comparison, the holotype of D. nigripalatum was an adult male of 548 mm TL. The new species further differs from D. nigripalatum in the following characteristics: claspers of adult male shorter (inner length 5.9–6.4 vs. 8.8% TL) but slightly broader (base width 1.7–2.1 vs. 1.6% TL; pectoral and pelvic fins closer together (pectoral–pelvic space 21.2–26.1 vs. 27.2% TL); eyes narrower (eye height 0.7–1.3 vs. 2.2% TL); slightly more vertebrae (total centra 133–143 vs. 130; monospondylous centra 45–47 vs. 42).
4. Discussion
4.1. Relationships within the Carcharhiniformes
All members of the Dichichthyidae family were previously placed in the genus Parmaturus within the family Pentanchidae. The family Pentanchidae was resurrected by [6], which separated off those genera that lack supraorbital crests on the chondrocranium, i.e., Apristurus, Asymbolus, Bythaelurus, Cephalurus, Figaro, Galeus, Halaelurus, Haploblepharus, Holohalaelurus, Parmaturus, and Pentanchus. The genera which possess supraorbital crests were retained in the Scyliorhinidae family, i.e., Atelomycterus, Aulohalaelurus, Cephaloscyllium, Poroderma, Schroederichthys, and Scyliorhinus. The discovery of supraorbital crests on the chondrocranium through micro-CT scanning of D. bigus and D. melanobranchus in this study immediately contradicts the familial placement of these species if they were to remain in Parmaturus. Likewise, the molecular results also found that D. melanobranchus and D. satoi n. sp. come out in different places in the tree to P. pilosus and P. xaniurus (Figure 5).
The type species of the genus Parmaturus Garman, 1906 [16], by subsequent designation, is P. pilosus Garman, 1906 [16]. Thus, based on molecular results and lack of supraorbital crests, P. pilosus and P. xaniurus are retained in Parmaturus. With the reallocation of D. albimarginatus, D. bigus, D. melanobranchus, and D. nigripalatum to Dichichthys, the genus Parmaturus is now restricted to P. angelae Soares, de Carvalho, Schwingel and Gadig, 2019 [51]; P. albipenis Séret and Last, 2007 [25]; P. campechiensis Springer, 1979 [2]; P. macmillani Hardy, 1985 [52]; P. pilosus; P. xaniurus.
4.2. Intraspecific Variation
Dichichthys bigus and D. nigripalatum are represented by only a single specimen, a gravid female and an adult male, respectively; thus, no information is available on intraspecific variation. The holotype of D. albimarginatus is an adult male, and the only other specimen is the juvenile from Papua New Guinea, D. cf. albimarginatus. These two specimens differ in numerous morphological features (see in the Remarks section for D. albimarginatus above), leading to the juvenile specimen being considered a distinct species [37,38]. However, since the intraspecific variation is poorly known in Dichichthys, it is tentatively assigned to D. cf. albimarginatus pending further taxonomic investigation when additional specimens are obtained. The paucity of material for these two species precludes the construction of a dichotomous key.
A key feature that differs between the juvenile D. cf. albimarginatus specimen and the holotype of D. albimarginatus is the morphology of the lateral trunk denticles. The juvenile trunk denticles are mostly more upright and more widely spaced than in the adult holotype, but several different denticle types are apparent on the dorsolateral trunk skin sample examined (Figure 8). The first type are smaller, upright, unicuspid, bristle-like denticles with a single median ridge on the crown; the second type are slightly larger tricuspid denticles with a single median ridge on the crown; the third, less common, type are larger, broader tricuspid denticles with two median ridges on the crown (Figure 8). The smaller, bristle-like, unicuspid denticles are likely the juvenile type, which then become tricuspid as the shark grows. The larger tricuspid denticles with two median ridges more closely resemble adult denticles. The same ontogenetic differences in denticle morphology were found in D. melanobranchus. Chan [19] stated that the denticles on the body of the juvenile holotype (235 mm TL) of D. melanobranchus were bristle-like, and the illustrations provided show similar unicuspid, upright body denticles as was observed in the juvenile D. cf. albimarginatus specimen. Likewise, [49] provided an image of the dermal denticles from the dorsal surface in front of the first dorsal fin from a 148 mm TL individual, which are all bristle-like, unicuspid, and upright (see Figure 3 in [49]), with no evidence of tricuspid denticles forming. In contrast, the adult D. melanobranchus examined in this study had tricuspid denticles with two median ridges on the crowns, which more closely resemble the third, rarer type of denticles on the juvenile D. cf. albimarginatus (Figure 8).
The only two known juveniles of D. melanobranchus, the 235 mm TL holotype [19] and the 148 mm TL Taiwanese specimen [49], differ substantially via a number of characteristics from the adult specimens examined in this study, e.g., shorter pre-fin lengths (pre-first dorsal length 42.6–45.9% vs. 52.5–53.9% TL; pre-second dorsal length 57.4–59.6% vs. 67.6–69.6% TL; prepelvic length 39.0% vs. 46.9–49.5% TL; preanal length 49.2–51.1% vs. 61.1–62.3% TL) and shorter second dorsal fins (base 9.4–9.5% vs. 8.2–8.8% TL). All these characteristics also differ between the juvenile and the adult male holotype of D. albimarginatus, suggesting they represent ontogenetic differences between small juveniles and adults for this genus.
Several morphometric characteristics differed slightly between the three smallest specimens (632–642 mm TL) and the remaining larger specimens (>830 mm TL) of D. satoi n. sp.: head more depressed (head height 5.1–7.8 vs. 8.9–12.7% TL); pre-second dorsal length, 65.2–66.6 vs. 67.1–68.7% TL; tail slightly longer (dorsal–caudal margin 18.7–19.7 vs. 16.3–18.2% TL); preanal length, 58.1–59.3 vs. 60.1–62.1% TL. However, except for the above differences, the morphometrics of the types of D. satoi n. sp. varied little.
The 148 mm TL D. melanobranchus recorded by [49] is likely a newly hatched individual based on its small size and the presence of two rows of enlarged denticles running parallel to each other from the posterior head to the first dorsal fin. These enlarged denticle rows have been observed in numerous other late-term embryo and newly hatched scyliorhinoids, e.g., Apristurus ovicorrugatus [53], Bythaelurus bachi [54], and Poroderma africanum [55]. While it has been hypothesized that these double rows of enlarged denticles are used to help the embryo hatch from the egg case, [56] theorized that they act as “initiator” rows which trigger denticle patterning around them, a process like the development of feathers in chicks. It is possible that, at least in some species, these enlarged denticles may also assist with emergence from the egg case during hatching, e.g., prevent the embryo from sliding back when trying to push through the ends of the egg case. These enlarged rows of denticles were not visible on the 235 mm TL D. melanobranchus holotype, suggesting they disappear not long after hatching has occurred.
4.3. Reproduction
The assignment of a reproductive mode to Dichichthys is complicated based on the information currently available. Multiple specimens of Dichichthys satoi n. sp. contained three egg cases, two in one uterus and a single egg case in the other (Figure 29a). The two egg cases occupied all available space of one uterus, whilst the other uterus had a single egg case positioned just posterior to the oviducal gland, with the posterior half of the uterus empty. Thus, it is conceivable that D. satoi n. sp. could have up to four egg cases per pregnancy. In the right uterus, with two egg cases, the posterior egg case had the fibrous material from the anterior end wrapped around the tendrils on the posterior end of the anteriormost egg case. Examination of the contents of the posteriormost egg case (considered to be the oldest egg case out of the three) revealed a large reserve of yolk with no macroscopic evidence of embryonic development.
There are three modes of oviparity known in cartilaginous fishes, i.e., short single oviparity, sustained single oviparity, and multiple oviparity [57], with all three represented in catsharks. In the two single oviparous modes, a single egg case is produced in each uterus, where they are then deposited immediately (short single) or retained, allowing most of the embryonic development to occur internally before deposition (sustained oviparity). Most catshark species exhibit short single oviparity [57], and the egg case occupies most of the available space in the uterus, e.g., Bythaelurus dawsoni [58]. In this mode of oviparity, females may lay multiple pairs of egg cases over a certain period. For example, a Cephaloscyllium isabella held in an aquarium in Wellington, New Zealand, deposited 13 viable eggs over a period of several months (E. Howard, pers obs.). In multiple oviparous species, multiple eggs are retained in both uteri for part of the gestation period and individual egg cases are much smaller than the available space in each uterus. For example, Halaelurus maculosus specimens were recorded as having 3–6 egg cases in each uterus [59].
Based on the above oviparous modes, D. satoi n. sp. fits the multiple oviparity mode based on having more than one egg case in numerous specimens in at least one uterus. However, there is no indication of retained oviparity allowing embryonic development to occur in utero. The presence of many large oocytes in the functional ovary (Figure 29a) suggests that the egg cases are deposited regularly and not retained for long periods. Thus, D. satoi n. sp. may exhibit a fourth type of oviparity, a short multiple oviparity where multiple egg cases are present in uteri, but they are not retained for a period of time to allow some embryonic development to occur. More information is required on the reproduction of members of this genus to confirm this.
No reproductive data are available for D. albimarginatus and D. nigripalatum. The holotype of D. bigus was dissected to allow for the removal of two egg cases that could be felt through the abdomen. A single egg was present in each uterus of the D. bigus gravid female holotype, suggesting single short oviparity. However, it is possible that this 710 mm TL specimen only recently became mature and larger adults could also exhibit multiple oviparity, thus not ruling out D. bigus being a multiple oviparous species. No gravid females of D. melanobranchus were examined, but it has been observed to be a multiple oviparous species (K. Sato pers. comm., Okinawa Churaumi Aquarium).
4.4. Egg Case Morphology
Egg cases can be a valuable taxonomic characteristics for distinguishing between species [60] and families [61]. Egg cases are known from three of the five Dichichthys species, i.e., D. bigus, D. melanobranchus, and D. satoi n. sp., and are characterized by having an asymmetrical shape (one lateral edge longer than the other, giving the egg case a curved or banana-shaped appearance) and longitudinal pliable ridges on both dorsal and ventral surfaces. Since the egg cases of these three species are very similar in their morphology, it is considered likely that the egg cases of the remaining two species, D. albimarginatus and D. nigripalatum, share similar characteristics and will be easily distinguishable as belonging to the family Dichichthyidae.
Egg cases of the family Dichichthyidae are unique from other oviparous shark egg cases in their morphology and structure, being formed of several layers: an outer fibrous mat encapsulating the whole egg case (only partially encapsulating in D. bigus egg cases), a second layer of pliable, prominent ridges formed of tightly packed zig-zag like fibres, and an innermost layer of thin, fragile shell-like material. Some species do share similarities with Dichichthyidae in individual features, such as Chiloscyllium and Hemiscyllium shark egg cases being encapsulated partially or fully with an outer fibrous mat. Several other catsharks’ egg cases possess prominent ridges, i.e., Cephaloscyllium laticeps (Duméril, 1853) [62], Apristurus ovicorrugatus (White, O’Neill, Devloo-Delva, Nakaya, and Iglésias, 2023) [53], and Bythaelurus canescens (Günther, 1878) [63]. However, the ridges of these species are made of the same hard, collagenous material of the egg case structure and are not soft and pliable like the ridges of Dichichthyidae egg cases. Despite the egg cases of some species sharing some characteristics with Dichichthyidae egg cases, the unique egg case structure is not known from other shark species known to date.
Egg case ridges have been theorized to have a number of functions, including (1) to act as anti-foulant, making it difficult for invertebrates such as sponges and barnacles to attached to the egg; (2) as a means to reduce predation, making it difficult for gastropods to bore through the thick and textured egg casing; (3) by improving the hydrodynamic flow over the egg case which may assist with respiration of the developing embryo; (4) to improve the strength of the egg case making it more resistant to mechanical shock [53]. Although the ridges on dichichthyid egg cases are made up of densely packed fibres, it is possible they serve the same function as in other ridged shark egg cases. The presence of attachment fibres on the anterior end, fibrous mat, and tendrils on the posterior ends of dichichthyid egg cases suggest that they attach their eggs to benthic structures and/or sessile macroinvertebrates, such as corals and sponges. Similar ‘attachment features’ (tendrils and attachment fibres) are present in the egg cases of many catshark species. For example, the egg cases of Schroederichthys bivius (Smith, 1838 in [64]) possess tendrils and attachment fibres which entangle on coral, sponges and algae [65]. More investigation and in situ observation of dichichthyid egg cases are needed to provide better insights into the function of their unique structure.
4.5. Parasites
The Dichichthys bigus individual observed by a deepwater ROV off northern Queensland had several isopods attached to it and a number of raised nodular or cyst-like lesions (Figure 12d). Without being able to conduct a biopsy and histological preparation of these lesions, the cause of them is not known. Possible causes of these lesions are:
- Viral infection, e.g., oncogenic virus-like papilloma-inducing viruses [66], epidermal hyperplasia retrovirus [67], or lymphocystis virus causing fibroblast hyperplasia [68];
- Skin irritation caused by an allergic or chemical exposure response. Chemical exposure is unlikely, given the depth and remote location. Allergic reaction to parasite bites is a possibility;
- Parasitic disease, e.g., metazoan trematode forming cysts (e.g., [69]);
- Bacterial infections, e.g., mycobacteria, can cause lumps of inflammatory cells (granulomas) (R. Chong and P. Quezada Rodriguez, CSIRO, pers. comm.);
- Fungal infection, although rare in sharks (R. Chong, CSIRO, pers. comm.).
No such lesions were apparent on any specimens of Dichichthys examined in this study nor on any of the several hundred catsharks present in the CSIRO Australian National Fish Collection. Thus, this appears to be an unusual finding and it is likely that this individual was in a poor condition.
5. Conclusions
The western Pacific Ocean is known as a biodiversity hotspot, particularly within the Coral Triangle region of Papua New Guinea, Indonesia, and the Philippines, including a large number of chondrichthyan species [33]. The increase in deep-water surveys and specimen sampling in the region, combined with innovations in molecular analysis and CT scanning, has permitted comprehensive analysis of rare species with a greater degree of certainty, as has been covered in this paper. We anticipate that integrated approaches such as those in this paper will become the new norm in new species descriptions and generic revisions.
The new family of carcharhiniform sharks described in this paper lays the foundation for a taxonomic revision of the catshark families and genera. Multiple paraphyletic groups (families and genera) are in urgent need of revision, but the limited specimens available for some of these groups are a hindrance to the full resolution of their systematics.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/fishes9040121/s1, Table S1: Summary information of the specimens of Dichichthys examined in this study.
Author Contributions
Conceptualization, W.T.W.; methodology, W.T.W. and G.J.P.N.; data capture and curation, W.T.W., A.L.S., H.L.O. and G.J.P.N.; writing—original draft preparation, W.T.W., A.L.S., H.L.O. and G.J.P.N.; writing—review and editing, W.T.W.; project administration, W.T.W. and G.J.P.N.; funding acquisition, G.J.P.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research and authors W.T.W. and H.L.O. was funded by the CSIRO National Research Collections Australia. The sequence data were collected through a US National Science Foundation grant to G.J.P.N. (DEB 1541556). One of us (A.L.S.) was supported (in part) by the NZ National Institute of Water and Atmospheric Research Ltd. via the Oceans Centre SSIF programme Protecting Marine Biodiversity subcontract for fundamental knowledge of marine biodiversity with the Museum of New Zealand Te Papa Tongarewa.
Institutional Review Board Statement
Ethical review and approval were waived for this study since all specimens examined in this study are from natural history museum collections and were not collected specifically for this study.
Informed Consent Statement
Not applicable.
Acknowledgments
Thanks to research staff and collection managers for access to specimens and for providing images and/or data: A. Graham (CSIRO); J. Maclaine (Natural History Museum, London); K. Sato (Okinawa Churashima Foundation, Japan); and C. Struthers, J. Barker, S. Kortet and T. Linley (Museum of New Zealand Te Papa Tongarewa, Wellington). Thanks also to E. Gumina (CSIRO) for capturing SEM imagery; K. Goumann (Central Science Laboratory, UTas) for providing training and access to SEM; Fahmi (Research Centre for Oceanography, Jakarta) for providing fresh images of the D. nigripalatum holotype; L. Yang (UF) for helping with the molecular analyses and submitting sequences to GenBank; E. Stanley (UF) for capturing CT scans of two of the species; A. Hosie (WAM) and D. Barton (CSU) for help identifying the parasites from underwater imagery; R. Chong and P. Rodriguez Quezada (CSIRO) for providing insights into the skin condition of one of the sharks imaged underwater; R. Beaman (JCU) for providing underwater images; underwater imagery courtesy of the Schmidt Ocean Institute with thanks to all those onboard the RV Falkor, in particular, the operators of ROV SuBastian; P. McMillan (Museum of New Zealand Te Papa Tongarewa, Wellington) and T.J. Verhoeff (Tasmanian Museum and Art Gallery) for identification of prey items of one of the D. satoi n. sp. paratypes. Edward Howard (Wellington Marine Education Centre) provided the observation on the Cephaloscyllium isabella eggs.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gill, T. Analytical synopsis of the order Squali; a revision of the nomenclature of the genera. Ann. Lyceum Nat. Hist. 1862, 7, 367–413. [Google Scholar] [CrossRef]
- Springer, S. A revision of the catsharks, family Scyliorhinidae. NOAA Tech. Rep. NMFS Circ. 1979, 422, 1–152. [Google Scholar] [CrossRef]
- Compagno, L.J.V. Sharks of the Order Carcharhiniformes; Princeton University Press: Princeton, NJ, USA, 1988; pp. 1–486. [Google Scholar]
- White, E.G. A classification and phylogeny of the elasmobranch fishes. Am. Mus. Novit. 1936, 837, 1–16. [Google Scholar]
- Smith, H.M. Description of a new notidanoid shark from the Philippine Islands representing a new family. Proc. U. S. Nat. Mus. 1912, 41, 489–491. [Google Scholar] [CrossRef]
- Iglésias, S.P.; Lecointre, G.; Sellos, D.Y. Extensive paraphylies within sharks of the order Carcharhiniformes inferred from nuclear and mitochondrial genes. Mol. Phylo. Evol. 2005, 34, 569–583. [Google Scholar] [CrossRef]
- Last, P.R.; Stevens, J.D. Sharks and Rays of Australia, 2nd ed.; CSIRO Publishing: Melbourne, Australia, 2009; pp. 1–644. [Google Scholar]
- Ebert, D.A.; Fowler, S.; Compagno, L. Sharks of the World: A Fully Illustrated Guide; Wild Nature Press: Plymouth, UK, 2013; pp. 1–528. [Google Scholar]
- Soares, K.D.A.; Mathubara, K. Combined phylogeny and new classification of catsharks (Chondrichthyes: Elasmobranchii: Carcharhiniformes). Zool. J. Linn. Soc. 2022, 195, 761–814. [Google Scholar] [CrossRef]
- Garman, S. The Plagiostomia (shark, skates and rays). Mem. Mus. Comp. Zool. 1913, 36, 1–515. [Google Scholar] [CrossRef]
- Whitley, G.P. Taxonomic notes on sharks and rays. Aust. Zoologist 1939, 9, 227–262. [Google Scholar]
- Bigelow, H.B.; Schroeder, W.C. Cephalurus, a new genus of scyliorhinid shark with redescription of the genotype, Catulus cephalus Gilbert. Copeia 1941, 1941, 73–76. [Google Scholar] [CrossRef]
- Whitley, G.P. Studies in ichthyology. No. 2. Rec. Aust. Mus. 1929, 16, 211–240. [Google Scholar] [CrossRef][Green Version]
- Rafinesque, C.S. Caratteri di Alcuni Nuovi Generi e Nuove Specie di Animali e Piante Della Sicilia, con Varie Osservazioni Sopra i Medisimi; Per le stampe di Sanfilippo: Palermo, Italy, 1810. [Google Scholar]
- Fowler, H.W. Descriptions of new fishes obtained 1907–1910, chiefly in the Philippine Islands and adjacent seas. Proc. Acad. Nat. Sci. Phila. 1934, 85, 233–367. [Google Scholar]
- Garman, S. New Plagiostomia. Bull. Mus. Comp. Zool. 1906, 46, 203–208. [Google Scholar]
- White, W.T.; Fahmi, F.; Weigmann, S. A new genus and species of catshark (Carcharhiniformes: Scyliorhinidae) from eastern Indonesia. Zootaxa 2019, 4691, 444–460. [Google Scholar] [CrossRef] [PubMed]
- Sato, K. Skeletal morphology and systematics of Parmaturus melanobranchus (Scyliorhinidae). In Proceedings of the 8th Indo Pacific Fish Conference and 2009 ASFB Workshop and Conference, Fremantle, Australia, 31 May–5 June 2009. [Google Scholar]
- Chan, W.L. New sharks from the South China Sea. J. Zool. 1966, 148, 218–237. [Google Scholar] [CrossRef]
- Naylor, G.J.P.; Caira, J.N.; Jensen, K.; Rosana, K.A.M.; White, W.T.; Last, P.R. A DNA sequence-based approach to the identification of shark and ray species and its implications for global elasmobranch diversity and parasitology. Bull. Am. Mus. Nat. Hist. 2012, 367, 1–263. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, C.H. Scientific results of explorations by the U. S. Fish Commission Steamer Albatross. No. XXII. Descriptions of thirty-four new species of fishes collected in 1888 and 1889, principally among the Santa Barbara Islands and in the Gulf of California. Proc. U. S. Nat. Mus. 1892, 14, 539–566. [Google Scholar] [CrossRef]
- Jordan, D.S.; Richardson, R.E. A catalogue of the fishes of the island of Formosa, or Taiwan, based on the collections of Dr. Hans Sauter. Mem. Carnegie Mus. 1909, 4, 159–204. [Google Scholar] [CrossRef]
- Nakaya, K.; Sato, K.; Kawauchi, J.; Stewart, A.L. 14 Family Scyliorhinidae. In The Fishes of New Zealand; Roberts, C.D., Stewart, A.L., Struthers, C.D., Eds.; Te Papa Press: Wellington, New Zealand, 2015; pp. 75–89. [Google Scholar]
- Compagno, L.J.V. Sharks of the World: An Annotated and Illustrated Catalogue of Shark Species Known to Date. Bullhead, mackerel and carpet sharks (Heterodontiformes, Lamniformes and Orectolobiformes); FAO: Rome, Italy, 2001; Volume 2, pp. 1–269. [Google Scholar]
- Séret, B.; Last, P.R. Four new species of deep-water catsharks of the genus Parmaturus (Carcharhiniformes: Scyliorhinidae) from New Caledonia, Indonesia and Australia. Zootaxa 2007, 1657, 23–39. [Google Scholar] [CrossRef]
- Fahmi, F.; Ebert, D.A. Parmaturus nigripalatum n. sp., a new species of deep-sea catshark (Chondrichthyes: Carcharhiniformes: Scyliorhinidae) from Indonesia. Zootaxa 2018, 4413, 531–540. [Google Scholar] [CrossRef]
- O’Neill, H.L.; Avila, C.; White, W.T. Description of the egg cases and juvenile colouration in two catsharks of the genus Atelomycterus (Carcharhiniformes: Scyliorhinidae). J. Fish Biol. 2020, 97, 1724–1732. [Google Scholar] [CrossRef]
- White, W.T.; O’Neill, H.L.; Cleeland, J.; Lamb, T.D.; Iglésias, S.P. Further description of the Kerguelen sandpaper skate Bathyraja irrasa (Rajiformes: Arhynchobatidae) based on additional specimens including egg cases and embryos. J. Fish Biol. 2022, 101, 659–675. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Press: Cold Spring Harbor, NY, USA, 1989; Volume 1, pp. 1–1659. [Google Scholar]
- Swofford, D. PAUP*. Phylogenetic Analysis Using Parsimony (* and Other Methods). Version 4; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A.; Hoover, P.; Rougement, J. A rapid bootstrap algorithm for the RAxML web-servers. Sys. Biol. 2008, 57, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Sabaj, M.H. Codes for natural history collections in ichthyology and herpetology. Copeia 2020, 108, 593–669. [Google Scholar] [CrossRef]
- Grandperrin, R.; Bargibant, G.; Menou, J.L. Campagne HALICAL 1 de Pêche à la Palangre de Fond Dans le Nord et sur la Ride des Loayauté, en Nouvelle-Calédonie. N.O. Alis, 21 Novembre–1er Décembre et 12–23 Décembre: Rapport Final; ORSTOM: Nouméa, New Caledonia, 1995; pp. 1–67. [Google Scholar]
- Weigmann, S. Annotated checklist of the living sharks, batoids and chimaeras (Chondrichthyes) of the world, with a focus on biogeographical diversity. J. Fish Biol. 2016, 88, 837–1037. [Google Scholar] [CrossRef] [PubMed]
- Ebert, D.A.; Dando, M.; Fowler, S. Sharks of the World: A Complete Guide; Princeton University Press: Princeton, NJ, USA, 2021; pp. 1–607. [Google Scholar]
- White, W.T.; Ko’ou, A. An annotated checklist of the chondrichthyans of Papua New Guinea. Zootaxa 2018, 4411, 1–82. [Google Scholar] [CrossRef] [PubMed]
- White, W.T.; Baje, L.; Sabub, B.; Appleyard, S.A.; Pogonoski, J.J.; Mana, R.R. Sharks and Rays of Papua New Guinea; Australian Centre for International Agricultural Research: Canberra, Australia, 2018; pp. 1–327. [Google Scholar]
- White, W.T.; Mana, R.R.; Naylor, G.J.P. Description of a new species of deepwater catshark Apristurus yangi n.sp. (Carcharhiniformes: Pentanchidae) from Papua New Guinea. Zootaxa 2017, 4320, 25–40. [Google Scholar] [CrossRef]
- Last, P.R.; Stevens, J.D. Sharks and Rays of Australia; CSIRO: Melbourne, Australia, 1994; pp. 1–513. [Google Scholar]
- Last, P.R.; Pogonoski, J.J.; Gledhill, D.C.; White, W.T.; Walker, C.J. The deepwater demersal ichthyofauna of the western Coral Sea. Zootaxa 2014, 3887, 191–224. [Google Scholar] [CrossRef]
- Ebert, D.A.; White, W.T.; Ho, H.C.; Last, P.R.; Nakaya, K.; Seret, B.; Straube, N.; Naylor, G.J.P.; Carvalho, M.R.d. An annotated checklist of the chondrichthyans of Taiwan. Zootaxa 2013, 3752, 279–386. [Google Scholar] [CrossRef]
- Chu, Y.T.; Meng, Q.W.; Liu, J.X. Description of a new species of Scyliorhinidae from China. Acta Zootax. Sin. 1983, 8, 104–107. [Google Scholar]
- Compagno, L.J.V. FAO Species Catalogue. Sharks of the World, An Annotated and Illustrated Catalogue of Shark Species Known to Date; FAO: Rome, Italy, 1984; Volume 4, Part 2 (Carcharhiniformes). [Google Scholar]
- Compagno, L.J.V.; Niem, V.H. Scyliorhinidae: Catsharks. In The Living Marine Resources of the Western Central Pacific. FAO Species Identification Guide for Fishery Purposes. Vol. 2. Cephalopods, Crustaceans, Holothurians and Sharks; Carpenter, K.E., Niem, V.H., Eds.; FAO: Rome, Italy, 1998; pp. 1279–1292. [Google Scholar]
- Compagno, L.J.V. Checklist of living elasmobranchs. In Sharks, Skates, and Rays: The Biology of Elasmobranch Fishes; Hamlett, W.C., Ed.; Johns Hopkins University Press: Baltimore, MY, USA, 1999; pp. 471–498. [Google Scholar]
- Yano, K. Chondrichthyans of the Ryukyu Islands, Japan. In Proceedings of the 5th Indo-Pacific Fish Conference, Nouméa, New Caledonia, 3–8 November 1997; Séret, B., Sire, J.Y., Eds.; Societe Francaise d’Ichthyologie & Institut de Recherche pour le Developpement: Paris, France, 1999; pp. 351–365. [Google Scholar]
- Gledhill, D.C.; Last, P.R.; White, W.T. Resurrection of the genus Figaro Whitley (Carcharhiniformes: Scyliorhinidae) with the description of a new species from northeastern Australia. In Descriptions of New Australian Chondrichthyans; Last, P.R., White, W.T., Pogonoski, J.J., Eds.; CSIRO Marine and Atmospheric Research Paper 022; CSIRO: Hobart, Australia, 2008; pp. 179–187. [Google Scholar]
- Lee, P.F.; Shao, K.T. New record of the rare shark Parmaturus melanobranchius (Scyliorhinidae) from Taiwan. Taiwanica 2010, 55, 386–390. [Google Scholar] [CrossRef]
- Yano, K.; Kugai, K. Deep-sea chondrichthyans collected from the waters around the Okinawa Islands: Results of catch analysis of bottom longlines. Bull. Seikai Nat. Fish. Res. Inst. 1993, 71, 51–65. [Google Scholar]
- Soares, K.D.A.; Carvalho, M.R.d.; Schwingel, P.R.; Gadig, O.B.F. A new species of Parmaturus (Chondrichthyes: Carcharhiniformes: Scyliorhinidae) from Brazil, southwestern Atlantic. Copeia 2019, 107, 314–322. [Google Scholar] [CrossRef]
- Hardy, G.S. A new species of catshark in the genus Parmaturus Garman (Scyliorhinidae) from New Zealand. N. Z. J. Zool. 1985, 12, 119–124. [Google Scholar] [CrossRef]
- White, W.T.; O’Neill, H.L.; Devloo-Delva, F.; Nakaya, K.; Iglésias, S.P. What came first, the shark or the egg? Discovery of a new species of deepwater shark by investigation of egg case morphology. J. Fish Biol. 2023, 103, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Weigmann, S.; Ebert, D.A.; Clerkin, P.J.; Stehmann, M.F.W.; Naylor, G.J.P. Bythaelurus bachi n. sp., a new deep-water catshark (Carcharhiniformes, Scyliorhinidae) from the southwestern Indian Ocean, with a review of Bythaelurus species and a key to their identification. Zootaxa 2016, 4208, 401–432. [Google Scholar] [CrossRef] [PubMed]
- Human, B.A. A taxonomic revision of the catshark genus Poroderma Smith, 1837 (Chondrichthyes: Carcharhiniformes: Scyliorhinidae). Zootaxa 2006, 1229, 1–32. [Google Scholar] [CrossRef]
- Cooper, R.L.; Thiery, A.P.; Fletcher, A.G.; Delbarre, D.J.; Rasch, L.J.; Fraser, G.J. An ancient Turing-like patterning mechanism regulates skin denticle development in sharks. Sci. Adv. 2018, 4, eaau5484. [Google Scholar] [CrossRef]
- Nakaya, K.; White, W.T.; Ho, H.C. Discovery of a new mode of oviparous reproduction in sharks and its evolutionary implications. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Francis, M.P. Distribution and biology of the New Zealand endemic catshark, Halaelurus dawsoni. Environ. Biol. Fish. 2006, 75, 295–306. [Google Scholar] [CrossRef]
- White, W.T.; Last, P.R.; Stevens, J.D. Halaelurus maculosus n. sp. and H. sellus n. sp., two new species of catshark (Carcharhiniformes: Scyliorhinidae) from the Indo-West Pacific. Zootaxa 2007, 1639, 1–21. [Google Scholar] [CrossRef]
- Flammang, B.E.; Ebert, D.A.; Cailliet, G.M. Egg cases of the genus Apristurus (Chondrichthyes: Scyliorhinidae): Phylogenetic and ecological implications. Zoology 2007, 110, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Mabragaña, E.; Figueroa, D.E.; Scenna, L.B.; Díaz de Astarloa, J.M.; Colonello, J.H.; Delpiani, G. Chondrichthyan egg cases from the south-west Atlantic Ocean. J. Fish Biol. 2011, 79, 1261–1290. [Google Scholar] [CrossRef]
- Duméril, A.H.A. Monographie de la tribu des Scylliens ou Roussettes (poissons plagiostomes) comprenant deux espèces nouvelles. Rev. Mag. Zool. (Sér. 2) 1853, 5, 8–25, 73–87, 119–130. [Google Scholar]
- Günther, A. Preliminary notices of deep-sea fishes collected during the voyage of H.M.S. ‘Challenger’. Ann. Mag. Nat. Hist. (Ser. 5) 1878, 2, 17–28, 179–187, 248–251. [Google Scholar] [CrossRef]
- Müller, J.; Henle, F.G.J. Systematische Beschreibung der Plagiostomen; Veit und Comp: Berlin, Germany, 1838; pp. 1–28. [Google Scholar]
- Vazquez, D.M.; Belleggia, M.; Schejter, L.; Mabragana, E. Avoiding being dragged away: Finding egg cases of Schroederichthys bivius (Chondrichthyes: Scyliorhinidae) associated with benthic invertebrates. J. Fish Biol. 2018, 92, 248–253. [Google Scholar] [CrossRef]
- Dill, J.A.; Camus, A.C.; Leary, J.H.; Ng, T.F.F. Microscopic and molecular evidence of the first elasmobranch adomavirus, the cause of skin disease in a giant guitarfish, Rhynchobatus djiddensis. mBio 2018, 9, e00185-18. [Google Scholar] [CrossRef]
- Chong, R.S.M. Chapter 76—Oncogenic finfish viral diseases. In Aquatic Pathophysiology. Volume I. Finfish Diseases; Kibenge, F.S.B., Baldisserotto, B., Chong, R.S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 839–843. [Google Scholar]
- Volpatti, D.; Ciulli, S. Chapter 15—Lymphocystis viral disease. In Aquatic Pathophysiology. Volume I. Finfish Diseases; Kibenge, F.S.B., Baldisserotto, B., Chong, R.S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 201–216. [Google Scholar]
- Chong, R.S.M. Chapter 46—Digenetic trematode infections. In Aquatic Pathophysiology. Volume I. Finfish Diseases; Kibenge, F.S.B., Baldisserotto, B., Chong, R.S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 569–590. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).