Abstract
The shape of sagitta otoliths was used to compare individuals of little tunny (Euthynnus alleteratus) harvested on board commercial fishing vessels from the coastal areas along the Eastern Atlantic, including the Mediterranean Sea. Fish sampling and selection was designed to cover possible seasonal changes and tuna size. The research encompassed both morphometric and shape analyses of left sagittal otoliths extracted of 504 fish specimens. Four shape indices (Circularity, Roundness, Rectangularity, and Form-Factor) were significantly different between two groups, showing a statistical differentiation between two clear spatial units. The degree of divergence was even more pronounced along the rostrum, postrostrum, and excisura of the generated otolith outlines between these two groups. One group corresponds to the samples from the coastal areas in the Northeast Temperate Atlantic and Mediterranean Sea (NETAM Area) and a second group from the coastal areas off the Eastern Tropical Atlantic coast of Africa (ETA Area). This study is the first to use otolith shape to differentiate tunas from separate spatial units. These results could be used to re-classify previously collected samples and to correct time series of data collected.
Key Contribution:
The analysis of the shape of sagitta otoliths of Euthynnus alleteratus statistically differentiated two spatial groups within the Eastern Atlantic. The results have implications for the spatial management of this species by ICCAT and could be used to separate historical samples.
1. Introduction
An accurate understanding of the fish population structure is of vital importance for sustainable management [1]. The incomprehension of the population structure of exploited species can lead to dramatic changes in biological attributes, productivity rates, and genetic diversity, as well as overfishing and depletion of the less productive population units [2,3]. To initially investigate the population structure of marine fish species, it is necessary to successfully determine the discrimination of the populations [4]. Several techniques have been used to identify stock limits, such as tagging experiments [5], analyses of spatial and temporal variation of genetic or morphometric markers [6,7,8], differentiation of life-history variables [9], parasites composition [10,11], and contaminant concentrations [12].
Otolith-based research is an important tool that provides information on the population biology and life history of fish that is otherwise extremely difficult to collect [13]. Otolith research can be categorized into several distinct themes: (1) age and growth estimation and validation; (2) chemical composition; (3) historical comparisons using ancient otoliths; (4) otolith identification from intestinal contents of marine species in diet-based studies; (5) species identification, especially among cryptic species or in particular environments; and (6) shape analysis for stock structure and fisheries management [14,15,16,17,18,19,20,21].
Otolith morphology study can be a powerful tool for fish stock identification purposes, in particular for stocks that are likely to have spent a significant part of their lives in different environments and therefore may provide an indirect basis for potential stock separation [4,22,23]. Otolith shape analysis has recently gained interest among fisheries biologists, as it is a particularly powerful tool to investigate the stock discrimination in fish species, since several studies show evidence that it is controlled by both genetic and environmental factors [24,25,26], and thus highly variable between species and populations [17,27,28]. Although this technique has been widely used for discriminating diverse species, it has not been frequently applied in tuna and small tuna species [29,30,31].
The group of small tunas include a large number of species [32], mainly from the coastal areas [33]. The genus Euthynnus is one of the best-known species groups and is composed of three species: Euthynnus lineatus, Euthynnus affinis, and Euthynnus alletteratus. The little tunny (LTA)—Euthynnus alletteratus—is distributed throughout tropical and temperate zones of the Atlantic Ocean, including the Gulf of Mexico and the Mediterranean Sea. It is a commercially important tuna species in regional fishing communities due to the large volume of their catches [34], and is mainly exploited by gears such as gillnets, handline, traps, and purse seines [35].
The International Commission for the Conservation of Atlantic Tunas (ICCAT) is the organization responsible for the stock assessment and management of the populations of tunas and tuna-like species across the Atlantic Ocean and its adjacent seas [36]. This includes small tuna species such as Euthynnus alletteratus. According to the ICCAT official catch statistics, this species account for a significant proportion of the total small tuna species production, representing in the last years around 15% of the total catch [37]. Diverse biological information on this species is available focusing on age and growth [38,39,40,41,42,43,44], reproduction [45,46,47,48,49,50,51], and stock assessment [32,35]. However, concerning stock structure, there are some knowledge gaps with a lack of concise information on this species [43,44]. At present, there are no clear stock boundaries defined for some small tuna species such as little tunny in the Atlantic Ocean. Generally, five stocks unit areas are defined by ICCAT for data collection and management purposes: Mediterranean Sea, Southwest Atlantic, Southeast Atlantic, Northwest Atlantic, and Northeast Atlantic [35]. Therefore, the advance in the knowledge of the stocks structure of this species will improve the understanding and management of its fishery in the future.
In this research, the main focus was to evaluate population differences based on the shape of the sagittal otolith for little tunny captured along the coastal areas of Eastern Atlantic Ocean and Mediterranean Sea. In the same way, the usefulness of otoliths was validated as an applicable technique in small tunas, so as to generate correct management recommendations on these fisheries.
2. Materials and Methods
2.1. Tuna Sampling and Otoliths Collection
Little tunny samples were collected between 2017 and 2021 by observers on board commercial fishing vessels of gillnets, trawlers, handlines, traps, and purse seines on the Atlantic and Mediterranean, including Malta, Portugal, Spain, Senegal, Côte d’Ivoire, and Gabon coastal waters. Fish selection was based on the capture area during all potential months to cover possible seasonal changes and tuna size. Sagittal otoliths were extracted from 504 fishes at the laboratory, were cleaned with ultrapure water, allowed to dry, and stored in Eppendorfs. Straight fork length (SFL) was measured for each specimen to the nearest mm ranging from 219 mm to 987 mm (mean 407 mm; SD 135) (Table 1). The left sagitta was used for otolith morphometry and shape analysis.

Table 1.
Summary of length samples (cm) of little tunny collected across various areas and countries.
2.2. Otoliths Image Processing and Shape Analysis
For imaging, otoliths were photographed individually using a digital video camera mounted on a binocular microscope (Nikon SMZ1270, Tokyo, Japan) under reflected light and dark field. Each otolith was oriented with the sulcus side facing up and the rostrum pointing to the left. In some cases, to improve the image quality of the otoliths, the software ImageJ 1.53 t was used [52]. All images were stored in JPEG format with file sizes ranging from 107 to 790 kb. The images were imported into the “shapeR" package [52] for R [53], and were analyzed under the same threshold level (0.2) to generate the otolith morphometrics including length, width, area, and perimeter (Figure 1).
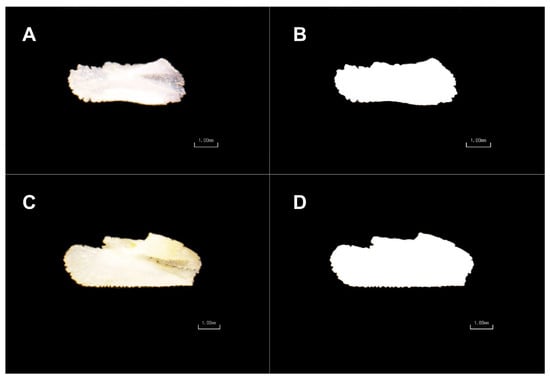
Figure 1.
Original and processed images of two little tunny sagittal otolith from Portugal—58.9 cm SFL (A,B) and Côte d’Ivoire—67.9 cm SFL (C,D).
2.3. Preliminary Data Analysis
Preliminary analyses were carried out comparing otolith shapes collected in coastal waters of different countries. To examine and compare the variation in otolith outline of each country, the mean shape was plotted using the “shapeR" package [54]. Standardized wavelet coefficients represented the otolith shape. Standardization of the wavelet coefficient uses the straight fork length of the fish to remove the allometric effect of growth on the otolith, but is unable to correct any ontogenetic changes which an otolith may experience across sizes/ages [25]. This adjustment for allometric relationships with fish length is also implemented in the “shapeR” package [52]. The standardized wavelet coefficients were visually inspected for normality before further statistical analyses. Canonical Analysis of Principal Coordinates (CAP) was conducted using the “capscale” function of the “vegan” package [55] on standardized wavelet coefficients.
Hierarchical cluster analysis was performed to evaluate the similarity and diversity of country samples based on the averages of the CAP ordination and the mean of six otolith morphological indices (Figure 2A). For cluster analysis, Euclidean distance measures and Ward linkage were used. The six common shape indices calculated using the otolith morphometrics were: Circularity, Roundness, Rectangularity, Form Factor, Aspect Ratio, and Ellipticity (Table 2).

Figure 2.
Hierarchical clustering based on the averages of CAP ordination and the otolith morphological indices (A). Canonical analysis of principal coordinates of the Wavelet coefficients for six country areas. CAP1 and CAP2 are the first and second discriminant axis, respectively. The group centroids represent the mean canonical value for each country samples analyzed (B). Average shape outline of otoliths for each country area. The numbers 0, 90, 180, and 270 represent angle in degrees (°) (C).

Table 2.
Otolith morphological indices calculated from the measurement data. OA = otolith area (mm2), OL = otolith length (mm), OP = otolith perimeter (mm), OW = otolith width (mm).
The ordination of the averages in each country area was visually assessed using the first two canonical axes (CAP1 and CAP2) (Figure 2B). Finally, the average shape outline of otoliths for each country area was plotted to explore the relationship between otolith shape and country [56] (Figure 2C).
The preliminary analysis statistically separated two groups that corresponded to distinct spatial areas. The similarities obtained between individuals captured in nearby areas highlighted the clear differentiation between two groups. The first group included samples collected in the Northeast Temperate Atlantic and the Mediterranean Sea (Portugal, Spain, and Malta) called the NETAM Area (n = 164) and the second group included samples collected in the Eastern Tropical Atlantic coast of Africa (Senegal, Côte d’Ivoire, and Gabon), which was called the ETA Area (n = 340) (Table 1; Figure 3).
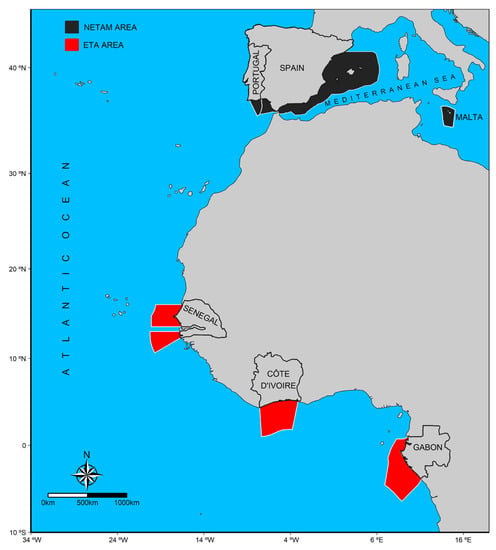
Figure 3.
Map showing area where little tunny individuals were collected. The two distinct groups identified in the preliminary analysis (in black and red) correspond to samples collected in the Northeast Temperate Atlantic and Mediterranean Sea (NETAM Area) and in the Eastern Tropical Atlantic (ETA Area) respectively.
2.4. Data Analysis
In light of the existence of two statistically distinct groups, specific analyses were carried out to compare them. A univariate analysis of variance (ANOVA) was used to test for differences in the shape indices between individuals of two delimited areas (NETAM and ETA). Additionally, a t-test analysis was used to assess the differences in Wavelet coefficients between samples from both areas.
The variation in otolith shape was examined by plotting the mean shape of each area. To estimate which part of the otolith outline contributed most to the difference between the potential groups, the mean and standard deviation of the coefficients were plotted against the angle using “plotCI” function from the “gplots” package [57] as recommended by Libungan et al. (2016) [58]. The proportion of variation within groups along the outline was summarized with intraclass correlation (ICC). Canonical Analysis of Principal coordinates (CAP) was performed on the standardized wavelet coefficients to explore the relationships between otolith shape and geographical area. The ordination of the averages in each group area (NETAM and ETA) was graphically examined along the first two canonical axes (CAP1 and CAP2). The canonical scores were further tested for significance (α = 0.05) using ANOVA-like permutation tests with 1000 permutations.
3. Results
The one-way ANOVA test used to compare shape indices between the delimited areas revealed differences (Table 3). In effect, examination of the mean otolith shape demonstrated that there were dissimilarities among both areas in the study. In this work, the differences were observed for four shape indices. The sagittae otoliths from the NETAM Area were significantly different from those from the ETA Area in Circularity, Roundness, Rectangularity, and Form-Factor (p-value < 0.05). In contrast, Aspect Ratio and Ellipticity were not significantly different. Among shape indices analyzed in this work, Circularity, Form-Factor, Roundness, and Rectangularity were the most efficient variables in distinguishing both delimited areas.

Table 3.
One-way analyses of variance (ANOVA) to test differences in otolith shape indices between Northeast Temperate Atlantic together with the Mediterranean Sea (NETAM Area) and Eastern Tropical Atlantic (ETA Area) samples of Euthynnus alletteratus.
The results of ANOVA-like permutation test using the Wavelet distances demonstrated significant differences between areas (p < 0.05). These differences were mainly at the excisura, rostrum, and postrostrum projections (Figure 4), which was further confirmed by examining variability in the mean Wavelet coefficients and the proportion of variation between both groups summarized with the ICC (Figure 5). Furthermore, almost 60% of the Wavelet coefficients revealed significant differences (Supplementary Figure S1). The otoliths from little tunnies captured in the ETA Area were less indented at the level of the excisura compared to those captured in the NETAM Area, but largest at the level of the postrostrum. This comparison of the otolith shape showed a large variation along the outline of the otolith at 0–40°, 120–210°, and 330–360° angles.

Figure 4.
Average shape of otoliths for the two sampling areas in the study. The numbers 0, 90, 180, and 270 represent the angle in degrees (°) on the outline which correspond to Figure 5. Northeast Temperate Atlantic and Mediterranean Sea (NETAM Area). Eastern Tropical Atlantic (ETA Area). The central crossmap indicates the position of the otoliths: anterior (A); dorsal (D); posterior (P); ventral (V).
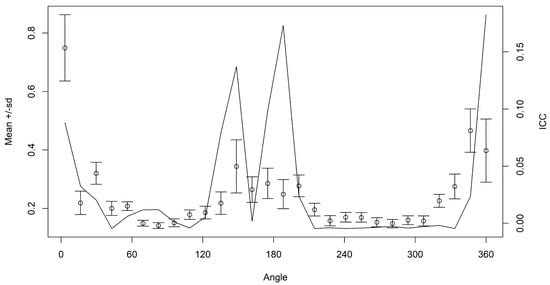
Figure 5.
Mean and standard deviation (dots and whiskers) of the Wavelet coefficients for all combined otoliths and the proportion of variance within groups for the intraclass correlation (ICC, black solid line).
Analyzing the canonical scores for both areas revealed the largest differences between areas. The first two discriminating axes of the CAP analysis based on the Wavelet coefficients explained 36.6% of the variation between the two species group (CAP 1: 11.6%, CAP 2: 25%), demonstrating a clear difference between NETAM and ETA (Figure 6).

Figure 6.
Canonical analysis of principal coordinates of the Wavelet coefficients for the two sampled areas. CAP1 and CAP2 are the first and second discriminant axis, respectively. The group centroids represent the mean canonical value for each area analyzed. Northeast Temperate Atlantic and Mediterranean Sea (NETAM Area). Eastern Tropical Atlantic (ETA Area).
4. Discussion
Otolith shape analysis statistical differences between the two groups provided a clear indication of the differentiation between these two spatial units of Euthynnus alletteratus present in the Atlantic Ocean. The most significant variation was observed in the shape-related morphometric indices, such as Circularity, Roundness, Rectangularity, and Form-Factor, which effectively differentiated the samples from NETAM and ETA areas (Table 3). The divergence was further evident along the rostrum, postrostrum, and excisura of the generated otolith outlines, as shown in Figure 4. These results were also supported by the proportion of variance within groups for the intraclass correlation (Figure 5).
This discreteness can be attributed to genetic isolation as well as the differences in environmental conditions between two delimited regions [59,60] creating phylogeographical breaks [61]. Another possible explanation for the differentiation of the species could be due to latitudinal isolation due to lack of migration patterns, geographic distance, and differences in oceanographic characteristics [62,63], which would also prove the pattern observed in the genus Euthynnus in the Atlantic [44]. This situation can lead to a biological specialization, which is linked to a differentiation in growth, reproductive, and morphological aspects [64].
Biological differences between both areas analyzed in this work have been poorly documented. In the genetic aspect, differentiating results could indicate the presence of separate species [65,66]. Olle et al. (2022) observed that the mtDNA CR divergence between the two areas studied of E. alletteratus was nearly 20 times larger than the CR divergence between E. lineatus (Pacific Ocean) and E. affinis (Indian Ocean), and similar to the distance that separates E. alletteratus respectively from E. affinis and from E. lineatus [65]. Regarding growth, almost all the works published so far come from very specific areas and mostly from the Mediterranean [39,40,42,43]. However, comparing one of the few studies in progress developed in the ETA area, specifically on the coast of Senegal [67], and comparing the length at age information with the values of other individuals in the Mediterranean Sea, differences are observed. For the same age, the values from Senegal presented smaller lengths compared to those from the Mediterranean and the Western Atlantic [41,44,68]. Similarly, reproduction has been poorly analyzed in this species. If the existing information between ETA and NETAM areas is compared, in a study carried out in the ETA area, a very extensive spawning period is observed, which occupies practically the whole year [47]. However, in the Mediterranean Sea, the period is quite seasonal and limited, coinciding with the warmer period, between the months of June and August [49,50,51,69]. However, both biological aspects must be studied in depth in the future. On the other hand, morphometric differences were observed between individuals from the Tunisian coast compared to others from the ETA area [70]. This evidence agrees with the spatial structure we propose in this study. Similarly, species differentiation along Atlantic Ocean have been documented for several fish genera such as Lepidopus spp. (Ward et al. 2008), Auxis spp. [71,72], Thunnus spp. [73], Scomber spp. [74,75], Trachurus spp. [76], Zeus spp. [77], and Diplodus spp. [78].
There is abundant scientific literature that applies otolith shape analysis as a stock differentiator or population structure descriptor [79,80,81,82,83,84]. This technique has also been widely used for the differentiation between species [85,86,87,88,89,90,91], demonstrating that it is an efficient technique for this type of analysis [28]. The shape of the otolith is known to vary depending on the ecological, evolutionary, and phylogenetic characteristics of each species [92]. This variation is particularly evident in coastal species that inhabit dynamic environments, such as E. alletteratus, and can be observed in the morphometry of their otoliths. The high differentiation and classification rates observed for our research-collected otoliths indicate that, as previously confirmed by genetic analyses, it follows that there are clear otolith shape differences between the Northeast Temperate Atlantic together with the Mediterranean Sea and Eastern Tropical Atlantic areas with a high degree of confidence. A pattern that is repeated in this type of study in the differentiation of species from shape otoliths, which is also fulfilled in our work, is that at least three otolith morphological descriptors analyzed show significant variations between the groups of individuals analyzed and 25% of the otolith outlines present great divergences [85,86,87,88,89,90,91]. Perhaps these are results to consider when carrying out an analysis of this type for the differentiation between species. This may indicate certain isolation among localities that are nearby each other geographically or that there are natural environmental barriers that prevent mixing between both areas [93]. This situation is not common in pelagic fish species and even less in tuna, since they tend to migrate over medium or long distances, both along coastal areas and on open sea [94,95,96,97]. There are cases of restricted geographic expansion in some tuna species in the Atlantic waters, such as Thunnus atlanticus, which is distributed exclusively in tropical and subtropical waters of the Western Atlantic Ocean, ranging from the mid-Atlantic region of the United States east coast to northern Brazil, including the Gulf of Mexico [98]. In the same way, Auxis thazard, although it can be found throughout the Atlantic Ocean, is not distributed along the Mediterranean Sea, where Auxis rochei is dominant [71]. However, there are records mainly from the Strait of Gibraltar, the area where water masses interchanges between the Atlantic Ocean and the Mediterranean Sea [72].
From the analysis in the paper, the differentiation results are similar to other previously published results for different species of one same genus [27,91,99,100,101]. Some studies have been carried out using otolith shape analysis in tunas and small tunas, mainly for stock delimitation [29,30,31]. To our knowledge, however, this is the first published study to use otolith shape to validate tuna spatial units’ differentiation that might correspond to different species. This could be applied to other genera with several species (e.g., Thunnus spp. or Auxis spp.) and would help to improve the accuracy of fisheries monitoring and facilitate re-classification of previously collected samples where the identification to the species level is problematic. Applying the technique to observer-sourced otolith collections would also improve confidence in datasets for analyses of the biological and ecological differences between species [102].
Otolith shape analysis complements the genetics study already published on stock structure of this species [65]. The findings from our research on little tunny in the Eastern Atlantic have unveiled a new perspective. There are clear indications of a species-level segregation between two distinct regions, which are presently regarded as a single species, challenging the existing geographic division at the stock level proposed by ICCAT [35]. This discovery holds significant implications for both the collection of scientific data and commercial fishing data, necessary for fisheries management. Furthermore, future investigations should focus on exploring new biological parameters, such as reproduction and growth, in both defined areas. A comparative analysis of these parameters could provide valuable insights into potential species-specific differences [103,104]. Moreover, to enhance our understanding of this phenomenon, additional samples are needed to gather comprehensive information from intermediate zones such as the waters of Morocco and Mauritania, as well as unexplored regions such as the Eastern Mediterranean and the Northeastern Atlantic.
5. Conclusions
This study demonstrates that the fish otolith shape can be utilized to validate the differentiation of tuna species. In addition to the limited literature available on the shape otoliths in tunas, no reports have been found regarding otolith asymmetries in these species. To address this issue, it is important to conduct a comprehensive comparative analysis of asymmetry in the left and right pairs of sagittal otoliths in the future. This analysis would greatly contribute to our understanding of this aspect and help fill this knowledge void. It is manifest that the pattern presented in this work does not comply with the current single accepted species Euthynnus alletteratus distributed along the Atlantic Ocean. A revised classification, considering the observed genetic and morphological evidence, should rather characterize the species of genus Euthynnus occupying the Atlantic Ocean, distinguishing Eastern Tropical Atlantic individuals as a new species of little tunny. To enhance the analysis in the future, it is crucial to include individuals from previously unexplored regions, such as the Eastern Mediterranean or the Western Atlantic, as well as unanalyzed intermediate zones such as the coasts of Mauritania or Morocco. By expanding the geographical scope to encompass these regions, we could gain valuable insights into little tunny populations and their distribution in previously understudied areas. It is imperative to approach this issue rigorously, as it can lead to significant consequences for fisheries management.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes8060317/s1, Figure S1: Median (white bar) and inter-quartile bounds (box) of Wavelet descriptors for the Northeast Temperate Atlantic together with the Mediterranean Sea (NETAM Area) and the Eastern Tropical Atlantic (ETA Area) of little tunny samples analyzed. Significant differences of t-test analysis between areas by descriptors are included in each graph.
Author Contributions
Conceptualization, R.M.-L. and P.G.L.; methodology, R.M.-L.; software, R.M.-L.; validation, G.B.d.S. and P.G.L.; formal analysis, R.M.-L.; investigation, R.M.-L.; resources, R.M.-L., F.N.S., D.N.C., D.A., D.M. and A.M.-G.; data curation, R.M.-L.; writing—original draft preparation, R.M.-L. and P.G.L.; writing—review and editing, F.N.S., D.N.C., D.A., D.M., A.M.-G., G.B.d.S. and J.M.S.G.; supervision, P.G.L. and J.M.S.G.; project administration, G.B.d.S. and P.G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ICCAT Small Tunas Year Program (SMTYP) and partially by the European Union through the EU Grant Agreement No. S12.819116—Strengthening the scientific basis for decision-making in ICCAT.
Institutional Review Board Statement
Not applicable. All fish sampling involves deceased individuals obtained from the commercial fishing fleet, specifically purchased at the fish market. The Institutional Review Board approval is not applicable for this research.
Data Availability Statement
The raw data that support this study are available from the corresponding author upon reasonable request.
Acknowledgments
This work was carried out within the IPMA Portuguese National Program for Biological Sampling (PNAB), integrated in the EU Data Collection Framework (DCF). The authors are grateful to all people and members of the ICCAT Small Tuna Species Group who were of great help with the data and sampling collection. This work was carried out under the provision of the ICCAT Small Tunas Year Program (SMTYP). The contents of this paper do not necessarily reflect the point of view of ICCAT, which has no responsibility over them, and in no ways anticipate the Commission’s future policy in this area. We express our gratitude to three anonymous referees whose valuable comments significantly enhanced the quality of our paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Saltalamacchia, F.; Berg, F.; Casini, M.; Davies, J.C.; Bartolino, V. Population Structure of European Sprat (Sprattus sprattus) in the Greater North Sea Ecoregion Revealed by Otolith Shape Analysis. Fish. Res. 2022, 245, 106131. [Google Scholar] [CrossRef]
- Cadrin, S.X.; Karr, L.A.; Mariani, S. (Eds.) Stock Identification Methods: Applications in Fishery Science, 2nd ed.; Academic Press: London, UK; Waltham, MA, USA; San Diego, CA, USA, 2014. [Google Scholar]
- Begg, G.A.; Friedland, K.D.; Pearce, J.B. Stock Identification and Its Role in Stock Assessment and Fisheries Management: An Overview. Fish. Res. 1999, 43, 1–8. [Google Scholar] [CrossRef]
- Begg, G.A.; Waldman, J.R. An Holistic Approach to Fish Stock Identification. Fish. Res. 1999, 43, 35–44. [Google Scholar] [CrossRef]
- Goethel, D.R.; Quinn, T.J.; Cadrin, S.X. Incorporating Spatial Structure in Stock Assessment: Movement Modeling in Marine Fish Population Dynamics. Rev. Fish. Sci. 2011, 19, 119–136. [Google Scholar] [CrossRef]
- Utter, F.; Ryman, N. Genetic Markers and Mixed Stock Fisheries. Fisheries 1993, 18, 11–21. [Google Scholar] [CrossRef]
- Reiss, H.; Hoarau, G.; Dickey-Collas, M.; Wolff, W.J. Genetic Population Structure of Marine Fish: Mismatch between Biological and Fisheries Management Units. Fish Fish. 2009, 10, 361–395. [Google Scholar] [CrossRef]
- Laikre, L.; Palm, S.; Ryman, N. Genetic Population Structure of Fishes: Implications for Coastal Zone Management. Ambio J. Hum. Environ. 2005, 34, 111–119. [Google Scholar] [CrossRef]
- Begg, G.A.; Hare, J.A.; Sheehan, D.D. The Role of Life History Parameters as Indicators of Stock Structure. Fish. Res. 1999, 43, 141–163. [Google Scholar] [CrossRef]
- Lester, R.J.G. Reappraisal of the Use of Parasites for Fish Stock Identification. Mar. Freshw. Res. 1990, 41, 855–864. [Google Scholar] [CrossRef]
- Timi, J.T. Parasites as Biological Tags for Stock Discrimination in Marine Fish from South American Atlantic Waters. J. Helminthol. 2007, 81, 107–111. [Google Scholar] [CrossRef]
- Dickhut, R.M.; Deshpande, A.D.; Cincinelli, A.; Cochran, M.A.; Corsolini, S.; Brill, R.W.; Secor, D.H.; Graves, J.E. Atlantic Bluefin Tuna (Thunnus thynnus) Population Dynamics Delineated by Organochlorine Tracers. Environ. Sci. Technol. 2009, 43, 8522–8527. [Google Scholar] [CrossRef] [PubMed]
- Begg, G.A.; Campana, S.E.; Fowler, A.J.; Suthers, I.M. Otolith Research and Application: Current Directions in Innovation and Implementation. Mar. Freshw. Res. 2005, 56, 477–483. [Google Scholar] [CrossRef]
- Kalish, J.M. Otolith Microchemistry: Validation of the Effects of Physiology, Age and Environment on Otolith Composition. J. Exp. Mar. Biol. Ecol. 1989, 132, 151–178. [Google Scholar] [CrossRef]
- Campana, S.E.; Neilson, J.D. Microstructure of Fish Otoliths. Can. J. Fish. Aquat. Sci. 1985, 42, 1014–1032. [Google Scholar] [CrossRef]
- Campana, S.E. Otolith Science Entering the 21st Century. Mar. Freshw. Res. 2005, 56, 485–495. [Google Scholar] [CrossRef]
- Campana, S.E.; Casselman, J.M. Stock Discrimination Using Otolith Shape Analysis. Can. J. Fish. Aquat. Sci. 1993, 50, 1062–1083. [Google Scholar] [CrossRef]
- D’Iglio, C.; Natale, S.; Albano, M.; Savoca, S.; Famulari, S.; Gervasi, C.; Lanteri, G.; Panarello, G.; Spanò, N.; Capillo, G. Otolith Analyses Highlight Morpho-Functional Differences of Three Species of Mullet (Mugilidae) from Transitional Water. Sustainability 2021, 14, 398. [Google Scholar] [CrossRef]
- Campana, S.E. Long-Term Shifts in Otolith Age Interpretations. Fish. Res. 2023, 263, 106681. [Google Scholar] [CrossRef]
- Korostelev, N.B.; Frey, P.H.; Orlov, A.M. Using Different Hard Structures to Estimate the Age of Deep-Sea Fishes: A Case Study of the Pacific Flatnose, Antimora microlepis (Moridae, Gadiformes, Teleostei). Fish. Res. 2020, 232, 105731. [Google Scholar] [CrossRef]
- Ibáñez, C.M.; Riera, R.; Leite, T.; Díaz-Santana-Iturrios, M.; Rosa, R.; Pardo-Gandarillas, M.C. Stomach Content Analysis in Cephalopods: Past Research, Current Challenges, and Future Directions. Rev. Fish Biol. Fish. 2021, 31, 505–522. [Google Scholar] [CrossRef]
- Mahé, K.; Ider, D.; Massaro, A.; Hamed, O.; Jurado-Ruzafa, A.; Gonçalves, P.; Anastasopoulou, A.; Jadaud, A.; Mytilineou, C.; Elleboode, R.; et al. Directional Bilateral Asymmetry in Otolith Morphology May Affect Fish Stock Discrimination Based on Otolith Shape Analysis. ICES J. Mar. Sci. 2019, 76, 232–243. [Google Scholar] [CrossRef]
- Hüssy, K.; Mosegaard, H.; Albertsen, C.M.; Nielsen, E.E.; Hemmer-Hansen, J.; Eero, M. Evaluation of Otolith Shape as a Tool for Stock Discrimination in Marine Fishes Using Baltic Sea Cod as a Case Study. Fish. Res. 2016, 174, 210–218. [Google Scholar] [CrossRef]
- Cardinale, M.; Doering-Arjes, P.; Kastowsky, M.; Mosegaard, H. Effects of Sex, Stock, and Environment on the Shape of Known-Age Atlantic Cod (Gadus morhua) Otoliths. Can. J. Fish. Aquat. Sci. 2004, 61, 158–167. [Google Scholar] [CrossRef]
- Hüssy, K. Otolith Shape in Juvenile Cod (Gadus morhua): Ontogenetic and Environmental Effects. J. Exp. Mar. Biol. Ecol. 2008, 364, 35–41. [Google Scholar] [CrossRef]
- Vignon, M.; Morat, F. Environmental and Genetic Determinant of Otolith Shape Revealed by a Non-Indigenous Tropical Fish. Mar. Ecol. Prog. Ser. 2010, 411, 231–241. [Google Scholar] [CrossRef]
- Afanasyev, P.K.; Orlov, A.M.; Rolsky, A.Y. Otolith Shape Analysis as a Tool for Species Identification and Studying the Population Structure of Different Fish Species. Biol. Bull. 2017, 44, 952–959. [Google Scholar] [CrossRef]
- Ponton, D. Is Geometric Morphometrics Efficient for Comparing Otolith Shape of Different Fish Species? J. Morphol. 2006, 267, 750–757. [Google Scholar] [CrossRef]
- Brophy, D.; Haynes, P.; Arrizabalaga, H.; Fraile, I.; Fromentin, J.M.; Garibaldi, F.; Katavic, I.; Tinti, F.; Karakulak, F.S.; Macías, D. Otolith Shape Variation Provides a Marker of Stock Origin for North Atlantic Bluefin Tuna (Thunnus thynnus). Mar. Freshw. Res. 2015, 67, 1023–1036. [Google Scholar] [CrossRef]
- Duncan, R.; Brophy, D.; Arrizabalaga, H. Otolith Shape Analysis as a Tool for Stock Separation of Albacore Tuna Feeding in the Northeast Atlantic. Fish. Res. 2018, 200, 68–74. [Google Scholar] [CrossRef]
- de Souza Corrêa, G.M.; Coletto, J.L.; Castello, J.P.; Miller, N.R.; de Almeida Tubino, R.; Neto, C.M.; da Costa, M.R. Identification of Fish Stock Based on Otolith as a Natural Marker: The Case of Katsuwonus pelamis (Linnaeus, 1758) in the Southwest Atlantic Ocean. Fish. Res. 2022, 255, 106436. [Google Scholar] [CrossRef]
- Pons, M.; Kell, L.; Rudd, M.B.; Cope, J.M.; Lucena Frédou, F. Performance of Length-Based Data-Limited Methods in a Multifleet Context: Application to Small Tunas, Mackerels, and Bonitos in the Atlantic Ocean. ICES J. Mar. Sci. 2019, 76, 960–973. [Google Scholar] [CrossRef]
- Collette, B.B.; Nauen, C.E. FAO Species Catalogue: Vol. 2 Scombrids of the World—An Annotated and Illustrated Catalogue of Tunas, Mackerels, Bonitos and Related Species Known to Date; FAO Fisheries Synopsis No. 125; FAO: Rome, Italy, 1983; 137p. [Google Scholar]
- Majkowski, J. Global Fishery Resources of Tuna and Tuna-like Species; FAO Fisheries Technical Paper 483; FAO: Rome, Italy, 2007. [Google Scholar]
- Lucena-Frédou, F.; Mourato, B.; Frédou, T.; Lino, P.G.; Muñoz-Lechuga, R.; Palma, C.; Soares, A.; Pons, M. Review of the Life History, Fisheries, and Stock Assessment for Small Tunas in the Atlantic Ocean. Rev. Fish Biol. Fish. 2021, 31, 709–736. [Google Scholar] [CrossRef]
- Levesque, J.C. International Fisheries Agreement: Review of the International Commission for the Conservation of Atlantic Tunas: Case Study—Shark Management. Mar. Policy 2008, 32, 528–533. [Google Scholar] [CrossRef]
- Anonymous. Report of the 2021 ICCAT Small Tunas Species Group Intersessional Meeting. Collect. Vol. Sci. Pap. ICCAT 2021, 78, 1–65. [Google Scholar]
- Cayré, P.; Diouf, T. Croissance de La Thonine Euthynnus alletteratus (Rafinesque, 1810) Etablie a Partir de Coupes Tranversales Du Premier Rayon de La Nageoire Dorsale; CRODT: Dakar, Senegal, 1980. [Google Scholar]
- El-Haweet, A.E.; Sabry, E.; Mohamed, H. Fishery and Population Characteristics of Euthynnus alletteratus (Rafinesque 1810) in the Eastern Coast of Alexandria, Egypt. Turk. J. Fish. Aquat. Sci. 2013, 13, 629–638. [Google Scholar] [CrossRef]
- Hajjej, G.; Hattour, A.; Hajjej, A.; Cherif, M.; Allaya, H.; Jarboui, O.; Bouain, A. Age and Growth of Little Tunny, Euthynnus alletteratus (Rafinesque, 1810), from the Tunisian Mediterranean Coasts. Cah. Biol. Mar. 2012, 53, 113–122. [Google Scholar] [CrossRef]
- Johnson, A.G. Comparison of Dorsal Spines and Vertebrae as Ageing Structures for Little Tunny, Euthynnus alletteratus, from the Northeast Gulf of Mexico. In Proceedings of the International Workshop on Age Determination of Oceanic Pelagic Fishes: Tunas, Billfishes and Sharks, Southeast Fisheries Center, Miami Laboratory, National Marine Fisheries Service, NOAA, Miami, FL, USA, 15–18 February 1982; NOAA Technical Report NMFS 8. U.S. Department of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service: Silver Spring, MD, USA, 1983; pp. 111–115. [Google Scholar]
- Kahraman, A.E.; Oray, I.K. The Determination of Age and Growth Parameters of Atlantic Little Tunny Euthynnus alleteratus (Rafinesque, 1810) in Turkish Waters. Collect. Vol. Sci. Pap. ICCAT 2001, 52, 719–732. [Google Scholar]
- Valeiras, X.; Macías, D.; Gómez, M.J.; Lema, L.; Godoy, D.; Ortiz de Urbina, J.M.; de la Serna, J.M. Age and Growth of Atlantic Little Tuna (Euthynnus alletteratus) in the Western Mediterranean Sea. Collect. Vol. Sci. Pap. ICCAT 2008, 62, 1638–1648. [Google Scholar]
- Vieira, J.; Costa, P.A.S.; Braga, A.C.; São-Clemente, R.R.B.; Ferreira, C.E.L.; Silva, J.P. Age, Growth, and Maturity of Little Tunny, Euthynnus alletteratus (Rafinesque, 1810) in Southeastern Brazil. Lat. Am. J. Aquat. Res. 2021, 49, 773–787. [Google Scholar]
- Bahou, L.; d’Almeida, M.-A.; Koné, T.; Boua, C.A.; Séripka, G.D. Reproductive Biology and Histological Characteristics of Female Little Tunny Euthynnus alletteratus (Rafinesque, 1810) Caught on Continental Shelf of Côte d’Ivoire. Sci. J. Biol. Sci. 2016, 5, 88–102. [Google Scholar]
- Cruz-Castán, R.; Meiners-Mandujano, C.; Macías, D.; Jiménez-Badillo, L.; Curiel-Ramírez, S. Reproductive Biology of Little Tunny Euthynnus alletteratus (Rafinesque, 1810) in the Southwest Gulf of Mexico. PeerJ 2019, 7, e6558. [Google Scholar] [CrossRef] [PubMed]
- Gaykov, V.Z.; Bokhanov, D. V The Biological Characteristic of Atlantic Black Skipjack (Euthynnus alletteratus) of the Eastern Atlantic Ocean. Collect. Vol. Sci. Pap. ICCAT 2008, 62, 1610–1628. [Google Scholar]
- Hajjej, G.; Hattour, A.; Allaya, H.; Jarboui, O.; Bouain, A. Some Biological Parameters of the Little Tuna Euthynnus alletteratus (Rafinesque, 1810) in Tunisian Waters. Cah. Biol. Mar. 2011, 52, 33–40. [Google Scholar]
- Kahraman, A.E.; Alicli, T.Z.; Akayli, T.; Oray, I.K. Reproductive Biology of Little Tunny, Euthynnus alletteratus (Rafinesque), from the North-eastern Mediterranean Sea. J. Appl. Ichthyol. 2008, 24, 551–554. [Google Scholar] [CrossRef]
- Mohamed, H.; El-Haweet, A.E.; Sabry, E. Reproductive Biology of Little Tunny, Euthynnus alletteratus (Rafinesque 1810) in the Eastern Coast of Alexandria, Egypt. Egypt. J. Aquat. Biol. Fish. 2014, 18, 139–150. [Google Scholar]
- Saber, S.; De Urbina, J.O.; Lino, P.G.; Gómez-Vives, M.J.; Coelho, R.; Muñoz-Lechuga, R.; Macías, D. Biological Aspects of Little Tunny Euthynnus alletteratus from Spanish and Portuguese Waters. Collect. Vol. Sci. Pap. ICCAT 2018, 75, 95–110. [Google Scholar]
- Libungan, L.A.; Pálsson, S. ShapeR: An R Package to Study Otolith Shape Variation among Fish Populations. PLoS ONE 2015, 10, e0121102. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing: Version 4.2.2; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Libungan, L.; Pálsson, S. shapeR: Collection and Analysis of Otolith Shape Data; R Package Version 1.0-1; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package; R Package Version 2.4-2; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Anderson, M.J.; Willis, T.J. Canonical Analysis of Principal Coordinates: A Useful Method of Constrained Ordination for Ecology. Ecology 2003, 84, 511–525. [Google Scholar] [CrossRef]
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, W.H.A.; Lumley, T.; Maechler, M.; Magnusson, A.; Moeller, S.; et al. gplots: Various R Programming Tools for Plotting Data; R Package Version 3.1.3; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Libungan, L.; Slotte, A.; Otis, E.; Pálsson, S. Otolith Variation in Pacific Herring (Clupea pallasii) Reflects Mitogenomic Variation Rather than the Subspecies Classification. Polar Biol. 2016, 39, 1571–1579. [Google Scholar] [CrossRef]
- Palumbi, S.R. Genetic Divergence, Reproductive Isolation, and Marine Speciation. Annu. Rev. Ecol. Syst. 1994, 25, 547–572. [Google Scholar] [CrossRef]
- Ferguson, J.W.H. On the Use of Genetic Divergence for Identifying Species. Biol. J. Linn. Soc. 2002, 75, 509–516. [Google Scholar] [CrossRef]
- Patarnello, T.; Volckaert, F.A.M.J.; Castilho, R. Pillars of Hercules: Is the Atlantic–Mediterranean Transition a Phylogeographical Break? Mol. Ecol. 2007, 16, 4426–4444. [Google Scholar] [CrossRef]
- Kikuchi, E.; Cardoso, L.G.; Canel, D.; Timi, J.T.; Haimovici, M. Using Growth Rates and Otolith Shape to Identify the Population Structure of Umbrina canosai (Sciaenidae) from the Southwestern Atlantic. Mar. Biol. Res. 2021, 17, 272–285. [Google Scholar] [CrossRef]
- Tedesco, P.A.; Paradis, E.; Lévêque, C.; Hugueny, B. Explaining Global-scale Diversification Patterns in Actinopterygian Fishes. J. Biogeogr. 2017, 44, 773–783. [Google Scholar] [CrossRef]
- Turner, G.F. What Is a Fish Species? Rev. Fish Biol. Fish. 1999, 9, 281–297. [Google Scholar] [CrossRef]
- Ollé, J.; Vilà-Valls, L.; Alvarado-Bremer, J.; Cerdenares, G.; Duong, T.Y.; Hajjej, G.; Lino, P.G.; Muñoz-Lechuga, R.; Sow, F.N.; Diaha, N.C.; et al. Population Genetics Meets Phylogenetics: New Insights into the Relationships among Members of the Genus Euthynnus (Family Scombridae). Hydrobiologia 2022, 849, 47–62. [Google Scholar] [CrossRef]
- Alvarado Bremer, J.R.; Ely, B. Pronounced Levels of Genetic Differentiation among Two Trans-Atlantic Samples of Little Tunny (Euthynus alletteratus). Collect. Vol. Sci. Pap. ICCAT 1999, 49, 236–242. [Google Scholar]
- Cayré, P.M.; Diouf, T. Estimating Age and Growth of Little Tunny, Euthynnus alletteratus, off the Coast of Senegal Using Dorsal Fin Spine Sections. In Proceedings of the International Workshop on Age Determination of Oceanic Pelagic Fishes: Tunas, Billfishes and Sharks, Southeast Fisheries Center, Miami Laboratory, National Marine Fisheries Service, NOAA, Miami, FL, USA, 15–18 February 1982; NOAA Technical Report NMFS 8. U.S. Department of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service: Silver Spring, MD, USA, 1983; pp. 105–110. [Google Scholar]
- Adams, J.L.; Kerstetter, D.W. Age and Growth of Three Coastal-Pelagic Tunas (Actinopterygii: Perciformes: Scombridae) in the Florida Straits, USA: Blackfin Tuna, Thunnus atlanticus, Little Tunny, Euthynnus alletteratus, and Skipjack Tuna, Katsuwonus pelamis. Acta Ichthyol. Piscat. 2014, 44, 201–211. [Google Scholar] [CrossRef]
- Hajjej, G.; Hattour, A.; Allaya, H.; Jarboui, O.; Bouain, A. Biology of Little Tunny Euthynnus alletteratus in the Gulf of Gabes, Southern Tunisia (Central Mediterranean Sea). Rev. Biol. Mar. Oceanogr. 2010, 45, 399–406. [Google Scholar] [CrossRef]
- Hajjej, G.; Hattour, A.; Hajjej, A.; Allaya, H.; Jarboui, O.; Bouain, A. Morphological Variation of Little Tuna Euthynnus alletteratus in Tunisian Waters and Eastern Atlantic. Panam. J. Aquat. Sci. 2013, 8, 1–9. [Google Scholar]
- Orsi Relini, L.; Palandri, G.; Garibaldi, F.; Lanteri, L.; Cilli, G.; Ferrara, G.; Tinti, F. Towards a New Taxonomical Approach to Mediterranean Small Tuna of Genus Auxis. Biol. Mar. Mediterr. 2008, 15, 207–210. [Google Scholar]
- Ollé, J.; Macías, D.; Saber, S.; José Gómez-Vives, M.; Pérez-Bielsa, N.; Viñas, J. Genetic Analysis Reveals the Presence of Frigate Tuna (Auxis thazard) in the Bullet Tuna (Auxis rochei) Fishery of the Iberian Peninsula and the Western-Central Mediterranean Sea. Bull. Mar. Sci. 2019, 95, 317–325. [Google Scholar] [CrossRef]
- Kumar, G.; Kocour, M. Population Genetic Structure of Tunas Inferred from Molecular Markers: A Review. Rev. Fish. Sci. Aquac. 2015, 23, 72–89. [Google Scholar] [CrossRef]
- Catanese, G.; Manchado, M.; Infante, C. Evolutionary Relatedness of Mackerels of the Genus Scomber Based on Complete Mitochondrial Genomes: Strong Support to the Recognition of Atlantic Scomber colias and Pacific Scomber japonicus as Distinct Species. Gene 2010, 452, 35–43. [Google Scholar] [CrossRef]
- Infante, C.; Blanco, E.; Zuasti, E.; Crespo, A.; Manchado, M. Phylogenetic Differentiation between Atlantic Scomber colias and Pacific Scomber japonicus Based on Nuclear DNA Sequences. Genetica 2007, 130, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Karaiskou, N.; Triantafyllidis, A.; Triantaphyllidis, C. Shallow Genetic Structure of Three Species of the Genus Trachurus in European Waters. Mar. Ecol. Prog. Ser. 2004, 281, 193–205. [Google Scholar] [CrossRef]
- Ward, R.D.; Costa, F.O.; Holmes, B.H.; Steinke, D. DNA Barcoding of Shared Fish Species from the North Atlantic and Australasia: Minimal Divergence for Most Taxa, but Zeus faber and Lepidopus caudatus Each Probably Constitute Two Species. Aquat. Biol. 2008, 3, 71–78. [Google Scholar] [CrossRef]
- Bargelloni, L.; Alarcon, J.A.; Alvarez, M.C.; Penzo, E.; Magoulas, A.; Palma, J.; Patarnello, T. The Atlantic–Mediterranean Transition: Discordant Genetic Patterns in Two Seabream Species, Diplodus puntazzo (Cetti) and Diplodus sargus (L.). Mol. Phylogenet. Evol. 2005, 36, 523–535. [Google Scholar] [CrossRef]
- Bacha, M.; Jemaa, S.; Hamitouche, A.; Rabhi, K.; Amara, R. Population Structure of the European Anchovy, Engraulis encrasicolus, in the SW Mediterranean Sea, and the Atlantic Ocean: Evidence from Otolith Shape Analysis. ICES J. Mar. Sci. 2014, 71, 2429–2435. [Google Scholar] [CrossRef]
- Vieira, A.R.; Neves, A.; Sequeira, V.; Paiva, R.B.; Gordo, L.S. Otolith Shape Analysis as a Tool for Stock Discrimination of Forkbeard (Phycis phycis) in the Northeast Atlantic. Hydrobiologia 2014, 728, 103–110. [Google Scholar] [CrossRef]
- Moreira, C.; Froufe, E.; Vaz-Pires, P.; Correia, A.T. Otolith Shape Analysis as a Tool to Infer the Population Structure of the Blue Jack Mackerel, Trachurus picturatus, in the NE Atlantic. Fish. Res. 2019, 209, 40–48. [Google Scholar] [CrossRef]
- Mahe, K.; Oudard, C.; Mille, T.; Keating, J.; Gonçalves, P.; Clausen, L.W.; Petursdottir, G.; Rasmussen, H.; Meland, E.; Mullins, E.; et al. Identifying Blue Whiting (Micromesistius poutassou) Stock Structure in the Northeast Atlantic by Otolith Shape Analysis. Can. J. Fish. Aquat. Sci. 2016, 73, 1363–1371. [Google Scholar] [CrossRef]
- Stransky, C.; Murta, A.G.; Schlickeisen, J.; Zimmermann, C. Otolith Shape Analysis as a Tool for Stock Separation of Horse Mackerel (Trachurus trachurus) in the Northeast Atlantic and Mediterranean. Fish. Res. 2008, 89, 159–166. [Google Scholar] [CrossRef]
- Agüera, A.; Brophy, D. Use of Saggital Otolith Shape Analysis to Discriminate Northeast Atlantic and Western Mediterranean Stocks of Atlantic Saury, Scomberesox saurus saurus (Walbaum). Fish. Res. 2011, 110, 465–471. [Google Scholar] [CrossRef]
- Yu, X.; Cao, L.; Liu, J.; Zhao, B.; Shan, X.; Dou, S. Application of Otolith Shape Analysis for Stock Discrimination and Species Identification of Five Goby Species (Perciformes: Gobiidae) in the Northern Chinese Coastal Waters. Chin. J. Oceanol. Limnol. 2014, 32, 1060–1073. [Google Scholar] [CrossRef]
- He, T.; Cheng, J.; Qin, J.; Li, Y.; Gao, T. Comparative Analysis of Otolith Morphology in Three Species of Scomber. Ichthyol. Res. 2018, 65, 192–201. [Google Scholar] [CrossRef]
- Tuset, V.M.; Parisi-Baradad, V.; Lombarte, A. Application of Otolith Mass and Shape for Discriminating Scabbardfishes Aphanopus spp. in the North-eastern Atlantic Ocean. J. Fish. Biol. 2013, 82, 1746–1752. [Google Scholar] [CrossRef]
- Zhuang, L.; Ye, Z.; Zhang, C. Application of Otolith Shape Analysis to Species Separation in Sebastes spp. from the Bohai Sea and the Yellow Sea, Northwest Pacific. Environ. Biol. Fishes 2015, 98, 547–558. [Google Scholar] [CrossRef]
- Pavlov, D.A. Differentiation of Three Species of the Genus Upeneus (Mullidae) Based on Otolith Shape Analysis. J. Ichthyol. 2016, 56, 37–51. [Google Scholar] [CrossRef]
- L’Abée-Lund, J.H. Otolith Shape Discriminates between Juvenile Atlantic Salmon, Salmo salar L., and Brown Trout, Salmo trutta L. J. Fish Biol. 1988, 33, 899–903. [Google Scholar] [CrossRef]
- Morales, C.J.C.; Barnuevo, K.D.E.; Delloro, E.S., Jr.; Cabebe-Barnuevo, R.A.; Calizo, J.K.S.; Lumayno, S.D.P.; Babaran, R.P. Otolith Morphometric and Shape Distinction of Three Redfin Species under the Genus Decapterus (Teleostei: Carangidae) from Sulu Sea, Philippines. Fishes 2023, 8, 95. [Google Scholar] [CrossRef]
- Morawicki, S.; Solimano, P.J.; Volpedo, A.V. Unravelling Stock Spatial Structure of Silverside Odontesthes argentinensis (Valenciennes, 1835) from the North Argentinian Coast by Otoliths Shape Analysis. Fishes 2022, 7, 155. [Google Scholar] [CrossRef]
- Selkoe, K.A.; Henzler, C.M.; Gaines, S.D. Seascape Genetics and the Spatial Ecology of Marine Populations. Fish Fish. 2008, 9, 363–377. [Google Scholar] [CrossRef]
- Ely, B.; Viñas, J.; Alvarado Bremer, J.R.; Black, D.; Lucas, L.; Covello, K.; Labrie, A.V.; Thelen, E. Consequences of the Historical Demography on the Global Population Structure of Two Highly Migratory Cosmopolitan Marine Fishes: The Yellowfin Tuna (Thunnus albacares) and the Skipjack Tuna (Katsuwonus pelamis). BMC Evol. Biol. 2005, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Fromentin, J.; Powers, J.E. Atlantic Bluefin Tuna: Population Dynamics, Ecology, Fisheries and Management. Fish Fish. 2005, 6, 281–306. [Google Scholar] [CrossRef]
- Gonzalez, E.G.; Beerli, P.; Zardoya, R. Genetic Structuring and Migration Patterns of Atlantic Bigeye Tuna, Thunnus obesus (Lowe, 1839). BMC Evol. Biol. 2008, 8, 252. [Google Scholar] [CrossRef]
- Vinas, J.; Bremer, J.A.; Pla, C. Phylogeography of the Atlantic Bonito (Sarda sarda) in the Northern Mediterranean: The Combined Effects of Historical Vicariance, Population Expansion, Secondary Invasion, and Isolation by Distance. Mol. Phylogenet. Evol. 2004, 33, 32–42. [Google Scholar] [CrossRef]
- Saillant, E.A.; Luque, P.L.; Short, E.; Antoni, L.; Reynal, L.; Pau, C.; Arocha, F.; Roque, P.; Hazin, F. Population Structure of Blackfin Tuna (Thunnus atlanticus) in the Western Atlantic Ocean Inferred from Microsatellite Loci. Sci. Rep. 2022, 12, 9830. [Google Scholar] [CrossRef]
- Vu, Q.T.; Kartavtsev, Y. Otolith Shape Analysis and Its Utilily for Identification of Two Smelt Species, Hypomesus japonicus and H. nipponensis (Osteichthyes, Osmeridae) from the Northwestern Sea of Japan with Inferences in Stock Discrimination of H. japonicus. Russ. J. Mar. Biol. 2020, 46, 431–440. [Google Scholar] [CrossRef]
- Tuset, V.M.; Rosin, P.L.; Lombarte, A. Sagittal Otolith Shape Used in the Identification of Fishes of the Genus Serranus. Fish. Res. 2006, 81, 316–325. [Google Scholar] [CrossRef]
- Torres, G.J.; Lombarte, A.; Morales-Nin, B. Sagittal Otolith Size and Shape Variability to Identify Geographical Intraspecific Differences in Three Species of the Genus Merluccius. J. Mar. Biol. Assoc. UK 2000, 80, 333–342. [Google Scholar] [CrossRef]
- Moore, B.R.; Parker, S.J.; Pinkerton, M.H. Otolith Shape as a Tool for Species Identification of the Grenadiers Macrourus caml and M. whitsoni. Fish. Res. 2022, 253, 106370. [Google Scholar] [CrossRef]
- Murua, H.; Rodriguez-Marin, E.; Neilson, J.D.; Farley, J.H.; Juan-Jordá, M.J. Fast versus Slow Growing Tuna Species: Age, Growth, and Implications for Population Dynamics and Fisheries Management. Rev. Fish Biol. Fish. 2017, 27, 733–773. [Google Scholar] [CrossRef]
- Farley, J.H.; Williams, A.J.; Hoyle, S.D.; Davies, C.R.; Nicol, S.J. Reproductive Dynamics and Potential Annual Fecundity of South Pacific Albacore Tuna (Thunnus alalunga). PLoS ONE 2013, 8, e60577. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).