Abstract
Fish can tolerate prolonged periods of fasting more easily than endothermic organisms. However, these fasting periods are associated with pronounced lipid and protein catabolism and body weight loss. We evaluated the use of body reserves, growth performance, and the histology of the intestines and muscles of Colossoma macropomum subjected to prolonged fasting for 45 days and refeeding for 14 days. We used 66 juvenile C. macropomum (71.78 ± 10.75 g) distributed in 10 tanks of 100 L in a recirculating aquaculture system (RAS) and kept 6 fish in a separate tank, considered the basal group. The fish were divided into two groups: fed (continuously fed for 59 days) and fasted/refed (subjected to fasting for 45 days and subsequently refed for 14 days). The tambaqui juveniles showed the mobilization of their body reserves during 45 days of fasting but with a large deficit in their growth performance. The 14-day refeeding period was sufficient for fish to restore their energy but insufficient for recovering most growth parameters.
Key Contribution:
Juvenile tambaquis mobilize body reserves throughout a prolonged fasting period of 45 days but quickly recover these stores after seven days of refeeding. A 14-day refeeding period was insufficient to promote complete compensatory growth in tambaquis after prolonged fasting.
1. Introduction
Many species of fish can mobilize their metabolic and body reserves such as glycogen, lipids, and proteins during a certain period of the year and may go days or even months without feeding due to fluctuations in the demand for food in nature or during the reproduction and spawning seasons when these animals do not feed [1]. However, important factors such as the species and the animals’ own physiological needs can influence this mobilization [2]. During refeeding, fish may exhibit hyperphagia, quickly restore their energy needs and body composition, and achieve compensatory growth, corresponding to an animal’s ability to grow rapidly after having gone through a period of total or partial food restriction [3,4].
Fasting and refeeding protocols in aquaculture have been applied in several ways, e.g., aiming to reduce costs of food and labor while still seeking to achieve compensatory growth. Thus, several studies use short and/or alternating cycles of fasting (fasting periods of less than 10 days) and refeeding [5,6,7,8,9] or longer cycles [10,11,12]. Long periods of fasting are associated with pronounced lipid and protein catabolism, and body weight loss may also occur [13].
Generally, the parameters most used in studies with fasting fish include blood and tissue metabolic variables and growth performance parameters, in which compensatory responses can be observed. In addition, histological evaluations, such as the influence of fasting on intestinal villi [14] and musculature [15], have also been used as a key method since the digestive tract is directly related to the behavioral characteristics and nutritional status of the fish [16], and the skeletal muscle tissue is the main part of the fish trunk, representing 40 to 60% of its body weight [17,18], and an important source of animal protein for human consumption [18]. Certain fasting situations can lead to substantial decreases in muscle fiber size in many fish species, such as Oreochromis niloticus [19], Ctenopharyngodon idellus [18], and Synechogobius hasta [20].
The tambaqui (Colossoma macropomum) is a native fish to the Amazon and Orinoco basins and is widely distributed in the tropical regions of South America [21]. It is the most commercially produced native species in Brazil, with a production that reached 94.5 thousand tons in 2021 [22]. It has fast growth, hardiness, and high meat quality [23] and easily adapts to farming systems, with wide acceptance for consumption in Brazil and countries such as Colombia, Peru, and Venezuela [24,25]. In addition, the tambaqui also has a great ability to adjust physiologically when subjected to short periods of fasting and refeeding [6,26]. However, little is still known about the physiological and growth responses that this species presents to prolonged periods of fasting. This study aims to evaluate the use of body reserves, growth performance, and intestinal villi and muscle fiber histologies of C. macropomum juveniles subjected to prolonged fasting, followed by refeeding.
2. Materials and Methods
2.1. Fish and Experimental Conditions
The experiment was carried out at the Aquaculture Laboratory (LAQUA) of the Veterinary School of the Federal University of Minas Gerais (UFMG), Belo Horizonte, Minas Gerais, Brazil, and followed the protocol approved by the Ethics Committee on Animal Use (CEUA, protocol 213/2020).
Sixty-six juveniles of C. macropomum (71.78 ± 10.75 g and 15.50 ± 0.61 cm) were acquired from the “Biofish Aquicultura” fish farm in Porto Velho, Rondônia, Brazil. Sixty fish were distributed in ten 100 L tanks mounted on a water recirculation system (RAS), and six fish were kept in a separate 100 L tank and considered the basal group. Soon after arriving at the laboratory, the animals underwent a 15-day acclimatization period, receiving a commercial extruded diet (Laguna Onívoros Alevinos, 2.6 mm, Socil: 36% crude protein, 7% ether extract, 5% crude fiber, 9% mineral matter, 1–1.8% calcium, and 1% phosphorus) twice a day (08:00 and 16:00).
During the acclimatization and experimental periods, the water quality parameters were measured weekly and presented the following values: temperature (28.07 ± 1.66 °C), dissolved oxygen (6.32 ± 0.69 mg/L), and oxygen saturation (79.55 ± 6.75%). These values were measured using a digital oximeter (YSI EcoSense®—DO200A, Yellow Springs, USA), pH 8.24 ± 0.43 was measured using a pH meter (KASVI®—model K39, São José dos Pinhais, Brazil), and the total ammonia (0.10 ± 0.12 mg/L) was measured using a commercial kit (Bioclin®, Belo Horizonte, Brazil). The limnological conditions of the experiment were in accordance with the requirements of the species in a growing environment [27,28].
2.2. Experimental Protocol
After acclimatization and before starting the experimental period, six juveniles corresponding to the baseline group were used for blood collection and subsequently euthanized with anesthetic overdose (285 mg/L of eugenol) for tissue removal and further analysis. The fish were divided into two experimental groups, with five replicates each, as follows:
- − Fed group: The fish were continuously fed a commercial diet (the same diet used in the acclimatization period) twice a day (08:00 and 16:00) until apparent satiation and for 59 days;
- − Fasted/Refed group: The fish were subjected to fasting for 45 days and subsequently refed with a commercial diet (the same diet used in the acclimatization period and for the fed group) twice a day (08:00 and 16:00) until apparent satiation for 14 days, totaling 59 experimental days.
The experiment was carried out using a completely randomized design (DIC), two groups (fed and fasted/refed), and five sampling periods (15, 30, 45, 52, and 59 experimental days, with 52 and 59 days corresponding to 7 and 14 days of refeeding, respectively.
2.3. Blood Parameters and Body Indices
Blood collections were performed at the beginning (baseline) and experimental days 15, 30, 45, 52, and 59. In each collection, six animals from each treatment (six fish from one tank per group were sampled) were contained in a damp cloth, and the blood was removed by puncturing the caudal vein using heparinized syringes. An aliquot of whole blood was separated to determine the hematocrit (%), using capillaries filled with 2/3 of blood, centrifuged for 10 min at 10,000 rpm (Micro Spin 1000), the hematocrit read in a microhematocrit, using an appropriate scale, and hemoglobin concentrations, measured by the cyanmethemoglobin reaction method, using a commercial kit (Bioclin®).
The remaining aliquot of blood was centrifuged for 10 min at 4000 rpm for plasma separation and determining glucose, triglyceride, and cholesterol concentrations, performed with the colorimetric method, using commercial kits (Bioclin®—Belo Horizonte, Brazil, accessed on www.bioclin.com.br) and spectrophotometer reading (Biochrom Libra S22, Cambridge, England). The total plasma protein concentrations were determined using a refractometer (RHC 200-ATC, Huake Instrument Co., Ltd., Zhejiang, China).
After blood sampling in each collection, the same fish were euthanized in an anesthetic overdose solution to remove and weigh the liver and mesenteric adipose tissue to determine the following biometric indices:
- − Hepatosomatic index (%) = (liver weight/body weight) × 100;
- − Mesenteric fat index (%) = (visceral fat weight/body weight) × 100.
2.4. Growth Performance
To evaluate the growth performance parameters, six fish from each experimental group were carefully removed from the tanks with a net and subsequently weighed (Balança Marte—AD5002), and the total length (ruler) was measured at the beginning and at days 15, 30, 45, 52, and 59 of the experiment. During sampling, the final weight (g), weight gain (g), specific growth rate (%/day), condition factor (g/cm3), and feed intake (g/fish) were determined and calculated. Weight gain, the specific growth rate, and the condition factor were calculated according to the following formulas:
- − Weight gain (g) = final weight − initial weight;
- − Specific growth rate (%/day) = 100 × [(ln final weight) − (ln initial weight)/days between samplings];
- − Condition factor (g/cm3) = 100 × [final weight/(final length)3].
2.5. Histological Analyses of the Foregut and White Muscle
For each collection, samples were also taken from the foregut and portions of the white muscle from the dorsal region of each animal for later histological evaluation. The samples were fixed in Bouin’s solution for 24 h. Then, they were washed in 70% alcohol for dehydration in an increasing alcoholic series, followed by a serial clearing of xylols, inclusion in histological paraffin, and sectioning to thicknesses of 2–3 μm. Three slides were made for each repetition. The slides were stained using the hematoxylin–eosin technique. The material was analyzed and photo-documented under a microscope (Nikon-E200 microscope, Nikon, Tokyo, Japan).
To determine the histochemical content of the foregut cells and structures, the slides were treated with periodic acid–Schiff (PAS), Alcian blue pH 2.5 (AB pH 2.5), and Alcian blue pH 0.5 (AB pH 0.5). For the histometric analysis, the length of 10 intestinal villi and the diameter of 20 muscle fibers, measured by slide, were considered. Histological and histochemical slides and measurements were performed using the Olympus CELLSens Standard software, with an Olympus SC-30 camera coupled to an Olympus—BX50 microscope.
2.6. Statistical Analysis
The experiment was conducted in a completely randomized design. Data normality and variance homoscedasticity were assessed using the Shapiro–Wilk and Levene tests, respectively, and then submitted to a two-way ANOVA followed by the Tukey test with a 5% probability for a comparison of the means to evaluate the experimental groups (fed and fasted groups) and sampling periods (0, 15, 30, 45, 52, and 59 days). All data are presented as the mean and the standard error of the mean (S.E.M.). All analyses were made using the statistical program SigmaPlot® Software, version 12.0.
3. Results
3.1. Hematological Parameters
Hemoglobin concentrations (Figure 1)showed an increase after 30 days in the fasted group compared to the fed group (p = 0.011). However, there were no statistical differences for the fasted/refed group in all collection times compared to the basal group. Significant differences for hematocrit were found on experimental days 52 and 59, with the refed group showing a lower percentage compared to the control group.
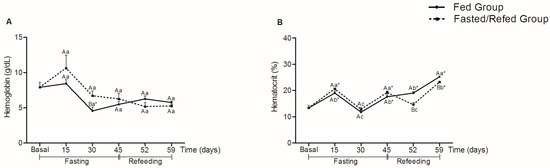
Figure 1.
Hemoglobin (g/dL) (A) and hematocrit (%) (B) in tambaqui juveniles submitted to fasting for 45 days and refed for 14 days or continuously fed during 59 days of the experiment. Capital letters indicate differences in the fasted/refed group and fed group within the same sampling period. Lowercase letters compare the fasted/refed group or fed group throughout the sampling period. Asterisks indicate differences between each group (fasted/refed and fed groups) and the basal group. Values are expressed as mean ± standard error mean (SEM). All comparisons were statistically different at p < 0.05.
3.2. Biochemical Parameters and Biometric Indices
In Figure 2, there was an increase in glucose levels after 30 days of fasting in the fasted/refed group compared to the fed group (p = 0.031). At 45 days of fasting, there was a decrease in the fasted/refed group compared to the fed (p = 0.036) and basal (p = 0.004) groups. However, seven days of refeeding was enough for the glucose levels of the fasted/refed group to be equal to the fed (p = 0.456) and basal (p = 0.523) groups.
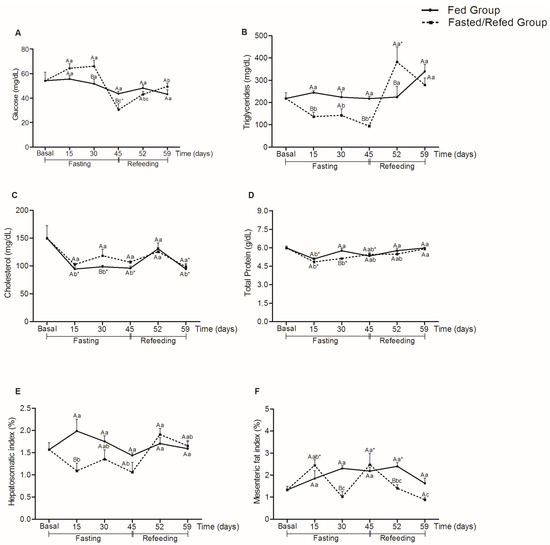
Figure 2.
Glucose (mg/dL) (A), triglycerides (mg/dL) (B), cholesterol (mg/dL) (C), total protein (g/dL) (D), hepatosomatic index (%) (E), and mesenteric fat index (%) (F) in tambaqui juveniles subjected to fasting for 45 days and refed for 14 days or continuously fed during the 59 days of experiment. Capital letters indicate differences in the fasted/refed group and fed group within the same sampling period. Lowercase letters compare the fasted/refed group or fed group throughout the sampling period. Asterisks indicate differences between each group (fasted/refed and fed groups) with basal. Values are expressed as mean ± standard error mean (SEM). All comparisons were statistically different at p < 0.05.
The triglyceride levels of the fasted/refed group decreased after 15 days of fasting (p < 0.011) and increased after 45 days of fasting (p = 0.004) and 7 days of refeeding (p = 0.001) compared to the fed group. However, these levels were equal to the fed (p = 0.149) and basal (p = 0.667) groups after 14 days of refeeding.
For cholesterol, there were no significant differences between the fasted/refed and fed groups at 15 (p = 1.000), 45 (p = 0.154), 52 (p = 0.672), and 59 days (p = 0.549). However, there was an increase in cholesterol levels of the fasted/refed group after 30 days of fasting compared to the fed group (p = 0.040), and both fasted/refed and fed groups showed a decrease in cholesterol levels compared to the basal group at 59 days (p = 0.028, fasted/refed group and p = 0.030, fed group). For total protein, there was a significant decrease in levels between fasted/refed and fed groups only at 30 days (p = 0.010). At days 15 (p < 0.001) and 30 (p = 0.004), there was a decrease in the total protein levels for the fasted/refed group compared to the basal group.
There was a decrease in the hepatosomatic index in the group subjected to 15 days of fasting compared to the fed group (p < 0.001). However, there were no significant differences for both groups and collection times compared to the basal group. For the mesenteric fat index, the decrease in relation to the fed occurred after 30 days of fasting (p < 0.001) and 52 days (7 days of refeeding) (p = 0.006). At days 15 (p = 0.026) and 45 (p = 0.003), there was an increase in the mesenteric fat index for the group subjected to fasting compared to the basal group.
3.3. Growth Performance
No mortalities were recorded during the entire experimental period. In Figure 3, the final weight and weight gain of the fasted/refed group were lower at day 45 compared to the fed group (p < 0.001, for the final weight and weight gain), remaining lower during the refeeding period of 7 (52 days) (p < 0.001, for final weight and weight gain) and 14 days (p = 0.030, for final weight and weight gain) (59 experimental days).
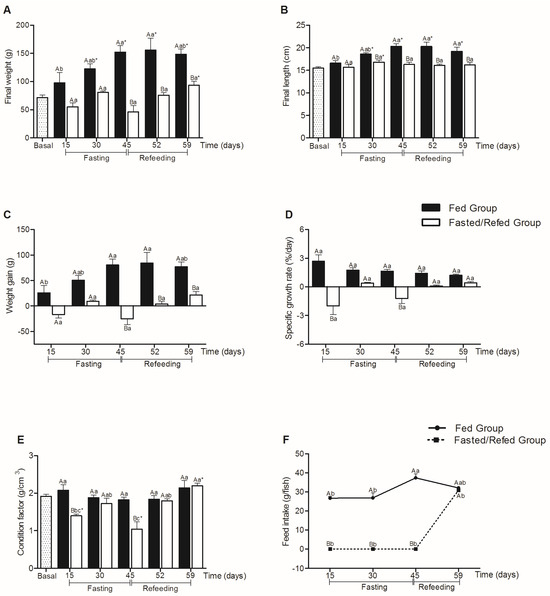
Figure 3.
Final weight (g) (A), final length (cm) (B), weight gain (g) (C), specific growth rate (%/day) (D), condition factor (g/cm3) (E), and feed intake (g/fish) (F) in tambaqui juveniles subjected to fasting for 45 days and refed for 14 days or continuously fed during 59 days of the experiment. Capital letters indicate differences in the fasted/refed group and fed group within the same sampling period. Lowercase letters compare the fasted/refed group or fed group throughout the sampling period. Asterisks marked in Figure (A,B,E) indicate differences between each group (fasted/refed and fed groups) with basal. Values are expressed as mean ± standard error mean (SEM). All comparisons were statistically different at p < 0.05.
It was observed that the fed group showed greater final weight and length throughout the experimental period when compared to the basal group, with the exception only at 15 days (p = 0.121 for final weight and p = 0.104 for final length). The fasted/refed group showed greater weight at the end of the 14-day refeeding period (59 experimental days) (p = 0.031) and greater length at 30 days (p = 0.042) when compared to the basal group.
The final length decreased after 30 days of fasting (p = 0.035) in relation to the fed group, remaining smaller until the end of the experimental period.
The specific growth rate was significantly lower after 15 (p < 0.001) and 45 (p < 0.001) days of fasting compared to the fed group. The refeeding period did not show significant differences between the groups (p > 0.05).
Compared to the fed group, the fasting group showed a significant reduction in the condition factor after 15 (p < 0.001) and 45 (p < 0.001) days, but no statistical differences were observed after the refeeding period. It is also observed that the condition factor was higher for the fasted/refed group at the end of the 14-day refeeding period (p = 0.013) compared to the basal group. Regarding feed intake, the group subjected to fasting did not statistically differ from the fed group after 14 days of refeeding (59 experimental days).
3.4. Histological Parameters of the Foregut and White Muscle
Histologically, no morphological alterations were observed in the foregut, as well as in the muscular organization of the animals submitted to fasting and subsequently refed compared to the fed group. The intestinal mucosa in both groups showed a simple prismatic epithelium with a brush border and goblet cells.
Regarding the length of intestinal villi (Figure 4), there was a decrease after 30 (p = 0.022) and 45 days (p < 0.0001) of fasting and 7 days of refeeding (52 days) (p = 0.040) compared to the fed group, but there were no significant differences present in the other collection times.
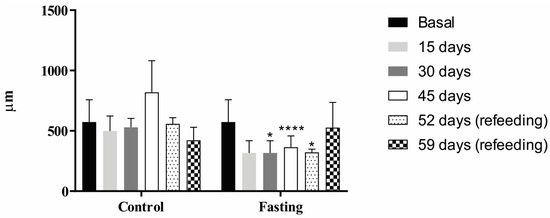
Figure 4.
Villi length (in micrometers, µm) in the foregut of tambaqui juveniles submitted to fasting for 45 days and refed for 14 days or continuously fed for 59 days of the experiment. The asterisks represent p < 0.05 (*) and p < 0.0001 (****).
Histochemical analysis of the foregut samples (Figure 5) also showed no difference in labeling between the fasted/refed and fed groups. However, positive stainings for PAS, AB pH 0.5, and AB pH 2.5 were observed in the goblet cells, indicating the presence of glycogen, neutral glycoproteins, sialomucins (PAS), sulfated glycoconjugates (AB pH 0.5), carboxylate acids, and sulfated glycoconjugates, including sialomucins (AB pH 2.5).
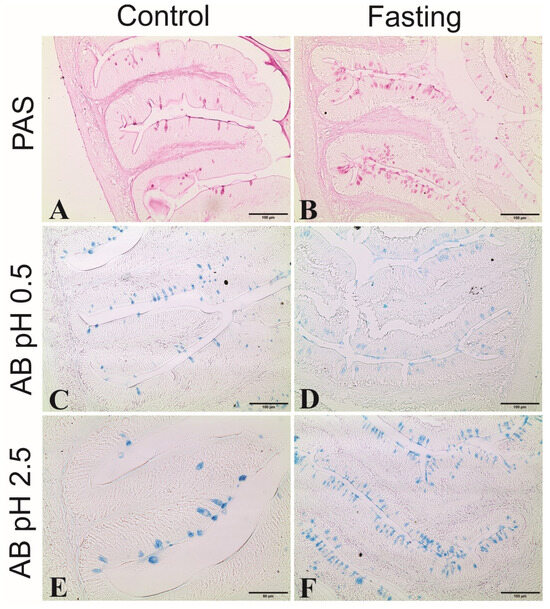
Figure 5.
Histochemical reactions in foregut samples of tambaquis. (A,C,E) represent cross-sections of villi from control fish (continuously fed); (B,D,F) represent cross-sections of fasting fish; (A,B) positive staining for periodic acid–Schiff (PAS) in goblet cells; (C,D) positive staining for Alcian Blue (AB) in pH 0.5 in goblet cells; (E,F) = positive staining for Alcian Blue (A,B) in pH 2.5 in goblet cells.
Regarding muscle fiber thickness (Figure 6), lower values were observed after 15 (p < 0.05), 30 (p < 0.001), and 45 (p < 0.005) days of fasting compared to the fed group. However, no significant differences were observed between groups during the refeeding period (after 52 and 59 experimental days) (p > 0.05).
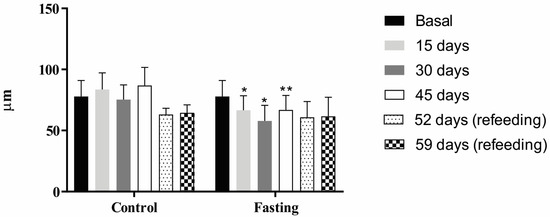
Figure 6.
Muscle fiber thickness (in micrometers, µm) of tambaqui juveniles submitted to fasting for 45 days and refed for 14 days or continuously fed for 59 days of the experiment. The asterisks represent p < 0.05 (*) and p < 0.01 (**).
4. Discussion
Hematological parameters are important indicators that can indicate the health conditions of animals [29] and are used to measure the ability to transport oxygen through the bloodstream in stressful situations, such as long fasting periods.
Hemoglobin and hematocrit did not show significant changes during the fasting period, with the only exception being hemoglobin at 30 days, in which there was a slight increase in its concentration for the fasted group compared to the fed group. However, for both parameters, the 45-day fasting period was not enough to promote major changes in these parameters since the values found in our study are like those found in other studies on the same species [6,26,30].
Glucose levels showed an increase after 30 days and subsequently a decrease between 30 and 45 days of fasting compared to the fed group. Glucose is the most important metabolite for providing energy to cells, and during periods of fasting, its demand can be readily met through the breakdown of liver glycogen (glycogenolysis) to regulate blood glucose homeostasis [31,32,33]. Although liver glycogen was not evaluated in the present study, we observed that the hepatosomatic index decreased after 15 days of fasting compared to the fed group, which may indicate that there was a breakdown of glycogen to maintain the blood glucose levels between 15 and 30 days of fasting. Liver glycogen can provide glucose for a limited time during food deprivation, which would appear to be less than two weeks without food for fish such as sunshine bass (Morone chrysops × Morone saxatilis) [34].
It is important to highlight that the hepatosomatic index is a good indicator of energy reserve use, such as glycogen and lipids in fish [35]. However, in this study, we observed that the possible breakdown of hepatic glycogen at 15 days was insufficient to maintain glucose levels throughout the fasting period, as there was a decrease in glucose levels between days 30 and 45 compared to the fed and basal groups.
During fasting periods, it is common to observe lipogenesis inhibition and lipolysis and β-oxidation stimulations of fatty acids from the fat reserves of mesenteric adipose tissue, liver, and muscle [1,8,36], such as in the maintaining of the triglyceride levels in the blood. In our study, it was observed that the decrease in the mesenteric fat index after 30 days of fasting was not enough to increase triglyceride levels. However, after seven days of refeeding, there was an increase comparable to the fed group, which may reflect the use of mesenteric fat reserves during this period, as well as the availability of lipids from the diet.
Cholesterol is a key factor during fasting, as it is a source of energy and a precursor in stress hormone synthesis, which can promote gluconeogenesis or help combat stressful conditions and thus prolong the longevity of fish [37]. We did not observe major changes in plasma cholesterol concentrations throughout the entire fasting and refeeding period, similar, for example, to studies with the species Acipenser baerii [38] and Oreochromis niloticus [19] subjected to different fasting and refeeding methods.
It is also interesting to note that a long fasting period of 45 days did not promote significant changes in the total protein levels in tambaqui juveniles, except for 30 days of fasting, compared to the fed and basal groups. Although there was a decrease in the total protein levels after 30 days of fasting, refeeding promoted a general recovery of these stores compared to the fed group.
The prolonged fasting period of 45 days resulted in a general decrease in growth performance, such as the reduction in final length from day 30, as well as the reduction in final weight, weight gain, and specific growth rate after 45 days of fasting. These responses corroborate other studies that also used long fasting periods for other fish species [39,40,41,42]. Furthermore, the 14-day refeeding period promoted greater final weight in the fasted/refed group when compared to the basal group, but this period was not enough for this group to reach the weight, length, and weight gain of the fed group.
The final length of the fed group showed little variation throughout the entire experimental period, as did the fasted/refed group, in which no change was observed even after refeeding. It is important to highlight that the tambaqui, in addition to species such as the pacu (Piaractus mesopotamicus), pirapitinga (Piaractus brachypomus), and their hybrids, are called round-shaped fish [43], as they have a rounded body shape. Therefore, measurements such as body height should also be taken into consideration to monitor the growth of these animals in captivity.
In a study on the interrelationships between morphometric variables and body performance in round fish, [44] found a high correlation (0.83) between body height and weight. Although we did not measure the body height of the tambaquis in the present study, this is a measurement that should be incorporated into future studies, which could provide better responses to the growth of these animals, such as under conditions of fasting and refeeding, for example.
It was also observed that at days 15 and 45 of fasting, there was a decrease in the condition factor compared to the fed group. The condition factor is an important indicator of the physiological state and health of fish [45,46] and has been evaluated in several studies using different feeding regimes [46,47,48,49] to explain energy reserve use by fish. Despite this decrease during the fasting period, the condition factor increased after refeeding, reaching values similar to the fed and basal groups, which corroborates other studies [50,51,52].
In the present study, the decrease in the hepatosomatic (at 15 days of fasting) and mesenteric fat (at 30 days of fasting) indices can be correlated with the decrease in the condition factor after 15 and 45 days for the fasting group compared to the fed group. Furthermore, despite observing condition factor recovery during the refeeding period (52 and 59 days), the weight and final length of the fish were not restored. Paralichthys olivaceus juveniles showed a decrease in body weight, as well as in the condition factor and hepatosomatic index after fasting [48], and the authors explained that this decrease resulted from using body energy necessary for basal metabolism and maintenance for survival even during fasting.
It was observed that, at the end of the 14-day refeeding period, the fasting group showed similar feed intake to fed fish, but this period was not enough for these animals to obtain complete compensatory growth, which corresponds to situations in which animals subjected to food deprivation can reach the same size, at the same age, as continuously fed animals [3].
Regarding the histological parameters, a reduction was observed in the length of the foregut villi after 30 and 45 days of fasting and after seven days of refeeding, similar to the results of other studies [53,54]. Intestinal villi are used as measures to assess the integrity of the mucosa, demonstrating the digestive and nutrient absorption capacity of fish [16]. In a study with juveniles of Rhamdia quelen, the authors observed that the epithelial area of the foregut of fish subjected to 30 days of fasting reduced by up to 70%, accompanied by a reduction of 15% in body weight [55]. Likewise, it is important to note in our study that the reduction in intestinal villi during fasting also promoted a reduction in the final weight and weight gain of fish after 45 days of fasting and in the final length of fish after 30 days of fasting. We also observed that even with the recovery in the villi length after 14 days of refeeding being like the fed group, these growth parameters remained low.
The morphological changes in the intestinal villi due to prolonged fasting were not accompanied by histochemical changes, as positive stainings for PAS, AB pH 0.5, and AB pH 2.5 were observed in the goblet cells of the intestines of both fasting and fed groups. These colors are related to the presence of glycogen, neutral glycoproteins and sialomucins (PAS), sulfated glycoconjugates (AB pH 0.5), carboxylate acids, and sulfated glycoconjugates, including sialomucins (AB pH 2.5), which are mucosubstances involved in processes such as lubrication, physical protection against pathogen invasion, and changes in the viscosity of the intestinal mucosa [56,57].
Our study also evaluated the impact of fasting on the thickness of muscle fibers in tambaqui juveniles, which showed a decrease in all fasting periods (15, 30, and 45 days) compared to the fed group but recovered after 14 refeeding days. However, despite this recovery in fiber thickness during refeeding, this period was insufficient for the fish to reach the same growth pattern as fed fish. In a study on O. niloticus juveniles, the authors reported muscle fiber atrophy in animals subjected to short fasting periods (5 or 10 days), characterized by changes in genes related to muscle growth (MyoD, myogenin, and myostatin). However, unlike the present study, a 10-week refeeding period promoted compensatory mass gain for this species [19].
5. Conclusions
Overall, 45 days of fasting allowed tambaqui juveniles to survive and mobilize part of their body reserves but with a large deficit in growth performance. The 14-day refeeding period was sufficient for these animals to restore their body needs but not enough to recover most growth parameters.
Author Contributions
Conceptualization, G.C.F. and R.K.L.; methodology, G.C.F., R.K.L., and L.d.A.P.; data collection: L.d.A.P., Y.P.A.S.A., M.P.S.A., P.E.C.M.d.O., G.C.F. and R.K.L.; formal analysis: L.d.A.P., Y.P.A.S.A., M.P.S.A., P.E.C.M.d.O., A.L.P., N.B., G.C.F. and R.K.L.; writing—original draft preparation: L.d.A.P., Y.P.A.S.A., M.P.S.A., P.E.C.M.d.O., G.C.F. and R.K.L.; formal analysis: L.d.A.P., Y.P.A.S.A., M.P.S.A., P.E.C.M.d.O., A.L.P., N.B., G.C.F. and R.K.L.; writing—review and editing, G.C.F., L.d.A.P. and R.K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Brazil—402952/2021-9 and CNPq-Brazil—316901/2021-0), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG-Brazil APQ-01531-21 and FAPEMIG-Brazil APQ-00645-22) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-Brazil). G.C.F. received research fellowships from CNPq (CNPq No. 316901/2021-0), and R.K.L. received research fellowships from CNPq (CNPq No. 308547/2018-7).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee on Animal Use (CEUA, protocol 213/2020) of Universidade Federal de Minas Gerais.
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the main text. Detailed numerical data will be made available to individuals upon request.
Acknowledgments
The authors thank Conselho Nacional de Desenvolvimento Científico e Tecnológico, Fundação de Amparo à Pesquisa do Estado de Minas Gerais and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior for financial support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Navarro, I.; Gutierrez, J. Fasting and starvation. Biochem. Mol. Biol. Fishes 1995, 4, 394–434. [Google Scholar]
- Dar, S.A.; Srivastava, P.P.; Varguese, T.; Rasool, S.I.; Anand, G.; Gupta, S.; Gireesh-Babu, P.; Krishna, G. Regulation of compensatory growth by molecular mechanism in Labeo rohita juveniles under different feeding regimes. Gen. Comp. Endocrinol. 2018, 261, 89–96. [Google Scholar]
- Ali, M.; Nicieza, A.; Wootton, R.J. Compensatory growth in fishes: A response to growth depression. Fish Fish. 2003, 4, 147–190. [Google Scholar] [CrossRef]
- Jobling, M. Are compensatory growth and catch-up growth two sides of the same coin? Aquac. Int. 2010, 18, 501–510. [Google Scholar] [CrossRef]
- Favero, G.C.; Santos, F.A.; Júlio, G.S.C.; Pedras, P.P.C.; Ferreira, A.L.; Silva, W.S.; Luz, R.K. Effects of short feed restriction cycles in Piaractus brachypomus juveniles. Aquaculture 2021, 536, 736465. [Google Scholar] [CrossRef]
- Assis, Y.P.A.S.; Porto, L.A.; Melo, N.F.A.C.; Palheta, G.D.A.; Luz, R.K.; Favero, G.C. Feed restriction as a feeding management strategy in Colossoma macropomum juveniles under recirculating aquaculture system (RAS). Aquaculture 2020, 529, 735689. [Google Scholar] [CrossRef]
- Blanquet, I.; Oliva-Teles, A. Effect of feed restriction on the growth performance of turbot (Scophthalmus maximus L.) juveniles under commercial rearing conditions. Aquac. Res. 2010, 41, 1255–1260. [Google Scholar]
- Hoseini, S.M.; Yousefi, M.; Rajabiesterabadi, H.; Paktinat, M. Effect of short-term (0–72 h) fasting on serum biochemical characteristics in rainbow trout Oncorhynchus mykiss. J. Appl. Ichthyol. 2014, 30, 569–573. [Google Scholar] [CrossRef]
- Takahashi, L.S.; Biller, J.D.; Criscuolo-Urbinati, E.; Urbinati, E.C. Feeding strategy with alternate fasting and refeeding: Effects on farmed pacu production. J. Anim. Physiol. Anim. Nutr. 2011, 95, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Rios, F.S.; Kalinin, A.L.; Rantin, F.T. The effects of long-term food deprivation on respiration and haematology of the neotropical fish Hoplias malabaricus. J. Fish Biol. 2002, 61, 85–95. [Google Scholar] [CrossRef]
- Bar, N. Physiological and hormonal changes during prolonged starvation in fish. Can. J. Fish. Aquatic Sci. 2014, 71, 1447–1458. [Google Scholar] [CrossRef]
- Furné, M.; Morales, A.E.; Trenzado, C.E.; García-Gallego, M.; Carmen Hidalgo, M.; Domezain, A.; Sanz Rus, A. The metabolic effects of prolonged starvation and refeeding in sturgeon and rainbow trout. J. Comp. Physiol. 2012, 182, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Farbridge, K.J.; Leatherland, J.F. Plasma growth hormone levels in fed and fasted rainbow trout (Oncorhynchus mykiss) are decreased following handling stress. Fish Physiol. Biochem. 1992, 10, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Piñeyro, J.I.G.; Alexandre, R.L.S.; Sousa, R.G.C. Aspectos histológico das vilosidades intestinais de Tambaquis (Colossoma macropomum, Cuvier, 1818) selvagens e de cultivo. Braz. J. Develop. 2020, 6, 51832–51839. [Google Scholar] [CrossRef]
- Forgati, M. Crescimento Muscular Compensatório e Metabolismo Energético de Cyprinus carpio Realimentados Após Privação de Alimento. AGRIS. 2011. Available online: https://agris.fao.org/search/en/providers/122415/records/647368c253aa8c89630d60b3 (accessed on 2 February 2024).
- Ferreira, C.M.; Antoniassi, N.A.B.; Silva, F.G.; Povh, J.A.; Potença, A.; Moraes, T.C.H.; Silva, T.K.S.T.; Abreu, J.S. Características histomorfométricas do intestino de juvenis de tambaqui após uso de probiótico na dieta e durante transporte. Pesq. Vet. Bras. 2014, 34, 1258–1260. [Google Scholar] [CrossRef][Green Version]
- Weatherley, A.H.; Gill, H.S. Dynamics of increase in muscle fibers in fishes in relation to size and growth. Cell. Mol. Life Sci. 1985, 41, 353–354. [Google Scholar] [CrossRef]
- Xu, Y.; Tan, Q.; Kong, F.; Yu, H.; Zhu, Y.; Yao, J.; Azm, F.R.A. Fish growth in response to different feeding regimes and the related molecular mechanism on the changes in skeletal muscle growth in grass carp (Ctenopharyngodon idellus). Aquaculture 2019, 512, 734295. [Google Scholar] [CrossRef]
- Nebo, C.; Gimbo, R.Y.; Kojima, J.T.; Overturf, K.; Dal-Pai-Silva, M.; Portella, M.C. Depletion of stored nutrients during fasting in Nile tilapia (Oreochromis niloticus) juveniles. J. Appl. Aquac. 2013, 30, 157–173. [Google Scholar] [CrossRef]
- Chen, X.; Xu, Y.; Cui, X.; Zhang, S.; Zhong, X.; Ke, J.; Wu, Y.; Lui, Z.; Wei, C.; Ding, Z.; et al. Starvation affects the muscular morphology, antioxidant enzyme activity, expression of lipid metabolism-related genes, and transcriptomic profile of javelin goby (Synechogobius hasta). Aquac. Nutr. 2022, 2022, 7057571. [Google Scholar] [CrossRef]
- Prado-Lima, M.; Val, A.L. Transcriptomic characterization of tambaqui (Colossoma macropomum, Cuvier, 1818) exposed to three climate change scenarios. PLoS ONE 2016, 11, e0152366. [Google Scholar] [CrossRef]
- IBGE (Brazilian Geography and Statistics Institute). Produção da Aquicultura. 2022. Available online: https://sidra.ibge.gov.br/tabela/3940#resultado (accessed on 13 October 2023).
- Araújo-Dairiki, T.B.; Chaves, F.C.M.; Dairiki, J.K. Seeds of sacha inchi (Plukenetia volubilis, Euphorbiaceae) as a feed ingredient for juvenile tambaqui, Colossoma macropomum, and matrinxã, Brycon amazonicus (Characidae). Acta Amaz. 2018, 48, 32–37. [Google Scholar] [CrossRef]
- Maia, E.L.; Rodriguez-Amaya, D.B. Fatty acid composition of the total, neutral and phospholipids of the Brazilian freshwater fish Colossoma macropomum. Dev. Food Sci. 1992, 29, 633–642. [Google Scholar]
- Woynárovich, A.; Anrooy, R.V. Field guide to the culture of tambaqui (Colossoma macropomum, Cuvier, 1816). In FAO–Fisheries and Aquaculture Technical Paper; Ed. 624; FAO: Rome, Italy, 2019. [Google Scholar]
- Roa, F.G.B.; Silva, S.S.; Hoshiba, M.A.; Silva, L.K.S.; Barros, A.F.; Abreu, J.S. Production performance of tambaqui juveniles subjected to short feed-deprivation and refeeding cycles. Bol. Inst. Pesca 2019, 45, 1–9. [Google Scholar] [CrossRef]
- Araújo-Lima, C.A.R.M.; Gomes, L.C. Criação do tambaqui. In Espécies Nativas para a Piscicultura no Brasil; Baldisserotto, B., Gomes, L., Eds.; Editora UFSM: Santa Maria, Brazil, 2005; pp. 175–202. [Google Scholar]
- Figueiredo, F.M.; Bomfim, S.C.; Lima, R.A.; Pontes, W.P.; Pontuschka, R.B.; Hurtado, F.B. Exploratory study of limnological parameters during the cycle of tambaqui fingerlings. Rev. Eletrônica Gest. Educ. Tecnol. Ambient. 2018, 22, 1–14. [Google Scholar] [CrossRef]
- Fazio, F. Fish hematology analysis as an important tool of aquaculture: A review. Aquaculture 2019, 500, 237–242. [Google Scholar] [CrossRef]
- Nakayama, C.L.; Silva, L.F.S.; Santos, F.A.C.; Boaventura, T.P.; Favero, G.C.; Palheta, G.D.A.; Melo, N.F.A.C.; Romano, L.A.; Luz, R.K. Zootechnical performance and some physiological indices of tambaqui, Colossoma macropomum juveniles during biofloc maturation and in different feed regimes. Agriculture 2022, 12, 1025. [Google Scholar] [CrossRef]
- Polakof, S.; Panserat, S.; Soengas, J.L.; Moon, T.W. Glucose metabolism in fish: A review. J. Comp. Physiol. 2012, 182, 1015–1045. [Google Scholar] [CrossRef]
- Polakof, S.; Panserat, S. How Tom Moon’s research highlighted the question of glucose tolerance in carnivorous fish. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2016, 199, 43–49. [Google Scholar] [CrossRef]
- Dai, Y.; Shen, Y.; Guo, J.; Yang, H.; Chen, F.; Zhang, W.; Wu, W.; Xu, X.; Li, J. Glycolysis and gluconeogenesis are involved of glucose metabolism adaptation during fasting and re-feeding in black carp (Mylopharyngodon piceus). Aquac. Fish. 2022, in press. [Google Scholar] [CrossRef]
- Davis, K.B.; Gaylord, T.G. Effect of fasting on body composition and responses to stress in sunshine bass. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 158, 30–36. [Google Scholar] [CrossRef]
- Alonso-Fernández, A.; Saborido-Rey, F. Relationship between energy allocation and reproductive strategy in Trisopterus luscus. J. Exp. Mar. Biol. Ecol. 2012, 416–417, 8–16. [Google Scholar] [CrossRef]
- Li, H.; Xu, W.; Jin, J.; Yang, Y.; Zhu, X.; Han, D.; Liu, H.; Xie, S. Effects of starvation on glucose and lipid metabolism in gibel carp (Carassius auratus gibelio var. CAS III). Aquaculture 2018, 496, 166–175. [Google Scholar] [CrossRef]
- Godavarthy, P.; Kumari, Y.S.; Bikshapathy, E. Starvation induced cholesterogenesis in hepatic and extra hepatic tissues of climbing Perch, Anabas testudineus (Bloch). Saudi J. Biol. Sci. 2012, 19, 489–494. [Google Scholar] [CrossRef]
- Jafari, N.; Falahatkar, B.; Sajjadi, M.M. The effect of feeding strategies and body weight on growth performance and hematological parameters of Siberian sturgeon (Acipenser baerii, Brandt 1869): Preliminary results. J. Appl. Ichthyol. 2019, 35, 289–295. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Khattab, Y.A.E.; Ahmad, M.H.; Shalaby, A.M.E. Compensatory growth, feed utilization, whole body composition, and hematological changes in starved juvenile Nile tilapia, Oreochromis niloticus (L.). J. Appl. Aquac. 2006, 18, 17–36. [Google Scholar] [CrossRef]
- Elbialy, Z.; Gamal, S.; Al-Hawary, I.; Shukry, M.; Salah, A.; Aboshosha, A.A.; Assar, D.H. Exploring the impacts of diferent fasting and refeeding regimes on Nile tilapia (Oreochromis niloticus L.): Growth performance, histopathological study, and expression levels of some muscle growth-related genes. Fish Physiol. Biochem. 2022, 48, 973–989. [Google Scholar] [CrossRef]
- Falahatkar, B. The metabolic effects of feeding and fasting in beluga Huso huso. Mar. Environ. Res. 2012, 82, 69–75. [Google Scholar] [CrossRef]
- Porto, L.A.; Assis, Y.P.A.S.; Amorim, M.P.S.; Luz, R.K.; Favero, G.C. Physiological responses to long fasting followed by refeeding in juveniles of pirapitinga, Piaractus brachypomus. Acta Amaz. 2023, 53, 187–195. [Google Scholar] [CrossRef]
- Valenti, W.C.; Barros, H.P.; Moraes-Valenti, P.M.; Bueno, G.W.; Cavalli, R.O. Aquaculture in Brazil: Past, present and future. Aquac. Rep. 2021, 19, 100611. [Google Scholar] [CrossRef]
- Neto, R.V.R.; Freitas, R.T.F.; Serafini, M.A.; Costa, A.C.; Freato, T.A.; Rosa, P.V.; Allaman, I.B. Interrelationships between morphometric variables and rounded fish body yields evaluated by path analysis. Rev. Bras. Zoot. 2012, 41, 1576–1582. [Google Scholar] [CrossRef]
- Lima-Junior, S.E.; Cardone, I.B.; Goitein, R. Determination of a method for calculation of Allometric Condition Factor of fish. Acta Sci. 2002, 24, 397–400. [Google Scholar]
- Rios, F.S.; Carvalho, C.S.; Pinheiro, G.H.D.; Donatti, L.; Fernandes, M.N.; Rantin, F.T. Utilization of endogenous reserves and effects of starvation on the health of Prochilodus lineatus (Prochilodontidae). Environ. Biol. Fish. 2011, 91, 87–94. [Google Scholar] [CrossRef]
- Eroldogan, O.T.; Kumlu, M.; Aktas, M. Optimum feeding rates for European sea bass Dicentrarchus labrax L. reared in seawater and freshwater. Aquaculture 2004, 231, 501–515. [Google Scholar] [CrossRef]
- Cho, S.H.; Lee, S.-M.; Park, B.H.; Ji, S.-C. Compensatory growth of juvenile olive flounder, Paralichthys olivaceus L., and changes in proximate composition and body condition indexes during fasting and after refeeding in summer season. J. World Aquac. Soc. 2006, 37, 168–174. [Google Scholar] [CrossRef]
- Bu, X.; Lian, X.; Zhang, Y.; Yang, C.; Cui, C.; Che, J.; Tang, B.; Su, B.; Zhou, Q.; Yang, Y. Effects of feeding rates on growth, feed utilization, and body composition of juvenile Pseudobagrus ussuriensis. Aquac. Int. 2017, 25, 1821–1831. [Google Scholar] [CrossRef]
- Bandeen, J.; Leatherland, J.F. Changes in the proximate composition of juvenile white suckers following re-feeding after a prolonged fast. J. World Aquac. Soc. 1997, 5, 327–337. [Google Scholar]
- Gaylord, I.G.; Gatlin, D.M., III. Assessment of compensatory growth in channel catfish Ictalurus punctatus R. and Associated changes in body condition indices. J. World Aquac. Soc. 2000, 31, 326–336. [Google Scholar] [CrossRef]
- Rueda, F.M.; Martinez, F.J.; Zamora, S.; Kentouri, M.; Divanach, P. Effect of fasting and refeeding on growth and body composition of red porgy, Pagrus pagrus L. Aquac. Res. 1998, 29, 447–452. [Google Scholar]
- Shen, Y.; Li, H.; Zhao, J.; Tang, S.; Zhao, Y.; Bi, Y.; Chen, X. The digestive system of mandarin fish (Siniperca chuatsi) can adapt to domestication by feeding with artificial diet. Aquaculture 2021, 538, 736546. [Google Scholar] [CrossRef]
- Zhou, R.; Wu, G.; Qu, L.; Zhong, X.; Gao, Y.; Ding, Z.; Xu, J.; Chen, X.; Chen, H. Effect of starvation on intestinal morphology, digestive enzyme activity and expression of lipid metabolism-related genes in javelin goby (Synechogobius hasta). Aquac. Res. 2022, 53, 87–97. [Google Scholar] [CrossRef]
- Henández, D.R.; Barrios, C.E.; Santinón, J.J.; Sánchez, S.; Baldisserotto, B. Effect of fasting and feeding on growth, intestinal morphology and enteroendocrine cell density in Rhamdia quelen juveniles. Aquac. Res. 2018, 49, 1512–1520. [Google Scholar] [CrossRef]
- Mello, G.C.G.; Santos, M.L.; Arantes, F.P.; Pessali, T.C.; Brito, M.F.G.; Santos, J.E. Morphological characterisation of the digestive tract of the catfish Lophiosilurus alexandri Steindachner, 1876 (Siluriformes, Pseudopimelodidae). Acta Zool. 2019, 100, 14–23. [Google Scholar] [CrossRef]
- Santos, M.L.; Arantes, F.P.; Pessali, T.C.; Santos, J.E. Morphological, histological and histochemical analysis of the digestive tract of Trachelyopterus striatulus (Siluriformes: Auchenipteridae). Zoologia 2015, 32, 296–305. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).