Abstract
This study was conducted to evaluate the effects of high salinity combined with stocking density on Litopenaeus vannamei. Three salinity gradients, namely, 28 g/L, 36 g/L, and 44 g/L, and two stocking densities, namely, 300 and 600 shrimp/m3, were used to analyze the synergistic effect of high salinity and stocking density on the growth performance, digestibility, and energy budgets of L. vannamei. The experimental testing period lasted 45 days. The research results showed that a salinity level of 36 g/L was the most suitable salinity level for shrimp growth under both high and low stocking densities. The body weight, specific growth rate, and relative weight gain of the shrimp in the 36 g/L salinity group were significantly higher than those in the other two salinity groups under both high and low stocking densities. The high-density farming group with 600 shrimp/m3 exhibited a significant inhibition of shrimp growth compared to the low-density group under the same salinity conditions. The activities of amylase, lipase, and protease in the high-density-group shrimp gradually decreased with an increase in salinity, and the three digestive enzymes had the same overall effect of changing trends. This indicates that under high-density farming conditions, the increase in salinity is not conducive to the digestive function of shrimps. At the same time, the proportion of respiratory energy to feeding energy gradually decreased in the high-density group and with the increase in salinity. However, under the same salinity conditions, the higher the stocking density, the higher the energy consumed by respiration compared to the low-density group. In addition, the expression of the growth-related gene’s small nuclear ribonucleoprotein polypeptide G (SNRPG) under high stocking density was significantly lower than that in the low-density group at a salinity of 28 g/L, and ribosomal protein L7 (RPL7) expression was also significantly lower under high stocking density than that in the low-density group at a salinity of 44 g/L. The expression levels of molting-related genes retinoid X receptor (RXR), ecdysone receptor (ECR), and ecdysone-induced protein 75 (E75) were significantly higher in the 36 g/L salinity group compared with the other two salinity groups under high-stocking-density treatment. The findings indicate that the synergistic effects of salinity and stocking density have a significant impact on the growth of L. vannamei, and excessive salinity would inhibit its growth in the process of high-density culturing.
Key Contribution:
Salinity and stocking density have significant synergistic impacts on the Pacific white shrimp. Excessive salinity and stocking density have negative effects on shrimp aquaculture.
1. Introduction
As a widely farmed aquatic economic species, the Pacific white shrimp (Litopenaeus vannamei) has been popular with consumers since it was introduced to China in 1988 [1]. It has a wide range of salinity characteristics, living in freshwater, brackish water, saltwater, and other water bodies, and it can be used for factory farming [2]. In intensive aquaculture, the determination of the maximum breeding capacity of a water body is particularly important to maximize the benefits per unit of water due to the restricted water area. High rearing density increases competition for food and living space between individuals and increases individual growth variability, and among crustaceans, it is generally accepted that culture density is negatively correlated with shrimp growth rate and weight gain rate [3]. Shrimp growth is affected by culture density in two main ways. On the one hand, the process of increasing culture density leads to lower dissolved oxygen levels and higher accumulation of toxic substances such as inorganic phosphorus and ammonia and nitrogen in the water environment, and these physicochemical factors generate stress on shrimp [4,5]. For example, it has been found that culture density significantly affects bioflocs and various environmental factors, and related studies have shown that culture density indirectly affects aquatic organisms by influencing environmental factors such as DO, pH, TAN, NO2-N, and NO3-N in the water column [6,7,8]. On the other hand, from the behavior and physiology analysis of prawns, the increase in culture density will compress the survival space of the shrimp, and the frequent displacement of shrimp leads to frequent exhibition of fighting behaviors and an increase in the rate of interspecific mutilation [9,10,11]. Due to the high activity, the proportion of energy consumed through respiration increases, while the proportion of energy required for growth decreases as a percentage of the energy produced by decomposing feed, in turn leading to a decrease in growth efficiency [3,12]. High-density culturing can have an impact on the normal growth and survival of shrimp [13,14].
Environmental factors affect the adaptability of organisms to changes in environmental conditions [15,16,17,18]. Salinity is a fundamental environmental factor in mariculture, and it has a more significant influence in intensive culture patterns. Abnormal changes in salinity can affect many physiological functions of shrimp [19,20,21,22]. Lemos found that the energy efficiency of Farfantepenaeus paulensis reached a maximum at a salinity level of 25 g/L [23]. It has been shown that increasing salinity within a certain range enhanced the growth of the Pacific white shrimp [24]. In addition, studies have shown that shrimp grow better at a salinity level of 20 g/L than at 13 g/L [25]. Salinity stress causes changes in the digestive function of an organism by affecting osmotic pressure and thus the activity of related digestive enzymes.
The activity of pepsin, tryptase, and alkaline phosphatase in Macrobrachium mipponensis is best at a salinity of 14‰ when compared with salinity levels of 7‰ and 20‰ [26]. The cited experiment also showed that the mRNA expression in the hepatopancreas of L. vannamei also decreased under low-salt conditions [20]. In addition, the activity of trypsin in shrimp decreased at higher salinity, whereas chymotrypsin activity was reversed [27]. In addition to salinity stress, studies have confirmed that the activities of trypsin, amylase, and lipase of Palaemonetes sinensis were significantly reduced under high-density culturing [3]. It has been found that crustaceans use more energy for growth under suitable salinity conditions. Furthermore, salinity can affect the physiological activity of crustaceans in conjunction with environmental factors such as temperature and density [28,29,30].
The above studies show that salinity and culture density are significant factors in L.vannamei culturing. Asia has vast salt fields and high-salinity environments that can be developed for shrimp farming. However, excessive salinity and culture density may have adverse effects on shrimp. Therefore, it is necessary to explore suitable farming salinity and density. In our study, we aimed to analyze the combined effects of the growth, digestion, and energy balance of whiteleg shrimp under high salinity and culture density stress to provide basic data for intensive culture and to promote the healthy culturing of shrimp with high productivity and efficiency.
2. Materials and Methods
2.1. Experimental Animals
The experimental shrimp were obtained from a commercial farm in Haiyang Shandong Province. The average weight was 2.10 ± 0.20 g. The shrimp were cultured in tanks with seawater (salinity 23 g/L, pH 7.5 ± 0.5) and kept at 28 ± 0.5 °C for seven days before the formal experiment. The tanks used were cylindrical PVC tanks with a radius of 90 cm and a height of 100 cm. Feeding was conducted three times a day (8:00, 14:00, and 22:00). Half the volume of the aquaculture water body was changed every day, and the dissolved oxygen level was not less than 6 mg/L. Qingdao Agriculture University’s Animal Experiment Ethics Committee approved all treatments in this study.
2.2. Experimental Design and Samples Collection
After acclimation, the shrimp were randomly divided into 6 groups, and each group contained three replicates. The stocking densities were set at 300 shrimp/m3 and 600 shrimp/m3, and each stocking density had three salinity levels: 28 g/L, 36 g/L, and 44 g/L. Among them, the one with a stocking density of 300 shrimp/m3 and a salinity of 28 g/L was set up as a control group. The salinity of these experimental groups was increased by 3–4 g/L/day to reach 36 g/L and 44 g/L, respectively. The salinity conditions required for the experiments were induced via sea salt addition. The YSI 556MPS Handheld Multiparameter Meter (YSI, Inc., Yellow Springs, OH, USA) was used for seawater parameter detection. The experiment lasted 45 days. Half the volume of farmed water was changed every day. The shrimp were fed twice a day (at 8:00 and 20:00), regularly providing an excess of feed. The excess feed left by the shrimp was collected after 1.5 h of feeding, and feces and shrimp shells were collected after 4 h of feeding. The collected samples were dried at 70 °C and stored for nitrogen and energy balance measurement. The culture experiment lasted for 45 days, and the body length and weight of 6 shrimp in each box were randomly measured weekly. Hepatopancreas, muscle, and stomach were collected at 0 d and 45 d, and a total of nine shrimp were sampled per treatment and per time point (three replicates, with three shrimp per replicate). Tissues were frozen in liquid nitrogen rapidly and stored at −80 °C.
2.3. Determination of Enzyme Activity
The hepatopancreas and muscle were made into 10% tissue homogenate in PBS. The homogenate was centrifuged at 4 °C at 12,000 rpm, and the supernatant was collected and used for the determination of enzyme activity. The activity levels of enzymes such as pepsin, amylase, and lipase were detected using commercial assay kits (Nanjing Jiancheng Institute, Nanjing, China). The experimental method was executed according to the instructions in the kits’ manuals.
2.4. Quantitative Real-Time PCR
In order to investigate the effects of high salinity and stocking density stress on genes expression related to growth, molting, and energy budget, three growth-related genes, namely, SNRPG, L18, and RPL7; three molting-related genes, i.e., RXR, ECR, and E75; and an energy-related gene, ATP-α subunit, were selected for quantitative real-time PCR (qPCR) analysis. The β-actin gene of L. vannamei was selected as an internal standard. Information on the primers used for qPCR is shown in Table 1.

Table 1.
Information on primers used in this study.
The total RNA of the samples was extracted using TRIzol Reagent (Vazyme, Nanjing, China). The cDNA was synthesized using a PrimeScript™ RT Reagent Kit (Vazyme, Nanjing, China). The qPCR reaction was carried out according to the manufacturer’s instructions included with the ChamQ™ Universal SYBR® qPCR Master Mix Kit (Vazyme, Nanjing, China). The qPCR reaction was performed using a CFX96™ Real-Time System (BIO-Rad, Hercules, CA, USA). The 2−ΔΔCT comparative CT method was used for data analysis [31].
2.5. Growth Performance
The weight gain rate (WGR), specific growth rate (SGR), survival rate (SR), and length gain rate (LGR) were calculated with the following equations:
where Wt is final mean body weight (wet weight), W0 is initial mean body weight (wet weight), t is the number of days in culture, Nt is the final number of shrimp, N0 is the initial number of shrimp, Lt is final mean body length, and L0 is initial mean body length [1,32].
WGR (%) = [Wt − W0]/W0 × 100%,
SGR (%/d) = [ln Wt – ln W0]/t (d) × 100%,
SR (%) = Nt/N0 × 100%,
LGR (%) = [(Lt − L0)/L0] × 100%,
2.6. Nitrogen Balance
The nitrogen content of feed, whole shrimp, shells, and feces (nitrogen content = protein content × 0.16) was determined using a Kjeldahl azotometer. The nitrogen balance equation is shown below [33,34]:
where CN is the intake of feed nitrogen (mg·g−1·d−1), GN is the nitrogen accumulated in whole shrimp (mg·g−1·d−1), EN is molting material consumed (mg·g−1·d−1), UN is nitrogen lost in excretion (mg·g−1·d−1), FN is nitrogen excreted in feces (mg·g−1·d−1), and AN is absorbed nitrogen (mg·g−1·d−1).
CN = GN + EN + UN + FN = AN + FN,
2.7. Energy Balance Measurement
Feed, whole shrimp, feces, and molting shells were first dried at 70 °C. Then, an IKA-C200 (IKA, Staufen, Germany) was used to measure gross energy. The energy budget was calculated according to the following formula:
where C is the energy consumed through feeding (kJ), G is the energy spent on growth (kJ), F is the energy lost in feces (kJ), U is the energy lost in excretion (kJ), E is the energy spent on exuviae (kJ), and R is the energy expenditure for respiration (kJ). The estimate of U was based on the nitrogen budget equation, A = G + R, wherein A is assimilation energy (kJ·g−1·d−1) [24,35].
C = G + E + F + R + U,
The estimation of U was based on the nitrogen budget equation:
where CN is the nitrogen consumed from feed (g), FN is the nitrogen lost in feces (g), GN is the nitrogen deposited in a shrimp’s body (g), EN is the nitrogen lost through molting (g), and 24.83 is the energy content in excreted nitrogen per gram (KJ/g) [32,36].
U = (CN − GN − FN − EN) × 24.83,
The value of respiratory energy (R) was calculated using the following equation [37]:
R = C − G − F − E − U.
2.8. Statistical Analysis
All data for the experiments are expressed as means with standard deviations. A two-way ANOVA followed by Tukey’s honest significant difference test (p < 0.05) were used to analyze significant differences between the observations of the control and test groups. The statistical analyses were conducted using SPSS 18.
3. Results
3.1. Digestive Enzyme
The activity of pepsin in the hepatopancreas significantly decreased in the high-density treatment as salinity increased (Figure 1A), while pepsin activity in the low-density treatment showed a change rule consisting of a gradual decrease followed by a gradual increase (p < 0.05). Among the stocking density experimental treatments, the activity of pepsin in the 28 g/L and 36 g/L salinity treatments was significantly higher in the high-density (600 shrimp/m3) group than in the low-density (300 shrimp/m3) group, while the opposite was true in the 44 g/L salinity treatments (p < 0.05). As shown in Table 2, salinity and culture density had a significant interactive effect on the activity of pepsin (p < 0.05).

Figure 1.
Enzyme activities related to digestion-related parameters in the hepatopancreas: (A) the activity of pepsin; (B) the activity of amylase; (C) the activity of lipase. Asterisks indicate significant differences among different culture densities at the same salinity (p < 0.05). Capital letters indicate significant differences in the different salinities under high-culture-density conditions (p < 0.05). Lowercase letters indicate significant differences in the different salinities under low-culture-density conditions (p < 0.05) (the same letters or no letter indicate that there are no significant differences, with p > 0.05). The asterisk (*) indicates significant differences between high-density and low-density treatments under the same salinity conditions (p < 0.05).

Table 2.
Summary of two-way analysis of variance: the effects of salinity (28 g/L, 36 g/L, and 44 g/L) and density (300 shrimp/m3 and 600 shrimp/m3) on growth performance, enzyme activity, and gene expression.
The amylase activity significantly decreased with increasing treatment salinity in the high-density treatments, while amylase activity in the low-density treatments significantly increased (p < 0.05) (Figure 1B). The amylase activity in the low-density treatments was significantly higher than that in the high-density group in the 44 g/L salinity group (p < 0.05). In terms of amylase activity, salinity and stocking density interacted significantly (p < 0.05) (Table 2).
Among all the salinity groups, the activity of lipase was significantly higher in the high-density group than in the control group (p < 0.05) (Figure 1C), and it was significantly lower (p < 0.05) in the high-culture-density treatment under a salinity of 44 g/L.
3.2. Growth
The effects of salinity and stocking density stress on the growth results are shown in Figure 2. The shrimps’ body weight (wet and dry) (Figure 2A,E), body length (Figure 2B), specific growth rate (Figure 2C), and relative weight gain rate (Figure 2D) showed a change rule consisting of a gradual increase followed by a gradual decrease as salinity increases. Almost all the growth indicators of the 36 g/L salinity group were higher than those of the other two salinity groups. And all the growth indicators in the high-density group were significantly lower than those in the control group. Our analysis showed that salinity and stocking density interacted significantly with respect to the growth traits of L. vannamei (Table 2). Under both stocking density conditions, the salinity of 36 was found to be the most favorable for shrimp growth.
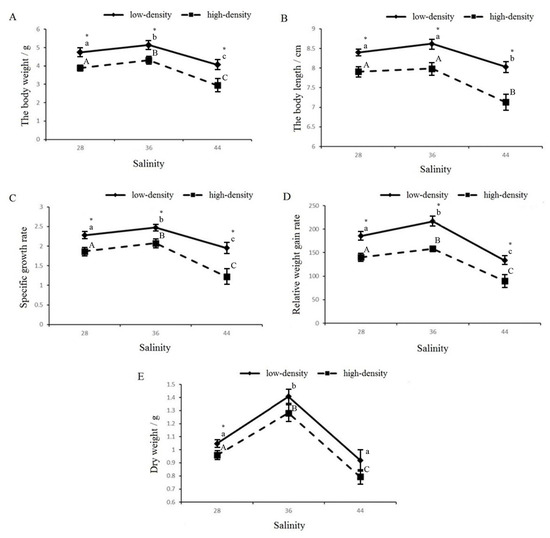
Figure 2.
The growth-related parameters under different salinities and types of stocking density stress: (A) body weight (wet weight); (B) body length; (C) specific growth rate; (D) relative weight gain; (E) body weight (dry weight). Capital letters indicate significant differences in the different salinities under high-culture-density conditions (p < 0.05). Lowercase letters indicate significant differences in the different salinities under low-culture-density conditions (p < 0.05) (the same letters or no letters indicate that there are no significant differences, with p > 0.05). The asterisk (*) indicates significant differences between the high-density and low-density treatments under the same salinity conditions (p < 0.05).
3.3. Growth-Related Genes
The expression of SNRPG under conditions of high salinity and stocking density stress is shown in Figure 3A. In the low-density treatment, the expression of SNRPG changed significantly as salinity increased, showing a change rule consisting of decreasing and then increasing (p < 0.05). In the 28 g/L and 36 g/L salinity groups, the expression of SNRPG was significantly affected by both high and low densities (p < 0.05). As shown in Table 2, salinity and stocking density interacted significantly with regard to the expression of SNRPG (p < 0.05).
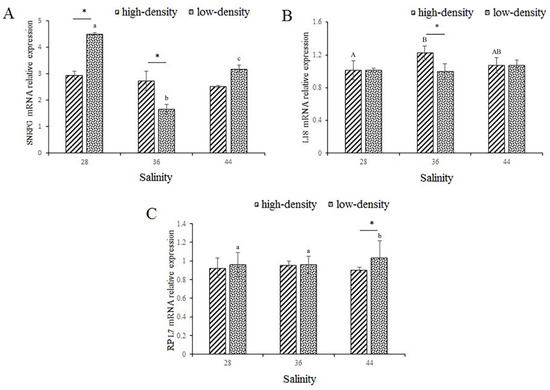
Figure 3.
The expression of SNRPG (A), L18 (B), and RPL7 (C) growth-related genes in muscle. SNRPG, small nuclear ribonucleoprotein polypeptide G; L18, ribosomal protein L18; RPL7, ribosomal protein RPL7. Capital letters indicate significant differences in the different salinities under high-culture-density conditions (p < 0.05). Lowercase letters indicate significant differences in the different salinities under low-culture-density conditions (p < 0.05) (the same letters or no letters indicate that there are no significant differences, with p > 0.05). The asterisk (*) indicates significant differences between high-density and low-density treatments under the same salinity conditions (p < 0.05).
The expression of L18 under conditions of high salinity and stocking density stress is shown in Figure 3B. In the salinity of 36 g/L group, the expression of L18 reached a peak in the high-density treatments. In the salinity of 36 g/L group, high and low stocking densities had significant effects on the expression of L18, and the expression in the high-density group was significantly higher than that in the low-density group (p < 0.05).
The expression of RPL7 under conditions of high salinity and stocking density stress is shown in Figure 3C. In the high-density treatments, there was no significant effect on the expression of RPL7 with the change in salinity. However, in the low-density group, the expression of RPL7 increased significantly as salinity increased, and significant differences in the expression of RPL7 occurred between the high- and low-density groups only under the conditions of the high-salinity treatment (p < 0.05).
3.4. Molting-Related Genes
The expression of RXR under conditions of high salinity and stocking density stress is shown in Figure 4A. In the high-density treatments, the expression of RXR showed a significant increase followed by a decrease as salinity increased (p < 0.05). However, the expression of RXR was significantly reduced under the low-density treatment (p < 0.05). Among the salinity 28 g/L groups, the expression of RXR in the low-density group was significantly higher than that in the high-density group (p < 0.05).
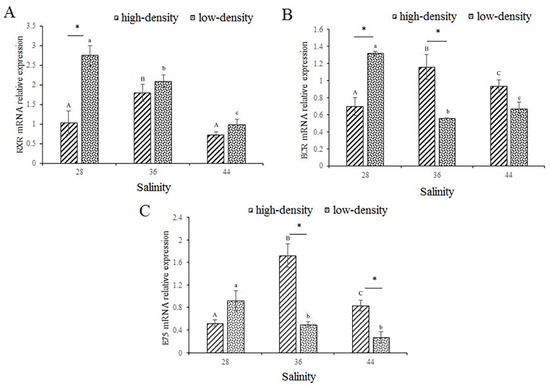
Figure 4.
The expression of RXR (A), ECR (B), and E75 (C) molting-related genes in muscle. RXR, retinoid X receptor; ECR, ecdysone receptor; E75, ecdysone-induced protein 75. Capital letters indicate significant differences in the different salinities under high-culture-density conditions (p < 0.05). Lowercase letters indicate significant differences in the different salinities under low-culture-density conditions (p < 0.05) (the same letters or no letters indicate that there are no significant differences, with p > 0.05). The asterisk (*) indicates significant differences between high-density and low-density treatments under the same salinity conditions (p < 0.05).
The expression of ECR under high salinity and stocking density stress is shown in Figure 4B. In the high-density group, the expression of ECR first increased and then decreased significantly as salinity increased, but it was significantly decreased in the low-density group (p < 0.05). The expression of ECR was significantly higher under the low-density-culture conditions than that under the high-density-culture conditions in the 28 g/L salinity group; however, the situation was reversed in the 36 g/L salinity group.
The expression of E75 under high salinity and stocking density stress is shown in Figure 4C. Under high-density stress, the expression of E75 increased with salinity and decreased significantly after reaching a peak at a salinity of 36 g/L (p < 0.05); under the low-density treatments, they decreased gradually. In the 36 g/L and 44 g/L salinity groups, the expression of E75 was significantly higher under high-density stress than that under low-density stress (p < 0.05). Table 2 shows that salinity and culture density interacted significantly regarding the expression of the three genes (p < 0.05). The three molt-related genes tested showed increased activity in the high-density experimental group at a salinity of 36.
3.5. Energy-Related Genes
The expression of the ATP-α subunit gene under high salinity and stocking density stress is shown in Figure 5. The expression of the ATP-α gene showed a gradual decrease followed by a gradual increase as salinity increased. The expression of the ATP-α subunit gene was significantly higher under low-density stress than high-density stress in the 28 g/L salinity group; however, the situation was reversed in the 44 g/L salinity group (p < 0.05). The expression level of the ATP-α subunit gene was significantly lower at a salinity of 36 compared to the other two salinities, in both high and low-density farming conditions. This suggests that at a salinity of 36, the shrimp have the lowest energy expenditure.
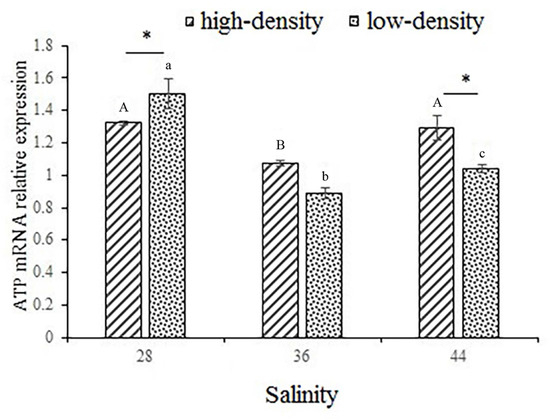
Figure 5.
The expression of ATP-α subunit energy-related gene in muscle. Capital letters indicate significant differences in the different salinities under high-culture-density conditions (p < 0.05). Lowercase letters indicate significant differences in the different salinities under low-culture-density conditions (p < 0.05) (the same letters or no letters indicate that there are no significant differences, with p > 0.05). The asterisk (*) indicates significant differences between high-density and low-density treatments under the same salinity conditions (p < 0.05).
3.6. Energy Balance
As can be seen in Table 3, energy allocation was significantly influenced by salinity and culture density. With the increase in salinity, the ratio of energy for growth and the excretion of fecal energy for feed intake increased significantly in the high-density group; however, the higher the salinity, the lower the energy used for growth. The proportion of energy consumed by respiration and excretion gradually decreased as salinity increased, while the proportion of molting energy first decreased and then increased (p < 0.05). In the low-density group, the proportion of growth energy decreased significantly as salinity increased, and the proportions of respiratory and molting energy decreased significantly (p < 0.05), while there was no significant difference in excretory energy. In a comparison of high and low densities, it was noted that the 28 g/L salinity group’s stocking densities had a significant effect on growth energy, respiratory energy, excretory energy, and molting energy (p < 0.05). In the 36 g/L salinity group, stocking densities only had significant effects on respiratory energy, while the other energy shares were not significantly different. In the 44 g/L salinity group, stocking densities had significant effects on the growth energy and respiratory energy of L. vannamei but no significant differences were found in other energy shares. This means that whether it is high-density or low-density farming, shrimp can obtain a more favorable energy supply for growth under the salinity of 36. This suggests that the salinity of 36 is more beneficial for the growth of shrimp, enabling them to utilize energy more effectively to support their growth.

Table 3.
The effect of density on the energy budget of Litopenaeus vannamei under different salinities.
4. Discussion
Shrimp, a high-quality source of protein, are experiencing an increasing demand worldwide. Determining how to enhance shrimp production is an urgent priority. Expanding farming areas and increasing stocking density are two highly important measures. The vast salt fields and high-salinity water areas in the world are a treasure trove awaiting development. High stocking density is employed to maximize production and optimize land and water resource utilization, and the Pacific white shrimp is an excellent breed known for its broad salt tolerance and high resistance to various stressors. Thus, we aimed to investigate the impact of two crucial factors, high salinity and stocking density, on L. vannamei.
4.1. Effects of High Salinity and Stocking Density Stress on Digestive Enzyme Activity
The ability of organisms to digest feed and absorb nutrients is mainly reflected by their digestive enzyme activity in vivo. Most of the previous research reports focused on the digestive capacity of L. vannamei under low-salt conditions [38]. The activities of trypsin, amylase, superoxide dismutase, and catalase in L. vannamei under a salinity of 3.0 g/L were higher than those in the salinity group of 17 g/L [3,39]. Previous research has shown that under low-salt conditions of 5 g/L, 10 g/L, and 15 g/L, the activities of amylase, lipase, and trypsin of L. vannamei decrease with increasing salinity [40]. This pattern still holds true in the high-salt testing conducted in this study. The present study obtained similar results: the activities of pepsin, amylase, and lipase in shrimp showed an overall weakening trend as salinity increased. It was also found that there was an overall effect among the three enzymes; i.e., the trend of the three enzyme activities showed consistency, and this trend was more significant in the high-density groups. However, under the high-density-culture and high-salinity conditions, the activities of the three enzymes were higher in the high-density group than those in the low-density group. This observation contradicts the trend observed in previous studies on Palaemonetes sinensis [3]. It is speculated that the possible reason for this is that high salinity breaks the balance of the original density stress, resulting in the high-density population being more inclined to seek food to maintain their own metabolism under high-salinity conditions.
4.2. Effects of High Salinity and Culture Density Stress on Growth
It is generally believed that when seawater salinity is in the optimal salinity range of shrimp or close to the isosmotic point, it is most conducive to the growth of shrimp [41,42]. Shrimp require more energy for the regulation of osmotic pressure balance in their bodies, and the energy that would have been used for growth is consumed, resulting in the inhibition of the growth, digestion, and immunity of these organisms [43,44,45]. Culture density is another influencing factor that has been reported both at home and abroad. The growth of shrimp will be inhibited when the stocking density is too high, which can lead to a lower weight gain rate and a reduced survival rate [46]. In this study, the low-density-cultures of L. vannamei were superior to the high-density cultures in terms of body weight and specific growth rate, a finding similar to the results of previous studies. In both farming densities, the growth performance of 36 g/L salinity shrimps was superior to the other two salinities. Previous studies have shown that the growth of L. vannamei is best at salinities around 33–40 g/L [46,47]. The results of this study are similar to those of the previous study. Therefore, the optimal farming salinity for L. vannamei would be within a range centered around 36 g/L.
Ribosomal proteins are involved in various processes and play important roles in the growth of organisms [48]. SNRPG (Small nuclear ribonucleoprotein polypeptide G) is mainly involved in the processing of mRNA during the translation phase of proteins and promotes protein synthesis [49]. RPL7 (ribosomal protein L7) is the large subunit protein of ribosomal organelles, and RPL7 is mainly involved in gonadal development in mammals and fish [50]. L18 (Ribosomal protein L18) is a component of the large ribosomal subunit, and its expression is relatively stable in different growth stages or different tissues of Anastrepha obliqua and Portunus trituberculatus [51,52]. In this study, three genes were selected for an analysis of the growth traits of L. vannamei under high salinity and stocking density stress. It was found that the expression of SNRPG, RPL7, and L18 was generally higher in the low-density treatments than in the high-density treatments. The potential reason could be that high stocking density induces chronic stress, triggering physiological and oxidative stress, resulting in increased energy consumption and growth inhibition. Similar findings have also been observed in studies on fish [53].
Molting is crucial for the growth of shrimp. Investigating the physiological processes involved in molting can provide insights into the growth statuses of shrimp. The synthesis of ecdysteroids during molting is subject to the combined action of several hormones, occurring through a series of processes such as the transmission of transcription factors such as RXR (retinoid X receptor), ECR (ecdysone receptor), and E75 to finally complete molting [54]. In our study, it was observed that in the low-density group, the expression levels of three molting-related genes decreased with increasing salinity. This suggested that the increase in salinity inhibited the expression of molting-related genes, which was detrimental to shrimp molting and growth. Similar patterns have also been observed in studies on juvenile shrimp, indicating that changes in salinity have a significant impact on their molting and growth [24]. However, a new pattern emerged in the high-density treatment, revealing that the expression levels of all three molting-related genes in the salinity 36 g/L experimental group were higher than the other two salinity groups. This suggested that selecting an appropriate salinity level was beneficial for the molting and growth of shrimp under high-density farming conditions. In the exploration of inland ground saline water aquaculture, it has been found that selecting an appropriate salinity level under specific stocking density conditions is beneficial for shrimp growth [55].
4.3. Effects of High Salinity and Culture Density Stress on Energy Balance
Crustaceans have a good ability to regulate osmotic pressure [56]. This regulation exhibits duality. When the salinity is higher than that of crustaceans in vivo, excess salt needs to be secreted out of the body in order to keep the water in the body from being lost; on the contrary, the excess water needs to be excreted from the body. The processes of salt secretion and water loss require a great deal of energy consumption. Studies have shown that the respiratory metabolism of shrimp and crabs consumes the least amount of energy at the isotonic point [42]. The effect of density on the energy of aquatic organisms under the conditions of artificial culturing is mainly due to the increased movement and struggling of the shrimp in an attempt to obtain food and coordinate interspecific struggles, resulting in an increased respiratory metabolic capacity and a reduction in the energy used for growth [57]. In this study, the results showed that the high-density-culture group used significantly more energy for respiration than the low-density-culture group, similar to the conclusions obtained in the above-mentioned study. This meant that excessively high stocking density resulted in unnecessary energy consumption, which was detrimental to the growth of shrimp. This conclusion has also been validated in studies on tiger shrimp Penaeus monodon [58]. Additionally, our research found that the order of energy types in the experiment as a percentage of the feeding energy was as follows: respiration consumption > growth accumulation > energy contained in feces > excretion consumption energy > molting consumption energy. This study provides valuable insights and references for future research on the impact of stocking density and salinity stress on shrimp energy balance, as well as the formulation of shrimp farming strategies.
5. Conclusions
Our research confirmed that the synergistic effect of salinity and stocking density on L. vannamei was significant. The synergistic effect of the two factors resulted in different changes in the growth performance, digestive capacity, and energy metabolism of shrimp. We recommend selecting a farming salinity within the range of 36 and its vicinity and avoiding excessively high breeding density (greater than 600) because excessively high salinity (44 g/L) could weaken the digestive capacity and affect the energy balance of shrimp, and an excessively high stocking density (600 shrimp/m3) could lead to a reduced growth rate of shrimp. The scientific and reasonable control of salinity and stocking density can effectively improve the efficiency of shrimp aquaculture. This study provides valuable experimental data for the development of the intensive culturing of L. vannamei under high-salinity stress.
Author Contributions
F.L.: conceptualization, formal analysis, writing—original draft, project administration, funding acquisition, writing—review and editing. J.S.: methodology, formal analysis, and visualization. J.L.: formal analysis and writing—original draft. L.S.: resources and data curation. C.L. and X.W.: investigation. L.Z. and P.H.: resources. Y.C. and R.W.: investigation and resources. Z.W.: funding acquisition, investigation, and methodology. Y.L.: conceptualization, supervision, project administration, funding acquisition and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the National Key Research and Development Program of China (2022YFD2400203); the National Science Foundation of China (31902407, 32102762); the earmarked fund for the Modern Agro-industry Technology Research System in Shandong Province (SDAIT-13-03); the Key R&D Program of Shandong Province (2021LZGC027); the High-level Talents Research Fund of Qingdao Agricultural University (665/1122015); and the “First Class Fishery Discipline” program in Shandong Province.
Institutional Review Board Statement
All treatments in this study were undertaken strictly in accordance with the guidelines of the Animal Experiment Ethics Committee of Qingdao Agriculture University, which also approved the protocol in May 2020 (Approval Code: 2020-026).
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to express our gratitude to Changyi Haijingzhou Biotechnology Co., Ltd. (Weifang, China) for providing the experimental animals for this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cheng, K.M.; Hu, C.Q.; Liu, Y.N.; Zheng, S.X.; Qi, X.J. Effects of dietary calcium, phosphorus and calcium/phosphorus ratio on the growth and tissue mineralization of Litopenaeus vannamei reared in low-salinity water. Aquaculture 2006, 251, 472–483. [Google Scholar] [CrossRef]
- Laramore, S.; Laramore, C.R.; Scarpa, J. Effect of Low Salinity on Growth and Survival of Postlarvae and Juvenile Litopenaeus vannamei. J. World Aquac. Soc. 2001, 32, 385–392. [Google Scholar] [CrossRef]
- Dong, J.; Zhao, Y.Y.; Yu, Y.H.; Sun, N.; Li, Y.D.; Wei, H.; Yang, Z.Q.; Li, X.D.; Li, L. Effect of stocking density on growth performance, digestive enzyme activities, and nonspecific immune parameters of Palaemonetes sinensis. Fish Shellfish Immunol. 2017, 73, 37–41. [Google Scholar] [CrossRef]
- Fleckenstein, L.J.; Kring, N.A.; Tierney, T.W.; Fisk, J.C.; Lawson, B.C.; Ray, A.J. The effects of artificial substrate and stocking density on Pacific white shrimp (Litopenaeus vannamei) performance and water quality dynamics in high tunnel-based biofloc systems. Aquac. Eng. 2020, 90, 102093. [Google Scholar] [CrossRef]
- Ma, H.; Lv, M.; Lin, Y.; Chen, X.; Wang, D.; Du, X.; Li, J. Prawn (Macrobrachium rosenbergii)–plant (Hydrilla verticillata) co-culture system improves water quality, prawn production and economic benefit through stocking density and feeding regime manage. Aquac. Res. 2020, 51, 2169–2178. [Google Scholar] [CrossRef]
- Edwards, R.R.C. Field experiments on growth and mortality of Penaeus vannamei in a Mexican coastal lagoon complex. Estuar. Coast. Mar. Sci. 1977, 5, 107–121. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Wang, Q. The effects of dissolved oxygen concentration and stocking density on growth and non-specific immunity factors in Chinese shrimp, Fenneropenaeus chinensis. Aquaculture 2006, 256, 608–616. [Google Scholar] [CrossRef]
- Williams, A.S.; Davis, D.A.; Arnold, C.R. Density-Dependent Growth and Survival of Penaeus setiferus and Penaeus vannamei in a Semi-Closed Recirculating System. J. World Aquac. Soc. 1996, 27, 107–112. [Google Scholar] [CrossRef]
- Arnold, S.J.; Sellars, M.J.; Crocos, P.J.; Coman, G. Response of juvenile brown tiger shrimp (Penaeus esculentus) to intensive culture conditions in a flow through tank system with three-dimensional artificial substrate. Aquaculture 2005, 246, 231–238. [Google Scholar] [CrossRef]
- da Costa, F.P.; Gomes, B.S.F.d.F.; Pereira, S.D.d.N.A.; de Fátima Arruda, M. Influence of stocking density on the behaviour of juvenile Litopenaeus vannamei (Boone, 1931). Aquac. Res. 2016, 47, 912–924. [Google Scholar] [CrossRef]
- Zhang, B.; Wenhui, L.; Wang, Y.; Xu, R. Effects of artificial substrates on growth, spatial distribution and non-specific immunity factors of Litopenaeus vannamei in the intensive culture condition. Turk. J. Fish. Aquat. Sci. 2010, 10, 491–497. [Google Scholar] [CrossRef]
- Chunhou, L.I.; Qin, H.; Jia, X.; Tian, L. The effect of density on energy conversion efficiency of juvenile shrimp Litopenaeus vannamei. South China Fish. Sci. 2006, 2, 30–33. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chen, J.-C.; Chen, Y.-Y.; Yeh, S.-T.; Chen, L.-L.; Huang, C.-L.; Hsieh, J.-F.; Li, C.-C. Crowding of white shrimp Litopenaeus vananmei depresses their immunity to and resistance against Vibrio alginolyticus and white spot syndrome virus. Fish Shellfish Immunol. 2015, 45, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Mena-Herrera, A.; Gutierrez-Corona, C.; Linan-Cabello, M.; Sumano-Lopez, H. Effects of stocking densities on growth of the Pacific white shrimp (Litopenaeus vannamei) in earthen ponds. Isr. J. Aquac. Bamidgeh 2006, 58, 205–213. [Google Scholar] [CrossRef]
- Abdullahi, B.A. The effect of temperature on reproduction in three species of cyclopoid copepods. Hydrobiologia 1990, 196, 101–109. [Google Scholar] [CrossRef]
- Devreker, D.; Souissi, S.; Winkler, G.; Forget-Leray, J.; Leboulenger, F. Effects of salinity, temperature and individual variability on the reproduction of Eurytemora affinis (Copepoda; Calanoida) from the Seine estuary: A laboratory study. J. Exp. Mar. Biol. Ecol. 2009, 368, 113–123. [Google Scholar] [CrossRef]
- Marx, M.T.S.; Souza, C.d.F.; Almeida, A.P.G.; Descovi, S.N.; Bianchini, A.E.; Martos-Sitcha, J.A.; Martínez-Rodríguez, G.; Antoniazzi, A.Q.; Baldisserotto, B. Expression of Ion Transporters and Na+/K+-ATPase and H+-ATPase Activities in the Gills and Kidney of Silver Catfish (Rhamdia quelen) Exposed to Different pHs. Fishes 2022, 7, 261. [Google Scholar] [CrossRef]
- Ly, K.V.; Murungu, D.K.; Nguyen, D.P.; Nguyen, N.A.T. Effects of Different Densities of Sea Grape Caulerpa lentillifera on Water Quality, Growth and Survival of the Whiteleg Shrimp Litopenaeus vannamei in Polyculture System. Fishes 2021, 6, 19. [Google Scholar] [CrossRef]
- Danilo, C.; Marc, A.C.; Peter, T.; Elena, G.; Peter, T. Salinity modulates the energy balance and reproductive success of co-occurring copepods Acartia tonsa and A. clausi in different ways. Mar. Ecol. Prog. 2006, 312, 177–188. [Google Scholar] [CrossRef]
- Gao, W.; Tian, L.; Huang, T.; Yao, M.; Hu, W.; Xu, Q. Effect of salinity on the growth performance, osmolarity and metabolism-related gene expression in white shrimp Litopenaeus vannamei. Aquac. Rep. 2016, 4, 125–129. [Google Scholar] [CrossRef]
- Ydj, A.; Rs, B.; Mi, C.; Ja, B.; Dsb, A.; Pcs, A. Effect of low salinity on the growth and survival of juvenile pacific white shrimp, Penaeus vannamei: A revival. Aquaculture 2019, 515, 734561. [Google Scholar] [CrossRef]
- Ruan, W.; Dong, Y.; Lin, Z.; He, L. Molecular Characterization of Aquaporins Genes from the Razor Clam Sinonovacula constricta and Their Potential Role in Salinity Tolerance. Fishes 2022, 7, 69. [Google Scholar] [CrossRef]
- Lemos, D.; Phan, V.N.; Alvarez, G. Growth, oxygen consumption, ammonia-N excretion, biochemical composition and energy content of Farfantepenaeus paulensis Pérez-Farfante (Crustacea, Decapoda, Penaeidae) early postlarvae in different salinities. J. Exp. Mar. Biol. Ecol. 2001, 261, 55–74. [Google Scholar] [CrossRef]
- Feng, C.; Tian, X.; Dong, S.; Su, Y.; Ma, S. Effects of frequency and amplitude of salinity fluctuation on the growth and energy budget of juvenile Litopenaeus vannamei (Boone). Aquac. Res. 2008, 39, 1639–1646. [Google Scholar] [CrossRef]
- Zhang, S.; Dong, S.L. The effects of food and salinity on energy budget of juvenile shrimp of Penaeus chinensis. J. Dalian Fish. Univ. 2002, 17, 227–233. [Google Scholar] [CrossRef]
- Wang, W.; Sun, R.; Wang, A.; Lei, B.; Wang, P. Effect of different environmental factors on the activities of digestive enzymes and alkaline phosphatase of Macrobrochium nipponense. Chin. J. Appl. Ecol. 2002, 13, 1153–1156. [Google Scholar] [CrossRef]
- Tsai, I.H.; Lu, P.J.; Chuang, J.L. The midgut chymotrypsins of shrimps (Penaeus monodon, Penaeus japonicus and Penaeus penicillatus). Biochim. Biophys. Acta 1991, 1080, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Araneda, M.; Gasca-Leyva, E.; Vela, M.A.; Dominguez-May, R. Effects of temperature and stocking density on intensive culture of Pacific white shrimp in freshwater. J. Therm. Biol. 2020, 94, 102756. [Google Scholar] [CrossRef]
- Tsai, S.J.; Chen, J.C. Acute toxicity of nitrate on Penaeus monodon juveniles at different salinity levels. Aquaculture 2002, 213, 163–170. [Google Scholar] [CrossRef]
- Wiesepape, L.M. Effects of temperature and salinity on thermal death in postlarval brown shrimp, Penaeus aztecus. Physiol. Zool. 1972, 45, 22–33. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Hou, C.; Wang, F.; Dong, S.; Zhu, Y. The effects of different Ca2+ concentration fluctuation on the moulting, growth and energy budget of juvenile Litopenaeus vannamei (Boone). Aquac. Res. 2011, 42, 1453–1459. [Google Scholar] [CrossRef]
- Pei, S.; Dong, S.; Wang, F.; Tian, X.; Gao, Q. Effects of density on variation in individual growth and differentiation in endocrine response of Japanese sea cucumber (Apostichopus japonicus Selenka). Aquaculture 2012, 356–357, 398–403. [Google Scholar] [CrossRef]
- Yuan, X.; Yang, H.; Wang, L.; Zhou, Y.; Zhang, T.; Liu, Y. Effects of aestivation on the energy budget of sea cucumber Apostichopus japonicus (Selenka) (Echinodermata: Holothuroidea). Acta Ecol. Sin. 2007, 27, 3155–3161. [Google Scholar] [CrossRef]
- Zhu, C.; Dong, S.; Wang, F.; Huang, G. Effects of Na/K ratio in seawater on growth and energy budget of juvenile Litopenaeus vannamei. Aquaculture 2004, 234, 485–496. [Google Scholar] [CrossRef]
- Fang, W.; Dong, S.; Huang, G.; Wu, L.; Shen, M. The effect of light color on the growth of Chinese shrimp Fenneropenaeus chinensis. Aquaculture 2003, 228, 351–360. [Google Scholar] [CrossRef]
- Zhang, E.; Dong, S.; Wang, F.; Tian, X.; Gao, Q. Effects of l-tryptophan on the performance, energy partitioning and endocrine response of Japanese sea cucumber (Apostichopus japonicus Selenka) exposed to crowding stress. Aquac. Res. 2018, 49, 471–479. [Google Scholar] [CrossRef]
- Gao, W.; Tan, B.; Mai, K.; Chi, S.; Liu, H.; Dong, X.; Yang, Q. Profiling of differentially expressed genes in hepatopancreas of white shrimp (Litopenaeus vannamei) exposed to long-term low salinity stress. Aquaculture 2012, 364–365, 186–191. [Google Scholar] [CrossRef]
- Li, E.; Chen, L.; Zeng, C.; Yu, N.; Xiong, Z.; Chen, X.; Qin, J.G. Comparison of digestive and antioxidant enzymes activities, haemolymph oxyhemocyanin contents and hepatopancreas histology of white shrimp, Litopenaeus vannamei, at various salinities. Aquaculture 2008, 274, 80–86. [Google Scholar] [CrossRef]
- Long, L.; Liu, H.; Lu, S. Effects of Low Salinity on Growth, Digestive Enzyme Activity, Antioxidant and Immune Status, and the Microbial Community of Litopenaeus vannamei in Biofloc Technology Aquaculture Systems. J. Mar. Sci. Eng. 2023, 11, 2076. [Google Scholar] [CrossRef]
- Erchao, L.; Leticia, A.; Liqiao, C.; Qin, J.G.; Alain, V.W. Characterization and Tissue-Specific Expression of the Two Glutamate Dehydrogenase cDNAs in Pacific White Shrimp, Litopenaeus Vannamei. J. Crustac. Biol. 2009, 29, 379–386. [Google Scholar] [CrossRef][Green Version]
- Pillai, B.R.; Diwan, A.D. Effects of acute salinity stress on oxygen consumption and ammonia excretion rates of the marine shrimp Metapenaeus monoceros. J. Crustac. Biol. 2002, 22, 45–52. [Google Scholar] [CrossRef]
- Huong, D.T.; Yang, W.J.; Okuno, A.; Wilder, M.N. Changes in free amino acids in the hemolymph of giant freshwater prawn Macrobrachium rosenbergii exposed to varying salinities: Relationship to osmoregulatory ability. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 128, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.J.; Neill, W.H.; Lawrence, A.L.; Gatlin, D.M. Effect of salinity and body weight on ecophysiological performance of the Pacific white shrimp (Litopenaeus vannamei). J. Exp. Mar. Biol. Ecol. 2009, 380, 119–124. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, J.-C. Effects of pH, temperature and salinity on immune parameters of the freshwater prawn Macrobrachium rosenbergii. Fish Shellfish Immunol. 2000, 10, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Sookying, D.; Silva, F.S.D.; Davis, D.A.; Hanson, T.R. Effects of stocking density on the performance of Pacific white shrimp Litopenaeus vannamei cultured under pond and outdoor tank conditions using a high soybean meal diet. Aquaculture 2011, 319, 232–239. [Google Scholar] [CrossRef]
- Ponce-Palafox, J.; Martinez-Palacios, C.A.; Ross, L.G. The effects of salinity and temperature on the growth and survival rates of juvenile white shrimp, Penaeus vannamei, Boone, 1931. Aquaculture 1997, 157, 107–115. [Google Scholar] [CrossRef]
- Wada, Y.; Ohsawa, S.; Igaki, T. Yorkie ensures robust tissue growth in Drosophila ribosomal protein mutants. Development 2021, 148, 198705. [Google Scholar] [CrossRef]
- Mabonga, L.; Kappo, A.P. The oncogenic potential of small nuclear ribonucleoprotein polypeptide G: A comprehensive and perspective view. Am. J. Transl. Res. 2019, 11, 6702–6716. Available online: https://pubmed.ncbi.nlm.nih.gov/31814883 (accessed on 2 January 2024).
- Ortiz-Rivas, B.; Jaubert-Possamai, S.; Tanguy, S.; Gauthier, J.P.; Tagu, D.; Claude, R. Evolutionary study of duplications of the miRNA machinery in aphids associated with striking rate acceleration and changes in expression profiles. BMC Evol. Biol. 2012, 12, 216. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Liu, Y. Gene expression profiles of the swimming crab Portunus trituberculatus exposed to salinity stress. Mar. Biol. 2011, 158, 2161–2172. [Google Scholar] [CrossRef]
- Nakamura, A.M.; Chahad-Ehlers, S.; Lima, A.L.A.; Taniguti, C.H.; Sobrinho, I.; Torres, F.R.; de Brito, R.A. Reference genes for accessing differential expression among developmental stages and analysis of differential expression of OBP genes in Anastrepha obliqua. Sci. Rep. 2016, 6, 17480. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Wang, L.; Hou, Y.; Feng, W.; Li, B.; Zhu, J. Effects of Stocking Density on the Growth Performance, Physiological Parameters, Redox Status and Lipid Metabolism of Micropterus salmoides in Integrated Rice-Fish Farming Systems. Antioxidants 2022, 11, 1215. [Google Scholar] [CrossRef] [PubMed]
- King-Jones, K.; Thummel, C.S. Nuclear receptors—A perspective from Drosophila. Nat. Rev. Genet. 2005, 6, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Chadha, N.K.; Prakash, S.; Pavan-Kumar, A.; Harikrishna, V.; Gireesh-Babu, P.; Krishna, G. Salinity, stocking density, and their interactive effects on growth performance and physiological parameters of white-leg shrimp, Penaeus vannamei (Boone, 1931), reared in inland ground saline water. Aquac. Int. 2023. [Google Scholar] [CrossRef]
- Panikkar, N.K. Osmotic behaviour of shrimps and prawns in relation to their biology and culture. Food Agric. Organ. United Nations 1968, 2, 527–538. [Google Scholar]
- Armstrong, J.D.; Griffiths, S.W. Density-dependent refuge use among over-wintering wild Atlantic salmon juveniles. J. Fish Biol. 2001, 58, 1524–1530. [Google Scholar] [CrossRef]
- Arnold, S.J.; Sellars, M.J.; Crocos, P.J.; Coman, G.J. Intensive production of juvenile tiger shrimp Penaeus monodon: An evaluation of stocking density and artificial substrates. Aquaculture 2006, 261, 890–896. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).