Abstract
Amphipods are frequently used as bioindicators of water quality in experimental or behavior trials. Thus, it is a group considered suitable for use as a model organism in tests with essential oils (EOs). This study evaluated the time required for anesthesia induction and recovery of the amphipod crustacean H. bonariensis exposed to the essential oils of Aloysia triphylla (EOAT) and Lippia alba (EOLA), and their major compounds citral and linalool, respectively. In addition, we evaluated the locomotor activity of amphipods using ANY-maze® software. Mortalities were observed at concentrations of 100 and 200 µL/L of citral (50.0 ± 0.39%) and 750 µL/L of EOLA (66.7 ± 0.33%). Except for linalool, increased concentrations of the compounds of the essential oils decreased the time for sedation and anesthesia induction. There were differences for the induction of anesthesia (p < 0.05) and recovery (p < 0.05) between EOLA and linalool treatments, but not between that for EOAT and citral. Reduced locomotor activity and longer time and episodes of freezing were observed in animals exposed to EOAT. The EOs and their major compounds induced anesthesia and affected the locomotor activity of H. bonariensis, Therefore, EOAT and linalool are recommended for anesthesia of this species. EOAT can also be utilized in long-term exposure.
Key Contribution:
Amphipod Hyalella bonariensis can be used as a model to test new anesthetics for crustaceans. The essential oil of Aloysia triphylla and linalool can be used as anesthetics for this species.
1. Introduction
The genus Hyalella (Smith, 1874; Hyalellidae, Amphipoda) includes about 91 described species distributed from Patagonia to Canada []. These amphipods consist of freshwater and some marine species that live in the benthic environment, which is usually associated with algae or sediment []. Amphipods are important species in the trophic chain and are used as bioindicators of water quality in ecotoxicological trials due to their high sensitivity to environmental impacts and contamination, short life cycles, easy sampling, and simple laboratory maintenance [,,,].
Behavioral changes provide important tools on the ecological and health status of animals [,]. For example, changes in locomotion, swimming speed, feeding, and ventilation frequency may indicate neurotoxic actions or interference in the neuromuscular transmission by various substances in experimental assays [,]. Stress affects the locomotor capacity [], social interactions, and escape from predators [], as observed in experiments with the amphipod Gammarus fossarum [] and crayfish (Procambarus clarkii) []. Observations of individuals of a species can contribute to understanding the relationship between environmental factors and populations, which would support management, either for conservation at the environmental level or for standardization of laboratory protocols [].
A variety of natural and synthetic anesthetic substances have been investigated to reduce metabolism and stress in crustacean species []. Previous studies have reported the use of tricaine methanesulfonate (MS-222), Aqui-STM [], 2-phenoxyethanol [], and quinaldine [] on crustaceans. However, these compounds were found to be neither sufficiently safe nor effective for crustaceans. Essential oils (EOs) have been widely employed with a variety of invertebrates, including amphipods and shrimp, mainly because they have therapeutic properties, are easily accessible, and biodegrade in the environment. Clove oil (Eugenia caryophyllata); the EOs of Lippia alba (EOLA), Aloysia triphylla (EOAT), and Melaleuca alternifolia; and some major EO compounds, such as eugenol, terpinen-4-ol, linalool, and citral, have been found to present sedative, anesthetic, and antioxidant properties in some species, including Daphnia magna [], Gammarus minus [], Litopenaeus vannamei [], Macrobrachium rosenbergii [], and Neohelice granulata [].
In small invertebrates, anesthetics can be used for short-term immobilization, such as for in vivo studies, microscopic analysis, the application of sensors in physiological assessments, and the manipulation of species in the wild. We hypothesized that H. bonariensis [] may be suitable for use as a behavioral model in EOs tests. Therefore, the aim of this study was to determine the time required for anesthesia induction and the recovery of H. bonariensis exposed to EOAT and EOLA and their major compounds citral (mix of neral and geranial) and linalool (mix of S-(+) and R-(−) isomers) and evaluated their effects on the locomotor activity of amphipods using ANY-maze® video monitoring software. Our hypothesis is that the EOs and compounds tested will induce anesthesia and reduce locomotor activity of H. bonariensis.
2. Materials and Methods
2.1. Animals
Specimens of H. bonariensis (5 mm) were collected in Santa Maria municipality in the central region of Rio Grande do Sul, South Brazil. The crustaceans were collected with a hand net (mesh of 250 μm) and transported to the laboratory in 150 mL plastic bottles with a maximum of five individuals each. In a laboratory maintained at a temperature of 20 °C and a photoperiod-controlled room (12 L:12 D), the animals were acclimatized in continuously aerated 5 L aquaria with leaves and sediment in the bottom. They were left in acclimatization conditions for at least one week before study.
2.2. Essential Oils and Major Constituents
Lippia alba (Mill.), N. E. Brown (Verbenaceae), and A. triphylla (L’Herit) Britton (Verbenaceae) were obtained from plants cultivated at the Universidade Federal de Santa Maria campus at Frederico Westphalen, Rio Grande do Sul. The EO extraction was performed via hydrodistillation as described by []. Linalool and citral were acquired from the company Sigma-Aldrich (Burlington, MA, USA). The major components identified for the essential oil of L. alba (EOLA) were linalool (59.8%), cineole (10.29%), germacrene D (6.49%), germacrene B (4.78%), and β-caryophyllene (3.64%). The essential oil of A. triphylla (EOAT) was composed mainly of β-citral (45.59%), p-menth-1-ene (20.26%), β-caryophyllene (6.02%), and caryophyllene oxide (4.3%).
2.3. Anesthesia Induction and Recovery
For the determination of the anesthetic activity, 112 amphipods (n = 8 animals per concentration and anesthetic) were randomly divided and placed into 50 mL beakers (n = 2 amphipods per beaker). Animals were exposed to the following concentrations: clean dechlorinated tap water (control) or solutions containing ethanol (6750 µL/L, equivalent to the highest concentration used to dilute EOLA) with either (i) 250, 500, or 750 µL/L of EOLA; (ii) 150, 300, or 500 µL/L of EOAT; (iii) 100, 200, or 400 µL/L of linalool; or (iv) 100, 200, or 400 µL/L of citral. After the exposure, animals were transferred to containers free of anesthetics to determine recovery time. The anesthetics were diluted in absolute ethanol at a ratio of 1:10 before being added to the test beakers. Anesthetic induction and recovery were evaluated according to []: partial loss of equilibrium (stage 1—sedation), total loss of equilibrium and no reaction to external stimuli (stage 2—anesthesia), and recovery of equilibrium and body movement (stage 3—recovery). Each amphipod was tested only once. The maximum observation time for sedation or anesthesia induction was 30 min. Induction time and recovery time were recorded using a digital stopwatch (expressed in seconds). The studied concentrations were selected according to preliminary tests to observe if the EOs and compounds could induce sedation and/or anesthesia using n = 3 for each concentration, from 25 to 750 µL/L of each EO and compound.
2.4. Locomotor Activity
Forty amphipods (n = 5 animals per concentration and anesthetic) were transferred individually to transparent aquaria containing 40 mL aerated freshwater (±24 °C). The following treatments were tested: (i) clean dechlorinated tap water (control), (ii) 1800 µL/L of ethanol, (iii) 75 µL/L of EOAT, (iv) 100 and 200 µL/L of EOLA, or (v) 50 and 75 µL/L of linalool. The animals were exposed to anesthetic baths in each treatment for 5 min. In this second experiment, concentrations were chosen based on the results of the anesthesia experiments. The concentrations used were the lowest concentrations required for anesthesia induction. The aquarium test was divided into four different virtual zones (A—upper side; B—bottom side; C—right side; D—left side) to delimit the locomotor activity and location of the animals.
Animal movements were recorded for 5 min with a digital camera (Sony Cyber-shot DSC-H300, Campinas, SP, Brazil). Digital analysis of the videos was performed using ANY-maze® software (Stoelting CO, Wood Dale, IL, USA) with the aim of scoring the following behavioral parameters: total distance traveled (m); mean speed (m/s), maximum speed (m/s); absolute turning angle; freezing episodes (duration of time not moving) as time freezing (s); number of crossings between the tank zones; number of entries in each virtual zone (upper/bottom and right/left) and dwelling time in each zone (upper/bottom and right/left) (Table 1). The videos for each anesthetic were analyzed separately.

Table 1.
Description of the behavioral features analyzed by ANY-maze® software.
2.5. Statistical Analysis
The data were expressed as the mean ± SE. The homoscedasticity of variances was verified with the Levene’s test, and normality was assessed using the Kolmogorov–Smirnov test. The significant difference between the time needed for anesthesia induction and the concentration of the anesthetic were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s test. The total distance traveled, mean speed, maximum speed, absolute turning angle, freezing episodes, number of crossings between the tank zones, number of entries in each virtual zone, and dwelling time in each zone data were compared by using one-way ANOVA followed by Tukey’s test, or the Kruskal–Wallis test followed by Dunn’s post hoc test. Analyses were performed using the Statistical version 7.0 (StatSoft, Tulsa, OK, USA) software, and the minimum significance level was set at p < 0.05.
The graphics of total distance traveled, mean speed, maximum speed, absolute turning angle, freezing episodes, and time freezing were performed using Graph Pad Prism 6 (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Anesthesia Induction and Recovery with EOLA and Linalool
Mortality occurred at 66.7 ± 0.33% at 750 µL/L of EOLA, mainly during the recovery time. Increasing concentrations of EOLA decreased the time required for the induction of sedation and anesthesia stages, but this relationship was not observed for linalool (Figure 1a,b). The EOLA concentration of 750 µL/L shortened the time for the induction of stages 1 (less than 1 min) (F = 11.883; p = 0.0026) and 2 of anesthesia (F = 12.039; p = 0.0024) compared with 250 or 500 µL/L of EOLA. The time for the induction of stages 2 of anesthesia with 250 or 500 µL/L of EOLA was 3.27 ± 0.08 min and 2.15 ± 0.04 min, respectively. However, the recovery time was significantly shorter following exposure to the lower EOLA concentrations (F = 13.802; p = 0.001) (Figure 1a). There were no significant differences with stages 1 and 2 of anesthesia between 100 and 200 μL/L of linalool. The concentration of 400 μL/L of linalool reduced the time required for the induction of anesthesia (stage 2) (F = 8.431; p = 0.015) and increased recovery time (F = 2.795; p = 0.010) (Figure 1b). Amphipods anesthetized with 500 and 750 μL/L of EOLA showed a lower time for the induction of anesthesia (stage 2) than those anesthetized with 100 and 200 μL/L of linalool. Recovery from anesthesia was significantly longer at 750 μL/L of EOLA compared with all EOLA and linalool treatments.
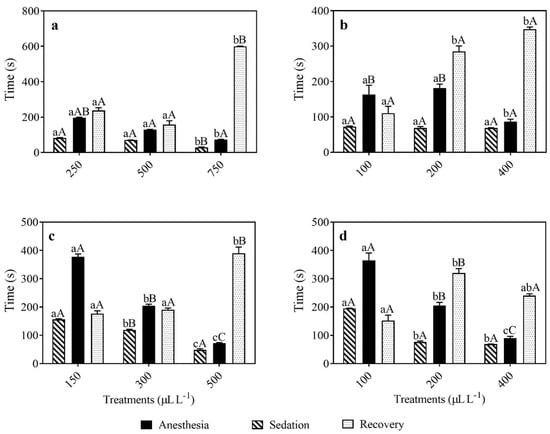
Figure 1.
Time required for anesthesia induction and recovery of the amphipod H. bonariensis exposed to L. alba EO (a), linalool (b), A. triphylla EO (c), and citral (d). Different lowercase letters above the bars indicate significant differences in the time required for anesthesia induction or recovery between concentrations for the same anesthetics or major compounds. Different uppercase letters indicate significant differences in the time required for anesthesia induction or recovery between the anesthetics and their major compounds (L. alba X linalool; A. triphylla X citral) (p < 0.05). Data are presented as mean ± SEM (n = 8).
3.2. Anesthesia Induction and Recovery with EOAT and Citral
Mortality was 50.0 ± 0.39% at the concentrations of 100 or 200 µL/L of citral. Increasing concentrations of EOAT and citral decreased the time required for the induction of sedation and anesthesia stages. Amphipods anesthetized with EOAT demonstrated a reduced time for the induction of stages 1 (F = 11.925; p = 0.0001) and 2 (F = 15.358; p = 0.008) with higher concentrations. The EOAT concentration of 500 μL/L induced fast sedation (1.15 ± 0.01 min) and anesthesia (1.87 ± 0.11 min). However, the recovery time was significantly lower for 150 and 300 μL/L EOAT (F = 12.194; p = 0.002) (Figure 1c). The concentration of 100 μL/L of citral promoted the longest time required for the induction of anesthesia stages 1 (F = 18.925; p = 0.0008) and 2 (F = 15.358; p = 0.008) (3.26 ± 0.03 min and 7.00 ± 0.18 min, respectively). The shortest times for the induction of anesthesia were observed at 400 μL/L of citral. The recovery times were significantly higher for 200 μL/L of citral compared to 100 μL/L of citral (7.33 ± 0.14 min and 5.11 ± 0.60 min, respectively) (F = 11.794; p = 0.003) (Figure 1d). The concentration of 150 μL/L of EOAT and 100 μL/L of citral induced longer sedation (stage 1) and anesthesia (stage 2) compared to the other EOAT and citral treatments. No differences were found for the induction of anesthesia stage 1 and stage 2 between the EOAT and citral. The recovery times were longer at 200 μL/L of citral and 500 μL/L EOAT.
3.3. Locomotor Activity
EOAT resulted in the most distinct behavioral parameters in relation to swimming speed, line crossings, distance traveled, and freezing compared to the control groups. EOLA and linalool treatments resulted in locomotor activity that was similar to that of the control group, with the exception of the maximum speed observed at 200 µL/L. Greater agitation and slight loss of equilibrium were observed for the ethanol group at the initial time. Linalool caused agitation of the animals throughout the time of exposure.
The concentration of 75 µL/L of EOAT resulted in lower mean speed compared to the control group (F = 1.1583; p = 0.006) (Figure 2a). The values of maximum speed at 75 µL/L of EOAT and 200 µL/L of EOLA were significantly lower than those of the control (F = 6.096; p = 0.001) (Figure 2b). There were no significant differences in absolute turn angle between concentrations of EOs and major compounds in relation to the control and ethanol groups (F = 0.504; p = 0.070) (Figure 2c). The concentration of 75 µL/L of EOAT resulted in a higher time of freezing compared to all other samples evaluated (F = 2.899; p = 0.0007) (Figure 2d), while freezing episodes showed no differences between treatments with EOs and major compounds compared to the control group. (Figure 2e).
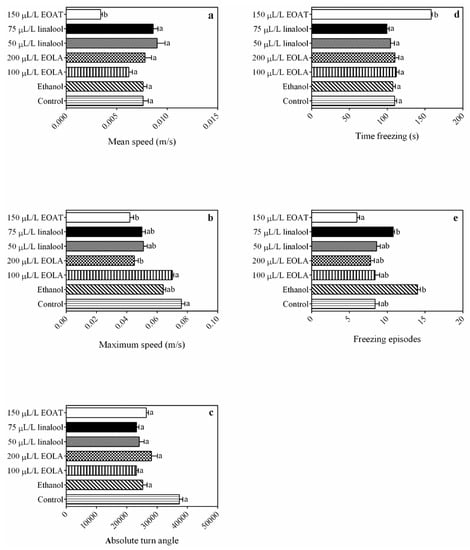
Figure 2.
Locomotor parameters and comparison between immobility periods observed in H. bonariensis groups during behavioral trial. Mean speed (a). Maximum speed (b). Absolute turning angle (c). Time of freezing (d). Freezing episodes (e). Different letters on the right side of the bars indicate significant differences between anesthetic concentrations (p < 0.05; by one-way ANOVA followed by Tukey’s or Kruskal–Wallis tests; n = 5 per group).
The total distance that the amphipods travelled between each zone of the aquarium was significantly lower for those exposed to 150 µL/L of EOAT when compared to the control groups (F = 1.161; p = 0.011) (Figure 3a). The number of crossings observed between the different tank zones was similar for the treatments with the addition of EOs or major compounds compared to the control group (Figure 3b).
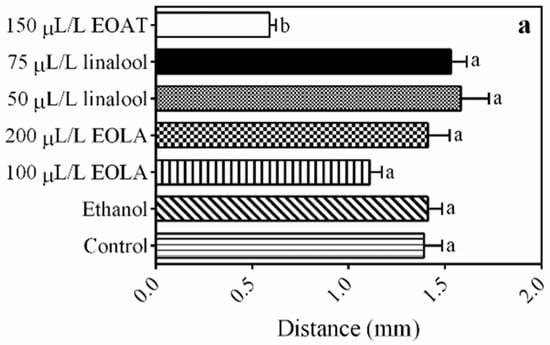
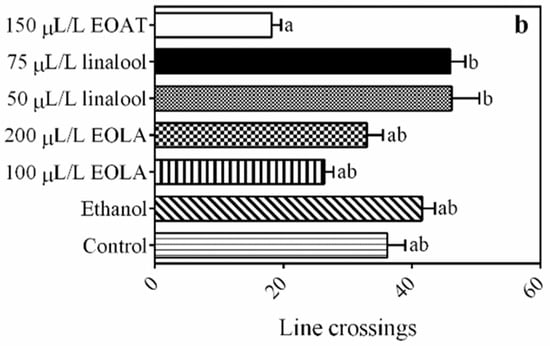
Figure 3.
Tank exploration of H. bonariensis exposed to control, ethanol, and 75 µL/L of A. triphylla EO (EOAT). Concentrations of 50 and 75 µL/L of linalool and 100 and 200 µL/L of L. alba EO (EOLA). Distance (a). Line crossings (b). Different letters indicate significant difference in length of stay at the aquarium zones between the different treatments (p < 0.05; by one-way ANOVA followed by Tukey’s or Kruskal–Wallis tests; n = 5 per group).
Overall, there were no differences in the number of entries between zones in each treatment. The amphipods submitted to both concentrations of linalool showed a higher number of entries for the treatments with EOLA and ethanol when compared to the control group. Amphipods exposed to EOAT exhibited a decreased number of entries to all zones compared with those of the control (Figure 4). There were no preferences for different zones observed between all Eos and major compounds. The animals exposed to 50 µL/L of linalool, 75 µL/L of EOAT, and 100 and 200 µL/L of EOLA remained for a longer time at the bottom of the aquarium (zone B) than the control group did. In contrast, amphipods of the control and ethanol groups stayed for longer periods on the right side (zone C) but without differences between zones A and B for the ethanol group. In the control group, amphipods spent most of the time swimming in the upper zone (zone A) compared with the bottom zone (zone B).
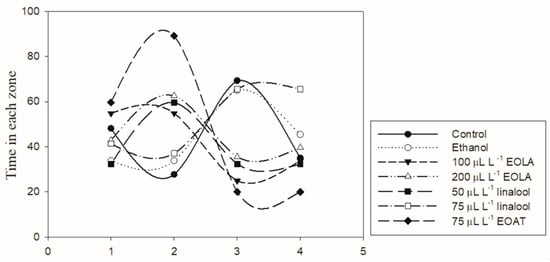
Figure 4.
Comparative analysis of the time spent in each zone of aquarium for the amphipod H. bonariensis during exposure to control, ethanol, and 75 µL/L of A. triphylla EO (EOAT). Concentrations of 50 and 75 µL/L of linalool and 100 and 200 µL/L of L. alba EO (EOLA).
4. Discussion
4.1. Anesthesia Induction and Recovery
Different compositions of EOs may result in distinct pharmacological effects during anesthesia. These differences in composition are influenced by environmental conditions, soil cultivation, collection season, genotypic variations, and extraction method []. In the present study, linalool and β-citral were identified as the primary constituents of EOLA and EOAT, representing 59.80% and 45.59%, respectively. The results demonstrated that linalool and citral alone were effective on the amphipods, H. bonariensis, as sedative and anesthetic substances. Additionally, EOAT and citral were equally successful in anesthesia induction, but EOLA was less efficient than linalool.
In this research, we found that the concentration of EOLA directly influenced the time required to induce anesthesia in H. bonariensis. The shrimp L. vannamei anesthetized with EOLA presented similar responses []. However, the time taken by the amphipod to recover from anesthesia was longer with the increased EOLA concentrations. Moreover, the higher EOLA concentration induced anesthesia faster but resulted in a toxic effect. The EOLA was more effective in terms of speed of anesthesia induction and recovery times for H. bonariensis than has been observed for L. vannamei and F. paulensis exposed to a similar concentration of 500 µL/L of EOLA (16 and 30 min, respectively) [,]. In general, small crustaceans have a higher sensitivity to anesthesia due to greater gill surface area in relation to body size [,].
Linalool occurs naturally in two isomeric forms, which differ according to carbon 3 chirality, characterized by the levorotatory form (3R-(−)-linalool or licareol) and the dextrorotatory form (3S-(+)-linalool or coriandrol) [,]. According to some studies, S-(+)- and R-(−)- linalool presented biological differences [,]. Silva et al. [] did not observe differences in the induction times for stage 2 of anesthesia for silver catfish Rhamdia quelen exposed to both isomers. In comparison with EOLA, linalool induces anesthesia and recovery with a longer time at a concentration of 200 µL/L. On the other hand, the concentration of 400 µL/L of linalool led to a faster recovery without causing mortality. Both linalool and EOLA induced anesthesia within the indicated time frame for crustaceans (3–5 min) [,]. However, linalool has been shown to be safer for use in studies with H. bonariensis.
The pharmacological action of EOs could be a direct effect of major compounds, interactions among active substances, or the synergistic activity between constituents []. Our results indicated that there was no significant difference in time to induce anesthesia between EOAT and citral. The concentrations of 150 µL/L of EOAT and 100 µL/L induced anesthesia at above the time range recommended (higher than 5 min). The mortality of the amphipods anesthetized with 100 or 200 µL/L of citral can be related to the longer time of exposure compared to those exposed at 400 µL/L of citral. Anesthesia with the citral chemotype of L. alba is not recommended for R. quelen because it caused a stressful condition []. These results support the hypothesis that the final effect of the EOAT is the result of the synergism of its different components.
4.2. Locomotor Activity
Anesthetic substances may result in behavioral alterations, including locomotor performance, swimming velocity, reduction in complex or aggressive movements, and stimulation of the frequency of stationary behaviors [,,,]. The different methods that were used for the behavior assessment indicated that EOLA and linalool concentrations used in the locomotor experiment did not result in significant behavioral changes. However, parameters of speed, distance traveled, freezing behavior, and number of entries in the different zones of the aquarium showed changes in response to EOAT, mainly compared to the control group.
The reduction in mean velocity, maximum velocity, number of crossings, and distance traveled in the aquarium by amphipods exposed to EOAT was related to the increase in time without moving. The decrease in maximum velocity at 100 µL/L of EOLA and 75 µL/L of EOAT may be explained by the interaction on the γ-aminobutyric acid (GABA) receptor complex [] or the inhibition of locomotor activity due to depressive action on neuromuscular synapses, respectively []. Furthermore, recent studies have revealed the involvement between a GABA neurotransmitter and metabotropic glutamate receptors in the regulation of anxiolytic effects in invertebrates [,]. A decrease in the swimming velocity was also observed in D. magna exposed to clove oil [] and L. vannamei anesthetized with Cymbopogon citratus EO [].
The Hyalella genus is an essential part of the benthic macrofauna in aquatic environments [,,]. Its population distribution is regulated mainly by the presence or absence of aquatic macrophytes in rivers or lakes []. Light stimuli in the eyes of amphipods are associated with escape behavior to avoid stressful or dangerous situations []. Thus, the presence of substrates or refuge structures determines the behavioral pattern of H. bonariensis in both natural and artificial environments. We observed that the addition of EOs can help prevent negative effects caused by the absence of a substrate in the laboratory or transport this species to other places. Moreover, this hypothesis is confirmed by the higher activity of the control group near the upper zone of the aquarium. The results suggest that the EOs prevent alterations in the natural tendency of the location or distribution of amphipods in the bottom of the aquarium.
In crustaceans, stress behavior results in increased aggressiveness and locomotor activity as a primary response. Chronic stress provokes an increase in metabolic consumption of energy by organisms, compromising health status, reproduction, foraging behavior, and the sociability of the animals [,,]. Some amphipod species increase investment in essential behavior, such as mating behavior or food-intake rates, as a form of reducing the state of vulnerability and utilize a protective or defensive response to stressful experiences [,,,]. This type of behavior variation in amphipods influences other behaviors, such as swimming patterns and the response of escape from predators. These events can trigger consequences at the individual or population level, endangering energetic transference within important aquatic food webs in stream ecosystems [,,].
The EOAT and EOLA induced anxiolytic behavior in zebrafish Danio rerio and R. quelen through an increase in swimming activity in the upper section of the tank, without altering locomotion []. Consistently, EOLA and 50 µL/L of linalool did not influence the swimming pattern of H. bonariensis, but EOAT was responsible for the reduced exploratory activity of amphipods in the present experiment. These results do not demonstrate the anxiolytic action of EOs on H. bonariensis, but further studies are needed to demonstrate if this effect is related to the protection of stress.
The evaluation of the effects of natural products on aquatic animals are important for reducing the impact of chemical products in aquaculture. As demonstrated in the current study, some of the products tested provoked mortality at some of the concentrations tested. The amphipod H. bonariensis proved to be an interesting model for testing natural products, complementing other aquatic organisms traditionally used in toxicological tests, such as zebrafish embryos [,].
5. Conclusions
In conclusion, our study provides information regarding the anesthetic action of EOs and two major EO compounds on the behavioral activity of the amphipod H. bonariensis. The concentrations of 250 or 500 µL/L of EOLA; 100, 200, or 400 µL/L of linalool; 150, 300, or 500 µL/L of EOAT; and 400 µL/L of citral were effective in the induction of sedation and anesthesia in H. bonariensis. Due to their toxic natures, EOA concentrations higher than 500 µL/L and citral are not recommended as an anesthetic for this species. EOAT is recommended for behavioral and long-exposure tests and could also potentially be utilized in the transport, immobilization into laboratory analyses, or collection of these amphipods.
Author Contributions
Conceptualization, S.S. and B.B.; methodology, A.J.B., B.O.C. and B.M.H.; software, A.J.B.; validation, A.J.B. and B.B.; formal analysis, A.J.B. and B.B.; investigation, A.J.B.; resources, S.S., B.O.C., B.M.H. and B.B.; data curation, A.J.B. and B.B.; writing—original draft preparation, A.J.B., S.S., B.O.C., B.M.H. and B.B.; writing—review and editing, A.J.B., S.S., B.O.C., B.M.H. and B.B.; visualization, A.J.B., S.S., B.O.C., B.M.H. and B.B.; supervision, B.B.; project administration, B.B.; funding acquisition, B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS/CAPES, process 19/2551-0000655-1, Brazil); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (finance code 001, CAPES, Brazil), which provided a doctoral fellowship to A. J. Becker; and Conselho Nacional de Desenvolvimento Tecnológico (CNPq, Brazil), which awarded research fellowships to S. Santos, B.H. Heinzmann, B.O. Caron, and B. Baldisserotto.
Institutional Review Board Statement
Ethical review and approval were waived for this study because in Brazil, invertebrate experiments do not require approval by the ethics committee.
Data Availability Statement
Data are available upon request to the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Limberger, M.; Santos, S.; Castiglioni, D.S. Hyalella luciae (Crustacea, Amphipoda, Hyalellidae)—A new species of freshwater amphipod from Southern Brazil. Zootaxa 2022, 5174, 568–582. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, D.D.; Ozga, A.V.; Rodrigues, S.G.; Bueno, A.A.D. Population dynamics of a freshwater amphipod from South America (Crustacea, Amphipoda, Hyalellidae). Nauplius 2016, 24, e2016028. [Google Scholar] [CrossRef]
- Duan, Y.; Guttman, S.I.; Oris, J.T. Genetic differentiation among laboratory populations of Hyalella azteca: Implications for toxicology. Environ. Toxicol. Chem. 1997, 16, 691–695. [Google Scholar] [CrossRef]
- Neuparth, T.; Costa, F.O.; Costa, M.H. Effects of temperature and salinity on life history of the marine amphipod Gammarus locusta. Implications for ecotoxicological testing. Ecotoxicology 2002, 11, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, D.D.S.; Bond-Buckup, G. Reproductive strategies of two sympatric species of Hyalella Smith, 1874 (Amphipoda, Dogielinotidae) in laboratory conditions. J. Nat. Hist. 2007, 41, 1571–1584. [Google Scholar] [CrossRef]
- Ding, Y.; Weston, D.P.; You, J.; Rothert, A.K.; Lydy, M.J. Toxicity of sediment-associated pesticides to Chironomus dilutus and Hyalella azteca. Arch. Environ. Contam. Toxicol. 2011, 61, 83–92. [Google Scholar] [CrossRef]
- Barr, S.; Laming, P.R.; Dick, J.T.A.; Elwood, R.W. Nociception or pain in a decapod crustacean? Anim. Behav. 2008, 75, 745–751. [Google Scholar] [CrossRef]
- Elwood, R.W. Pain and suffering in invertebrates? ILAR J. 2011, 52, 175–184. [Google Scholar] [CrossRef]
- De Lange, H.J.; Peeters, E.T.H.M.; Lürling, M. Changes in ventilation and locomotion of Gammarus pulex (Crustacea, Amphipoda) in response to low concentrations of pharmaceuticals. Hum. Ecol. Risk Assess. 2009, 15, 111–120. [Google Scholar] [CrossRef]
- Bownik, A. Clove essential oil from Eugenia caryophyllus induces anesthesia, alters swimming performance, heart functioning and decreases survival rate during recovery of Daphnia magna. Turk. J. Fish. Aquat. Sci. 2015, 15, 157–166. [Google Scholar] [CrossRef]
- Marks, I.M.; Nesse, R.M. Fear and fitness: An evolutionary analysis of anxiety disorders. Ethol. Sociobiol. 1994, 15, 247–261. [Google Scholar] [CrossRef]
- Fossat, P.; Bacqué-Cazenave, J.; De Deurwaerdère, P.; Delbecque, J.P.; Cattaert, D. Anxiety-like behavior in crayfish is controlled by serotonin. Science 2014, 344, 1293–1297. [Google Scholar] [CrossRef] [PubMed]
- Perrot-Minnot, M.J.; Banchetry, L.; Cézilly, F. Anxiety-like behaviour increases safety from fish predation in an amphipod crustacea. R. Soc. Open Sci. 2017, 4, 171558. [Google Scholar] [CrossRef] [PubMed]
- Bacqué-Cazenave, J.; Cattaert, D.; Delbecque, J.P.; Fossat, P. Social harassment induces anxiety-like behaviour in crayfish. Sci. Rep. 2017, 7, 39935. [Google Scholar] [CrossRef]
- Boyd, W.A.; Brewer, S.K.; Williams, P.L. Altered Behaviour of Invertebrates Living in Polluted Environments. In Behavioural Ecotoxicology; Dell’Omo, G., Ed.; John Wiley & Sons: Chichester, UK, 2002; pp. 93–336. [Google Scholar]
- Valente, C.D. Anaesthesia of decapod crustaceans. Vet. Anim. Sci. 2022, 16, 100252. [Google Scholar] [CrossRef] [PubMed]
- Coyle, S.D.; Dasgupta, S.; Tidwell, J.H.; Beavers, T.; Bright, L.A.; Yasharian, D.K. Comparative efficacy of anesthetics for the freshwater prawn Macrobrachium rosenbergii. J. World Aquac. Soc. 2005, 36, 282–290. [Google Scholar] [CrossRef]
- Jensen, M.A.; Fitzgibbon, Q.P.; Carter, C.G.; Adams, L.R. Recovery periods of cultured spiny lobster, Sagmariasus verreauxi juveniles: Effects of handling, force feeding, exercising to exhaustion and anaesthesia on oxygen consumption and ammonia-N excretion rates. Aquaculture 2013, 410, 114–121. [Google Scholar] [CrossRef]
- Guzmán-Sáenz, F.M.; González-Alanís, P.; Martínez, J.G.S.; Salazar, G.G.; Guzmán, G.A.; Perez-Castañeda, R. Uso de diferentes fármacos para anestesiar camarones Litopenaeus vannamei Boone en prácticas de acuacultura. REDVET R. Eléctron. Vet. 2010, 11, 1–9. Available online: http://www.veterinaria.org/revistas/redvet/n030310/031009.pdf (accessed on 10 February 2023).
- Venarsky, M.P.; Vilhelm, F.M. Use of clove oil to anaesthetize freshwater amphipods. Hydrobiologia 2006, 568, 425–432. [Google Scholar] [CrossRef]
- Parodi, T.V.; Cunha, M.A.; Heldwein, C.G.; Souza, D.M.; Martins, A.C.; Garcia, L.O.; Junior, W.W.; Monserrat, J.M.; Schmidt, D.; Caron, B.O.; et al. The anesthetic efficacy of eugenol and the essential oils of Lippia alba and Aloysia triphylla in post-larvae and subadults of Litopenaeus vannamei (Crustacea, Penaeidae). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2012, 155, 462–468. [Google Scholar] [CrossRef]
- Saydmohammed, M.; Pal, A.K. Anesthetic effect of eugenol and menthol on handling stress in Macrobrachium rosenbergii. Aquaculture 2009, 298, 162–167. [Google Scholar] [CrossRef]
- Souza, C.F.; Lima, T.; Baldissera, M.D.; Geihs, M.A.; Maciel, F.E.; Nery, L.E.; Santos, R.C.V.; Raffin, R.P.; Heinzmann, B.M.; Caron, B.O.; et al. Nanoencapsulated Melaleuca alternifolia essential oil exerts anesthetic effects in the brachyuran crab using Neohelice granulata. An. Acad. Bras. Ciências 2018, 90, 2855–2864. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.F.; Araujo, P.B.; Bond-Buckup, G. New species and new reports of Hyalella (Crustacea, Amphipoda, Dogielinotidae) from Argentina. Zootaxa 2008, 1760, 24–36. [Google Scholar] [CrossRef]
- Gobbo-Neto, L.; Lopes, N.P. Plantas medicinais: Fatores de influência no conteúdo de metabólitos secundários. Quim. Nova 2007, 30, 374–381. [Google Scholar] [CrossRef]
- Becker, A.J.; Vaz, L.J.; Garcia, L.O.; Wasielesky, W.; Heinzmann, B.M.; Baldisserotto, B. Anesthetic potential of different essential oils for two shrimp species, Farfantepenaeus paulensis and Litopenaeus vannamei (Decapoda, Crustacea). Cienc. Rural 2021, 51, e20200793. [Google Scholar] [CrossRef]
- Li, Y.; Liang, S.; She, Q.; Han, Z.; Li, Y.; Li, X. Influence of temperature and size on menthol anaesthesia in Chinese grass shrimp Palaemonetes sinensis (Sollaud, 1911). Aquac. Res. 2018, 49, 2091–2098. [Google Scholar] [CrossRef]
- Sugawara, Y.; Hara, C.; Tamura, K.; Fujii, T.; Nakamura, K.; Masujima, T.; Aoki, T. Sedative effect on humans of inhalation of essential oil of linalool: Sensory evaluation and physiological measurements using optically active linalools. Anal. Chim. Acta 1998, 365, 293–299. [Google Scholar] [CrossRef]
- Sousa, D.P.; Nóbrega, F.F.; Santos, C.C.M.P.; Almeida, R.N. Anticonvulsant activity of the linalool enantiomers and racemate: Investigation of chiral influence. Nat. Prod. Commun. 2010, 5, 1847–1851. [Google Scholar] [CrossRef]
- Hutt, A.G.; O’Grady, J. Drug chirality: A consideration of the significance of the stereochemistry of antimicrobial agents. J. Antimicrob. Chemother. 1996, 37, 7–32. [Google Scholar] [CrossRef]
- Mitra, S.; Chopra, P. Chirality and anaesthetic drugs: A review and an update. Indian J. Anaesth. 2011, 55, 556–562. [Google Scholar] [CrossRef]
- Silva, L.; Balconi, L.S.; Gressler, L.T.; Garlet, Q.I.; Sutili, F.J.; Vargas, A.P.; Baldisserotto, B.; Morel, A.F.; Heinzmann, B.M. S-(+)-and R-(−)-linalool: A comparison of the in vitro anti-Aeromonas hydrophila activity and anesthetic properties in fish. An. Acad. Bras. Ciênc. 2017, 89, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.J.; Ramos, P.B.; Monserrat, J.M.; Wasielesky, W.; Baldisserotto, B. Behavioural and biochemical responses in adult Pacific white shrimp, Litopenaeus vannamei, exposed to the essential oil of Cymbopogon citratus. Aquac. Res. 2021, 52, 6205–6217. [Google Scholar] [CrossRef]
- Heldwein, C.G.; Silva, L.L.; Gai, E.Z.; Roman, C.; Parodi, T.V.; Bürger, M.E.; Baldisserotto, B.; Flores, E.M.M.; Heinzmann, B.M. S-(+)-Linalool from Lippia alba: Sedative and anesthetic for silver catfish (Rhamdia quelen). Vet. Anaesth. Analg. 2014, 41, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.F.; Descovi, S.; Baldissera, M.D.; Bertolin, K.; Bianchini, A.E.; Mourão, R.H.V.; Schmidt, D.; Heinzmann, B.M.; Antoniazzi, A.; Baldisserotto, B.; et al. Involvement of HPI-axis in anesthesia with Lippia alba essential oil citral and linalool chemotypes: Gene expression in the secondary responses in silver catfish. Fish Physiol. Biochem. 2019, 45, 155–166. [Google Scholar] [CrossRef]
- Ozeki, M. The effects of eugenol on the nerve and muscle in crayfish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 1975, 50, 183–191. [Google Scholar]
- Bownik, A. Protective effects of ectoine on physiological parameters of Daphnia magna subjected to clove oil-induced anaesthesia. Turk. J. Fish. Aquat. Sci. 2016, 16, 691–701. [Google Scholar] [CrossRef]
- Cowing, D.; Powell, A.; Johnson, M. Evaluation of different concentration doses of eugenol on the behaviour of Nephrops norvegicus. Aquaculture 2015, 442, 78–85. [Google Scholar] [CrossRef]
- Heldwein, C.G.; Silva, L.L.; Reckziegel, P.; Barros, F.M.C.; Bürger, M.E.; Baldisserotto, B.; Mallmann, C.A.; Schmidt, D.; Caron, B.O.; Heinzmann, B.M. Participation of the GABAergic system in the anesthetic effect of Lippia alba (Mill.) NE Brown essential oil. Braz. J. Med. Biol. Res. 2012, 45, 436–443. [Google Scholar] [CrossRef]
- Swanson, C.J.; Bures, M.; Johnson, M.P.; Linden, A.M.; Monn, J.A.; Schoepp, D.D. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat. Rev. Drug Discov. 2005, 4, 131–144. [Google Scholar] [CrossRef]
- Kruschwitz, L.G. Environmental factors controlling reproduction of the amphipod Hyalella azteca. Proc. Okla. Acad. Sci. 1978, 21, 16–21. [Google Scholar]
- Muskó, I.B. Qualitative and quantitative relationships of Amphipoda (Crustacea) living on macrophytes in Lake Balaton (Hungary). In Trophic Relationships in Inland Waters; Biró, P., Talling, J.F., Eds.; Developments in Hydrobiology; Springer: Dordrecht, The Netherlands, 1990. [Google Scholar] [CrossRef]
- Wellborn, G.A. Determinants of reproductive success in freshwater amphipod species that experience different mortality regimes. Anim. Behav. 1995, 50, 353–363. [Google Scholar] [CrossRef]
- Stom, D.I.; Zhdanova, G.O.; Saksonov, M.N.; Balayan, A.E.; Tolstoy, M.Y. Light avoidance in Baikalian amphipods as a test response to toxicants. Contemp. Probl. Ecol. 2017, 10, 77–83. [Google Scholar] [CrossRef]
- Fossat, P.; Bacqué-Cazenave, J.; De Deurwaerdère, P.; Cattaert, D.; Delbecque, J.P. Serotonin, but not dopamine, controls the stress response and anxiety-like behavior in the crayfish Procambarus clarkii. J. Exp. Biol. 2015, 218, 2745–2752. [Google Scholar] [CrossRef] [PubMed]
- Crook, R.J.; Dickson, K.; Hanlon, R.T.; Walters, E.T. Nociceptive sensitization reduces predation risk. Curr. Biol. 2014, 24, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.J.; Kwan, G.T.; Gallup, J.; Tresguerres, M. Acute fluoxetine exposure alters crab anxiety-like behaviour, but not aggressiveness. Sci. Rep. 2016, 6, 19850. [Google Scholar] [CrossRef] [PubMed]
- Dianne, L.; Perrot-Minnot, M.J.; Bauer, A.; Guvenatam, A.; Rigaud, T. Parasite-induced alteration of plastic response to predation threat: Increased refuge use but lower food intake in Gammarus pulex infected with the acanthocephalan Pomphorhynchus laevis. Int. J. Parasitol. 2014, 44, 211–216. [Google Scholar] [CrossRef]
- Dunn, A.M.; Dick, J.T.A.; Hatcher, M.J. The less amorous Gammarus: Predation risk affects mating decisions in Gammarus duebeni (Amphipoda). Anim. Behav. 2008, 76, 1289–1295. [Google Scholar] [CrossRef]
- Mohammad, F.; Aryal, S.; Ho, J.; Stewart, J.C.; Norman, N.A.; Tan, T.L.; Eisaka, A.; Claridge-Chang, A. Ancient anxiety pathways influence Drosophila defense behaviors. Curr. Biol. 2016, 26, 981–986. [Google Scholar] [CrossRef]
- MacNeil, C.; Dick, J.T.; Elwood, R.W. The dynamics of predation on Gammarus spp. (Crustacea: Amphipoda). Biol. Rev. 1999, 74, 375–395. [Google Scholar] [CrossRef]
- Bossus, M.C.; Guler, Y.Z.; Short, S.J.; Morrison, E.R.; Ford, A.T. Behavioural and transcriptional changes in the amphipod Echinogammarus marinus exposed to two antidepressants, fluoxetine and sertraline. Aquat. Toxicol. 2014, 151, 46–56. [Google Scholar] [CrossRef]
- Worischka, S.; Schmidt, S.I.; Hellmann, C.; Winkelmann, C. Selective predation by benthivorous fish on stream macroinvertebrates—The role of prey traits and prey abundance. Limnologica 2015, 52, 41–50. [Google Scholar] [CrossRef]
- Junior, G.B.; Abreu, M.S.; Rosa, J.G.S.; Pinheiro, C.G.; Heinzmann, B.M.; Caron, B.O.; Baldisserotto, B.; Barcellos, L.J.G. Lippia alba and Aloysia triphylla essential oils are anxiolytic without inducing aversiveness in fish. Aquaculture 2018, 482, 49–56. [Google Scholar] [CrossRef]
- Capatina, L.; Boiangiu, R.S.; Dumitru, G.; Napoli, E.M.; Ruberto, G.; Hritcu, L.; Todirascu-Ciornea, E. Rosmarinus officinalis essential oil improves scopolamine-induced neurobehavioral changes via restoration of cholinergic function and brain antioxidant status in zebrafish (Danio rerio). Antioxidants 2020, 9, 62. [Google Scholar] [CrossRef]
- Capparucci, F.; De Benedetto, G.; Natale, S.; Pecoraro, R.; Iaria, C.; Marino, F. Evaluation of anaesthetic effect of commercial basil Ocimum basilicum on zebrafish (Danio rerio) embryos. Fishes 2022, 7, 318. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).