Abstract
The aim of this study was to clarify the “morphological-trait–body weight” correlation, gonadal development characteristics, and pleopod (main edible part) nutrient composition of the whelk (Volutharpa perryi perryi). Live body mass (BM), soft tissue mass (STM), and eight other morphological traits of the whelk were measured, and path coefficients, correlation indices (R2), and coefficients of determination were then calculated. Gonadal development characteristics were investigated by histological observation. Pleopod nutrient composition was analyzed by standard biochemical assays. The results indicated that (1) shell aperture width (SAW) and body whorl height (BWH) were positively correlated with both live BM and STM (p < 0.01), and shell height (SH) was positively correlated with both live BM and STM (p < 0.01) in male whelks; (2) similar gonadal development characteristics were observed in both female and male whelks; and (3) pleopod nutrient composition was consistent in both female and male whelks, whereas sex-specific variation in pleopod nutrient content was observed in the whelks. The observations in this study will provide theoretical support for the development of the whelk aquaculture industry.
Keywords:
whelk; morphological traits; correlation analysis; gonad development; pleopod nutrient composition Key Contribution:
Morphological trait correlations of whelks were analyzed. Gonadal development characteristics of whelks were investigated. Pleopod nutrient compositions of whelks were determined.
1. Introduction
The whelk (Volutharpa perryi perryi) is a deep-sea species that is naturally distributed in the soft mud or sandy seafloor of the northern Yellow Sea in China [1,2,3]. This species is gonochoristic with sex-specific organ and reproductive characteristics. Female reproductive organs mainly include the ovary, oviduct, and albumin glandbursa copulatrix. The male reproductive organ (vas deferens) is located at the bottom of the mantle and bulges at the ends to form the copulatory organ, located on the right side of the back of the head [1,2]. Over the past several decades, this species has been considered as an inexpensive alternative to the Pacific abalone (Haliotis discus, an expensive seafood) due to the appearance of its pleopod being similar to the abalone’s, and hence the name “false abalone”, seen in markets and restaurants [3,4,5]. Recently, with the discovery of the commercial value of the whelk in medicine and disease therapy [3,4,5,6,7], the demand for whelks has increased dramatically. In this context, the development of artificial breeding and aquaculture of whelks is necessary to meet the increased demand for this species.
In this study, we aimed to (1) explore the relationships among the morphological traits of the whelk in different sexes; (2) clarify gonadal development characteristics of the whelk; and (3) determine the nutrient composition and content of the whelk in different sexes. To this end, path correlation analysis, histological observation, and biochemical assays were employed. We analyzed the correlations among four morphological traits and performed a multiple regression analysis. The results from this study will not only address the knowledge gap concerning the basic biology of the whelk but will also provide theoretical support for artificial breeding and the development of the whelk aquaculture industry.
2. Materials and Methods
2.1. Animals and Sampling
Individual whelks were collected from the sea areas of the Oceanic Island in Dalian, Liaoning Province, China (123°28′ E, 39°01′ N). The collected specimens were transported to the Key Laboratory of Mariculture and Stock Enhancement at the Ministry of Agriculture of the North China Sea at Dalian Ocean University, Dalian, China. All of the specimens were kept in a circulating seawater tank (~1000 L) under natural light and allowed to acclimate to standard laboratory conditions (temperature: 8.37 ± 0.06 °C; pH: 7.83 ± 0.01; salinity: 29.97 ± 0.15 practical salinity units, PSU). Seawater was sand-filtered and continuously aerated. All of the specimens were fed scallops and frozen fish every 3 days.
2.2. Trait Measurements and Gonadal Histological Analysis
For trait measurement, a total of 60 individuals (30 individuals of each sex) of the same age were selected in July 2021. Shell height (SH), shell width (SW), shell aperture height (SAH), shell aperture width (SAW), body whorl height (BWH), body whorl width (BWW), spiral whorl height (SWH), and spiral whorl width (SWW) were measured using a digital display electronic Vernier caliper that had a precision of 0.01 mm (16EW; Mahr GmbH, Germany) (Figure 1). Live body mass (BM) and soft tissue mass (STM) were measured using a digital balance (0.01 g precision; JJ3OO, Changshu Shuangjie Testing Instrument Factory, Jiangsu, China). Specimens were dried with paper towels and then weighed individually using a digital balance (0.01 g precision; JJ300, Changshu Shuangjie Testing Instrument Factory, Jiangsu, China).
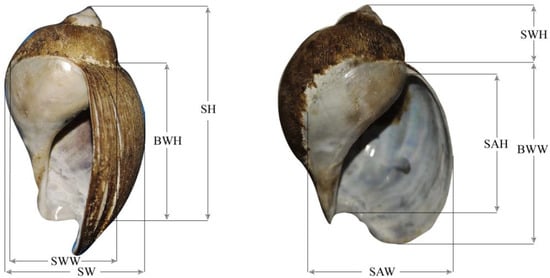
Figure 1.
Morphology parameters of the whelk. SH: shell height; SW: shell width; SAH: shell aperture height; SAW: shell aperture width; BWH: body whorl height; BWW: body whorl width; SWH: spiral whorl height; SWW: spiral whorl width.
For the gonadal development analysis, one-year sampling was performed for both male and female whelks. From July 2021 to July 2022, five individuals of each sex at the same age per month were selected, and the gonad tissue of each individual was dissected and weighed. The gonadosomatic index (GI) was calculated using the following formula:
GI = 100 × (gonadal weight/soft tissue mass)
Histological analysis was performed to ascertain the presence of reproductive cells in the gonads of the whelk. Gonadal samples were fixed for 24 h in Bouin’s fluid following the method of Feldman et al. [7] and then embedded in paraffin wax. Tissues sections (4 μm thick) were stained with hematoxylin and eosin (HE) using routine methods. Histological observations of the gonads were performed under an optical microscope (Leica, Germany).
2.3. Pleopod Nutrient Composition and Content Detection
A total of 60 individuals (30 individuals of each sex) of the same age were selected in July 2021. Amino acid extraction was performed by using hydrochloric acid (HCL) hydrolysis, following the method of Tsugita et al. [8] and the national standards of China (GB /T18246-2000), and the amino acid composition was then determined using an automatic amino acid analyzer (Hitachi L-8800, Tokyo, Japan). Amino acid scores (AASs) refer to amino acid ratios (mg of an essential amino acid in 1.0 g of test protein/mg of the same amino acid in 1.0 g of the FAO/WHO reference pattern) [9,10]. AAS is a widely used method to evaluate the nutritional value of sample proteins, which is not only suitable for the evaluation of single-sample proteins, but also for the evaluation of mixed-sample proteins. Herein, AASs were calculated to determine the first and second limiting amino acids. Fatty acid detection was performed following the method of Salimon et al. [11], and the components of fatty acids were determined using a GC-9A gas chromatograph (Shimadzu, Japan). Crude fat refers to the crude mixture of fat-soluble material present in samples [12]. Monosaccharides were analyzed following the method of Zhang et al. [13] and the national standards of China (GB/T 33108-2016), and the monosaccharides were determined using a liquid chromatograph (Agilent 1200, Santa Clara, CA, USA). Crude polysaccharide detection was performed using the method described by Zhang et al. [13], and then the composition of crude polysaccharides was determined using a visible light spectrophotometer (Agilent Technologies 8435, Santa Clara, CA, USA).
2.4. Data Analysis
All data were expressed as the mean ± standard deviation (SD), and all statistical analyses were performed with Excel 2010 (Microsoft, Redmond, WA, USA) and SPSS 22.0 software (IBM, Shanghai, China). Since normal distribution of data is the necessary basis of the linear regression model [14], all trait measurement data were converted into standard normal variables (base-2 logarithms) using Excel 2010 (Microsoft, Redmond, WA, USA) according to the method described by Slifker et al. [15]. Normal distributions of variables were then analyzed by Kolmogorov–Smirnov tests. Correlation coefficients and path coefficients were calculated according to the methods described by Jiang [16]. Herein, the path coefficient refers to the ratio of standard deviation due to a given cause to the total standard deviation of the effect [17]. The correlation coefficients and path coefficients were then used to calculate the determination coefficient (di) and the co-determination coefficient (dij) to determine the direct and indirect effects of morphological traits on quality traits. In this study, the determination coefficient refers to the proportion of explained variance present in the data [18].
Formulae for calculating the determination coefficient (di) of a single independent variable with respect to the dependent variable and the co-determination coefficient (dij) of two independent variables with respect to the dependent variable were as follows:
where rij is the correlation coefficient between i and j; Pi is the path coefficient of i; and Pj is the path coefficient of j.
The statistical significance of differences between morphological traits and quality traits in the multiple regression analysis of whelks was determined using ANOVA. We employed a t-test for determining differences in the correlation of traits and nutrient composition (AA, FA and saccharides) of different sexes. The p-value was automatically calculated using SPASS 22.0 software. The significance level was set at 0.05 (α = 0.05).
3. Results
3.1. Summary Statistics of Measured Traits
The mean, SD, and coefficient of variation (CV) of each measured trait are shown in Table S1. The CV is a normalized measure of variability and is considered an important index reflecting the selection potential of different traits [19]. In this study, the CVs of BM, STM, and SWH were generally greater than those of other measured traits in both female and male whelks, and the CVs of SH were the lowest in both sexes. Specifically, the CV from high to low in females was in the order STM > BM > SWH > SWW > SW > SAH > SAW > BWW > BWH > SH, while the CV from high to low in males was as follows: SWH > STM > SWW > BM > SAH > SAW > BWW > SW > BWH > SH. The highest CV was observed in the STM of females (12.15%). In males, SWH had the highest CV (9.73%), and SD (0.31) values and STM ranked second (CV: 4.42%; SD: 0.19). Since normal distribution of data is the necessary basis of the linear regression model [14], all trait measurement data were converted into standard normal variables (base-2 logarithms). Further, K-S normal distribution test results showed that the significance levels of BM and STM in females were 0.12 and 0.12, respectively, and in males they were 0.20 and 0.20, respectively, indicating that all data were normally distributed. Subsequent correlation analysis and path analysis could thus be performed on standard normal variables (transformed data).
3.2. Correlation and Multiple Regression Analyses
There were significant correlations between the morphological traits SH, SW, BWH, BWW, and SWW, and quality traits in both sexes (p < 0.01; Table 1). Of all pairs of measured traits, BM was most highly correlated with STM in both females and males. SH was also highly correlated with BM and STM in all specimens examined. In females, the correlation between morphometric traits and quality traits was strongest for BWH (R2 = 0.93), followed by BWW (R2 = 0.92) and SWW (R2 = 0.89) (Table 2). For males, the correlation between phenotypic traits and quality traits was strongest for SH (R2 = 0.79), followed by BWH (R2 = 0.66) and BWW (R2 = 0.59) (Table 2).

Table 1.
Correlations among the morphological traits of female and male whelks (n = 30).

Table 2.
Path analysis of morphological traits on two qualitative traits of female and male whelks.
Correlation analysis is a tool for understanding the relationship between two variables [20]. Multiple regression equations are based on correlation analysis to determine how different variables have an impact on a given variable [21]. Path analysis is based on the results of both correlation analysis and multiple regressions [22]. In this study, path analysis on correlation coefficients was also carried out to quantify the direct and indirect effects of the measured traits on BM and STM. As shown in Table 2, BWH had strong direct and indirect effects on both BM and STM in females. In addition, SH had a significant direct influence on BM and STM in males, indicating that SH might be considered as the primary breeding trait when BM and STM are the target traits for male parental selection. Further analysis showed that in females, the single parameter determination coefficient with respect to BM and STM was higher for BWH than for other morphological traits. BWH with SAW had the largest co-determination coefficients for BM; meanwhile, BWH with SW had the largest co-determination coefficients for STM. In males, the single parameter determination coefficient with respect to BM and STM was higher for SH than for other morphological traits (Table 3). Based on the results of Table 3, the following equations describing BM and STM in females and males in July (a post-spawning phase) were produced by stepwise regression analysis:

Table 3.
Determination coefficients of various morphological traits on two qualitative traits of female and male whelks.
For females:
For males:
3.3. Gonadal Development Characteristics
Histological observations showed that the gonad development cycle of the whelk was 1 year and that this could be generally divided into five stages: the proliferating stage (stage I), the growing stage (stage II), the maturing stage (stage III), the spawning stage (stage IV), and the resting stage (stage V) (Figure 2 and Figure 3A,B). Reproductive cells were initially present in autumn, and matured in spring (Figure 2). The highest GIs (female: 29.26%; male: 27.00%) were observed in spring and the lowest GIs (female: 15.47%; male: 14.40%) were observed in summer (Figure 3C).
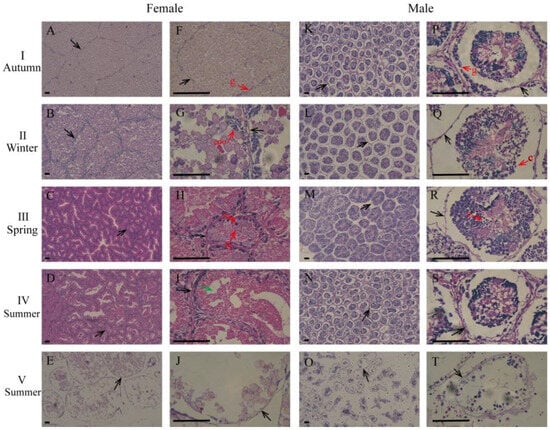
Figure 2.
Gonadal developmental characteristics of female and male whelks according to season. (A–J) female gonad; (K–T) male gonad. The black arrows represent the follicular wall and the green arrow represents the post-ovulation cavity. I: proliferating stage; II: growing stage; III: maturing stage; IV: spawning stage; V: resting stage. c: spermatocytes; epo: early prophasic oocytes; fg: full-grown oocytes; g: oogonia and spermatogonia; n: nucleus; z: spermatozoa. Scale bar: 50 μm.
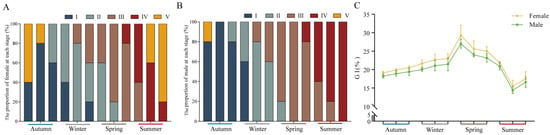
Figure 3.
Frequency of gonadal developmental stages and gonadosomatic index of female and male whelks according to season. (A) Female gonad; (B) male gonad. (C) The whelk. gonadosomatic index (GI). I: proliferating stage; II: growing stage; III: maturing stage; IV: spawning stage; V: resting stage.
3.4. Pleopod Nutrient Composition Analysis
The contents of 17 kinds of amino acids (AAs) in pleopod specimens were determined in this study. In addition to tryptophan (Trp), which was destroyed during acid hydrolysis, eight essential amino acids (EAAs) were determined to account for 29.04 ± 1.47% (females) and 28.28 ± 1.88% (males) of the total AAs in the examined pleopod specimens. In general, the content of detected EAAs in female pleopods was significantly higher than that of males, except for isoleucine (Ile) (p < 0.01). The content of Ile in male pleopods was significantly higher than that in female pleopods (p < 0.01) (Figure 4A). As for females, the percentages of EAAs ranked from high to low were as follows: leucine (Leu) (7.16 ± 0.32%) > lysine (Lys) (5.03 ± 0.28%) > valine (Val) (3.98 ± 0.17%) > threonine (Thr) (3.95 ± 0.18%) > Ile (2.84 ± 0.13%) > phenylalanine (Phe) (2.66 ± 0.14%) > methionine (Met) (1.81 ± 0.20%) > histidine (His) (1.61 ± 0.07%). The AASs ranked from high to low were as follows: Thr (21.67) > Leu (16.10) > Lys (14.38) > His (13.37) > Val (13.63) > Ile (12.98) > Met (10.77) > Phe (8.89). Phe and Met were then identified as the first and second limiting AAs in females, respectively (Figure 4B). As for males, the percentages of EAAs ranked from high to low were as follows: Leu (7.14 ± 0.44%) > Lys (4.88 ± 0.34%) > Val (4.01 ± 0.20%) > Thr (3.81 ± 0.26%) > Ile (3.00 ± 0.16%) > Phe (2.47 ± 0.17%) > His (1.58 ± 0.10%) > Met (1.38 ± 0.23%). The AASs ranked from high to low were as follows: Thr (20.29) > Leu (15.59) > Lys (13.55) > His (13.17) > Val (13.36) > Ile (13.34) > Phe (8.03) > Met (8.01). In this context, Met and Phe were identified as the first and second limiting AAs in males, respectively (Figure 4B).
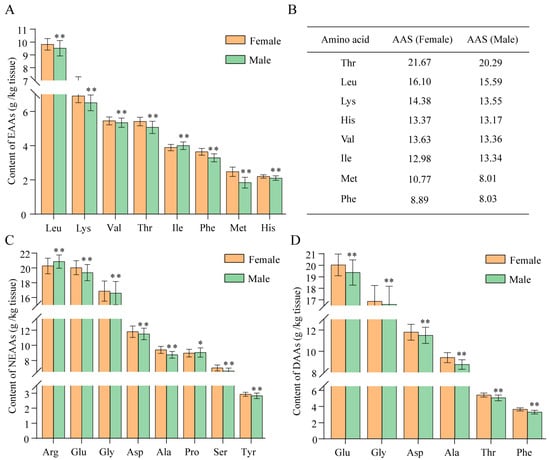
Figure 4.
Types and contents of amino acids in pleopods of female and male whelks. (A) Types and contents of essential amino acids (EAAs). (B) Amino acid score (AAS) of EAAs. (C) Types and contents of nonessential amino acids (NEAAs). (D) Types and contents of delicious amino acids (DAAs). Leu: leucine; Lys: lysine; Val: valine; Thr: threonine; Ile: Isoleucine; Phe: phenylalanine; Met: methionine; His: histidine; Arg: arginine; Glu: glutamate; Gly: glycine; Asp: aspartate; Ala: alanine; Pro: proline; Ser: serine; Tyr: tyrosine. *: p < 0.05 vs. female. **: p < 0.01 vs. female.
The contents of eight non-essential amino acids (NEAAs), namely, arginine (Arg), aspartate (Asp), glutamic acid (Glu), glycine (Gly), alanine (Ala), serine (Ser), proline (Pro) and tyrosine (Tyr), were also determined. In general, there were significant differences in pleopod NEAA content between females and males. The contents of Arg and Pro in males were significantly greater than those in females (Arg: p < 0.01, Pro: p = 0.0368), and the contents of the remaining six detected NEAAs were significantly greater in females than in males (p < 0.01; Figure 4C). Specifically, for females, the content percentages of NEAAs ranked from high to low were as follows: Arg (14.79 ± 0.75%) > Glu (14.61 ± 0.68%) > Gly (12.31 ± 0.98%) > Asp (8.60 ± 0.54%) > Ala (6.85 ± 0.34%) > Pro (6.55 ± 0.36%) > Ser (5.12 ± 0.29%) > Tyr (2.13 ± 0.10%) (Figure 4C). For males, the content percentages of NEAAs ranked from high to low were as follows: Arg (15.66 ± 0.66%) > Glu (14.54 ± 0.81%) > Gly (12.46 ± 1.17%) > Asp (8.63 ± 0.57%) > Pro (6.79 ± 0.45%) > Ala (6.57 ± 0.33%) > Ser (4.96 ± 0.29%) > Tyr (2.12 ± 0.14%). Arg was the most abundant NEAA in both female and male pleopods, being 20.27 ± 1.03 g/kg (females) and 20.86 ± 0.88 g/kg (males) (Figure 4C).
The contents of six delicious amino acids (DAAs), namely, Glu, Asp, Tyr, Ala, Phe, and Gly, in pleopods of different sex of the whelks were also determined. In general, the contents of the six DAAs in female pleopods were significantly higher than those in males (p < 0.01; (Figure 4D)). The total average amount of DAAs in female pleopods was 64.67 ± 3.76 g/kg, accounting for 47.17 ± 2.74% of total AAs; the total average amount of DAAs in male pleopods was 62.56 ± 4.20 g/kg, accounting for 46.27 ± 3.16% of total AAs. Among the examined DAAs, the content of Glu was the highest in both female pleopods (20.04 ± 0.93 g/kg) and male pleopods (18.80 ± 0.02 g/kg) (Figure 4D).
The percentages of saturated fatty acids (SFAs), unsaturated fatty acids (UFAs), and crude fat in female pleopods were 16.96 ± 0.73%, 21.65 ± 1.55%, and 43.84 ± 12.31%, respectively. The percentages of SFAs, UFAs, and crude fat in male pleopods were 19.52 ± 4.34%, 27.08 ± 3.69%, and 52.75 ± 15.28%, respectively (Figure 5A). In general, male pleopods had higher amounts of SFAs (except for myristic acid), UFAs (except for methyl arachidonic acid), and crude fat than female pleopods (p < 0.01; Figure 5B). As for SFAs, the contents of polyunsaturated fatty acids (PUFAs) were higher than those of monounsaturated fatty acids (MUFAs) in both female and male pleopods. Palmitic acid (C16:0) and stearic acid (C18:0) were identified as the main SFAs in female and male pleopods. Eicosapentaenoic acid (C20:5n3, EPA), α-Linolenic acid methyl ester (C18:3n3), and docosahexaenoic acid (C22:6n3, DHA) were the main PUFAs, and methyl oleate (C18:1n9c) was the main MUFA in both female and male pleopods. The PUFA/MUFA ratios (P/M) were 4.33 ± 0.36 (females) and 4.95 ± 0.25 (males) (Figure 5B).
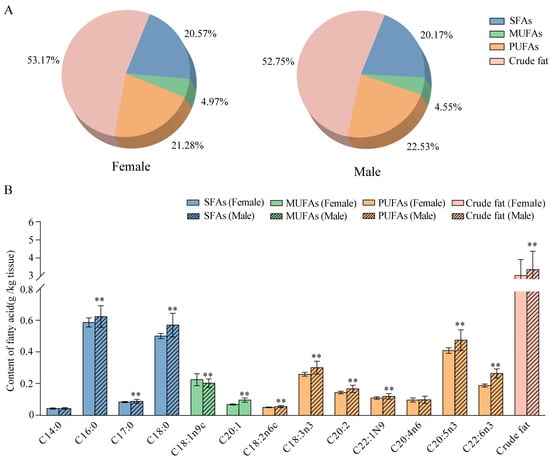
Figure 5.
Types and contents of fatty acids in pleopods of female and male whelks. (A) Contents of saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), polyunsaturated fatty acids (PUFAs), and crude fat in the pleopods of female and male whelks. (B) Fatty acid and crude fat contents of female and male whelks. **: p < 0.01 vs. female.
Monosaccharide and crude polysaccharide content analyses showed that the contents of monosaccharides and crude polysaccharides in pleopods were 45.07 ± 2.25 g/kg (females) and 48.60 ± 2.69 g/kg (males) (Figure 6). The percentages of monosaccharides in female pleopods from high to low were as follows: glucose (96.04 ± 4.04%) > galactose (1.07 ± 0.25%) > mannose (0.81 ± 0.15%) > xylose (0.64 ± 0.26%) > fucose (0.50 ± 0.07%) > ribose (0.15 ± 0.02%) > glucuronic acid (0.13 ± 0.09%) > arabinose (0.04 ± 0.02%). The percentages of monosaccharides in male pleopods from high to low were as follows: glucose (96.06 ± 4.86%) > galactose (1.11 ± 0.24%) > xylose (0.71 ± 0.18%) > mannose (0.56 ± 0.10%) > fucose (0.52 ± 0.08%) > ribose (0.21 ± 0.05%) > glucuronic acid (0.08 ± 0.04%) > arabinose (0.04 ± 0.02%). The contents of mannose, glucuronic acid, and arabinose in female pleopods were significantly higher than those in males (p < 0.01), whereas the contents of glucose, galactose, fucose, xylose, ribose, and crude polysaccharides in female pleopods were significantly lower than those in males (p < 0.01).
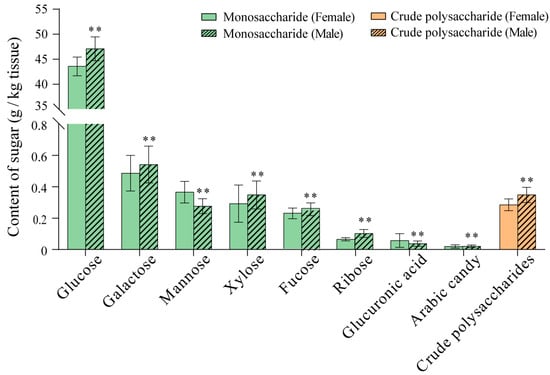
Figure 6.
Types and contents of saccharides in pleopods of female and male whelks. **: p < 0.01 vs. female.
4. Discussion
The relationships between quality traits and morphological traits, gonad development, the gamete maturation cycle, and nutrient requirements are all basic fundamental aspects of artificial breeding and aquaculture of commercial shellfish [23,24,25,26,27,28,29]. In the current study, we clarified the relationships between qualitative traits (BM and STM) and eight phenotypic traits, the characteristics of gonad development and the gamete maturation cycle, and the pleopod nutrient composition of the whelk in both sexes, for the first time.
It is well accepted that phenotypic traits are important determinants of commercial traits (especially in weight production) and are subject to natural selection and sex differences [30,31,32,33,34,35]. In this study, high CVs of BM and STM were observed in females, and high CVs of SWH and STM were observed in males, indicating that STM could be an important trait with greater selection potential in both female and male selective breeding of the whelk. This observation was consistent with the results from Babylonia areolata [36], Babylonia lutosa [37], and Glossaulax reiniana [38]. Interestingly, the CV of SWH (a morphometric trait) was greater than those of the qualitative trait (BW and STM in this study) in male whelks, indicating sex-specific differences in the CVs of morphological traits. This observation was in contrast to the hypothesis of qualitative traits always having greater selection potential than phenotypic traits [30]. Clearly, further research is needed to determine whether SWH has strong selection potential in male whelk breeding. The correlation analysis showed that all measured phenotypic traits were significantly correlated with both BM and STM in females, a result similar to the correlation results for the gastropod mollusk Pomacea canaliculate [39]. In males, only five measured phenotypic traits (SH, SW, BWH, BWW, and SWW) were significantly correlated with both BM and STM. These observations, on the one hand, suggest that SH, SW, BWH, BWW, and SWW could be potential target traits for the artificial selection of the whelk; on the other hand, the results suggest that there is a sex-specific difference in the relationship between phenotypic traits and commercial traits in whelks. Interestingly, sex-specific variation in the relationship between phenotypic traits and commercial traits has also been observed in the arthropod Penaeus chinensis [40], whereas no apparent sex-specific variation in the relationship between phenotypic traits and commercial traits was observed in the bivalve mollusks Chlamys farreri [41] or Mactra chinensis [42]. Combining the above previous observations with the results of the correlation analysis in this study, we thus assume that species-specific variation in the correlations between phenotypic traits and commercial traits should be paid more attention to in developing breeding programs for aquatic organisms.
Path analysis is a widely used method to identify the direct effects of one trait on another in developing breeding programs [16,43]. In this study, BWH had strong direct and indirect effects on BW and STM in female whelks, whereas the direct effects of SH on BW and STM were great in males. Interestingly, the results for females obtained in this study are similar to the path analysis results of our previous study [44], whereas the results for males were slightly different. This may have been due to the fact that our previous study did not accurately identify the sexes, resulting in a higher ratio of females than males among specimens. Moreover, we also found that the results for males obtained in this study were similar to the path analysis results for B. lutosa [37] and P. canaliculata [39], while the results for females were slightly different. Further investigation is needed to clarify the reasons for this pattern. To sum up, all of the above results indicate that BWH and SH could be considered as the primary breeding traits when BW and STM are the target traits for female and male parental selection, respectively. However, we should notice that the body morphological-trait variables were only determined in July (the post-spawning phase), and correlations between body morphological traits and their reproductive stages were not yet established. Therefore, further efforts should be paid, accordingly, so that we can accurately determine periods when they are more likely to be collected or captured without affecting the spawning times and development of the whelk.
The spawning of mature gametes is one of the key steps determining the success of artificial breeding. Histological analysis revealed that the gonad development cycle of the whelk was about 1 year and that this could be generally divided into five stages. This result is consistent with the gonad development stages of other economically important shellfish such as Neverita didyma [45], Modiolus modiolus [46], Mizuhopecten yessoensis [47], Scapharca subcrenata [48], Meretrix lamarkii [49], Lutraria sieboldii [50], and Mercenaria mercenaria [51]. The duration of the development and growth stages of the whelk was about 6 months, much longer than those of N. didyma (3 months) [45], M. yessoensis (3 months) [47], Coelomactra antiquata (3 months) [52], S. subcrenata (4 months) [48], and M. mercenaria (4 months) [51]. As for the maturing and spawning stages, the duration of these two stages was about 4 months, similar to those of N. didyma [45], M. yessoensis [47], S. subcrenata [48], M. lamarkii [49], and C. antiquate [52], but longer than that of M. mercenaria (3 months) [51]. In addition, GI determination revealed that the highest GI of the whelk was observed in spring. GI is not only an important indicator reflecting the degree of gonadal maturity, but also an index to estimate the period of the spawning season in aquatic animals [53]. Typically, spawning often occurs when the aquatic animal has the highest GI and spawning gradually ends as the GI decreases; we thus assume that the optimal spawning season for the whelk occurs around spring. Compared with other economically important shellfish, the breeding season of the whelk is similar to that of L. sieboldii [50] but different from those of M. yessoensis (March) [47], S. subcrenata (June) [48], and M. mercenaria (June) [51], indicating a species-specific seasonal variation in gonadal maturity and breeding in economically important shellfish.
The pleopod (muscle tissue) is not only the main organ for the whelk to store nutrients and initiate movement, but is also the main edible part (commercial trait) of the species. Therefore, a better understanding of the nutritional composition and content of the pleopod tissue of the whelk can not only enable us to more comprehensively evaluate its nutritional value, but can also provide certain reference materials for the future development of artificial compound feed for this species. In this study, we determined the composition and content of pleopod nutrients in July, which will provide some information for us to fully understand the overall annual (or seasonal) changes in the composition and content of pleopod nutrients in the future.
In terms of AAs, Leu was identified as the most abundant EAA in the pleopod of the whelk, followed by Lys, similar to the results of pleopod nutritional analyses from Haliotis discus hannai [54], Neptunea cumingii [55], Babylonia areolate [56], and Babylonia formosae habei [57]. Notably, the percentages of both Leu and Lys were higher than those in H. discus hannai [56], N. cumingii [55], B. areolate [56], and B. formosae habei [56], indicating a potentially better nutritional value of pleopods of the whelk compared to those of other commercial gastropod species from the point of view of the percentage of EAAs. In addition, taste is an important index reflecting the quality of seafood [57]. Typically, the composition and contents of DAAs (the AAs having delicious and palatable characteristics) are always considered major factors affecting the taste of food [58,59]. In this study, six typical DAAs were identified in the pleopods of the whelk, among which Glu accounted for the highest percentage. Compared with other economically important shellfish, the percentage of Glu in the pleopods of the whelk (above 14%) was higher than that in B. areolate (11.47%) [56], B. formosae habei (11.41%) [56], and N. cumingii (10.00%) [57]. Since Glu interacting with specific taste cells on the tongue is a major component of umami taste [60], we thus assume that the pleopods of the whelk may have a more delicious taste than B. areolate, B. formosae habei, and N. cumingii. In addition to focusing on the nutritional value of pleopods, we also focused on the nutritional requirements based on our AAS analysis data. The results showed that Phe and Met were the first and second limiting AAs in female whelks, respectively, while Met and Phe were the first and second limiting AAs in males, respectively. This observation suggests that Phe and Met should be supplemented in artificial breeding of the whelk. Moreover, we also noticed that there was species-specific limiting AA variation; specifically, the first and second limiting AAs in H. discus hannai were Lys and Trp, respectively [61]; the first and second limiting AAs in N. cumingii were Trp and (Met + Tys), respectively [56]; the first and second limiting AAs were Val and Iso, respectively, in B. areolate and B. formosae habei [56], and the first and second limiting AAs in Placopecten magellanicu were Val and (Met + Tys), respectively [62].
For fatty acids, a total of 13 kinds of fatty acids were detected in the pleopods of the whelk, fewer than those in H. discus hannai (16) [61], N. cumingii (16) [57], B. areolate (15) [56], and B. formosae habei (15) [56], indicating an apparent species-specific fatty acid component. In terms of SFAs, the percentages of C17:0 and C18:0 in the pleopods of the whelk were higher than those in N. cumingii [55], B. areolate [56], B. formosae habei [56], and H. discus hannai [61]. As for UFAs, the percentages of MUFAs in the pleopods of the whelk were higher than those in B. formosae habei (9.4%) [56], and the percentages of PUFAs in the pleopods of the whelk were higher than those in B. areolate (25.00%) [56] and B. formosae habei (31.80%) [56]. Notably, the percentages of both EPA and DHA in the pleopods of the whelk were all higher than those in N. cumingii [55], H. discus hannai [61], B. formosae habei [56], and B. areolate [56]. It has been well documented that EPA and DHA are two important PUFAs playing vital roles in inhibiting prostaglandin synthesis and platelet agglutination, reducing neutral lipids in blood, providing anti-atherosclerosis activity, and enhancing immune function [63]. From the above comparisons among the whelk and other shellfish, we conclude that the whelk pleopods could be important sources of DHA and EPA supplementation.
Monosaccharides are important molecules involved in cell metabolism and are potential biomarkers, making them one of the core concerns for the healthcare of human beings [64,65,66]. Moreover, monosaccharides can also give the food a sweet taste, making the food taste better [67,68,69]. Therefore, the type and content of monosaccharides are also one of the important indicators to evaluate the nutritional or medical value of the commercial traits of shellfish [70,71,72,73]. In this study, a total of eight kinds of monosaccharides were detected in the pleopods of the whelk, more than in Bullacta exarata (6) [74], H. discus hannai (4) [75], and N. didyma (6) [76]. This observation apparently reflected species-specific variation in polysaccharide type. However, we noted that this monosaccharide-type variation might be caused by different tissue sampling. Specifically, in this study, we collected pleopods as specimens to perform polysaccharide detection and analysis, while in B. exarata [74] and N. didyma [76], soft tissue (except the shell) was collected for polysaccharide detection and analysis, and gonad tissue was collected for polysaccharide detection and analysis of H. discus hannai [75]. Therefore, further research and exploration are needed to determine whether there are tissue-specific differences in monosaccharide types in the same shellfish species. As for the contents of monosaccharides, glucose was the most abundant monosaccharide in the pleopods of the whelk, similar to that in soft tissues of B. exarata [74], Crassostrea virginica [77], and Glossaulax didyma [78] and in muscle tissues of H. discus hannai [79]. In the gonads of H. discus hannai, glucose was identified as the second most abundant monosaccharide [75], and thus we assume that there may be species-specific and tissue-specific variation in the content of monosaccharides in shellfish species. In the future, we can collect samples from different seasons for nutrient composition analysis to explore the influence of seasons on nutrient composition. Taken together, we conclude that the pleopods of the whelk are a good food source with multiple monosaccharides and superior taste. Therefore, the whelk is a species of shellfish with high economic value and thus has the potential for large-scale artificial breeding.
5. Conclusions
In this study, the main morphological traits that affected the quality traits of the whelk in different sexes were identified. Sex-specific gonadal developmental characteristics and the gamete maturation cycle of the whelk were clarified. The composition and content of pleopod nutrients were determined and compared. All data obtained in this study will not only enrich the basic biology of the whelk but will also provide theoretical support for artificial breeding and the development of the whelk aquaculture industry.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes9020072/s1, Table S1. Descriptive statistics of the morphological traits of female and male whelks (n = 30).
Author Contributions
Y.Z. and Y.C. conceived and designed the experiments. L.Y., W.Y., S.H., T.Z. and Z.H. performed the experiments. D.Y. collected samples. Y.Z., L.Y., W.Y. and Y.C. analyzed the data. Y.Z. and L.Y. drafted the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key R & D Program of China (No. 2022YFD2400302).
Institutional Review Board Statement
The experimental protocol was designed in accordance with the recommendations of the Regulations of the Laboratory Animal—Guideline for Ethical Review of Animal Welfare (National Standards of P. R. China, GB/T 35823—2018) and reviewed and approved by the animal care and use committee of Dalian Ocean University (DLOU-2023006).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Yamazaki, T.; Sonoda, T.; Nobetsu, T.; Goshima, S. Contribution to the Knowledge of the Taxonomy of the Japanese Species of Volutharpa (Gastropoda: Buccinidae). Venus 2018, 76, 1–18. [Google Scholar] [CrossRef]
- Ikuta, K.; Nakahara, M. Uptake, Retention and Excretion 54Mn by a Perry Whelk Volutharpa ampullacea perryi. Nippon. Suisan. Gakkaishi 1986, 52, 1853–1859. [Google Scholar] [CrossRef][Green Version]
- He, S.; Sun, X.; Du, M.; Chen, H.; Tan, M.; Sun, H.; Zhu, B. Effects of Muscle Protein Denaturation and Water Distribution on the Quality of False Abalone (Volutharpa ampullacea perryi) during Wet Heating. J. Food Process. Eng. 2018, 42, e12932. [Google Scholar] [CrossRef]
- Zhu, B.; Dong, X.; Sun, L.; Xiao, G.; Chen, X. Effect of Thermal Treatment on the Texture and Microstructure of Abalone Muscle (Haliotis discus). Food Sci. Biotechnol. 2011, 20, 1467–1473. [Google Scholar] [CrossRef]
- Sarmadi, B.H.; Ismail, A. Antioxidative Peptides from Food Proteins: A Review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef]
- He, S.; Zhang, Y.; Sun, H.; Du, M.; Qiu, J.; Tang, M.; Sun, X.; Zhu, B. Antioxidative Peptides from Proteolytic Hydrolysates of False Abalone (Volutharpa ampullacea perryi): Characterization, Identifcation, and Molecular Docking. Mar. Drugs 2019, 17, 116. [Google Scholar] [CrossRef]
- Feldman, A.T.; Wolfe, D. Tissue Processing and Hematoxylin and Eosin Staining. Methods Mol. Biol. 2014, 1180, 31–43. [Google Scholar] [CrossRef]
- Tsugita, A.; Scheffler, J.J. A Rapid Method for Acid Hydrolysis of Protein with a Mixture of Trifluoroacetic Acid and Hydrochloric Acid. Eur. J. Biochem. 1982, 124, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Consultation, J.F.W.E. Protein Quality Evaluation; Food and Agriculture Organization of the United Nations: Rome, Italy, 1991; Volume 51, pp. 1–66. [Google Scholar]
- Yang, F.; Huang, X.; Zhang, C.; Zhang, M.; Huang, C.; Yang, H. Amino Acid Composition and Nutritional Value Evaluation of Chinese Chestnut (Castanea mollis sima Blume) and Its Protein Subunit. RSC Adv. 2018, 8, 2653–2659. [Google Scholar] [CrossRef]
- Salimon, J.; Omar, T.A.; Salih, N. An Accurate and Reliable Method for Identification and Quantification of Fatty Acids and Trans Fatty Acids in Food Fats Samples Using Gas Chromatography. Arab. J. Chem. 2017, 10, 1875–1882. [Google Scholar] [CrossRef]
- Shin, J.M.; Hwang, Y.O.; Tu, O.J.; Jo, H.B.; Kim, J.H.; Chae, Y.Z.; Rhu, K.H.; Park, S.K. Comparison of Different Methods to Quantify Fat Classes in Bakery Products. Food Chem. 2013, 136, 703–709. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Liu, X.; Chen, M.; Bai, B.; Yang, Y.; Bo, T.; Fan, S. The Separation, Purification, Structure Identification, and Antioxidant Activity of Elaeagnus umbellata Polysaccharides. Molecules 2023, 28, 6468. [Google Scholar] [CrossRef]
- Alexopoulos, E.C. Introduction to Multivariate Regression Analysis. Hippokratia 2010, 14 (Suppl. S1), 23–28. [Google Scholar]
- Slifker, J.; Shapiro, S.S. The Johnson System: Selection and Parameter Estimation. Technometrics 1980, 22, 239–246. [Google Scholar] [CrossRef]
- Jiang, W.; Ma, H.Y.; Ma, C.Y.; Li, S.J.; Liu, Y.X.; Qiao, Z.G.; Ma, L.B. Characteristics of Growth Traits and Their Effects on Body Weight of G1 Individuals in the Mud Crab (Scylla paramamosain). Genet. Mol. Res. 2014, 13, 6050–6059. [Google Scholar] [CrossRef]
- Gaur, A. Correlation and path coefficient analysis. 2018. [Google Scholar] [CrossRef]
- Di, B.A. Coefficient of Determination (R2). In Encyclopedia of Statistics in Quality and Reliability; Ruggeri, F., Kenett, R.S., Faltin, F.W., Eds.; Wiley: Chichester, UK, 2008. [Google Scholar] [CrossRef]
- Jin, W.; Bai, Z.; Fu, L.; Zhang, G.; Li, J. Genetic Analysis of early Growth Traits of the Triangle Shell Mussel, Hyriopsis Cumingii, as An Insight for Potential Genetic Improvement to Pearl Quality and Yield. Aquac. Int. 2012, 20, 927–933. [Google Scholar] [CrossRef]
- Lindley, D.V. Regression and Correlation Analysis. In Time Series and Statistics; Eatwell, J., Milgate, M., Newman, P., Eds.; Palgrave Macmillan: London, UK, 1990. [Google Scholar] [CrossRef]
- Krzywinski, M.; Altman, N. Multiple Linear Regression. Nat. Methods 2015, 12, 1103–1104. [Google Scholar] [CrossRef]
- Sripathi, R.; Kakani, V.G.; Wu, Y. Genotypic Variation and Trait Relationships for Morphological and Physiological Traits among New Switchgrass Populations. Euphytica 2013, 191, 437–453. [Google Scholar] [CrossRef]
- Wang, W.; Ma, C.Y.; Chen, W.; Ma, H.Y.; Zhang, H.; Meng, Y.Y.; Ni, Y.; Ma, L.B. Optimization of Selective Breeding through Analysis of Morphological Traits in Chinese Sea Bass (Lateolabrax maculatus). Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Brosse, S.; Charpin, N.; Su, G.; Toussaint, A.; Herrera-R, G.A.; Tedesco, P.A.; Villéger, S. Fishmorph: A Global Database on Morphological Traits of Freshwater Fishes. Glob. Ecol. Biogeogr. 2021, 30, 2330–2336. [Google Scholar] [CrossRef]
- Steinbach, C.; Císař, P.; Šauer, P.; Klicnarová, J.; Schmidt-Posthaus, H.; Golovko, O.; Kocour, K.H. Synthetic Progestin Etonogestrel negatively Affects Mating Behavior and Reproduction in Endler’s Guppies (Poecilia wingei). Sci. Total Environ. 2019, 663, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Rozenfeld, C.; García-Carpintero, V.; Pérez, L.; Gallego, V.; Herranz-Jusdado, J.G.; Tveiten, H.; Johnsen, H.K.; Fontaine, R.; Weltzien, F.A.; Cañizares, J.; et al. Cold Seawater Induces Early Sexual Developmental Stages in the BPG Axis of European Eel Males. BMC Genom. 2019, 20, 597. [Google Scholar] [CrossRef] [PubMed]
- Jobling, M. National Research Council (NRC): Nutrient Requirements of Fish and Shrimp. Aquacult. Int. 2012, 20, 601–602. [Google Scholar] [CrossRef]
- Shang, G.; Mao, Y.; Wang, X.; Liu, M.; Wang, Y.; Wang, G.; Li, J. Cyp17a Effected by Endocrine Disruptors and Its Function in Gonadal Development of Hyriopsis cumingii. Gen. Comp. Endocrinol. 2022, 323-324, 114028. [Google Scholar] [CrossRef]
- Prato, E.; Fanelli, G.; Parlapiano, I.; Biandolino, F. Bioactive Fatty Acids in Seafood from Ionian Sea and Relation to Dietary Recommendations. Int. J. Food Sci. Nutr. 2020, 71, 693–705. [Google Scholar] [CrossRef]
- Newkirk, G.F. Review of the Genetics and the Potential for Selective Breeding of Commercially Important Bivalves. Aquaculture 1980, 19, 209–228. [Google Scholar] [CrossRef]
- Jourdan, J.; Piro, K.; Weigand, A.; Plath, M. Small-scale Phenotypic Differentiation along Complex Stream Gradients in a Non-native Amphipod. Front. Zool. 2019, 16, 29. [Google Scholar] [CrossRef]
- Muto, N.; Kawasaki, T.; Kakioka, R.; Nagano, A.J.; Shimizu, Y.; Inose, S.; Shimizu, Y.; Takahashi, H. Genetic Architectures of Postmating Isolation and Morphology of Two highly Diverged Rockfishes (genus Sebastes). J. Hered. 2023, 114, 231–245. [Google Scholar] [CrossRef]
- Loukovitis, D.; Sarropoulou, E.; Batargias, C.; Apostolidis, A.P.; Kotoulas, G.; Tsigenopoulos, C.S.; Chatziplis, D. Quantitative Trait Loci for Body Growth and Sex Determination in the Hermaphrodite Teleost Fish Sparus aurata L. Anim. Genet. 2012, 43, 753–759. [Google Scholar] [CrossRef]
- Tanyaros, S.; Tarangkoon, W. Variability in Larval Period, Post-setting Growth and Survival of the Oyster Crassostrea Belcheri Produced by Gamete Stripping Method. Agric. Nat. Resour. 2016, 50, 295–298. [Google Scholar] [CrossRef]
- Hornick, K.M.; Plough, L.V. Tracking Genetic Diversity in a Large-scale Oyster Restoration Program: Effects of Hatchery Propagation and Initial Characterization of Diversity on Restored vs. Wild Reefs. Heredity 2019, 123, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yu, G.; Wang, J.Y.; Yan, J.X.; Yang, R.; Wu, K.C. Path Analysis of the Effects of Morphometric Attributes on the Body Weight of 7-month-old Babylonia areolate. Mar. Sci. 2017, 41, 82–88. [Google Scholar]
- Qin, Z. Studies on the Genetic Diverisity and Morphology of Babylonia lutosa. Master’s Thesis, Hunan Agricultural University, Changsha, China, 2014. [Google Scholar]
- Zhao, L.; He, Y.; Yang, F.; Nie, H.; Yan, X. Correlation and Path Analysis of Morphological and Weight Traits in Marine Gastropod Glossaulax reiniana. Chin. J. Ocean. Limnol. 2014, 32, 821–827. [Google Scholar] [CrossRef]
- Li, X.Y.; Luo, D.; Gu, D.E.; Xu, M.; Mu, X.D.; Zhang, J.E.; Hu, Y.C. The Relationship between Morphological Characters and Body Mass of Different Shell-Colored Apple Snail Pomacea canaliculata in Different Shell-Color. J. Biosaf. 2012, 21, 287–290. [Google Scholar]
- Zheng, L.; He, Y.Y.; Wang, Q.; Yu, A.; Xie, Y.J. Relationship between Morphological Traits and Body Weight of Chinese Shrimp Fenneropenaeus chinensis of Different Genders. Fish. Sci. 2023, 42, 566–574. [Google Scholar] [CrossRef]
- Zhao, C.N.; Yu, T.; Zheng, Y.; Li, B.; Wang, X.; Cai, Z.; Wang, X.; Ren, L.; Xu, S.; Wu, N.; et al. Correlation and Path Analysis of Traits of Male and Female Chlamys farreri with Different Shell Colors. J. Fish. Sci. China 2023, 30, 268–283. [Google Scholar]
- Xiao, L.Y.; Ma, G.F.; Guo, W.X.; Yan, X.W.; Yang, F.; Zhang, G.F. Correlation and Path Analysis to Quantitative Traits of Mactra chinensis in Different Sexes. Chin. Agric. Sci. Bull. 2012, 28, 115–119. [Google Scholar]
- de Assis Lago, A.; Reis-Neto, R.V.; Rezende, T.T.; da Silva Ribeiro, M.C.; de Freitas, R.T.F.; Hilsdorf, A.W.S. Quantitative Analysis of Black Blotching in a Crossbred Red Tilapia and Its Effects on Performance Traits Via a Path Analysis Methodology. J. Appl. Genet. 2019, 60, 393–400. [Google Scholar] [CrossRef]
- Han, S.R.; Song, M.Y.; Zhao, T.J.; Zou, Y.; Chang, Y.Q.; Zhan, Y.Y. Biological Research Progress on Sea Snail Volutharpa ampullacea Perryi: A Review. Chin. J. Fish. 2021, 34, 90–95. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, Z.X. Histological of Gonad and Reproductive Cycle of Neverita didyma. Trans. Oceanol. Limnol. 2009, 2, 67–72. [Google Scholar] [CrossRef]
- Ning, J.H.; Chang, Y.Q.; Song, J.; Hu, P.; Jing, C.C. Gonadal Development and the Reproductive Cycle of Modiolus modiolus. J. Fish. Sci. China 2015, 22, 469–477. [Google Scholar]
- Gao, Y.M.; Tian, B.; Yu, Y.G.; Sun, X.Q.; Ma, R.H. The Gonadal Development and Reproductive Cycle of Japanse scallop Patinopecten yessoensis in Tahe Bay in Dalian. J. Dalian Ocean. Univ. 2007, 22, 335–339. [Google Scholar]
- Yan, B.L.; Xu, X.H.; Zheng, J.S.; Xu, G.C.; Shi, D.Q.; Zhu, Z.Q. Study on the Development of Sex Gland and Reproductive Cycle of Scapharca subcrenata. Trans. Oceanol. Limnol. 2005, 92–98. [Google Scholar]
- Shao, Y.; Zhang, J.; Fang, J.; Xiao, G.; Teng, S.; Chai, X. Reproductive Cycle and early Development of Meretrix lamarkii (Veneroida: Veneridae) Under Artificial Conditions. J. Fish. Sci. China 2017, 24, 82–90. [Google Scholar] [CrossRef]
- Cao, F.; Liu, Y.; Zhang, C.; Luo, J.; Liu, Z. Study on the Development of Sex Gland and Reproductive Cycle of Lutraria sieboldii. Oceanol. Limnol. Sin. 2012, 43, 976–982. [Google Scholar]
- Lin, Z.H.; Shan, L.Z.; Chai, X.L.; Ying, X.P.; Fang, J.; Zhang, J.M.; Zhang, Y.P. The Reproductive biology of Hard Clam Mercenaria mercenaria (Linnaeus, 1758). Oceanol. Limnol. Sin. 2005, 36, 430–436. [Google Scholar]
- Liu, D.J.; Xie, K.E. Reproductive Biology of Coelomactra antiquata. Chin. J. Zool. 2003, 38, 10–15. [Google Scholar]
- Granado-Lorencio, E.C. Seasonal Changes in Condition, Nutrition, Gonad Maturation and Energy Content in Barbel, Barbus sclateri, Inhabiting a Fluctuating River. Environ. Biol. Fishes 1997, 50, 75–84. [Google Scholar]
- Shi, L.; Hao, G.X.; Chen, J.; Ma, S.K.; Weng, W.Y. Nutritional Evaluation of Japanese Abalone (Haliotis discus hannai Ino) Pleopod: Mineral Content, Amino Acid Profile and Protein Digestibility. Food Res. Int. 2020, 129, 108876. [Google Scholar] [CrossRef]
- Hao, Z.L.; Wang, Y.; Yu, Y.Y.; Zhan, Y.Y.; Tian, Y.; Wang, L.; Mao, J.X.; Chang, Y.Q. Analysis and Evaluation of Nutritive Composition in the Pleopod of Neptunea arthritica cumingii Crosse (Gastropoda: Buccinidae). J. Dalian Univ. 2016, 37, 66–70. [Google Scholar]
- Xu, Y.B.; Shen, M.H.; Wei, Y.J.; Wang, D.X.; Ke, C.H. Analysis and Evaluation of Nutritional Composition of Babylonia areolata and Babylonia formosae habei. J. Oceanogr. Tai Wan Strait 2008, 27, 26–32. [Google Scholar]
- Zhu, S.; Zhu, L.; Ke, Z.; Chen, H.; Zheng, Y.; Yang, P.; Xiang, X.; Zhou, X.; Jin, Y.; Deng, S.; et al. A Comparative Study on the Taste Quality of Mytilus coruscus Under Different Shucking Treatments. Food Chem. 2023, 412, 135480. [Google Scholar] [CrossRef] [PubMed]
- Mouritsen, O.G.; Duelund, L.; Petersen, M.A.; Hartmann, A.L.; Frøst, M.B. Umami Taste, Free Amino Acid Composition, and Volatile Compounds of Brown Seaweeds. J. Appl. Phycol. 2019, 31, 1213–1232. [Google Scholar] [CrossRef]
- Dong, Z.G.; Zhang, M.; Wei, S.F.; Ge, H.X.; Li, X.M.; Ni, Q.G.; Ling, Q.F.; Li, Y. Effect of Farming Patterns on the Nutrient Composition and Farming Environment of Loach, Paramisgurnus dabryanus. Aquaculture 2018, 497, 214–219. [Google Scholar] [CrossRef]
- Yamamoto, T.; Inui-Yamamoto, C. The Flavor-enhancing Action of Glutamate and Its Mechanism involving the Notion of Kokumi. NPJ Sci. Food. 2023, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Chen, S.J.; Li, L.H.; Yang, X.Q.; Huang, H. Nutritional Analysis and Quality Evaluation of Four Kinds of Abalone Pleopod. Food Ferment. Industr. 2018, 44, 227–231. [Google Scholar] [CrossRef]
- Wang, H. Composition of Nutritional Composition for Rock Scallop (Crassadoma gigantea) and Sea Scallop (Placopecten magellanicus) and Cultured Scallops of Three Species. Master’s Thesis, Dalian Ocean University, Dalian, China, 2016. [Google Scholar]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Peng, Y.; Pan, B.; Wang, B.; Peng, D.H.; Guo, W. A LC-MS/MS Method to Simultaneously Profile 14 Free Monosaccharides in Biofluids. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2022, 1192, 123086. [Google Scholar] [CrossRef] [PubMed]
- Febbraio, M.A.; Karin, M. “Sweet death”: Fructose as a Metabolic Toxin that Targets the Gut-liver Axis. Cell Metab. 2021, 33, 2316–2328. [Google Scholar] [CrossRef]
- Ramdas Nayak, V.K.; Satheesh, P.; Shenoy, M.T.; Kalra, S. Triglyceride Glucose (TyG) Index: A Surrogate Biomarker of Insulin Resistance. J. Pak. Med. Assoc. 2022, 72, 986–988. [Google Scholar] [CrossRef]
- Ran, T.; Li, H.Z.; Liu, Y.; Tang, S.X.; Han, X.F.; Wang, M.; He, Z.X.; Kang, J.H.; Yan, Q.X.; Tan, Z.L. Expression of Genes Related to Sweet Taste Receptors and Monosaccharides Transporters Along the Gastrointestinal Tracts at Different Development Stages in Goats. Livest. Sci. 2016, 188, 111–119. [Google Scholar] [CrossRef]
- Wise, P.M.; Nattress, L.; Flammer, L.J.; Beauchamp, G.K. Reduced Dietary Intake of Simple Sugars Alters Perceived Sweet Taste Intensity but not Perceived Pleasantness. Am. J. Clin. Nutr. 2016, 103, 50–60. [Google Scholar] [CrossRef]
- Wilk, K.; Korytek, W.; Pelczyńska, M.; Moszak, M.; Bogdański, P. The Effect of Artificial Sweeteners Use on Sweet Taste Perception and Weight Loss Efficacy: A Review. Nutrients 2022, 14, 1261. [Google Scholar] [CrossRef]
- Liu, B.; Liu, H.; Ai, C.; Zhu, Z.; Wen, C.; Song, S.; Zhu, B. Distribution of Uronic Acid-containing Polysaccharides in 5 Species of Shellfishes. Carbohydr. Polym. 2017, 164, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Ma, Y.; Li, Y.; Liu, R.; Zeng, M. Mediation of the Microbiome-gut Axis by Qyster (Crassostrea gigas) Polysaccharides: A Possible Protective Role in Alcoholic Liver Injury. Int. J. Biol. Macromol. 2021, 182, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Sudirman, S.; Mao, C.F.; Kong, Z.L. Glycoprotein from Mytilus edulis Extract Inhibits Lipid Accumulation and Improves Male Reproductive Dysfunction in High-fat Diet-induced Obese Rats. Biomed. Pharmacother. 2019, 109, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Jiang, Q.; Yang, H.; Zhou, X.; Chen, Y.; Chen, H.; Liu, S.; Chen, L. A Review on Shellfish Polysaccharides: Extraction, Characterization and Amelioration of Metabolic Syndrome. Front. Nutr. 2022, 9, 974860. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wu, H.; Xia, Z.; Wang, C.; Cai, J.; Huang, Z.; Du, L.; Sun, P.; Xie, J. Partial Characterization, Antioxidant and Antitumor Activities of Three Sulfated Polysaccharides Purified from Bullacta exarata. J. Funct. Foods 2012, 4, 784–792. [Google Scholar] [CrossRef]
- Liu, N. Anti-Inflammatory, Antioxidant and Antilipidemic Activity of Polyaccharide Conjugates Isolated from Abalone Gonad. Master’s Thesis, Dalian University of Technology, Dalian, China, 2021. [Google Scholar] [CrossRef]
- Xing, X.X.; Zhao, X.; Li, D.J.; Yu, G.L.; Yin, X.H. Extraction, Separation and Structural Characterization of Polysaccharides from Neverita didyma. J. Mar. Drugs China 2013, 32, 15–22. [Google Scholar]
- Getachew, A.T.; Lee, H.J.; Cho, Y.J.; Chae, S.J.; Chun, B.S. Optimization of Polysaccharides Extraction from Pacific oyster (Crassostrea gigas) using Subcritical Water: Structural Characterization and Biological Activities. Int. J. Biol. Macromol. 2019, 121, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zhao, L.; Li, S.; Zhao, X.; Zhang, Q.; Xiong, Q. Preliminary Characterization and Immunostimulatory Activity of Polysaccharides from Glossaulax didyma. Food Chem. Toxicol. 2013, 62, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, J.; Li, D.; Wen, C.; Liu, H.; Song, S.; Zhu, B. Comparison of Polysaccharides of Haliotis discus hannai and Volutharpa ampullacea perryi by PMP-HPLC-MS(n) Analysis upon Acid Hydrolysis. Carbohydr. Res. 2015, 415, 48–53. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).