Abstract
Discus fish, Symphysodon spp., have a unique parental care strategy where the fry feed on their parents’ skin mucus after hatching. Here, lipidomics was employed to compare the skin mucus lipid profiles of male or female discus fish during parental and non-parental care. By multivariate statistical analysis, clear separations were found between parental and non-parental female and between parental and non-parental male discus. In the comparison between female discus in the parental and non-parental stages, a total of 107 differentially expressed lipids (DELs) were observed, of which 23 showed increased levels during parental care. For male discus, a total of 108 DELs were found, of which 46 displayed increased levels during parental care. The main DELs were phosphatidyl ethanolamine and phosphatidylinositol, mainly involved in arachidonic acid and sphingolipid metabolism pathways. Further, by comparing parental male and female discus, we found 47 DELs involved in the glycerophospholipid metabolism pathway. Diglyceride showed a higher concentration in the skin mucus of parental females, while phospholipids showed a higher level in that of parental males. Our results revealed changes in the skin mucus lipid profiles of discus fish during parental care, as well as sex-dependent differences between parental fish.
Key Contribution:
This work reveals the lipidomic changes that occur between the parental care and the non-parental care periods in discus fish. These results are helpful to gain further insights in the functional and regulatory aspects of skin mucus in discus during parental care.
1. Introduction
The discus fish Symphysodon spp., belonging to the cichlids, are native to the Amazon basin in South America. Interestingly, this species presents a unique parental care behavior, specifically, free-swimming fry bite the skin mucus of their parents as a post-hatching nutritional source [1], and this behavior was proved to be essential for the development of discus fry [2]. To date, many studies have investigated the mucus composition of parental discus fish due to the unique parental care behavior of this species, mainly employing the diverse omics. Chong et al. [3] found that the protein components in the mucus of the discus fish changed during the parental period, and nucleoside diphosphate kinase, heat shock proteins, C-type lectin, etc., were up-regulated or uniquely expressed in the parental mucus. Wen et al. [4] studied the metabolites in discus fish mucus and found that more thymidine was detected in the mucus of female fish during the parental period, while more gentiobiose, D-maltose, guanine and cytosine were present in the mucus of male fish, indicating that the mucus changes in parent and non-parent fish were related to gender. Lipids are important nutritional substances, and no research has been conducted on the lipids in the skin mucus of discus fish during the parental period.
The skin mucus of fish contains various bioactive substances [5], among which lipids are important nutrients [6]. They can regulate mucus elasticity, wettability and adhesiveness [7], as well as affect its antibacterial properties [8] and signal transduction functions [9]. At the same time, fry lack the ability to synthesize phospholipids de novo [10] and must ingest complete phospholipids from the outside world to cope with growth and development. Many studies showed that phospholipids play an important role in the growth and development of fry [11,12]. After the fry of the discus fish have absorbed the yolk nutrients, the parental body surface mucus becomes their main source of nutrition. We speculate that the parents will produce more lipid components to support the growth and development of the fry. Therefore, analyzing the lipid composition of the skin mucus of the discus fish and its changes can provide information not only on the physiological status of the parents but also on the effect of lipids on the growth and development of the fry. There are many methods for studying lipidomics. LC-MS/MS-based lipidomics is suitable to identify low-abundance lipid and can achieve the high-throughput and quantitative detection of hundreds of lipids [13]. For fish mucus, there are already some studies focusing on lipids, e.g., works on the effect of diet on the fatty acid profile of skin mucus in Sparus aurata [14], on the fatty acid and lipid class composition of skin mucus in Atlantic salmon [15], and on the lipid content and fatty acid composition of skin mucus in Seriola dumerili [16]. Few studies, however, used LC-MS/MS to study the lipids in fish mucus.
Lipids are important substances meeting the growth need of fry, but the lipidomics of the lipid supply transmitted by parents to fry discus fish through skin mucus still needs to be improved. In this study, parental/non-parental female and male discus fish were chosen as the experimental subject. By LC-MS/MS, we aimed to explore the composition characteristics and variation patterns of skin mucus lipids in discus fish during the parental care period. These results could help explain the unique parental care behavior of discus fish and further provide an important theoretical basis for their artificial breeding.
2. Materials and Methods
2.1. Experimental Fish
A total of six pairs (one male and one female for each pair) of adult discus fish were provided by the Fish Breeding Laboratory, Shanghai Ocean University (Shanghai, China). Their maintenance and care were described earlier [17]. Male and female fish start parental care after the eggs are fertilized. The fry start the free-swimming stage three days after hatching, and then feed on parental skin mucus.
2.2. Sample Collection
Mucus was collected from six males and six females involved in parental care on the seventh day of fry biting at mucus. After two weeks, the parents were separated from the larvae; we also sampled mucus from the same fish during the non-parental care phase [1]. Prior to sampling, the fish were anesthetized with buffered MS-222 (100 mg/L) [18,19] and then left for 10 s to eliminate the extra water. A small piece of degrease cotton (Sangon Biotech Co., Ltd., Shanghai, China) was placed on the surface of the fish, from the caudal peduncle to the head, to gently absorb the skin mucus. The cotton was then put into a 2 mL Eppendorf tube, frozen by liquid nitrogen and stored at −80 °C. During the parental period, 12 mucus samples from six parental males (P-Ms) and six parental females (P-Fs) were collected. Another 12 mucus samples from six non-parental males (NP-Ms) and six non-parental females (NP-Fs) were obtained during the non-parental care period.
2.3. Chemicals and Quality Control Samples
HPLC-grade acetonitrile (ACN), methanol (MeOH), isopropanol (IPA), dichloromethane (CH2Cl2) and tert-butyl methyl ether (MTBE) were purchased from Merck (Darmstadt, Germany). HPLC-grade formic acid (FA) and ammonium formate (AmFA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ultrapure water was obtained by a Milli-Q system (Millipore, Billerica, MA, USA). The lipid standards were purchased from Sigma-Aldrich or Avanti Polar Lipids (Alabaster, AL, USA). QC samples were equally mixed from the lipid extracts of each sample. Four identical QC samples were used to identify the lipids in discus fish mucus and to ensure the reliability of the experimental results.
2.4. Lipid Extraction Process
We homogenized 20 mg of each sample in 1 mL of a mixture including methanol, MTBE and the internal standard solution, using a steel ball. The mixture was whirled for 15 min. Then, 200 uL of water was added, and the mixture was whirled for 1 min and then centrifuged at 12,000 rpm at 4 °C for 10 min. We took 300 uL of the supernatant and subjected it to evaporation. The powder was dissolved in 200 uL of reconstituted solution and stored at −80 °C. Finally, the dissolved sample was analyzed by LC-MS/MS.
2.5. Lipid Data Processing
The sample extracts were analyzed using an LC-ESI-MS/MS system (UPLC, ExionLC AD, https://sciex.com.cn/ (accessed on 1 January 2024); MS, QTRAP® 6500+ System, https://sciex.com/ (accessed on 1 January 2024)). The detailed HPLC and ESI-MS/MS conditions are shown in Texts S1 and S2. The lipids were detected by MetWare (http://www.metware.cn/ (accessed on 1 January 2024)) based on the AB Sciex QTRAP 6500 LC-MS/MS platform.
2.6. Bioinformatic and Statistical Analyses
The data were processed by the SIMCA 14 software package (Umetrics, Umea, Sweden) for principal component analysis (PCA), orthogonal projections to latent structures discriminate analysis (OPLS-DA), and permutation tests of OPLS-DA.
We used the R package ‘ComplexHeatmap’ (v3.5.0.) to analyze hierarchical clusters and constructed volcano plots using the R package ‘ggplot2′ (v3.5.0). The SDPLs between the groups were screened based on the criteria of either VIP > 1 and FC > 2 (up-regulated) or FC < 0.5 (down-regulated). MetaboAnalyst 5.0 (http://www.metaboanalyst.ca (accessed on 1 January 2024)) was performed for lipid metabolism pathway analysis.
3. Results
3.1. Reliability of the Analytical Method
Four identical QC samples were obtained by equally mixing the lipid extracts from six P-F samples, six NP-F samples, six P-M samples and six NP-M samples. For the QC samples, the total ion chromatograms of the retention time and peak area overlapped well in both positive and negative ion modes, indicating excellent instrument stability (Figure S1). The score plots of principal component analysis with orthogonal signal correction (PCA-X) showed that all QC samples scored within two standard deviations, indicating that our data were reliable (Figure S2A). The QC samples were highly correlated in both positive and negative ion modes, indicating that the data quality was sufficient (Figure S2B). These results indicated that the analytical method used in this study was reliable.
3.2. Analysis of the Lipid Profiles of Discus Fish Skin Mucus
We identified a total of 577 lipids in the four groups of samples (NP-F, NP-M, P-F and P-M) and classified them into 25 subclasses, including 26 free fatty acids (FFAs), 6 monoglycerides (MGs), 18 diglycerides (DGs), 85 triglycerides (TGs), 84 phosphatidylcholine (PCs), 120 phosphatidyl ethanolamines (PEs), 8 phosphatidylinositols (PIs), 13 phosphatidylserines (PSs), 4 phosphatidylglycerols (PGs), 41 lyso-phosphatidylcholines (LPCs), 15 lyso-phosphatidylethanolamines (LPEs), 1 N-acyl phosphatidylethanolamine (LNAPE), 2 lyso-phosphatidic acids (LPAs), 2 lyso-phosphatidylglycerols (LPGs), 5 methanol phosphates (PMeOHs), 20 sphingomyelins (SMs), 2 sphingosines (SPHs), 4 bile acids (BAs), 23 acylcarnitines (CARs), 33 ceramides (Cers), 1 ceramide phosphate (CerP), 1 cholesterol, 1 coenzyme Q (CoQ), 24 eicosanoids and 14 hexosylceramides (HexCers) (Figure S3 and Table S1).
The sum of all lipids of the same subclass was used to estimate the content of each subclass. The top five substances in the NP-F group were DG (34.462%), BA (27.584%), MG (13.025%), eicosanoid (10.098%) and PC (6.989%) (Figure 1A). The top five substances in the NP-M group were DG (34.125%), BA (31.037%), MG (12.962%), eicosanoid (8.213%) and PC (6.108%) (Figure 1B). The top five substances in the P-F group were DG (46.431%), BA (20.445%), MG (14.562%), PC (7.204%) and FFA (4.434%) (Figure 1C). The top five substances in the P-M group were DG (38.244%), BA (21.269%), MG (17.243%), PC (8.552%) and eicosanoid (4.579%) (Figure 1D). Compared with NP-Fs, in the parental period, the proportions of DG, PC, FFA, PE and PI increased, among which, that DG increased significantly, while the proportions of BA and eicosanoid decreased. Compared with NP-Ms, in the parental period, the proportions of DG, MG, PC, PE, PS and PI increased, while the proportions of BA and eicosanoid decreased. Compared with P-Fs, the female skin mucus had higher proportions of DG and FFA, while the male skin mucus had higher proportions of MG, eicosanoid, PC, PE, PS and PI.
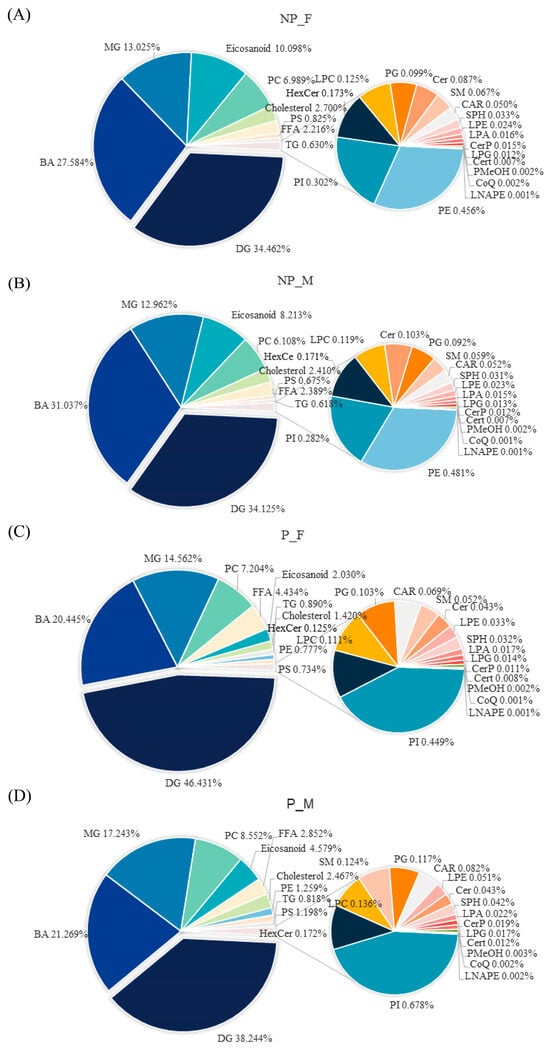
Figure 1.
The percentage composition of lipid subclasses in the NP-F group (A), NP-M group (B), P-F group (C) and P-M group (D).
In addition, we estimated the content of each subclass by considering the sum of all lipids in the same subclass. As shown in Figure 2, in the four groups of samples, the contents of eicosanoid, cholesterol, PE and PI changed significantly. Specifically, the contents of eicosanoid and cholesterol decreased in the parental period. The contents of eicosanoid and cholesterol in the P-F group were significantly lower than those in the NP-F and NP-M groups, and the content of eicosanoid in the P-M group was significantly lower than that in the NP-F group. On the contrary, the contents of PE and PI increased in the parental period. The contents of PE and PI in the P-M group were significantly higher than those in the NP-F and NP-M groups.
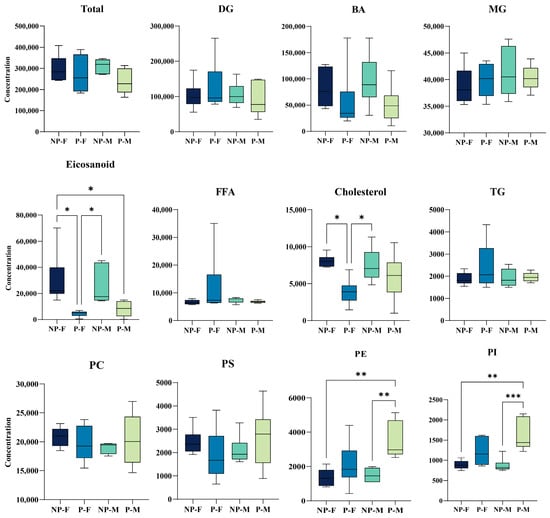
Figure 2.
Comparison of the levels of each lipid subclass between in NP-F and P-F, NP-M and P-M samples. * means p < 0.05, ** means p < 0.01, *** means p < 0.001.
3.3. PCA and OPLS-DA Analysis
Unsupervised PCA plots were generated to visualize the differences in lipids in all samples, showing that the samples from different periods were significantly separated within the same sex, while there was no obvious separation between different sexes in the same period. In addition, to analyze in more detail the variables that contributed to sample classification, we used supervised OPLS-DA. As shown in the OPLS-DA score plots, the lipid extracts of all sample groups were distinctly different (Figure 3B–E). However, the validity of OPLS-DA needed to be verified by some parameters. The cumulative values of R2Y and Q2 indicated the feasibility and high predictability of the model when analyzing differences between NP-Fs and P-Fs and between NP-Ms and P-Ms, while the permutation test showed the opposite results when comparing NP-Ms and NP-Fs and P-Ms and P-Fs (Figure S4A–D).
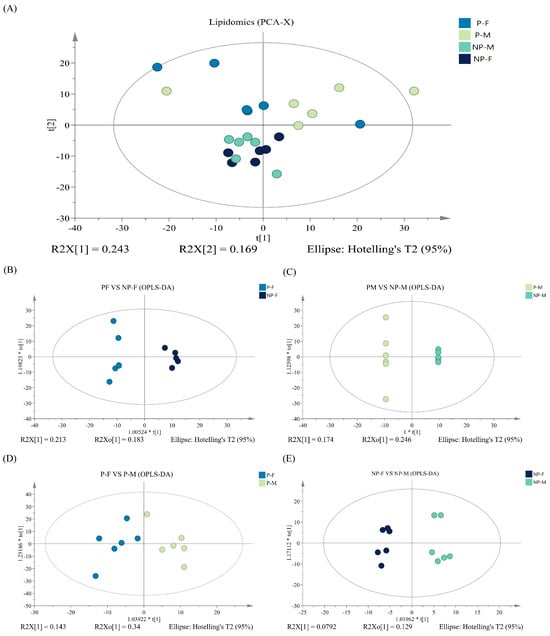
Figure 3.
PCA score maps of NP-F, P-F, NP-M and P-M samples (A), OPLS-DA score plots (B–E).
3.4. Differential Lipids
In this study, the criteria for selecting lipid species that were significantly different between the two groups were based on the prediction (VIP > 1) obtained from the OPLS-DA model and the fold change (FC ≥ 2 or ≤0.5) based on univariate statistical analysis. A total of 107 differential lipids were identified between NP-Fs and P-Fs (Table S2). A total of 108 differential lipids were identified between NP-Ms and P-Ms (Table S3). A total of 47 differential lipids were identified between P-Fs and P-Ms (Table S4). A total of seven differential lipids were identified between NP-Fs and NP-Ms (Table S5).
To better visualize these differences, a heat map with dendrograms showing the hierarchical clustering of DELs was generated. In the same-sex comparison, the parental females showed 23 lipids up-regulated and 84 lipids down-regulated compared to the non-parental females (Figure 4A). The parental males showed 46 lipids up-regulated and 62 lipids down-regulated compared to the non-parental males (Figure 4B). In the same-period comparison, the parental females showed 7 lipids up-regulated and 40 lipids down-regulated compared to the parental males (Figure 5A). In the non-parental period, the females showed three lipids up-regulated and four lipids down-regulated compared to the males (Figure 5B).
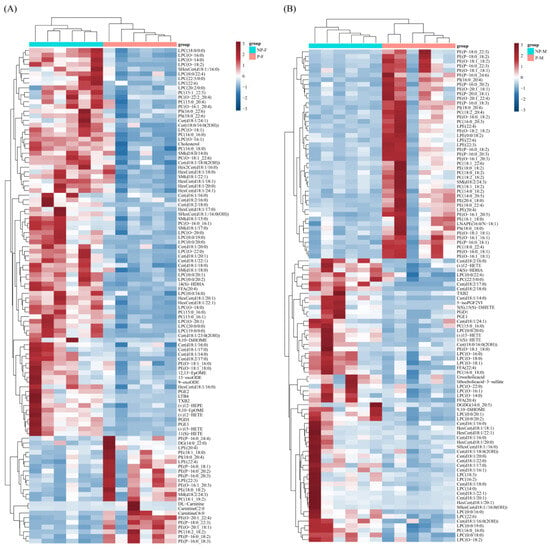
Figure 4.
Hierarchical clustering and heatmap depicting the significantly different lipids between NP-F and P-F samples (A) and NP-M and P-M samples (B).
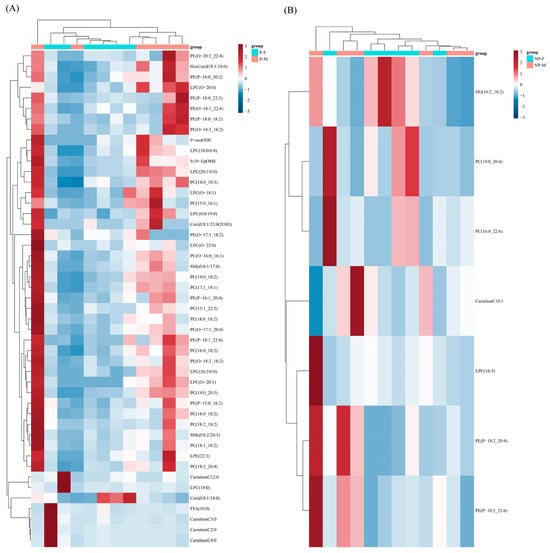
Figure 5.
Hierarchical clustering and heatmap depicting the significantly different lipids between P-F and P-M samples (A) and NP-F and NP-M samples (B).
3.5. Lipid Metabolism Pathways
In order to elucidate alterations in metabolic pathways involving lipids from the mucus samples of parental and non-parental discus fish, the DELs were mapped to the KEGG pathway database. As shown, the DELs between NP-Fs and P-Fs were involved in 72 lipid metabolic pathways (Table S6), the DELs between NP-Ms and P-Ms were involved in 46 lipid metabolic pathways (Table S7), the DELs between P-Ms and P-Fs were involved in 24 lipid metabolic pathways (Table S8), and the DELs between NP-Ms and NP-Fs were involved in 19 lipid metabolic pathways (Table S9). When further exploring the impact of these DELs, the lipid categories and their contents were transferred to MetaboAnalyst 5.0 to identify significantly correlated metabolic pathways. The most relevant fourteen pathways for P-Fs compared to NP-Fs were sphingolipid metabolism, linoleic acid metabolism, arachidonic acid metabolism, glycerophospholipid metabolism, alpha-linolenic acid metabolism, glycosylphosphatidylinositol (GPI) anchor biosynthesis, glycerolipid metabolism, ether lipid metabolism, phosphatidylinositol signaling system, inositol phosphate metabolism, biosynthesis of unsaturated fatty acids, primary bile acid biosynthesis, steroid biosynthesis and steroid hormone biosynthesis (Figure 6A). The most relevant eight pathways for P-Ms compared to NP-Ms were sphingolipid metabolism, arachidonic acid metabolism, glycerophospholipid metabolism, linoleic acid metabolism, alpha-linolenic acid metabolism, glycosylphosphatidylinositol (GPI) anchor biosynthesis, ether lipid metabolism and biosynthesis of unsaturated fatty acids (Figure 6B). Comparing P-Ms with P-Fs, the most relevant seven pathways identified were linoleic acid metabolism, glycerophospholipid metabolism, alpha-linolenic acid metabolism, glycosylphosphatidylinositol (GPI) anchor biosynthesis, sphingolipid metabolism, arachidonic acid metabolism and fatty acid biosynthesis (Figure 6C). Comparing NP-Ms with NP-Fs, the most relevant eight pathways identified were glycerophospholipid metabolism, linoleic acid metabolism, alpha-linolenic acid metabolism, glycosylphosphatidylinositol (GPI) anchor biosynthesis, glycerolipid metabolism, phosphatidylinositol signaling system, inositol phosphate metabolism and arachidonic acid metabolism (Figure 6D).
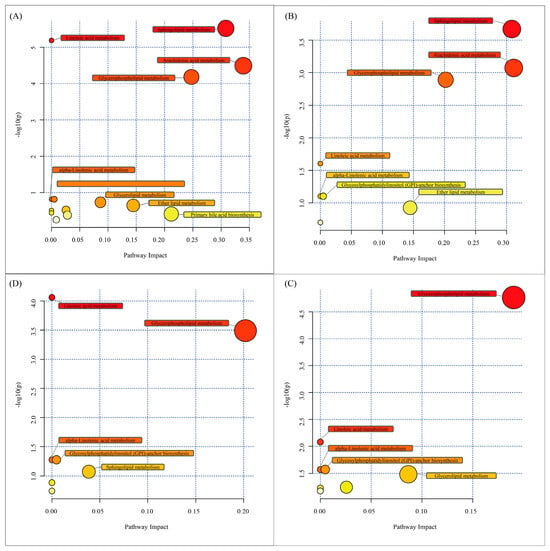
Figure 6.
Metabolomic view map of the significant metabolic pathways involving discus skin mucus lipids identified in NP-F and P-F samples (A), NP-M and P-M samples (B), P-F and P-M samples (C) and NP-F and NP-M samples (D). Bubble size is proportional to the impact of each pathway, and bubble colour indicates the degree of significance from the highest (red) to the lowest (white).
4. Discussion
So far, no study has been published on the lipids in the mucus of discus fish. We used a quantitative lipidomics method based on UHPLC-QTOF-MS to identify 577 lipids in the skin mucus of discus fish, which were divided into 25 subclasses. This study revealed for the first time the lipid content and composition of the body surface mucus of discus fish, as well as the changes in its lipid composition during the parental and non-parental phases. The large number of lipids identified in this study significantly improve our understanding of the composition of discus fish skin mucus, providing a basis for a better utilization of the lipids in the skin mucus.
We also compared the content of each lipid subclass in each group. In the skin mucus of discus fish, the content of PI was significantly higher during the parental period than during the non-parental period, while the content of eicosanoid and BA was significantly lower during the parental period than during the non-parental period. Although there was no significant difference, the proportions of DG, MG, PC, FFA, TG, PE, PG and CAR were also higher during the parental than during the non-parental period. These findings suggest that after the fry ingest the parental body surface mucus, the production of substances such as BA and eicosanoid in the newly formed mucus is reduced, resulting in a significantly lower content of these substances in the parental body surface mucus during the parental period than during the non-parental period. Conversely, those substances whose content showed no significant difference but increased in proportion or significantly increased (such as DG, PC, PE, PI, etc.), indicated that the parental discus increased the production of these substances, so that the rapidly regenerated mucus could always maintain them at a certain level. There are few studies on lipid subclass composition in fish mucus. Compared with the known skin mucus of Salmo salar, the lipid composition of the skin mucus of discus fish is significantly different. DG (34.12–46.43%) and BA (20.44–31.03%) are the two most abundant lipids in discus fish mucus, while cholesterol (22.3–26.9%) and PC (11.3–17.8%) are the most abundant substances in Salmo salar mucus [15]. Lewis [6] reported that phospholipids accounted for 48.8% and 62.4% of the total lipids in the skin mucus of the marine catfish Plotosus lineatus and the dusky flathead Platycephalus fuscus, respectively. DG is a neutral lipid that can provide energy. The high content of DG in skin mucus may be unique to discus fish, with the aim of providing high energy for the fry. Moreover, the proportion of DG increased further in the parental period, reaching 46.43% in the female parent fish, which also confirms this hypothesis.
Bile acids showed a high proportion in the body surface mucus lipids of discus fish. It is well known that BAs are strong olfactory stimuli for teleost fish [20,21,22]. At the same time, the extreme olfactory sensitivity of these fish to bile acids, as well as BA widespread distribution and chemical variation, have been considered to be related to BA role in fish behavior. For example, behavioral studies in yellow croaker showed that fry are attracted by intestinal bile acids, which affect their preference behavior [23]. Mexican cavefish feed on bat guano, possibly because bile substances are attractive to them [24]. Therefore, we speculate that the fry can immediately find and suck the parental body surface mucus after swimming, which may be due to the attraction exerted by bile acids. Some studies showed that adding bile acids to the feed can improve the growth performance of fish [25] and enhance the transport of fatty acids [26] and immunity [27].
Eicosanoids are bioactive signaling lipids derived from arachidonic acid and related polyunsaturated fatty acids (PUFAs) that regulate multiple homeostatic and inflammatory processes locally [28,29]. We observed a significant reduction in the content of eicosanoids in the mucus of the parent fish during the parental period. This may be because when the fry feed on the mucus of the parent fish, they also cause damage to the skin of the parent fish, which results in inflammation. The parent fish react to this to reduce the inflammation response. Among the lipids that showed significant differences between the non-parental group and the parental group, we observed that the levels of pro-inflammatory substances such as 12-hydroxyeicosatetraenoic acid (12-HETE), 15-hydroxyeicosatetraenoic acid (15-HETE), prostaglandin E1 (PGE1), thromboxane B2 (TXB2), etc., were significantly lower during the parental period than during the non-parental period, while those of the anti-inflammatory substance lipoxin B4 (LXB4) showed no significant difference. This suggests that the parent fish may reduce the activity of the COX enzyme and accelerate the production of LXB4 during the parental period [30]. Studies also found that in mammals, the level of LXB4 is significantly increased in various pathological conditions involving inflammatory stimuli, as a down-regulator of the inflammatory process [31]. In this study, lipoxin A4 (LXA4), one of the most effective anti-inflammatory substances [32], was detected in the non-parental group but was absent or present at very low levels in the parental group. This may indicate that LXA4 has a strong anti-inflammatory effect during the parental period in discus fish [33].
According to the different parental periods, we identified 107 and 108 DELs between NP-Fs and P-Fs and between NP-Ms and P-Ms, respectively, and most of these lipids were phospholipids. During the early rapid growth stage, the fry need a large amount of phospholipids, but studies have shown that freshwater and marine fish have limited ability to synthesize phospholipids de novo [34]; therefore, the fry need to intake complete phospholipids from the outside to meet the rapid growth demands of their body. Our study also confirmed this. In the mucus of the parent fish during the parental and non-parental periods, although a large amount of new mucus needs to be produced due to the fry feeding on it, a high phospholipid content in the new mucus was always maintained, and the phospholipid composition ratio was higher than that in the non-parental period. Among the lipids that showed significant differences between the non-parental group and the parental group, phospholipids were among the main ones. These findings indicate that changes in the synthesis and metabolism of phospholipids played an important role in the changes in mucus composition in the parental period of discus fish, and are of great significance for the growth and development of fry. Phospholipids have various functions for fish [10]. At present, the functions of the two subclasses of phosphatidylcholine and phosphatidylinositol seem to be clear, with PC promoting growth, and PI preventing deformities and exerting positive effects on survival [11]. The growth-promoting effect of PC may be due to its stimulation of the synthesis and secretion of the triacylglycerol-rich lipoprotein apoB48 [12]. The mechanism by which PI reduces fry malformation is still not fully understood. But one possible reason is that PI may affect of bone synthesis and mineralization [35]. Among the lipids that showed significant differences between the parental and the non-parental periods, PE was the most up-regulated substance in the parental period. PE is the main phospholipid component of biological membranes, second only to phosphatidylcholine, and plays an important role in various biological processes [36]. In addition, PE is essential for neural development [37]; so, we speculate that PE in the mucus of the parent fish may be closely related to the neural development of the fry.
PUFAs (polyunsaturated fatty acids) have been extensively studied in aquaculture due to their importance in the survival and development of teleosts [38]. Interestingly, in our study, we found that most of the differentially expressed phospholipids with unsaturated fatty acid chains were significantly up-regulated in the parental mucus, such as PC (O-18:1_20:4), PE (P-16:0_20:5), PE (P-18:0_22:6), PE (P-20:0_22:6), etc. EPA plays an important role in maintaining the normal color and immunity of fish [39]. Adding appropriate amounts of DHA and EPA in the feed can maintain the normal physiological functions in larvae and juvenile fish and promote growth [40]. Therefore, we speculate that the parent fish will produce more PUFAs during the parental period and transport them to the skin surface mucus, providing nutritional needs for the growth and development of the fry.
In the comparison between the parental and the non-parental periods within the same sex, arachidonic acid metabolism and sphingolipid metabolism were the most significantly correlated metabolic pathways. Arachidonic acid is normally used to produce biologically active prostaglandins [41]. In the P-F group, the reduction in arachidonic acid, PGE2, and LTB4 content may indicate that arachidonic acid metabolism was activated, thereby producing biologically active prostaglandins for discus fish fry [42], which is consistent with Wen’s study results [4]. Sphingolipid metabolism plays an important role in supporting cell growth and signal transduction [43]. In addition, in the NP-F and P-F groups, PC, PE, PS, and LPC participate in glycerophospholipid metabolism. In the parental group, the level of PE was significantly increased, but the content of PS was decreased. This may be due to an enhancement in the activity of the PS decarboxylase Psd1p in parent female discus fish [44], promoting the conversion of PS to PE. In summary, our study results support the hypothesis that changes in lipids in the surface mucus of discus fish during the parental period may affect the above metabolic pathways, thereby increasing the levels of specific lipids such as PE. We believe that the pathway analysis related to the DELs in the surface mucus of discus fish during the parental and the non-parental periods may improve our understanding of the changes and functions of “milk” during the parental period.
5. Conclusions
In this study, we used LC-MS/MS to analyze the lipids in discus fish skin mucus in different stages. A total of 26 subclasses and 577 lipids were identified in the compared groups. In the comparison between females in the two stages, 107 DELs were observed, of which 23 were up-regulated in the parental phase. For the males, 108 DELs were observed, including 46 that were up-regulated in the parental phase. The main DELs were PE and PI. In addition, in the comparison between females and males in the parental stage, we found 47 DELs, and diglyceride showed a higher proportion in the mucus of P-Fs, while phospholipids showed a higher level in the mucus of P-Ms. Our results revealed changes in the mucus in the parental and non-parental stages, as well as sex-specific differences in the mucus of parental fish. Our study results provide important lipidomics-level mechanistic insights for explaining the changes in skin mucus in discus fish during the parental period and provide a basis for further understanding the functions of skin mucus lipids.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes9010027/s1, Figure S1: Total ion chromatograms (TICs) of lipids in discus fish skin mucus. Positive ion mode (A). Negative ion mode (B); Figure S2: Reliability of the analytical methods. PCA-X score plots of QC samples (A). Correlation of QC samples (B); Figure S3: The lipid subclasses identified in the skin mucus of discus fish and the number of each subclass; Figure S4: Validation by OPLS-DA of of NP-F and P-F, NP-M and P-M samples (A–D); Table S1: Lipids contents in discus fish skin mucus (nmol/g); Table S2: Identification of 107 significantly different lipids between NP-F and P-F (nmol/g); Table S3: Identification of 107 significantly different lipids between NP-M and P-M (nmol/g); Table S4: Identification of 47 significantly different lipids between P-M and P-F (nmol/g); Table S5: Identification of 7 significantly different lipids between NP-M and NP-F (nmol/g); Table S6: Metabolic pathway identified from the significantly different lipids between NP-F and P-F; Table S7: Metabolic pathway identified from the significantly different lipids between NP-M and P-M; Table S8: Metabolic pathway identified from the significantly different lipids between P-M and P-F; Table S9: Metabolic pathway identified from the significantly different lipids between NP-M and NP-F; Text S1: HPLC conditions; Text S2: ESI-Q TRAP-MS/MS conditions.
Author Contributions
S.Z.: investigation, visualization, writing—original draft; B.W.: supervision, conceptualization, methodology, writing—review and editing; H.L.: writing—review and editing; J.G.: supervision; Z.C.: supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was supported by the Natural Science Foundation of Shanghai (20ZR1423600) and the Shanghai Sailing Program, China (19YF1419400).
Institutional Review Board Statement
All laboratory procedures involving animals were approved by the Animal Ethics Committee of Shanghai Ocean University (SHOU-DW-2019-013).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Buckley, J. Biparental mucus feeding: A unique example of parental care in an Amazonian cichlid. J. Exp. Biol. 2010, 213, 3787–3795. [Google Scholar] [CrossRef] [PubMed]
- Schutz, M.; Barlow, G.W. Young of the Midas cichlid get biologically active nonnutrients by eating mucus from the surface of their parents. Fish Physiol. Biochem. 1997, 16, 11–18. [Google Scholar] [CrossRef]
- Chong, K.; Joshi, S.; Jin, L.T.; Shu-Chien, A.C. Proteomics profiling of epidermal mucus secretion of a cichlid (Symphysodon aequifasciata) demonstrating parental care behavior. Proteomics 2006, 6, 2251–2258. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Zhou, J.Q.; Gao, J.Z.; Chen, H.R.; Shen, Y.Q.; Chen, Z.Z. Sex-dependent changes in the skin mucus metabolome of discus fish (Symphysodon haraldi) during biparental care. J. Proteom. 2020, 221, 103784. [Google Scholar] [CrossRef] [PubMed]
- Brinchmann, M.F. Immune relevant molecules identified in the skin mucus of fish using -omics technologies. Mol. Biosyst. 2016, 12, 2056–2063. [Google Scholar] [CrossRef]
- Lewis, R.W. Fish cutaneous mucus: A new source of skin surface lipid. Lipids 1970, 5, 947–949. [Google Scholar] [CrossRef]
- Galabert, C.; Jacquot, J.; Zahm, J.M.; Puchelle, E. Relationships between the lipid content and the rheological properties of airway secretions in cystic fibrosis. Clin. Chim. Acta Int. J. Clin. Chem. 1987, 164, 139–149. [Google Scholar] [CrossRef]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef]
- Matsumura, K.M.S.; Fusetani, N. Possible involvement of phosphatidylcholine in school recognition in the catfish, Plotosus lineatus. Zool. Sci. 2004, 21, 257–264. [Google Scholar] [CrossRef]
- Tocher, D.R.; Bendiksen, E.A.; Campbell, P.J.; Bell, J.G. The role of phospholipids in nutrition and metabolism of teleost fish. Aquaculture 2008, 280, 21–34. [Google Scholar] [CrossRef]
- Geurden, I.; Marion, D.; Charlon, N.; Coutteau, P.; Bergot, P. Comparison of different soybean phospholipidic fractions as dietary supplements for common carp, Cyprinus carpio, larvae. Aquaculture 1998, 161, 225–235. [Google Scholar] [CrossRef]
- Field, F.J.; Mathur, S.N. Intestinal lipoprotein synthesis and secretion. Prog. Lipid Res. 1995, 34, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Yin, Y.D.; Xu, M.M.; Wang, R.H.; Zhu, Z.J. Absolute quantitative lipidomics reveals lipidome-wide alterations in aging brain. Metabolomics 2018, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Torrecillas, S.; Montero, D.; Dominguez, D.; Robaina, L.; Izquierdo, M. Skin Mucus Fatty Acid Composition of Gilthead Sea Bream (Sparus aurata): A Descriptive Study in Fish Fed Low and High Fish Meal Diets. Fishes 2019, 4, 15. [Google Scholar] [CrossRef]
- Sprague, M.; Desbois, A.P. Fatty acid and lipid class composition in cutaneous mucus of Atlantic salmon, Salmo salar (L.). Aquac. Res. 2021, 52, 6808–6813. [Google Scholar] [CrossRef]
- Sato, S.; Hirayama, T.; Hirazawa, N. Lipid content and fatty acid composition of the monogenean Neobenedenia girellae and comparison between the parasite and host fish species. Parasitology 2008, 135, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Chen, Z.; Qu, H.; Gao, J. Growth and fatty acid composition of discus fish Symphysodon haraldi given varying feed ratios of beef heart, duck heart, and shrimp meat. Aquac. Fish. 2018, 3, 84–89. [Google Scholar] [CrossRef]
- Ekman, D.R.; Skelton, D.M.; Davis, J.M.; Villeneuve, D.L.; Cavallin, J.E.; Schroeder, A.; Jensen, K.M.; Ankley, G.T.; Collette, T.W. Metabolite Profiling of Fish Skin Mucus: A Novel Approach for Minimally-Invasive Environmental Exposure Monitoring and Surveillance. Environ. Sci. Technol. 2015, 49, 3091–3100. [Google Scholar] [CrossRef]
- Ivanova, L.; Tartor, H.; Grove, S.; Kristoffersen, A.B.; Uhlig, S. Workflow for the Targeted and Untargeted Detection of Small Metabolites in Fish Skin Mucus. Fishes 2018, 3, 21. [Google Scholar] [CrossRef]
- Giaquinto, P.C.; Hara, T.J. Discrimination of bile acids by the rainbow trout olfactory system: Evidence as potential pheromone. Biol. Res. 2008, 41, 33–42. [Google Scholar] [CrossRef]
- Hara, T.J. The diversity of chemical stimulation in fish olfaction and gustation. Rev. Fish Biol. Fish. 1994, 4, 1–35. [Google Scholar] [CrossRef]
- Zhang, C.; Brown, S.B.; Hara, T.J. Biochemical and physiological evidence that bile acids produced and released by lake char (Salvelinus namaycush) function as chemical signals. J. Comp. Physiol. B 2001, 171, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.J.; Zhang, X.L.; Yan, X.J. Intestinal Bile Acids Induce Behavioral and Olfactory Electrophysiological Responses in Large Yellow Croaker (Larimichthys crocea). Fishes 2023, 8, 26. [Google Scholar] [CrossRef]
- Kasumyan, A.O.; Vinogradskaya, M.I. Palatability of Bile Substances for Fish. J. Ichthyol. 2019, 59, 610–618. [Google Scholar] [CrossRef]
- Gao, Y.J.; Yao, Y.F.; Huang, J.; Sun, Y.J.; Wu, Q.J.; Guo, D.Q.; Wang, S.P. Effect of dietary bile acids supplementation on growth performance, feed utilization, intestinal digestive enzyme activity and fatty acid transporters gene expression in juvenile leopard coral grouper (Plectropomus leopardus). Front. Mar. Sci. 2023, 10, 1171344. [Google Scholar] [CrossRef]
- Li, T.; Yan, X.B.; Dong, X.H.; Pan, S.M.; Tan, B.P.; Zhang, S.; Suo, X.X.; Li, Z.H.; Huang, W.B.; Yang, Y.Z.; et al. Choline Alleviates Disorders of Lipid Metabolism in Hybrid Grouper (female Epinephelus fuscoguttatus × male E. lanceolatus) Caused by High-Lipid Diet. Aquac. Nutr. 2022, 2022, 8998849. [Google Scholar] [CrossRef]
- Adam, A.H.; Verdegem, M.; Soliman, A.A.; Zaki, M.; Khalil, R.H.; Nour, A.M.; Khaled, A.A.; Basuini MF, E.; Khalil, H.S. Effect of dietary bile acids: Growth performance, immune response, genes expression of fatty acid metabolism, intestinal, and liver morphology of striped catfish (Pangasianodon hypophthalmus). Aquac. Rep. 2023, 29, 101510. [Google Scholar] [CrossRef]
- Buczynski, M.W.; Dumlao, D.S.; Dennis, E.A. An integrated omics analysis of eicosanoid biology. J. Lipid Res. 2009, 50, 1015–1038. [Google Scholar] [CrossRef]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef]
- Han, X.; Chen, X.Y.; Sun, Y.J.; Nadler, J.L.; Bleich, D. Induction of cyclooxygenase-2 gene in Pancreatic β-cells by 12-lipoxygenase pathway product 12-hydroxyeicosatetraenoic acid. Mol. Endocrinol. 2002, 16, 2145–2154. [Google Scholar] [CrossRef]
- Wu, J.; Ding, D.H.; Li, Q.Q.; Wang, X.Y.; Sun, Y.Y.; Li, L.J. Lipoxin A4 Regulates Lipopolysaccharide-Induced BV2 Microglial Activation and Differentiation via the Notch Signaling Pathway. Front. Cell. Neurosci. 2019, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Das, U. Arachidonic Acid Has Anti-Inflammatory and Anti-Diabetic Actions In Vitro and In Vivo. Curr. Dev. Nutr. 2020, 4, 747. [Google Scholar] [CrossRef]
- Kronke, G.; Katzenbeisser, J.; Uderhardt, S.; Zaiss, M.M.; Scholtysek, C.; Schabbauer, G.; Zarbock, A.; Koenders, M.I.; Axmann, R.; Zwerina, J.; et al. 12/15-Lipoxygenase Counteracts Inflammation and Tissue Damage in Arthritis. J. Immunol. 2009, 183, 3383–3389. [Google Scholar] [CrossRef]
- Coutteau, P.; Geurden, I.; Camara, M.R.; Bergot, P.; Sorgeloos, P. Review on the dietary effects of phospholipids in fish and crustacean larviculture. Aquaculture 1997, 155, 149–164. [Google Scholar] [CrossRef]
- Sandel, E.; Nixon, O.; Lutzky, S.; Ginsbourg, B.; Tandler, A.; Uni, Z.; Koven, W. The effect of dietary phosphatidylcholine/phosphatidylinositol ratio on malformation in larvae and juvenile gilthead sea bream (Sparus aurata). Aquaculture 2010, 304, 42–48. [Google Scholar] [CrossRef]
- Vance, J.E. Thematic review series: Glycerolipids. Phosphatidylserine and phosphatidylethanolamine in mammalian cells: Two metabolically related aminophospholipids. J. Lipid Res. 2008, 49, 1377–1387. [Google Scholar] [CrossRef]
- Modica-Napolitano, J.S.; Renshaw, P.F. Ethanolamine and phosphoethanolamine inhibit mitochondrial function in vitro: Implications for mitochondrial dysfunction hypothesis in depression and bipolar disorder. Biol. Psychiatry 2004, 55, 273–277. [Google Scholar] [CrossRef]
- Torres, M.; Navarro, J.C.; Varo, I.; Agulleiro, M.J.; Morais, S.; Monroig, O.; Hontoria, F. Expression of genes related to long-chain (C18-22) and very long-chain (>C24) fatty acid biosynthesis in gilthead seabream (Sparus aurata) and Senegalese sole (Solea senegalensis) larvae: Investigating early ontogeny and nutritional regulation. Aquaculture 2020, 520. [Google Scholar] [CrossRef]
- Villalta, M.; Estevez, A.; Bransden, M.P.; Bell, J.G. Effects of dietary eicosapentaenoic acid on growth, survival, pigmentation and fatty acid composition in Senegal sole (Solea senegalensis) larvae during the Artemia feeding period. Aquac. Nutr. 2008, 14, 232–241. [Google Scholar] [CrossRef]
- Vizcaíno-Ochoa, V.; Lazo, J.P.; Barón-Sevilla, B.; Drawbridge, M.A. The effect of dietary docosahexaenoic acid (DHA) on growth, survival and pigmentation of California halibut Paralichthys californicus larvae (Ayres, 1810). Aquaculture 2010, 302, 228–234. [Google Scholar] [CrossRef]
- Liakh, I.; Pakiet, A.; Sledzinski, T.; Mika, A. Modern Methods of Sample Preparation for the Analysis of Oxylipins in Biological Samples. Molecules 2019, 24, 1639. [Google Scholar] [CrossRef] [PubMed]
- Grosvenor, C.E.; Picciano, M.F.; Baumrucker, C.R. Hormones and growth factors in milk. Endocr Rev 1993, 14, 710–728. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Xin, G.; Niannian, W.; Shiling, L.; Juan, D.; Zeliang, Q.; Junrong, Z.; Qingling, W. Evaluation of changes in egg yolk lipids during storage based on lipidomics through UPLC-MS/MS. Food Chem. 2023, 398, 133931. [Google Scholar]
- Tamura, Y.; Onguka, O.; Itoh, K.; Endo, T.; Iijima, M.; Claypool, S.M.; Sesaki, H. Phosphatidylethanolamine Biosynthesis in Mitochondria: Phosphatidylserine (PS) Trafficking is Independent of a PS Decarboxylase and Intermembrane Space Proteins Ups1p and Ups2p. J. Biol. Chem. 2012, 287, 43961–43971. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).