Abstract
Oxygen is crucial for the survival of marine species. Yet, the ocean has experienced a loss of approximately 2% of its oxygen inventory since the last century, resulting in adverse impacts on marine life and ecosystems. In particular, changes in the gap between the supply and demand for dissolved oxygen lead to physiological and ecological variations, which cause alterations in habitats and food webs for fish and ecosystem services. These changes vary over time and by region, and the heterogeneous characteristics of marine species bring about non-linear consequences to human society. Despite this, identifying the potential ripple effects of deoxygenation on human society is challenging due to the integrated impacts of other stressors, such as global warming and ocean acidification, and their varying changes depending on environmental conditions and regions, such as upwelling and eutrophication. Therefore, we conducted a literature review on ocean deoxygenation and its effects on fish dynamics and the ecosystem, with a focus on the environmental and societal impact, to present crucial considerations and pathways for future research on ocean deoxygenation. We found that quantitative approaches are necessary to assess the dynamic changes under deoxygenation, and the consequent effects on marine ecosystems should be verified to exploit the natural resources from the ocean. One of the most reliable approaches to quantifying the ripple impacts of deoxygenation is to model spatial and temporal changes with other climate stressors, forming a global network encompassing socio-economic and regional effects of this global change to facilitate and improve capabilities to address the impacts of ocean deoxygenation.
Key Contribution:
In this study, we evaluated the existing research on ocean deoxygenation and its effects on marine ecosystems and the services they provide to human societies. Our findings suggest that the scarcity of research on societal impacts may be attributed to the complex challenges posed by multiple climate stressors and differences in deoxygenation intensities by region and time. To address the remaining uncertainties regarding the societal impacts of deoxygenation, we suggest the development of models that incorporate spatio-temporal simulations of oxygen content and changes in marine ecosystems. Such models would provide a more comprehensive understanding of the potential consequences of deoxygenation and inform effective management strategies for marine ecosystems.
1. Introduction
Fishstocks and fisheries are crucial components of marine ecosystems, providing animal protein to 3.3 billion people and supporting the livelihoods of millions worldwide [1,2,3]. With an annual catch of approximately 100 million tonnes of marine fish, the industry generates about USD 150 billion in gross revenue and USD 450 billion in economic impacts [3,4]. However, the Global Fishing Index in 2021 revealed that 45% of assessed marine fish are overfished, posing a threat to sustainable exploitation. Climate change, including ocean deoxygenation, exacerbates this issue, further pressuring fish stocks and human welfare [5,6,7]. Therefore, it is imperative to investigate the relationship between climate change, in particular, ocean deoxygenation and the socio-economic impacts of fisheries to determine the optimal degree of fish stock exploitation that ensures sustainability for both humans and marine ecosystems. The objective of this review is to present crucial considerations and pathways for future research on ocean deoxygenation. The identification of potential impacts of deoxygenation on human society is constrained by the interplay of other climate stressors. Furthermore, the variability in the degree of deoxygenation across different environmental conditions and regions, such as upwelling and nutrient inputs from land, poses a challenge to predicting its potential effects.
Oxygen is crucial for the survival of aerobic organisms in marine ecosystems [8]; however, there has been a global decrease in ocean oxygen content since the mid-20th century [9], with a reduction of at least 2% in the global inventory [10]. This trend is projected to continue over the next few centuries, potentially leading to negative impacts on marine organisms and altering the ecosystem [11,12,13,14]. The reduction in oxygen in marine environments is primarily caused by two mechanisms, global warming and nutrient enrichment [15]. The rise in temperature has a direct effect on decreasing the solubility of oxygen, which, in turn, indirectly restricts the ventilation in the interior of the ocean [16]. Deoxygenation is observed in both the open ocean and the coastal-ocean continuum, extending up to the edge of the continental shelf and upwelling margins [8]. The mid-depth regions of the tropical Pacific, Atlantic, and Indian oceans, characterized by oxygen levels below 70 , have expanded to an extent equivalent to half the area of Canada [17]. In coastal waters, the discharge of nutrients has led to a more than tenfold increase in hypoxic areas, defined as regions with oxygen concentrations below 63 , compared to pre-1950 levels [18]. Ocean deoxygenation is considered one of the three major stressors, along with warming and ocean acidification, that are collectively referred to as the ‘deadly trio’ [19]. These stressors have been identified as leading to negative impacts on marine ecosystems [20]. Of the three stressors, deoxygenation is particularly concerning as it is likely to have the greatest impact on marine life and its ecosystem directly and indirectly, and leading to changes in the abundance and distribution of fish, which, in turn, affects fisheries [8,11,21].
This paper provides a review of the causes of deoxygenation and its impact on fish dynamics and marine ecosystems. It focuses on the analysis of deoxygenation from two key perspectives, natural and social science. The natural science aspect of the analysis examines the responses of fish to deoxygenation, changes in habitat, and alterations to the food-web. On the other hand, the social science component of the analysis discusses the impacts of deoxygenation on marine fisheries and changes in ecosystem services. By examining these factors, we identify potential directions for future research and modeling to better understand the potential effects of ocean deoxygenation on fish and fisheries management.
2. Methods
We conducted a literature review on ocean deoxygenation and its anticipated impacts on fish, fisheries, and marine ecosystem services. The search for relevant literature was restricted to the timeframe of 1985 to 2022, as no documents prior to 1980 were available online. The search was conducted through various search engines and citation databases, including Scopus, Google Scholar, Science Direct, scientific publications, non-governmental organizations, and technical reports, in order to gather a comprehensive collection of relevant documents. To streamline the search process, a comprehensive set of keywords and phrases were utilized, including “ocean deoxygenation”, “deoxygenation and fish”, “low oxygen effects on fish/fisheries/ecosystems”, “oxygen loss impacts on fish/fisheries/ecosystems”, and “hypoxia impacts in the ocean”. After conducting a thorough search, a total of 578 papers were found to be relevant to the topic of ocean deoxygenation. Out of these, 4 documents were screened and deemed to be of low relevance to our objective.
This literature review encompasses studies that investigate ocean deoxygenation and/or extreme oxygen loss events, which may be potentially associated with marine ecosystem services. This review presents empirical evidence on the relationship between the environment and marine fish in the context of a changing climate. As this topic is multidisciplinary, the articles included in this review are sourced from a range of research fields, including earth and planetary sciences, biological science, environmental science, biogeochemistry, physics, social and humanities, and economics.
We subsequently classified the documents according to the “Social Science” category, which encompasses the fields of social sciences and humanities, as well as economics, specifically pertaining to political, social, and cultural ramifications of ocean deoxygenation. The category of “Natural Science” pertains to the examination of the physical, ecological, and biological consequences of ocean deoxygenation, encompassing phenomena, such as matter, energy, and the principles of physics.
3. Trends in Ocean Deoxygenation Research
We compiled accessible documents including 574 research contributions that study the impacts of ocean deoxygenation on fish and fisheries. Based on the time span, there is a rapid increase in publications from the 2010s onward in natural science, while social science publications started increasing in 2021 (Figure 1). Studies on ocean deoxygenation recently had the attention of scientists, as 98% of the entire study has been conducted during the past decade. Despite the abundance of recent studies, research on the impacts of ocean deoxygenation lags behind that of other climate stressors, such as warming and acidification. Of particular concern is the fact that social science studies account for only 2% of the total research on ocean deoxygenation, indicating a significant gap in our understanding of the impact of this stress on human societies.
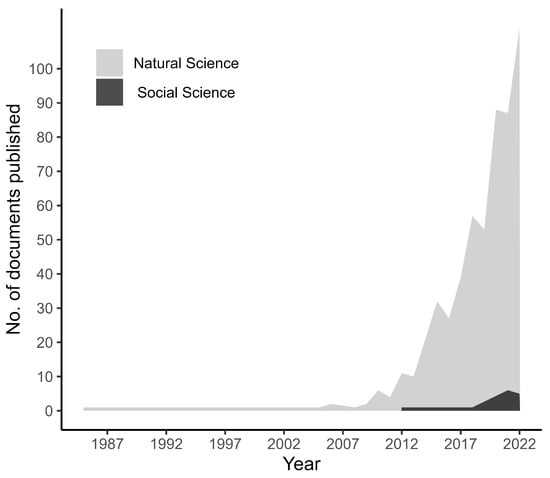
Figure 1.
The number of documents published in the fields of natural and social sciences on ocean deoxygenation up to the year 2022.
4. Ocean Deoxygenation Impacts on Natural Science
4.1. Deoxygenation in the World Ocean
Numerous regions across the globe have been identified as experiencing a decline in oxygen levels (Figure 2). The concentration of oxygen in the ocean is subject to modification through a range of physical and biogeochemical processes. The production of oxygen occurs through photosynthesis in the upper water column and through equilibration with the atmosphere. Conversely, oxygen is consumed through aerobic respiration by marine organisms.
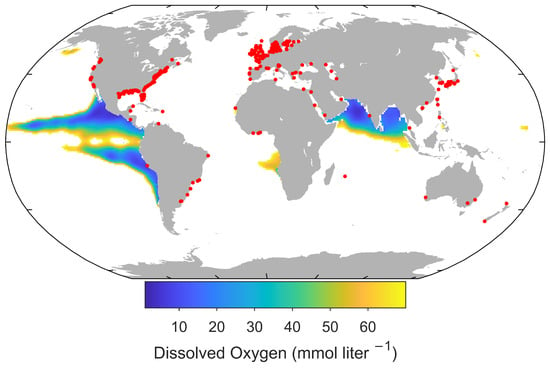
Figure 2.
Hypoxic areas (red dots; O < 63 O) and oxygen below 70 at depth of 200 m (colorbar). Dissolved oxygen was obtained from the World Ocean Atlas 2018 [22], and hypoxic areas were extracted from Diaz and Rosenberg 2008 [18].
Deoxygenation is a consequence of the disturbed balance between oxygen inflow from the atmosphere and outflow from the ocean. The replenishment of oxygen in the upper layer of the water column is facilitated by uptake from the atmosphere and photosynthesis. However, the water below the surface requires physical mechanisms to supplement oxygen. This process, referred to as ocean ventilation, involves the transfer of well-oxygenated water to mid- and deep-water layers. The ventilation process varies both vertically and horizontally and is influenced by various factors, such as advection, wind, tide, the inflow of rivers, and temperature [23].
Global warming is assessed as a primary cause of the widening gap between oxygen flows in the open ocean [13], reducing oxygen solubility and intensifying stratification near the surface water. This results in lower ventilation in line with increased respiration rates of marine organisms. Additionally, enhanced primary production by atmospheric inputs of nitrogen and iron also contributes to deoxygenation in the world ocean, producing more organic materials at the surface that are eventually consumed by mid-water microbial communities, increasing oxygen consumption [24,25]. As a result, Oxygen Minimum Zones (OMZs) are formed at mid-depth, primarily in Eastern boundary upwelling systems located in equatorial and coastal regions of the eastern Pacific and Atlantic oceans [26,27]. These systems convey oxygen-depleted and high-nutrient waters, which can affect natural oxygen depletion and the development of OMZs [8]. The strongest decreasing trend in oxygen levels at an OMZ is approximately 0.5 annually, detected over the Atlantic and equatorial eastern Pacific since the 1960s [17,25].
Anthropogenic nutrient enrichment, including human waste, sewage discharges, and agricultural fertilizer run-off, has led to eutrophication in coastal waters, resulting in deoxygenation in estuaries [15,23]. This is caused by increased primary and secondary production in surface waters, which consumes more oxygen and delays its mixing from the surface to the bottom. Currently, 65% of estuaries globally exhibit moderate or high eutrophication, and it is projected that over half of estuaries worldwide will worsen in the next two decades [28]. While deoxygenation varies regionally, it can impact nutrient budgets, biological productivity, and carbon fixation at regional to global scales [15]. Given that 98% of the global total catch comes from coastal waters (i.e., Economic Exclusive Zones) [29], understanding the local relationship between biogeochemistry and fisheries is crucial for assessing socio-economic impacts.
4.2. Fish Exposure to Deoxygenation
The primary productivity, nutrient and carbon cycling, and growth and environmental conditions of marine life are affected by changes in ocean oxygen content [13,30,31]. Deoxygenation creates an unequal status between limited oxygen supply from the water and biological oxygen demand for marine organisms [15]. In particular, fish are known to be highly susceptible to oxygen depletion compared to other marine organisms [32]. Perturbed oxygen balance in the ocean constrains growth, alters behavior, and increases mortality due to the limited capacity of marine fish to adapt to oxygen change [33,34]. Furthermore, the oxygen loss effect is expected to worsen in conjunction with other climate stressors, such as ocean warming and acidification, leading to a decline in fish populations [7,35,36].
The direct biological and physical impacts of deoxygenation on marine fish have been extensively studied and documented. Laboratory experiments have shown that low oxygen conditions can impair growth, with reductions in length and size of up to 89% observed in juvenile winter flounder, Atlantic menhaden, spot, and southern flounder [37,38,39,40]. Summer flounder have also been found to experience significant reductions in growth of up to 60% in lower oxygen conditions [41]. Field studies have further highlighted the significant gaps between sufficient and low-oxygen saturation for fish growth, with dab and plaice in Kattegat showing significantly lower weights in water with below 50% oxygen saturation [38].
In addition to impaired growth, deoxygenation has also been found to delay spawning and reduce reproduction rates due to the decline of energy circulation caused by hypoxia [42]. Repeated exposure to low oxygen can also alter immune responses, increase disease and worsen the eye vision of marine fish [43,44].
Although the physical responses of fish to insufficient oxygen content have been assessed, the impact on fish mortality is a complex issue due to the behavioral dynamics of fish, including habitat changes and cohort interactions, which vary depending on species and size. Consequently, there is a discrepancy among studies. For example, Breitburg (1994) found higher mortalities for two species, naked goby (demersal species) and anchovy (pelagic species), when exposed to lower oxygen concentration [45]; however, the author found that anchovies were more susceptible than naked goby, which contradicts the findings of other studies. Oxygen fluctuation has a significant influence on benthic fishes that differ from pelagic species [46,47,48]. This suggests that spatial approaches which consider the movements of fish would be required in combination with the impacts of deoxygenation to better understand the direct effect of hypoxia on fish mortality.
4.3. Impact on Habitat Displacement
Marine fish have relied on spatial movements as a survival strategy in response to environmental changes for centuries [49]. For instance, Wannamaker and Rice (2000) demonstrated that six estuarine species, including spot, pinfish, croaker, mullet, menhaden, and shrimp, could avoid low oxygen areas, indicating that increased hypoxic areas lead to habitat displacement [50]. The effects of movement extend beyond mere spatial distribution changes, with significant implications for mortality rates, migration patterns, and predator-prey dynamics as they adapt to new environments [51].
The extension of low-oxygen water has resulted in a reduction in oxygenated refugia, forcing fish to inhabit poor-conditioned habitats with low oxygen levels, and, as a result, fish have been observed to migrate towards shallower waters in search of oxygenated areas [52]. This has been attributed to the rising upper boundaries of oxygen minimum zones (OMZs) worldwide over the past few decades [53,54,55]. The vertical shoaling of upper layers has not only increased the vulnerability of pelagic fish to predators but has also made them more predictable and visible for fishing [25,51,56]. The expansion of OMZs has led to an estimated annual loss of vertical habitat of about 1 m year for blue marlin in the eastern tropical Atlantic, representing an overall habitat loss of 15% between 1960 and 2010 [55]. Additionally, tropical billfish and tuna, which demand high oxygen levels, are compressed in the near-surface by the lower oxygen levels, making them more vulnerable to overfishing by surface fishing gears [57]. The horizontal expansion of OMZs has also resulted in an increase in the geographical area encompassed by the OMZ upper boundary at a given depth [25]. This expansion has caused severe competition for prey in benthic areas extending the bottom of the OMZ, as fish are limited to horizontal movements to avoid low oxygen levels.
Habitat compression has been found to have negative impacts on fish populations, including increased natural and fishing mortalities, undernourished fish, and altered prey–predator relationships [51,58]. These effects can ultimately lead to reduced abundance, which hinders sustainable exploitation of fish [5,35]. Therefore, habitat compression, partly due to deoxygenation, poses a significant threat to both the environment and human society in terms of sustainable fisheries management [47,51].
4.4. Food Web Changes
The survival of marine predators is heavily influenced by the size, seasonal timing, and abundance of their prey [59]. However, the relationship between prey and predators can be altered by deoxygenation, leading to changes in food webs and ultimately reducing the survival rate, habitat, and mortality of marine species [17,60]. For instance, central mudminnows have been observed to modify their foraging behavior by avoiding hypoxic waters [61]. Similarly, Baltic cod have shifted their diets from benthic and pelagic prey to primarily targeting pelagic species due to the loss of benthic species caused by hypoxia-induced habitat degradation and, consequently, has led to a decline in the cod population [62].
Food webs exhibit diverse responses to environmental changes, which can complicate the prediction of changes in these webs. However, it is expected that benthic species, which have larger food webs compared to pelagic species [63,64], have likely altered their foraging behavior in response to deoxygenation before pelagic species.
5. Ocean Deoxygenation Impacts on Social Science
5.1. The Impacts on Marine Fisheries
The aforementioned ocean deoxygenation impacts would ultimately result in a detrimental impact on fisheries, thereby reducing the advantages that humans derive from them (see Table A1).
Deoxygenation has been found to have a significant impact on marine fisheries. Specifically, it has been observed that the abundance of large-bodied fish decreases while smaller-bodied fish become dominant in an epipelagic ecosystem [65]. This change has implications for the economy of the fishery, including changes in catch, cost of effort, and commercial value of fish [7,57]. Large-bodied fish are often highly valued, and their reduced abundance can lead to decreased catch and reduced commercial value. In contrast, smaller-bodied fish often command lower price in commercial markets, leading to changes in the cost of effort and profitability of the fishery. Therefore, the expected impacts of deoxygenation on marine fish are likely to have significant economic consequences for the fishing industry. The North Carolina shrimp fishery has already experienced a 12.9% annual loss in catch, amounting to USD 1.25 million (i.e., 13.4% of annual revenue), due to hypoxia from 1999 to 2005 [66,67]. This shortage of catch is likely to increase fishing costs, such as fuel consumption for longer fishing trips, and may lead to overfishing in an attempt to secure current revenue levels. Additionally, the body size of fish can affect fish price, meaning that even if the catch increases in some areas, revenue may still be lower due to the lower prices commanded by smaller-bodied fish [7,35]. In the Gulf of Mexico, for example, the catch composition has shifted towards smaller brown shrimp due to habitat compression and impaired growth caused by hypoxic water, resulting in a decrease in the relative price [68]. It is important to note that an increase in catch when the majority of the catch is small-bodied fish may not guarantee income growth and may lead to depletion of fish stocks.
In addition, areas with decreasing oxygen levels that support large-scale fisheries are expected to be economically susceptible. For example, sardines and anchovies in Peru and California waters, where a large OMZ has developed, are particularly vulnerable to changes in oxygen content [25,69]. In fact, it is predicted that anchovies will lose 50% of their habitat by 2100 due to lower oxygen level [70]. The United Nations Food and Agricultural Organization (FAO) has reported that around half of the world’s landings of anchovies and sardines are produced in these two areas. Considering the significant role that these fisheries play in providing sustenance and contributing to the global economy, the detrimental effects of oxygen depletion will have far-reaching and adverse consequences.
Commercial fishers typically exploit fish resources in more distant areas, while subsistence fishers tend to fish closer to home. This suggests that commercial fishery has a greater flexibility to adapt to changes in environmental conditions, such as those caused by climate change, which could have a more devastating impact on subsistence and artisanal fishers, with potentially catastrophic consequences for fishing communities worldwide [6]. Overall, ocean deoxygenation can have significant adverse effects on the economic structure of the fishing industry, which, in turn, has broader implications for the economy as a whole (Figure 3) [4].
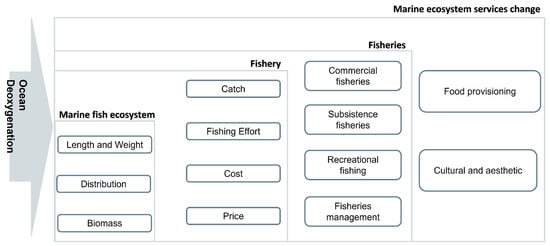
Figure 3.
Impact of ocean deoxygenation on marine ecosystem services: synthesized literature on societal impacts. The schematic diagram depicts the consequences of ocean deoxygenation on different levels of organizations interconnected, including marine fish ecosystems, fishery, fisheries, and, ultimately, marine ecosystem services.
5.2. Ecosystem Services Change
The response of an ecosystem to low dissolved oxygen is complex and non-linear, with significant feedback to other components of the Earth system, including those that impact human societies [71]. The structure of the ecosystem, including taxonomic composition, biodiversity, size and biomass of species, and vertical and geographical distribution, can be altered by changes in productivity resulting from habitat changes, bioturbation, colonization, resilience, trophic functions, symbiotic behaviors, and species interactions [23]. Due to the integrated nature of these processes, even small changes in marine ecosystem conditions can trigger significant alterations, which may be exacerbated by interactions with other stressors that perturb the ecosystem. In addition, even if the conditions that caused the initial deoxygenation are alleviated, the ecosystem may not return to its pre-disturbed state [15].
Ecosystem services can be simply defined as the benefits that people obtain from the outputs of ecosystems. The intermediate, as well as final marine ecosystem services can function as either end products or as inputs in the production of final goods and services derived from the ocean. Examples of end products include coastal tourism and recreational fisheries, while marine fish and aquaculture production are examples of goods and services that are produced using inputs from the marine ecosystem. These services can be classified into four primary categories, provisioning, regulating, supporting, and cultural (and/or aesthetic) [72].
The potential impacts of extreme and gradual deoxygenation on marine ecosystem services need to be urgently understood. However, the lack of consistent criteria defining oxygen changes and the lack of multidisciplinary studies that include all ‘deadly trio’ (i.e., warming, acidification, and deoxygenation) and the societal impacts make it challenging to interpret their effects comprehensively [73]. Nevertheless, according to global research on deoxygenation, there is a widespread agreement that the depletion of oxygen in the ocean has adverse effects on marine ecosystem services. Ocean deoxygenation is one of the main drivers of global biodiversity loss [74,75,76], and its impacts on marine ecosystem services, such as the potential for local or global extinction of exploited deep-sea fish stock, can lead to a loss of biodiversity and a reduction in the availability of seafood for human consumption [77]. Dead zones, characterized by low oxygen levels that render them uninhabitable for marine life, are a growing concern in coastal waters due to excessive nutrient levels that contribute to oxygen depletion and the emergence of waterborne diseases that pose a significant threat to human health [76]. To reduce, mitigate, and adapt to the societal impacts of these oxygen content changes, coherent actions are necessary, especially to address multiple stressors, such as the ‘deadly trio’ on continental margins [23]. Therefore, ecosystem assessments that examine the natural and social science implications of marine biogeochemical change are necessary to provide a broader accounting of how changing marine ecosystem services will affect human well-being [78].
Loss of oxygen in marine ecosystems has been studied regionally and has significant impacts on their biodiversity and the ecosystem services they generate. Gasbarro et al. (2022) predict that Lophelia pertusa, a deep Atlantic Ocean reef-forming coral, will experience significant reductions in climatic suitability due to the loss of oxygen, potentially impacting cold-water coral habitats in the south-east US margin [79]. The Baltic Sea has experienced bottom water deoxygenation due to anthropogenic pressure, leading to decreased water quality, loss of biodiversity, and reduced fish populations, impacting marine ecosystem services [80]. Orio et al. (2022) also found that the intensified expansion of the hypoxic zone in the Baltic Sea has negatively impacted ecosystem services, such as the decline in the maximum length of Baltic cod [81]. Torres et al. (2021) identify the projected rise of temperature and sea level, reduction in precipitation, increase in evapotranspiration, droughts, ocean acidification, and deoxygenation as the main threats to the marine ecosystem services in the Balearic Islands in the Mediterranean region, which has a high vulnerability of the tourism-based economy to climate change [82]. Vedor et al. (2021) show that the expanding hypoxia and fisheries in the eastern tropical Atlantic oxygen minimum zone have led to a decrease in the maximum dive depths of blue sharks due to decreasing dissolved oxygen, potentially increasing their vulnerability to surface fisheries, impacting marine ecosystem services [83]. Clarke et al. (2021) suggest that climate change will cause significant shifts in the habitat suitability of fish and invertebrate species in the Eastern Tropical Pacific, with some fisheries experiencing declines of up to 14%, impacting marine ecosystem services [84]. Vivekanandan et al. (2016) highlight the impacts of climate change on the Bay of Bengal Large Marine Ecosystem, affecting ocean productivity, habitats, and biological processes, and leading to changes in hydrology, physical threats to aquaculture facilities, and the prevalence or spread of known and new diseases of aquatic organisms, impacting traditional fisheries and inland and coastal aquaculture sectors [85]. These regional studies have highlighted the importance of adopting proactive management strategies for fish and their habitats. This involves making use of spatiotemporal predictions of distributions across various climate scenarios, as well as implementing effective conservation and marine resource management measures. Such measures are crucial for ensuring the sustainable management of fisheries in the long term [86]. Additionally, it is imperative to integrate climate change adaptation into decision-making and response initiatives to effectively address the challenges posed by climate change.
These global and regional studies highlighting the societal consequences of ocean deoxygenation concurs with negative consequences for the ecosystem, ranging from marine animals to human well-being. These literature pertaining to the impact of ocean deoxygenation on marine fish, fishery, fisheries, and marine ecosystem services was synthesized and presented in Figure 3. The figure underscores ocean deoxygenation and its interconnected impact on marine ecosystems and the services they provide. It highlights the various ways in which ocean deoxygenation affects marine fish, including physical, biological, and ecological changes within their ecosystems. These changes, in turn, have significant implications for the structure of fishery, fisheries, and management. We found that these studies frequently refer to the endangerment of provisioning services, particularly those related to fisheries production, due to the accelerated deoxygenation of the ocean. It is also crucial to note that the coastal communities, including the First Nations, will be at risk, as they play a significant role in providing cultural and aesthetic services. The impact of ocean deoxygenation on regulating and supporting services remains unexplored, likely due to the difficulty in defining these services by region or quantity, resulting in a limited number of studies on ocean deoxygenation [78,87].
6. Discussion and Conclusions
According to the deoxygenation projection models applied to the global ocean, it is highly probable that oxygen levels will decrease by about 4% under the RCP 8.5 (business as usual) scenario or 2% under the RCP 2.6 (high mitigation) scenario, in comparison to the levels recorded in 2000 [13]. Additionally, the general trend in the range shifts of epipelagic fishes is towards the poles [88].
However, regional trends in oxygen loss projections are subject to uncertainties. Compensatory mechanisms, such as increased oxygen concentrations due to lower oxygen consumption in response to primary and secondary production circumstances have been observed [89,90]. The impact of storms and hurricanes, particularly in tropical areas, on the duration, distribution, and intensity of OMZs can lead to unexpected results in the abundance of fish due to the mixing of organisms [18,91]. Furthermore, model resolution and time series may introduce biases. To effectively manage marine ecosystems, it is crucial to develop improved predictions on deoxygenation with lower uncertainties.
Despite the widespread occurrence of OMZs in various regions across multiple countries, there is a dearth of research on their societal effects on adjacent nations. The current knowledge gap between natural science and social science in relation to ocean deoxygenation, with only 2% of studies focusing on societal impacts, can be attributed to the intricate and multifaceted nature of the associated impacts. In addition, although extensive research conducted on simulating and projecting ocean loss, there remains a need for a regional-based, high-resolution model that incorporates both oxygen change and the marine ecosystem. The lack of such a model may impede accurate estimation of the societal ripple effects, as the uncertainties surrounding oxygen change remain a significant challenge.
The scarcity of societal studies on ocean deoxygenation may be due to the challenge of considering the multiple climate stressors. This makes it difficult to solely attribute the effects to deoxygenation. Identifying the impacts of deoxygenation presents a significant challenge due to the combined effects of other climatic stressors, as well as anthropogenic influences, such as overfishing and pollution [23,92]. Furthermore, the intensification of extremely low oxygen events further complicates the issue [93]. In doing so, the anticipated responses of the ecosystem change with co-occurring stressors in spatial and temporal approaches based on the scaling-up from data obtained in the laboratory and field studies in situ from the individual species level can be used to predict the socio-economic impacts of deoxygenation [15,94,95]. In order to accurately assess the impacts of deoxygenation on ecosystems and avoid misleading management policies, it is important to utilize scaling-up and quantitative approaches [96]. Additionally, it is important to consider the site-specific nature of fisheries and deoxygenation, as local conditions, such as authorization, management, commercial stocks, available vessels and ports, bathymetry, nutrient inputs, and biota can all impact these factors [8]. Given the strong links between primary and fisheries resources, it is crucial to improve our understanding of the effects of climate change on primary productivity at a regional scale relevant to fisheries [35,97]. Modeling spatial and temporal changes with other stressors can be a reliable approach to quantifying the ripple impacts of deoxygenation. Furthermore, forming a global network that encompasses socio-economic and regional effects would improve our capabilities to address the impacts of ocean deoxygenation.
Author Contributions
Conceptualization, H.K. and U.R.S.; methodology, H.K. and U.R.S.; software, H.K.; investigation, H.K. and A.C.F.; writing—original draft preparation, H.K.; writing—review and editing, H.K., A.C.F. and U.R.S.; visualization, H.K.; supervision, U.R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| OMZs | Oxygen Minimum Zones |
| FAO | The United Nations Food and Agricultural Organization |
Appendix A

Table A1.
Summary of studies that elucidate the societal impacts of ocean deoxygenation.
Table A1.
Summary of studies that elucidate the societal impacts of ocean deoxygenation.
| Study | Geographic Site | Method | Environmental Impact | Societal Impact |
|---|---|---|---|---|
| Gasbarro et al. (2022) [79] | Southeast US margin | Species distribution modeling and paleoclimate modeling | Potential climatic refugia for a reef-building cold-water coral based on its distribution and predicted future climate scenarios. | Cold-water coral ecosystems provide important habitat for a variety of commercially important fish species. The identification of areas where these corals are likely to persist can inform fisheries management decisions and potentially mitigate economic impacts of climate change on the fishing industry. |
| Borges et al. (2022) [73] | Global | Literature review | Low oxygen levels in marine ecosystems can lead to the death of marine organisms, which can cause a decline in biodiversity and ecosystem health. | 1. The loss of marine life due to low oxygen levels can have significant economic impacts on industries, such as fishing and tourism. 2. The neglect of research on the impacts of low oxygen on marine life highlights the need for policy changes to prioritize the protection and conservation of marine ecosystems. |
| Cheung et al. (2022) [77] | Global | Earth system models and a fuzzy logic expert system | The depletion of deep-sea demersal species could have a cascading effect on the marine ecosystem, leading to changes in predator–prey relationships and altering the food web. | 1. The vulnerability of deep-sea demersal species to ocean warming, deoxygenation, and acidification could have significant economic impacts on the fishing industry, as these species are often targeted for commercial fishing. 2. The depletion of deep-sea demersal species could have social impacts on communities that rely on fishing for their livelihoods, potentially leading to job losses and economic hardship. 3. The vulnerability of deep-sea demersal species to ocean warming, deoxygenation, and acidification could lead to changes in fisheries management policies, such as the implementation of stricter fishing quotas or the creation of marine protected areas. |
| Kaiser and Lerch (2022) [80] | Baltic Sea | The analysis of sedimentary faecal lipids as indicators of sewage pollution and population growth in the Baltic Sea since 1860 AD. | The impact of sewage pollution on the Baltic Sea ecosystem, which can lead to eutrophication, harmful algal blooms, and oxygen depletion, affecting marine life and water quality. | 1. The presence of sewage in the Baltic Sea can pose a risk to human health, as it can contain harmful pathogens and bacteria that can cause waterborne diseases. 2. The pollution of the Baltic Sea can have a significant economic impact on the fishing and tourism industries, as well as on the cost of water treatment and clean-up efforts. 3. Impact of population growth on sewage pollution, indicating the need for sustainable population management and wastewater treatment infrastructure to protect the environment and public health. |
| Orio et al. (2022) [81] | Baltic Sea | Generalized additive model | The decline in cod population can have a cascading effect on the entire ecosystem, leading to changes in the food web and potentially affecting other species. | 1. The decline in cod population with a size reduction can have significant economic impacts on the commercial fishing industry, and recreational fisheries. 2. The decline in cod population can have an impact on the cultural ecosystem services of communities that rely on cod fishing. 3. The findings of this study can inform policy decisions related to both reducing greenhouse gas emissions and fisheries management efforts in the Baltic Sea region. |
| Torres et al. (2021) [82] | Balearic Islands in the Mediterranean region | Literature review | The potential environmental impacts of climate change on the Balearic Islands, including deoxygenation, acidification, and sea level rise, increased frequency and intensity of extreme weather events, and changes in ecosystems and biodiversity. | 1. The potential economic impacts of climate change on the Balearic Islands, including reduced tourism revenue and increased costs for infrastructure maintenance and repair. 2. The paper provides guidance for policy design in Mediterranean regions, highlighting the need for coordinated action to mitigate and adapt to the impacts of climate change. |
| Pitcher et al. (2021) [74] | Global | Literature review | The impact of oxygen depletion on marine ecosystems, which can lead to the death of fish and other marine organisms. This can have a cascading effect on the food chain and ultimately impact the overall health of the ocean. | 1. The depletion of oxygen in coastal and open ocean waters can have significant economic impacts on industries, such as fishing and tourism. Reduced fish populations can lead to decreased catches and lower revenues for fisherfolks, while the degradation of marine ecosystems can deter tourists from visiting coastal areas. 2. Policy interventions may be necessary to address oxygen depletion in coastal and open ocean waters. This could include measures to reduce nutrient pollution and other human activities that contribute to oxygen depletion, as well as efforts to mitigate the impacts of climate change. |
| Altieri et al. (2021) [75] | Global (tropical ecosystems) | Literature review | 1. The potential impact of ocean deoxygenation on tropical ecosystems, which could lead to the loss of biodiversity in these regions. 2. Tropical ecosystems may be more resilient to ocean deoxygenation than previously thought, as they have the ability to adapt and adjust to changing oxygen levels. | 1. Loss of Biodiversity due to oxygen loss could have significant societal impacts, as many communities rely on these ecosystems for food, livelihoods, and cultural practices. 2. The loss of biodiversity in tropical ecosystems could also have implications for food security, as many communities rely on fish and other marine resources for sustenance. 3. Deoxygenation could also have economic impacts, particularly for industries that rely on marine resources. If these industries are negatively impacted, it could lead to job losses and economic instability in affected regions. |
| Vedor et al. (2021) [83] | Eastern tropical Atlantic Ocean | Generalized additive mixed model | Deoxygenation of the ocean could have wider impacts on marine ecosystems. As the shark is a top predator, its decline could have knock-on effects on other species in the food chain. | 1. Climate-driven deoxygenation increases the vulnerability of the ocean’s widest ranging shark to fishing. This could have a significant impact on the fishing industry, as the shark is a valuable catch for many fisherfolks. 2. The findings of the paper could have implications for conservation efforts aimed at protecting the shark. If the shark is becoming more vulnerable to fishing due to climate change, conservationists may need to adjust their strategies to ensure the species is adequately protected. |
| Clarke et al. (2021) [84] | Eastern Tropical Pacific | Ensemble simulations of climate change effects on fish and invertebrate species | Climate change is having significant impacts on living marine resources in the Eastern Tropical Pacific, including changes in species distribution, abundance, and productivity. Demersal species were projected to move into shallower waters. These impacts are likely to have significant ecological and economic consequences for the region. | Climate change is affecting the distribution and abundance of living marine resources in the Eastern Tropical Pacific, which has significant economic implications for the fishing industry and coastal communities that rely on these resources for their livelihoods. |
| Kirchman (2021) [76] | Global | Literature review | Dead zones can have a devastating impact on ecosystems, as they can lead to the death of marine life and the loss of biodiversity. This can have ripple effects throughout the food chain and impact other species that rely on the affected ecosystem. | 1. Dead zones can have a significant impact on the fishing industry, as fish and other marine life may be unable to survive in oxygen-depleted waters. This can lead to a decline in fish populations and a loss of income for fishermen and related industries. 2. Dead zones can also have political implications, as they can lead to conflicts over resource use and management. This can lead to tensions between different groups, such as fishermen, environmentalists, and policymakers, who may have different priorities and interests. |
| Sumaila (2018) [98] | Global | Literature review | Climate change is having a significant impact on marine ecosystems and world fisheries, leading to changes in the distribution and abundance of fish populations, as well as alterations in the timing of reproduction and migration patterns. | 1. Climate change is affecting the world fisheries industry, which is a major source of income for many countries. Changes in ocean temperatures, oxygen contents, and acidity levels are causing shifts in fish populations, leading to changes in fishing patterns and reduced catches. This can have a significant economic impact on fishing communities and the global seafood industry. 2. Climate change is affecting the livelihoods of millions of people who depend on fishing for their income and food security. Changes in fish populations and fishing patterns can lead to job losses and food shortages, particularly in developing countries. 3. Climate change is a global issue that requires international cooperation and political action. The impacts of climate change on marine ecosystems and world fisheries are likely to become a major political issue in the coming years, as countries seek to address the economic, environmental, and social impacts of climate change. |
| Shepherd et al. (2017) [99] | Global | Literature review | The paper highlights the impact of ocean deoxygenation on marine ecosystems, which can lead to the loss of biodiversity and the collapse of fisheries. | 1. The loss of fisheries and other marine resources can have significant economic consequences for coastal communities and the global economy. |
| Vivekanandan et al. (2016) [85] | Bay of Bengal | Literature review | Climate change effects in the Bay of Bengal Large Marine Ecosystem can also have significant environmental impacts, such as coral bleaching, sea level rise, ocean acidification, and deoxygenation. These impacts can affect the abundance and biodiversity of the ecosystem, as well as the livelihoods of those who depend on it. | 1. Climate change effects including warming, acidification, and deoxygenation in the Bay of Bengal Large Marine Ecosystem can lead to reduced catches and income for fishing communities particularly traditional fisheries. 2. Climate change effects in the Bay of Bengal Large Marine Ecosystem can also have social impacts, such as displacement of coastal communities, loss of cultural heritage and traditional knowledge, and increased vulnerability to natural disasters, such as cyclones and floods. 3. Governments and international organizations need to develop new policies and regulations to address the impacts of climate change on the ecosystem and the communities that depend on it. This can include measures to reduce greenhouse gas emissions, protect marine biodiversity, and support adaptation and resilience-building efforts. |
| Levin et al. (2015) [9] | Global | Literature review | The paper highlights the impact of human activities on dynamic continental margins, which can lead to environmental degradation. This can have a significant impact on the ecosystem and the overall health of the planet. | 1. The impact of resource depletion on dynamic continental margins can have a significant impact on the availability of resources for human consumption and can lead to economic and social challenges. 2. The impact of economic development on dynamic continental margins. This can have both positive and negative impacts on human societies, depending on how it is managed. |
| Cooley (2012) [78] | Global | Literature review | Changes in ocean biogeochemistry could have significant environmental impacts on marine ecosystems, including changes in the distribution and abundance of marine species, alterations in food webs, and changes in the physical and chemical properties of the ocean. | 1. Changes in ocean biogeochemistry could have significant economic impacts on communities that rely on fishing and other ocean-based industries. Changes in fish populations and the health of marine ecosystems could lead to job losses and decreased economic activity. 2. Changes in ocean biogeochemistry could also have health impacts on communities that rely on seafood as a primary source of protein. 3. Changes in ocean biogeochemistry could also have social impacts on communities that rely on the ocean for cultural and recreational activities. Changes in the health and composition of marine ecosystems could lead to the loss of cultural traditions and recreational opportunities, which could have negative impacts on community well-being. |
References
- Teh, L.C.L.; Sumaila, U.R. Contribution of marine fisheries to worldwide employment. Fish Fish. 2013, 14, 77–88. [Google Scholar] [CrossRef]
- Golden, C.D.; Koehn, J.Z.; Shepon, A.; Passarelli, S.; Free, C.M.; Viana, D.F.; Matthey, H.; Eurich, J.G.; Gephart, J.A.; Fluet-Chouinard, E.; et al. Aquatic foods to nourish nations. Nature 2021, 598, 315–320. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation: Towards blue transformation. In The state of World Fisheries and Aquaculture; FAO: Rome, Italy, 2022; Volume 2022. [Google Scholar] [CrossRef]
- Dyck, A.J.; Sumaila, U.R. Economic impact of ocean fish populations in the global fishery. J. Bioecon. 2010, 12, 227–243. [Google Scholar] [CrossRef]
- Lam, V.W.Y.; Cheung, W.W.L.; Sumaila, U.R. Marine capture fisheries in the Arctic: Winners or losers under climate change and ocean acidification? Fish Fish. 2016, 17, 335–357. [Google Scholar] [CrossRef]
- Sumaila, U.R.; Cheung, W.W.; Cury, M.P.; Tai, T. Climate change, marine ecosystems and global fisheries. In Building a Climate Resilient Economy and Society; Edward Elgar Publishing: Cheltenham, UK, 2017. [Google Scholar]
- Sumaila, U.R.; Tai, T.C.; Lam, V.W.Y.; Cheung, W.W.L.; Bailey, M.; Cisneros-Montemayor, A.M.; Chen, O.L.; Gulati, S.S. Benefits of the Paris Agreement to ocean life, economies, and people. Sci. Adv. 2019, 5, eaau3855. [Google Scholar] [CrossRef] [PubMed]
- Laffoley, D.; Baxter, J.M. Ocean Deoxygenation: Everyone’s Problem—Causes, Impacts, Consequences and Solutions; Full Report; IUCN: Gland, Switerland, 2019; p. 580. [Google Scholar] [CrossRef]
- Levin, L.A.; Breitburg, D.L. Linking coasts and seas to address ocean deoxygenation. Nat. Clim. Chang. 2015, 5, 401–403. [Google Scholar] [CrossRef]
- Schmidtko, S.; Stramma, L.; Visbeck, M. Decline in global oceanic oxygen content during the past five decades. Nature 2017, 542, 335–339. [Google Scholar] [CrossRef]
- Keeling, R.E.; Körtzinger, A.; Gruber, N. Ocean deoxygenation in a warming world. Annu. Rev. Mar. Sci. 2010, 2, 199–229. [Google Scholar] [CrossRef]
- Seibel, B.A. Critical oxygen levels and metabolic suppression in oceanic oxygen minimum zones. J. Exp. Biol. 2011, 214, 326–336. [Google Scholar] [CrossRef]
- Bopp, L.; Resplandy, L.; Orr, J.C.; Doney, S.C.; Dunne, J.P.; Gehlen, M.; Halloran, P.; Heinze, C.; Ilyina, T.; Séférian, R.; et al. Multiple stressors of ocean ecosystems in the 21st century: Projections with CMIP5 models. Biogeosciences 2013, 10, 6225–6245. [Google Scholar] [CrossRef]
- Oschlies, A. A committed fourfold increase in ocean oxygen loss. Nat. Commun. 2021, 12, 2307. [Google Scholar] [CrossRef] [PubMed]
- Breitburg, D.; Levin, L.A.; Oschlies, A.; Grégoire, M.; Chavez, F.P.; Conley, D.J.; Garçon, V.; Gilbert, D.; Gutiérrez, D.; Isensee, K.; et al. Declining oxygen in the global ocean and coastal waters. Science 2018, 359, eaam 7240. [Google Scholar] [CrossRef] [PubMed]
- Oschlies, A.; Brandt, P.; Stramma, L.; Schmidtko, S. Drivers and mechanisms of ocean deoxygenation. Nat. Geosci. 2018, 11, 467–473. [Google Scholar] [CrossRef]
- Stramma, L.; Schmidtko, S.; Levin, L.A.; Johnson, G.C. Ocean oxygen minima expansions and their biological impacts. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2010, 57, 587–595. [Google Scholar] [CrossRef]
- Diaz, R.J.; Rosenberg, R. Spreading dead zones and consequences for marine ecosystems. Science 2008, 321, 926–929. [Google Scholar] [CrossRef]
- Bijma, J.; Pörtner, H.O.; Yesson, C.; Rogers, A.D. Climate change and the oceans–what does the future hold? Mar. Pollut. Bull. 2013, 74, 495–505. [Google Scholar] [CrossRef]
- Noone, K.J.; Sumaila, U.R.; Diaz, R.J. Managing Ocean Environments in a Changing Climate: Sustainability and Economic Perspectives; Elsevier: Burlington, VT, USA, 2013. [Google Scholar] [CrossRef]
- Sampaio, E.; Santos, C.; Rosa, I.C.; Ferreira, V.; Pörtner, H.O.; Duarte, C.M.; Levin, L.A.; Rosa, R. Impacts of hypoxic events surpass those of future ocean warming and acidification. Nat. Ecol. Evol. 2021, 5, 311–321. [Google Scholar] [CrossRef]
- Garcia, H.; Weathers, K.; Paver, C.; Smolyar, I.; Boyer, T.; Locarnini, M.; Zweng, M.; Mishonov, A.; Baranova, O.; Seidov, D.; et al. World Ocean Atlas 2018, Volume 3: Dissolved Oxygen, Apparent Oxygen Utilization, and Dissolved Oxygen Saturation; NOAA/NESDIS National Centers for Environmental Information: Silver Spring, MD, USA, 2019; 38p. [Google Scholar]
- Levin, L.A. Manifestation, Drivers, and Emergence of Open Ocean Deoxygenation. Annu. Rev. Mar. Sci. 2018, 10, 229–260. [Google Scholar] [CrossRef]
- Chavez, F.P.; Messié, M.; Pennington, J.T. Marine primary production in relation to climate variability and change. Annu. Rev. Mar. Sci. 2011, 3, 227–260. [Google Scholar] [CrossRef]
- Gilly, W.F.; Beman, J.M.; Litvin, S.Y.; Robison, B.H. Oceanographic and biological effects of shoaling of the oxygen minimum zone. Annu. Rev. Mar. Sci. 2013, 5, 393–420. [Google Scholar] [CrossRef]
- Paulmier, A.; Ruiz-Pino, D. Oxygen minimum zones (OMZs) in the modern ocean. Prog. Oceanogr. 2009, 80, 113–128. [Google Scholar] [CrossRef]
- Grégoire, M.; Garçon, V.; Garcia, H.; Breitburg, D.; Isensee, K.; Oschlies, A.; Telszewski, M.; Barth, A.; Bittig, H.C.; Carstensen, J.; et al. A Global Ocean Oxygen Database and Atlas for Assessing and Predicting Deoxygenation and Ocean Health in the Open and Coastal Ocean. Front. Mar. Sci. 2021, 8, 724913. [Google Scholar] [CrossRef]
- Bricker, S.; Clement, C.G.; Pirhalla, D.E.; Orlando, S.P.; Farrow, D.R.G. National Estuarine Eutrophication Assessment: Effects of Nutrient Enrichment in the Nation’s Estuaries; Special Projects Office and the National Centers for Coastal Ocean Science: Silver Spring, MD, USA, 1999; Volume 71. [Google Scholar]
- Pauly, D.; Zeller, D.; Palomares, M. (Eds.) Sea around Us Concepts, Design and Data. 2022. Available online: http://www.seaaroundus.org/ (accessed on 1 April 2023).
- Deutsch, C.; Ferrel, A.; Seibel, B.; Pörtner, H.O.; Huey, R.B. Climate change tightens a metabolic constraint on marine habitats. Science 2015, 348, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Pauly, D.; Cheung, W.W.L. Sound physiological knowledge and principles in modeling shrinking of fishes under climate change. Glob. Chang. Biol. 2018, 24, e15–e26. [Google Scholar] [CrossRef] [PubMed]
- Vaquer-Sunyer, R.; Duarte, C.M. Thresholds of hypoxia for marine biodiversity. Proc. Natl. Acad. Sci. USA 2008, 105, 15452–15457. [Google Scholar] [CrossRef]
- Canfield, D.E.; Poulton, S.W.; Narbonne, G.M. Late-Neoproterozoic deep-ocean oxygenation and the rise of animal life. Science 2007, 315, 92–95. [Google Scholar] [CrossRef]
- Schurmann, H.; Steffensen, J.F. Lethal oxygen levels at different temperatures and the preferred temperature during hypoxia of the Atlantic cod, Gadus morhua L. J. Fish Biol. 1992, 41, 927–934. [Google Scholar] [CrossRef]
- Sumaila, U.R.; Cheung, W.W.L.; Lam, V.W.Y.; Pauly, D.; Herrick, S. Climate change impacts on the biophysics and economics of world fisheries. Nat. Clim. Chang. 2011, 1, 449–456. [Google Scholar] [CrossRef]
- Bindoff, N.L.; Cheung, W.W.L.; Kairo, J.G.; Arístegui, J.; Guinder, V.A.; Hallberg, R.; Hilmi, N.; Jiao, N.; Karim, M.S.; Levin, L.; et al. Changing Ocean, Marine Ecosystems, and Dependent Communities. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Portner, H.-O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegria, A., Nicolai, M., Okem, A., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2019; pp. 447–587. [Google Scholar]
- Bejda, A.J.; Phelan, B.A.; Studholme, A.L. The effect of dissolved oxygen on the growth of young-of-the-year winter flounder, Pseudopleuronectes americanus. Environ. Biol. Fishes 1992, 34, 321–327. [Google Scholar] [CrossRef]
- Petersen, J.K.; Pihl, L. Responses to hypoxia of plaice, and dab, Limanda limand, in the south-east Kattegat: Distribution and growth. Environ. Biol. Fishes 1995, 43, 311–321. [Google Scholar] [CrossRef]
- Taylor, J.; Miller, J.M. Physiological performance of juvenile southern flounder, Paralichthys lethostigma (Jordan and Gilbert, 1884), in chronic and episodic hypoxia. J. Exp. Mar. Biol. Ecol. 2001, 258, 195–214. [Google Scholar] [CrossRef] [PubMed]
- McNatt, R.A.; Rice, J.A. Hypoxia-induced growth rate reduction in two juvenile estuary-dependent fishes. J. Exp. Mar. Biol. Ecol. 2004, 311, 147–156. [Google Scholar] [CrossRef]
- Stierhoff, K.L.; Targett, T.E.; Miller, K.L. Ecophysiological responses of juvenile summer and winter flounder to hypoxia: Experimental and modeling analyses of effects on estuarine nursery quality. Mar. Ecol. Prog. Ser. 2006, 325, 255–266. [Google Scholar] [CrossRef]
- Landry, C.A.; Steele, S.L.; Manning, S.; Cheek, A.O. Long term hypoxia suppresses reproductive capacity in the estuarine fish, Fundulus grandis. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 148, 317–323. [Google Scholar] [CrossRef]
- Keppel, A.G.; Breitburg, D.L.; Wikfors, G.H.; Burrell, R.B.; Clark, V.M. Effects of co-varying diel-cycling hypoxia and pH on disease susceptibility in the eastern oyster Crassostrea virginica. Mar. Ecol. Prog. Ser. 2015, 538, 169–183. [Google Scholar] [CrossRef]
- McCormick, L.R.; Levin, L.A. Physiological and ecological implications of ocean deoxygenation for vision in marine organisms. Philos. Trans. Ser. A Math. Phys. Eng. Sci. 2017, 375, 20160322. [Google Scholar] [CrossRef]
- Breitburg, D.L. Behavioral response of fish larvae to low dissolved oxygen concentrations in a stratified water column. Mar. Biol. 1994, 120, 615–625. [Google Scholar] [CrossRef]
- Burton, D.T.; Richardson, L.B.; Moore, C.J. Effect of Oxygen Reduction Rate and Constant Low Dissolved Oxygen Concentrations on Two Estuarine Fish. Trans. Am. Fish. Soc. 1980, 109, 552–557. [Google Scholar] [CrossRef]
- Shimps, E.L.; Rice, J.A.; Osborne, J.A. Hypoxia tolerance in two juvenile estuary-dependent fishes. J. Exp. Mar. Biol. Ecol. 2005, 325, 146–162. [Google Scholar] [CrossRef]
- McAllen, R.; Davenport, J.; Bredendieck, K.; Dunne, D. Seasonal structuring of a benthic community exposed to regular hypoxic events. J. Exp. Mar. Biol. Ecol. 2009, 368, 67–74. [Google Scholar] [CrossRef]
- Pauly, D. Gasping Fish and Panting Squids: Oxygen, Temperature and the Growth of Water-Breathing Animals; Jorgensen, B.B., Ed.; International Ecology Institute: Oldendorf/Luhe, Germany, 2010. [Google Scholar]
- Wannamaker, C.M.; Rice, J.A. Effects of hypoxia on movements and behavior of selected estuarine organisms from the southeastern United States. J. Exp. Mar. Biol. Ecol. 2000, 249, 145–163. [Google Scholar] [CrossRef]
- Prince, E.D.; Goodyear, C.P. Hypoxia-based habitat compression of tropical pelagic fishes. Fish. Oceanogr. 2006, 15, 451–464. [Google Scholar] [CrossRef]
- Eby, L.A.; Crowder, L.B. Hypoxia-based habitat compression in the Neuse River Estuary: Context-dependent shifts in behavioral avoidance thresholds. Can. J. Fish. Aquat. Sci. 2002, 59, 952–965. [Google Scholar] [CrossRef]
- Bograd, S.J.; Castro, C.G.; Di Lorenzo, E.; Palacios, D.M.; Bailey, H.; Gilly, W.; Chavez, F.P. Oxygen declines and the shoaling of the hypoxic boundary in the California Current. Geophys. Res. Lett. 2008, 35, 409–420. [Google Scholar] [CrossRef]
- Whitney, F.; Gilbert, D.; Sinclair, A. Impacts of spreading hypoxia on coastal biota of the subarctic Pacific. Geochim. Cosmochim. Acta Suppl. 2009, 73, A1435. [Google Scholar]
- Stramma, L.; Prince, E.D.; Schmidtko, S.; Luo, J.; Hoolihan, J.P.; Visbeck, M.; Wallace, D.W.R.; Brandt, P.; Körtzinger, A. Expansion of oxygen minimum zones may reduce available habitat for tropical pelagic fishes. Nat. Clim. Chang. 2012, 2, 33–37. [Google Scholar] [CrossRef]
- Koslow, J.A.; Goericke, R.; Lara-Lopez, A.; Watson, W. Impact of declining intermediate-water oxygen on deepwater fishes in the California Current. Mar. Ecol. Prog. Ser. 2011, 436, 207–218. [Google Scholar] [CrossRef]
- Prince, E.D.; Luo, J.; Phillip Goodyear, C.; Hoolihan, J.P.; Snodgrass, D.; Orbesen, E.S.; Serafy, J.E.; Ortiz, M.; Schirripa, M.J. Ocean scale hypoxia-based habitat compression of Atlantic istiophorid billfishes. Fish. Oceanogr. 2010, 19, 448–462. [Google Scholar] [CrossRef]
- La, C.; Rice, J.A. Effects of hypoxia-induced habitat compression on growth of juvenile fish in the Neuse River Estuary, North Carolina, USA. Mar. Ecol. Prog. Ser. 2014, 497, 199–213. [Google Scholar] [CrossRef]
- Beaugrand, G.; Brander, K.M.; Alistair Lindley, J.; Souissi, S.; Reid, P.C. Plankton effect on cod recruitment in the North Sea. Nature 2003, 426, 661–664. [Google Scholar] [CrossRef]
- Pörtner, H.O. Oxygen- and capacity-limitation of thermal tolerance: A matrix for integrating climate-related stressor effects in marine ecosystems. J. Exp. Biol. 2010, 213, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Rahel, F.J.; Nutzman, J.W. Foraging in a Lethal Environment: Fish Predation in Hypoxic Waters of a Stratified Lake. Ecology 1994, 75, 1246–1253. [Google Scholar] [CrossRef]
- Casini, M.; Käll, F.; Hansson, M.; Plikshs, M.; Baranova, T.; Karlsson, O.; Lundström, K.; Neuenfeldt, S.; Gårdmark, A.; Hjelm, J. Hypoxic areas, density-dependence and food limitation drive the body condition of a heavily exploited marine fish predator. R. Soc. Open Sci. 2016, 3, 160416. [Google Scholar] [CrossRef] [PubMed]
- France, R.L. Differentiation between littoral and pelagic food webs in lakes using stable carbon isotopes. Limnol. Oceanogr. 1995, 40, 1310–1313. [Google Scholar] [CrossRef]
- Peterson, B.J. Stable isotopes as tracers of organic matter input and transfer in benthic food webs: A review. Acta Oecologica 1999, 20, 479–487. [Google Scholar] [CrossRef]
- Lefort, S.; Aumont, O.; Bopp, L.; Arsouze, T.; Gehlen, M.; Maury, O. Spatial and body-size dependent response of marine pelagic communities to projected global climate change. Glob. Chang. Biol. 2015, 21, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Smith, M.D.; Craig, J.K. Quantifying the Economic Effects of Hypoxia on a Fishery for Brown Shrimp Farfantepenaeus aztecus. Mar. Coast. Fish. 2010, 2, 232–248. [Google Scholar] [CrossRef]
- Huang, L.; Nichols, L.A.; Craig, J.K.; Smith, M.D. Measuring Welfare Losses from Hypoxia: The Case of North Carolina Brown Shrimp. Mar. Resour. Econ. 2012, 27, 3–23. [Google Scholar] [CrossRef]
- Smith, M.D.; Oglend, A.; Kirkpatrick, A.J.; Asche, F.; Bennear, L.S.; Craig, J.K.; Nance, J.M. Seafood prices reveal impacts of a major ecological disturbance. Proc. Natl. Acad. Sci. USA 2017, 114, 1512–1517. [Google Scholar] [CrossRef]
- Ekau, W.; Auel, H.; Pörtner, H.O.; Gilbert, D. Impacts of hypoxia on the structure and processes in pelagic communities (zooplankton, macro-invertebrates and fish). Biogeosciences 2010, 7, 1669–1699. [Google Scholar] [CrossRef]
- Howard, E.M.; Penn, J.L.; Frenzel, H.; Seibel, B.A.; Bianchi, D.; Renault, L.; Kessouri, F.; Sutula, M.A.; McWilliams, J.C.; Deutsch, C. Climate-driven aerobic habitat loss in the California Current System. Sci. Adv. 2020, 6, eaay3188. [Google Scholar] [CrossRef]
- Zhang, J.; Gilbert, D.; Gooday, A.J.; Levin, L.; Naqvi, S.W.A.; Middelburg, J.J.; Scranton, M.; Ekau, W.; Peña, A.; Dewitte, B.; et al. Natural and human-induced hypoxia and consequences for coastal areas: Synthesis and future development. Biogeosciences 2010, 7, 1443–1467. [Google Scholar] [CrossRef]
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Synthesis; Island Press: Washington, DC, USA, 2015. [Google Scholar]
- Borges, F.O.; Sampaio, E.; Santos, C.P.; Rosa, R. Impacts of Low Oxygen on Marine Life: Neglected, but a Crucial Priority for Research; University of Chicago Press: Chicago, IL, USA, 2022; Volume 243. [Google Scholar] [CrossRef]
- Pitcher, G.C.; Aguirre-Velarde, A.; Breitburg, D.; Cardich, J.; Carstensen, J.; Conley, D.J.; Dewitte, B.; Engel, A.; Espinoza-Morriberón, D.; Flores, G.; et al. System Controls of Coastal and Open Ocean Oxygen Depletion; Elsevier Ltd.: Amsterdam, The Netherlands, 2021; Volume 197. [Google Scholar] [CrossRef]
- Altieri, A.H.; Johnson, M.D.; Swaminathan, S.D.; Nelson, H.R.; Gedan, K.B. Resilience of Tropical Ecosystems to Ocean Deoxygenation; Elsevier Ltd.: Amsterdam, The Netherlands, 2021; Volume 36. [Google Scholar] [CrossRef]
- Kirchman, D.L. Dead Zones: The Loss of Oxygen from Rivers, Lakes, Seas, and the Ocean; Oxford University Press: Oxford, UK, 2021. [Google Scholar] [CrossRef]
- Cheung, W.; Wei, C.L.; Levin, L.A. Vulnerability of Exploited Deep-Sea Demersal Species to Ocean Warming, Deoxygenation, and Acidification; Springer Science and Business Media B.V.: Berlin/Heidelberg, Germany, 2022; Volume 105. [Google Scholar] [CrossRef]
- Cooley, S.R. How human communities could ‘feel’ changing ocean biogeochemistry. Curr. Opin. Environ. Sustain. 2012, 4, 258–263. [Google Scholar] [CrossRef]
- Gasbarro, R.; Sowers, D.; Margolin, A.; Cordes, E.E. Distribution and Predicted Climatic Refugia for a Reef-Building Cold-Water Coral on the Southeast US Margin; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2022; Volume 28. [Google Scholar] [CrossRef]
- Kaiser, J.; Lerch, M. Sedimentary Faecal Lipids as Indicators of Baltic Sea Sewage Pollution and Population Growth Since 1860 AD; Academic Press Inc.: Cambridge, MA, USA, 2022; Volume 204. [Google Scholar] [CrossRef]
- Orio, A.; Heimbrand, Y.; Limburg, K. Deoxygenation impacts on Baltic Sea cod: Dramatic declines in ecosystem services of an iconic keystone predator. Ambio 2022, 51, 626–637. [Google Scholar] [CrossRef]
- Torres, C.; Jordà, G.; de Vílchez, P.; Vaquer-Sunyer, R.; Rita, J.; Canals, V.; Cladera, A.; Escalona, J.M.; Miranda, M. Climate Change and Their Impacts in the Balearic Islands: A Guide for Policy Design in Mediterranean Regions; Reg Environ Change: Berlin/Heidelberg, Germany, 2021; Volume 21. [Google Scholar] [CrossRef]
- Vedor, M.; Queiroz, N.; Mucientes, G.; Couto, A.; Da Costa, I.; Dos Santos, A.; Vandeperre, F.; Fontes, J.; Afonso, P.; Rosa, R.; et al. Climate-Driven Deoxygenation Elevates Fishing Vulnerability for the Ocean’s Widest Ranging Shark; eLife Sciences Publications Ltd.: Cambridge, UK, 2021; Volume 10. [Google Scholar] [CrossRef]
- Clarke, T.M.; Reygondeau, G.; Wabnitz, C.; Robertson, R.; Ixquiac-Cabrera, M.; López, M.; Ramírez Coghi, A.R.; Del Río Iglesias, J.L.; Wehrtmann, I.; Cheung, W. Climate Change Impacts on Living Marine Resources in the Eastern Tropical Pacific. Divers. Distrib. 2021, 27, 65–81. [Google Scholar] [CrossRef]
- Vivekanandan, E.; Hermes, R.; O’Brien, C. Climate change effects in the Bay of Bengal Large Marine Ecosystem. Environ. Dev. 2016, 17, 46–56. [Google Scholar] [CrossRef]
- Sumaila, U.R. Infinity Fish: Economics and the Future of Fish and Fisheries: Economics and the Future of Fish and Fisheries; Academic Press: London, UK, 2021. [Google Scholar]
- Limburg, K.E.; Breitburg, D.; Swaney, D.P.; Jacinto, G. Ocean Deoxygenation: A Primer; Cell Press: Cambridge, MA, USA, 2020; Volume 2. [Google Scholar] [CrossRef]
- Jones, M.C.; Cheung, W.W.L. Multi-model ensemble projections of climate change effects on global marine biodiversity. ICES J. Mar. Sci. 2015, 72, 741–752. [Google Scholar] [CrossRef]
- Cabré, A.; Marinov, I.; Bernardello, R.; Bianchi, D. Oxygen minimum zones in the tropical Pacific across CMIP5 models: Mean state differences and climate change trends. Biogeosciences 2015, 12, 5429–5454. [Google Scholar] [CrossRef]
- Bopp, L.; Resplandy, L.; Untersee, A.; Le Mezo, P.; Kageyama, M. Ocean (de)oxygenation from the Last Glacial Maximum to the twenty-first century: Insights from Earth System models. Philos. Trans. Ser. A Math. Phys. Eng. Sci. 2017, 375, 20160323. [Google Scholar] [CrossRef]
- Naiman, R.J.; Alldredge, J.R.; Beauchamp, D.A.; Bisson, P.A.; Congleton, J.; Henny, C.J.; Huntly, N.; Lamberson, R.; Levings, C.; Merrill, E.N.; et al. Developing a broader scientific foundation for river restoration: Columbia River food webs. Proc. Natl. Acad. Sci. USA 2012, 109, 21201–21207. [Google Scholar] [CrossRef]
- Henson, S.A.; Beaulieu, C.; Ilyina, T.; John, J.G.; Long, M.; Séférian, R.; Tjiputra, J.; Sarmiento, J.L. Rapid emergence of climate change in environmental drivers of marine ecosystems. Nat. Commun. 2017, 8, 14682. [Google Scholar] [CrossRef] [PubMed]
- Köhn, E.E.; Münnich, M.; Vogt, M.; Desmet, F.; Gruber, N. Strong Habitat Compression by Extreme Shoaling Events of Hypoxic Waters in the Eastern Pacific. J. Geophys. Res. Ocean. 2022, 127, e2022JC018429. [Google Scholar] [CrossRef]
- Neilan, R.M.; Rose, K. Simulating the effects of fluctuating dissolved oxygen on growth, reproduction, and survival of fish and shrimp. J. Theor. Biol. 2014, 343, 54–68. [Google Scholar] [CrossRef] [PubMed]
- de Mutsert, K.; Steenbeek, J.; Lewis, K.; Buszowski, J.; Cowan, J.H.; Christensen, V. Exploring effects of hypoxia on fish and fisheries in the northern Gulf of Mexico using a dynamic spatially explicit ecosystem model. Ecol. Model. 2016, 331, 142–150. [Google Scholar] [CrossRef]
- Barnthouse, L. The strengths of the ecological risk assessment process: Linking science to decision making. Integr. Environ. Assess. Manag. 2008, 4, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Stock, C.A.; Alexander, M.A.; Bond, N.A.; Brander, K.M.; Cheung, W.W.; Curchitser, E.N.; Delworth, T.L.; Dunne, J.P.; Griffies, S.M.; Haltuch, M.A.; et al. On the use of IPCC-class models to assess the impact of climate on Living Marine Resources. Prog. Oceanogr. 2011, 88, 1–27. [Google Scholar] [CrossRef]
- Sumaila, U.R. Climate Change: Impact on Marine Ecosystems and World Fisheries; Ferranti, P., Berry, E.M., Anderson, J.R., Eds.; Encyclopedia of Food Security and Sustainability; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Shepherd, J.G.; Brewer, P.G.; Oschlies, A.; Watson, A.J. Ocean Ventilation and Deoxygenation in a Warming World: Introduction and Overview; Royal Society Publishing: London, UK, 2017; Volume 375. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).