Abstract
High environmental hydrogen peroxide (H2O2) has been demonstrated to be toxic for fish. However, the response mechanism of fish to chronic H2O2 exposure is not yet well understood. Therefore, this study aimed to investigate the alteration in ion transport in gills and analyzed the potential response mechanism after chronic H2O2 exposure. The common carps were exposed to 0, 0.25, 0.50, and 1.00 mM of H2O2 for 14 days. The histopathological evaluation results indicated that H2O2 exposure caused incomplete gill filament structure. In the plasma, H2O2 exposure suppressed the potassium (K+) concentration but increased sodium (Na+) concentration. In the gills, the calcium (Ca2+) level was raised, but the K+ and chlorine (Cl−) levels were decreased after H2O2 exposure. After 14 days of exposure, H2O2 prompted the activities of Ca2+/Mg2+-ATPase and H+/K+-ATPase but suppressed Na+/K+-ATPase activity in the gills. Gene transcription analysis showed that the ion-regulation-related genes including nkaa and rhbg were downregulated after H2O2 exposure. In addition, H2O2 exposure upregulated the mRNA levels of cam and camk II, indicating that the Ca2+ singling pathway was activated. In conclusion, our data showed that chronic H2O2 exposure altered gill structure and disturbed ion transport, which further negatively affected the equilibrium of ions and osmotic pressure.
Key Contribution:
Chronic H2O2 exposure injured the gill structure, decreased the Na+/K+-ATPase activity, and disturbed the ion balance. These results provide a valuable reference for the application of H2O2 in aquaculture and its toxicity evaluation in fish.
1. Introduction
Hydrogen peroxide (H2O2) is a common reactive oxygen species (ROS), distributed widely in natural water bodies such as rivers, lakes, and oceans [1]. There are four main sources of H2O2 in the aquatic environment: the product of photochemical reactions with dissolved organic matter, atmospheric wet or dry deposition, bacteria and algae secretion, and released by anthropogenic activities [2,3,4,5]. In aquaculture, H2O2 is frequently used as a therapeutic compound approved by the Food and Drug Administration of China, the USA, and Norway [6,7], which can effectively control fish diseases induced by bacteria and parasite infection [8]. Especially in the salmonid industry, it is applied to kill sea lice and treat amoebic gill disease [9,10]. Bechmann et al. [11] reported that the salmon farms of Norway consumed 135 million kilograms of H2O2 during 2009–2018. It is also a feasible algaecide which has been used to prevent cyanobacteria bloom and attenuate their toxins in aquatic environments [12,13]. These activities may lead to a short-term and/or repeated H2O2 accumulation in aquatic environments. Thus, it is categorized as one of the “Priority Assessment Chemical Substances” in some countries, such as Japan [1]. It has been reported that the H2O2 concentration may reach up to the micromolar level in aquatic ecosystems [14]. Currently available data show that H2O2 concentration is 0.09–3.2 μM in rivers, 0.00–5.31 μM in lakes, 0.06–0.45 μM in the open ocean, and 0–199 μM in rain [15,16,17,18,19]. This evidence indicates that it is possible to accumulate relatively higher concentrations of H2O2 in aquatic environments, which is harmful to the growth and survival of aquatic organisms [20].
Although H2O2 is considered to be an environment-friendly therapeutic compound in aquaculture, its accumulation has been affecting aquatic ecosystems due to oxidative stress to aquatic microorganisms [4]. H2O2 toxicity for fish has attracted much attention, and it varies considerably in different fish species, life stages, and temperatures. For instance, the 1 h-LC50 (median lethal concentration) value of H2O2 is 1640–2480 ppm in channel catfish (Ictalurus punctatus) and 183–260 ppm in rainbow trout (Oncorhynchus mykiss) at 22 °C [21]; the tolerance concentration was less than 0.335 mM in blue gourami (Trichogaster trichopterus) and 0.194 mM in suckermouth catfish (Hypostomus plecostomus) for 1 h of H2O2 exposure [7]. Meanwhile, the negative physiological effects, such as oxidative stress, immunosuppression, and tissues damage, on fish have been frequently reported in recent years under sub-lethal H2O2 exposure [22]. A 1 h exposure of H2O2 (50 ppm) induced a quick physiological stress response in sea bass (Dicentrarchus labrax) [23]. Similarly, short-term H2O2 exposure (1 h) resulted in an adverse immune response in olive flounder (Paralichthys olivaeceus) (100–500 ppm) and seabream (Sparus aurata) (50 ppm) [24,25]. H2O2 exposure also caused oxidative stress, leading to damage of the intestinal epithelial cells in Jian carp (Cyprinus carpio var. Jian) [26] and the liver in tilapia (Oreochromis niloticus) [27]. Our previous studies found that chronic H2O2 exposure (1 mM) affected the redox state, apoptosis, endoplasmic reticulum stress, immune response, autophagy, and brain function in common carp (Cyprinus carpio) [28,29,30]. However, the molecular mechanisms of chronic H2O2 exposure have not yet been clarified entirely in fish.
The gills of fish are crucial organs involved in multiple physiological activities, such as gas exchange, ion balance, energy metabolism, and detoxification [31]. They are also considered as a physical barrier and immune defense to prevent the invasion of pathogens in fish [32]. The gills are sensitive to changes in the water environment and more vulnerable to pollutants due to direct exposure to toxicants dissolved in water. Previous studies have showed that 50 ppm H2O2 exposure induced redox imbalance and lipid peroxidation in gills [33], while 200 mg/L H2O2 exposure resulted in gill damage of rainbow trout [34]. Meanwhile, H2O2 exposure modulated gill immune activity and disrupted the mucus covering of the gills of Atlantic salmon (Salmo salar) [35]. It is apparent that existing studies mainly focused on revealing the tissues damage, oxidative stress, and immune response of fish gills under a short-term H2O2 exposure but overlooked the changes in ion transport in H2O2-exposed fish gills.
The common carp (Cyprinus carpio) is a globally distributed (farmed or wild) and consumed fish species. It is also a frequently used model animal in the evaluation of the physiology, immunology, and toxicology of fish due to its easy adaptation to laboratory conditions. In this study, we exposed common carp to 0.25–1 mM of H2O2 for 14 days, and then analyzed the changes in the histomorphology, ion concentration, ion transport enzymes, and key genes related to ion regulation in the gills. To our knowledge, this is the first study to evaluate the effect of H2O2 exposure on ion transport in fish gills. These data may provide a valuable reference for the application of H2O2 in aquaculture.
2. Materials and Methods
2.1. Animals, Experiment Design, and Sampling
The common carp used in the experiment were provided by the farm of Freshwater Fisheries Research Center (Wuxi, China), with an average weight of 64 ± 5 g. They were temporarily raised in the circulating aquaculture system for two weeks to adapt to the lab conditions. During the temporary rearing period, a commercial diet (Tongwei, Chengdu, China) was fed at 2–3% of the total body weight of the fish twice a day.
After acclimation, the common carp were exposed to four concentrations of H2O2: 0 (normal control), 0.25, 0.50, and 1.00 mM, according to the 1 h LC50 and non-lethal concentration of H2O2 [30]. Each group included 30 individual fish kept in three tanks. The exposure time was 1 h per day for 14 consecutive days [20]. During the exposure period, the fish were fed with an appropriate diet to avoid the stress reaction caused by starvation. After 14 days, nine fish in each group (control group and H2O2-exposed groups) were picked at random and immediately anesthetized using 100 ppm of MS-222 (Sigma, St Louis, MO, USA) buffered with sodium bicarbonate [36,37]. Then, blood and gill tissues were collected in the shortest possible time. The blood was used to separate plasma by centrifugation (5000 r/min, 10 min, and 4 °C) [30]. All samples were stored in a −80 °C refrigerator until use. The flowchart of the experiments is shown in Figure 1.
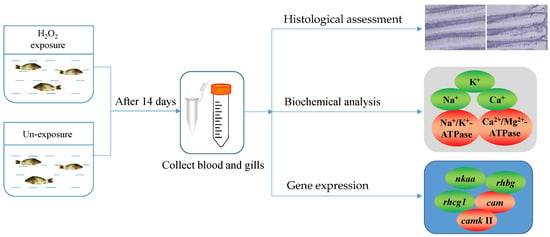
Figure 1.
Flowchart of the experiment.
2.2. Histological Evaluation of Gills
Common carp gills were fixed in neutral formalin solution (Solarbio, Beijing, China) for 24 h and dehydrated using an ethanol gradient (70%, 80%, 85%, 90%, 95%, and 100%). Dehydrated tissues were cleared by xylene (Sigam) and then embedded in paraffin (56–58 °C, Solarbio). The tissues were cut into 5–6 µm slices. The sections were stained with hematoxylin–eosin (H&E, Nanjing Jiancheng Bioengineering Institute, Nanjing, China) after deparaffinization and evaluated under a light microscope (Olympus, Tokyo, Japan). The histological changes were assessed via the method reported by Bernet et al. [38] and Nunes et al. [39]. The gill pathological changes included five reaction patterns (rp): circulatory disturbances, regressive changes, progressive changes, inflammation, and tumor. Each reaction pattern contained some alteration (alt), and each alteration was assigned to a value called the “importance factor (w)” ranging from 1 to 3. A “score value (a)” ranging from 0 to 6 was used to assess the extension of the pathological change. The “organ index (Iorg)” was calculated by the formula: . The high index represented a severe degree of damage to the gills.
2.3. Determination of Ion Content in Plasma and Gills
The levels of ions including sodium (Na+), potassium (K+), calcium (Ca2+), and chlorine (Cl−) in plasma and gills were tested using commercial kits according to the instructions of the manufacturer. The kits of Na+ (#C002-1-1), K+ (#C001-2-1), Ca2+ (#C004-2-1), and Cl− (#C003-2-1) were purchased from Nanjing Jiancheng Bioengineering Institute. Na+ was measured at 630 nm via reaction with 6-potassium antimony hydroxide [40]. K+ was tested at 440 nm by the sodium tetraphenylboron method [41]. Ca2+ was detected at 610 nm by the methylthymol blue method [42]. Cl− was determined at 505 nm using the mercuric thiocyanate method [43].
2.4. Determination of Ion-Transport-Related Enzymes in Gills
The levels of Na+/K+-ATPase, H+/K+-ATPase, and Ca2+/Mg2+-ATPase in the gills were measured using commercial kits (#A070-6 and #A069-1, Nanjing Jiancheng Biotechnology Institute, Nanjing, China). The ATPase activity was calculated via the inorganic phosphate (Pi) content produced from the ATP decomposition reaction under the Na+/K+-, H+/K+-, and Ca2+/Mg2+-ATPase-specific inhibitors [44]. The protein (#A045-4-2) content was evaluated by the bicinchoninic acid (BCA) method [45].
2.5. Real-Time Quantitative PCR
Total RNA of the gills was extracted according to a previous method described by Jia et al. [46]. About 0.06 g of common carp gill tissue was added to 1 mL of RNAiso Plus reagent (#9108, TaKaRa, Beijing, China) to homogenize. The homogenized mixture was used to extract the total RNA by centrifugation after adding chloroform and isopropyl alcohol (Sigma). The quality and quantity were evaluated by measuring OD260/OD280 value and gel electrophoresis. The total RNA was used to synthesize cDNA via reverse transcription with PrimeScriptTM RT Reagent (#RR047A, TaKaRa) according to the kit instructions. The reaction conditions were 37 °C for 15 min and 85 °C for 5 s.
The relative expression of target genes was detected by quantitative real-time PCR (qPCR) using TB GreenTM Premix EX TaqTM II kit (#RR820, TaKaRa) [47]. Briefly, the reaction mixture included 2 μL of cDNA, 12.5 μL of TB Green Premix Ex Taq II, 2 μL of specific primers, and 8.5 μL RNase-free water. The reaction conditions were as follows: 30 s at 95 °C for initial denaturation, 5 s at 95 °C for denaturation, and 1 min at 59–62 °C for annealing and extension, for a total of 40 cycles. The β-actin gene was used as an internal reference to calculate the relative mRNA level of target genes via the 2−ΔΔCq method, and the amplification efficiency of specific primers was approximately 100% [48]. The specific primers are shown in Table 1.

Table 1.
Specific primer sequences for qPCR in the study.
2.6. Statistical Analysis
All data were analyzed using the SPSS software package (24.0 version, Armonk, NY, USA), and the results are expressed as mean ± standard error (mean ± SE). The normal distribution and variance homogeneity were determined by the Shapiro–Wilk test and Levene test, respectively. The differences among groups were analyzed via one-way ANOVA with LSD multiple test (equal variances) or Tamhane’s T2 test (unequal variance). The difference between the control group and 1.00 mM H2O2 treatment was analyzed by independent-samples t test in histopathology assessment. The threshold value (p) was 0.05.
3. Results
3.1. Histopathology Observation of Gills after H2O2 Exposure
The histological alterations in the gills are shown in Figure 2. The gill from the control group had normal morphology, a complete structure, and regular branchial filaments (Figure 2A). In the H2O2-exposed group, gill filaments were irregular and incomplete. For example, atrophying and expanding gill filaments were found after H2O2 exposure. Some gill filaments and gill arches were damaged by H2O2 exposure (Figure 2B). Further, the gill pathological index was significantly higher in H2O2-exposed fish than in unexposed fish (Figure 2C).
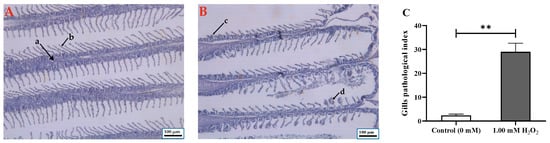
Figure 2.
Histological alterations in gill of Cyprinus carpio. (A) Control group (a, gill arch; b, gill filaments); (B) 1.00 mM H2O2-exposed group (c, atrophy; d, hypertrophy); (C) gill pathological index (mean ± SE, n = 9). The sections were stained with H&E and observed at 100 × original magnification. Bar = 100 μm. ** indicates significant difference between control group and H2O2-exposed group (p < 0.01, t test).
3.2. Changes in Ion Content in Plasma and Gills after H2O2 Exposure
In plasma, the levels of ions were significantly affected by H2O2 exposure (Figure 3). Compared with the control group (0 mM), the level of Na+ was markedly increased by 0.50 and 1.00 mM H2O2 treatments (p < 0.05, Figure 3A), but the level of K+ was markedly decreased by three concentrations of H2O2 exposure (p < 0.05, Figure 3B). The Ca2+ content was clearly increased by the 0.25 mM H2O2 treatment (p < 0.05) but returned to a normal level in the 0.50 and 1.00 mM H2O2 treatments (Figure 3C). The Cl− content did not show a significant change after H2O2 exposure relative to the control group (Figure 3D).
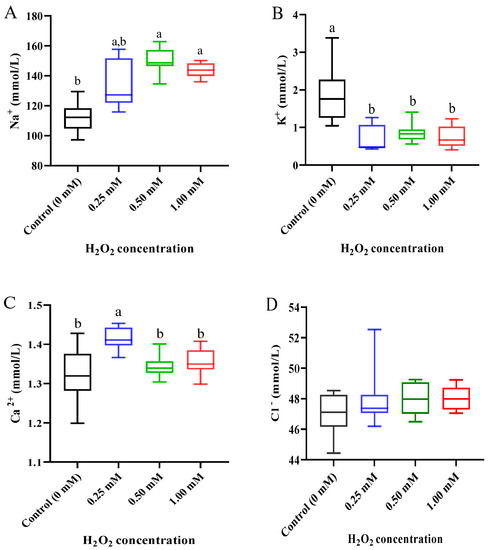
Figure 3.
Ion concentration in plasma of Cyprinus carpio under H2O2 exposure. (A) Sodium (Na+); (B) potassium (K+); (C) calcium (Ca2+); (D) chlorine (Cl−). Data are expressed as mean ± SE (n = 9). The different letters above each bar indicate a statistical significance among different concentrations of H2O2 exposure (p < 0.05).
In the gills, the K+ concentration reduced after H2O2 exposure, with a significant difference in the 1.00 mM H2O2-exposed group (p < 0.05, Figure 4A). The Ca2+ concentration was higher in the 1.00 mM H2O2 treatment than in the control group (p < 0.05, Figure 4B). Compared with the control group, three concentrations of H2O2 treatments caused a decrease in the Cl− content (p < 0.05, Figure 4C).
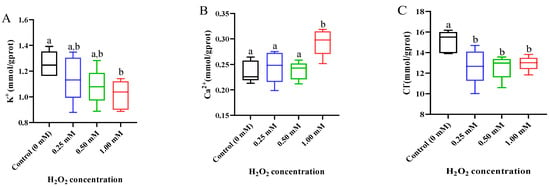
Figure 4.
Ion concentration in gills of Cyprinus carpio under H2O2 exposure. (A) Potassium (K+); (B) calcium (Ca2+); (C) chlorine (Cl−). Data are expressed as mean ± SE (n = 9). The different letters above each bar indicate a statistical significance among different concentrations of H2O2 exposure (p < 0.05).
3.3. Activities of Ion Transport Enzymes in Gills after H2O2 Exposure
After 14 days of exposure, the activities of ion transport enzymes in the gills showed significant differences in the higher H2O2-treated group (1.00 mM) but no obvious changes in the lower H2O2-treated group (0.25 mM) (Figure 5). Specifically, the activity of Na+/K+-ATPase was obviously decreased, but the activity of Ca2+/Mg2+-ATPase was distinctly raised in the 1.00 mM of H2O2-exposed group (p < 0.05, Figure 5A,B). The activity of H+/K+-ATPase was promoted by exposure to 0.50 and 1.00 mM of H2O2 compared to the control group (p < 0.05, Figure 5C).
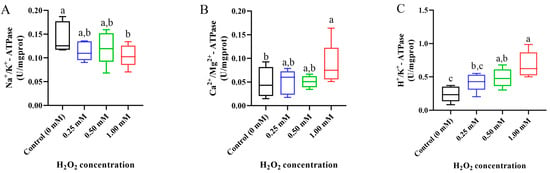
Figure 5.
Activities of Na+/K+-ATPase (A), Ca2+/Mg2+-ATPase (B), and H+/K+-ATPase (C) in gills of Cyprinus carpio under H2O2 exposure. Data are expressed as mean ± SE (n = 9). The different letters above each bar indicate a statistical significance among different concentrations of H2O2 exposure (p < 0.05).
3.4. Expression of Ion-Transport-Related Genes in Gills after H2O2 Exposure
The ion transport in the gills of common carp was evaluated by determining the gene expression of sodium/potassium-transporting ATPase subunit alpha (nkaa), rhesus glycoprotein B (rhbg), rhcg1, and rhcg2 (Figure 6). The nkaa expression displayed a downward trend, and the prominent downregulation was observed in the 1.00 mM of H2O2-exposed fish (p < 0.05, Figure 6A). After H2O2 exposure, the transcription level of rhbg was clearly downregulated at three doses of treatments (p < 0.05, Figure 6B), but the rhcg1 and rhcg2 expressions did not exhibit statistically significant differences compared with the control group (p > 0.05; Figure 6C,D).
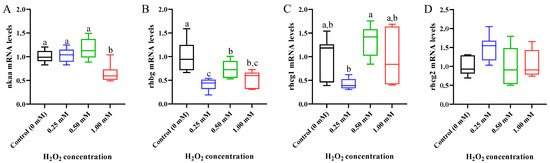
Figure 6.
Expression of ion-transport-related genes in gills of Cyprinus carpio under H2O2 exposure. (A) Sodium/potassium-transporting ATPase subunit alpha (nkaa); (B) rhesus glycoprotein B (rhbg); (C) rhesus glycoproteins C 1 (rhcg1) and (D) rhcg2. Data are expressed as mean ± SE (n = 9). The different letters above each bar indicate a statistical significance among different concentrations of H2O2 exposure (p < 0.05).
3.5. Expression of Calcium Signaling Pathway-Related Genes after H2O2 Exposure
It can be seen from Figure 7A that with the increase in H2O2 concentration, the mRNA level of calmodulin (cam) gradually increased and reached a significant level in the 0.50 and 1.00 mM H2O2-exposed common carp (p < 0.05). Similarly, the mRNA level of camk II (calmodulin-dependent protein kinase II) was significantly upregulated after 1.00 mM of H2O2 exposure compared to the control group (p < 0.05, Figure 7B).
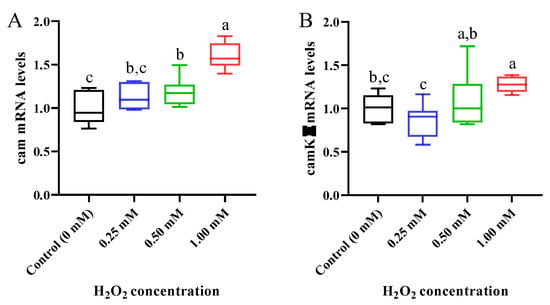
Figure 7.
Expression of calcium signaling pathway-related genes in gills of Cyprinus carpio under H2O2 exposure. (A) Calmodulin (cam); (B) calmodulin-dependent protein kinase II (camk II). Data are expressed as mean ± SE (n = 9). The different letters above each bar indicate a statistical significance among different concentrations of H2O2 exposure (p < 0.05).
4. Discussion
Fish live in complex aquatic environments where ionic and osmotic compositions are variable. To adapt to the changeable environment, fish form a series of adjustment mechanisms in the long-term evolution process. The gills play a critical role in adaptation to both acute and chronic changes in water condition [51]. Their response mechanisms are complex, involving variations in ion concentrations and multiple transport proteins [52]. Damage to gills adversely affects ionic transport and homeostasis in fish. It was reported that the fish gill was a target organ of multiple pollutants in the aquatic environment [53]. Early studies showed that gills were susceptible to H2O2 exposure in aquatic animals. For instance, the typical architecture of the gill was severely disrupted when the O. mykiss was exposed to 100–400 mg/L of H2O2 [34]. Clear evidence of gill damage was found in Pandalus borealis exposed to 1.5 mg/L of H2O2 for 1 h, and the adverse effects worsened with increasing H2O2 concentration [11]. In line with previous studies, our study also found damage to gill structure after 1.00 mM of H2O2 exposure for 14 days, which may influence gill physiological function, such as osmotic, ionic, and pH regulation. The damage was probably related to oxidative stress induced by H2O2 exposure [28].
In plasma, the alterations in ion concentrations such as Na+, K+, Ca2+, and Cl− may indicate a disturbance in acid–base balance and ion transport, which is closely related to gill function in fish [23,54]. Ana, et al. [23] found that short-term H2O2 exposure increased Na+, Mg2+, and Ca2+ concentrations but did not change K+ content in the plasma of D. labrax. Similarly, the Na+, K+, and Cl− concentrations were significantly increased in the plasma of S. salar at 6 and 12 h after 20 min of H2O2 exposure [55]. In H2O2-exposed Stizostedion vitreum, significant differences were only observed in the anion gap but not in the Na+, K+, Mg2+, or Ca2+ concentrations in plasma [56]. In this study, the Na+ and Ca2+ concentrations were distinctly increased, but the K+ concentration was distinctly decreased in the plasma of the common carp after H2O2 exposure. This evidence confirmed that H2O2 exposure caused plasma ion imbalance, which may further affect acid–base balance and oxygen transport. In addition, the ion concentrations in the gills of the common carp were also changed after H2O2 exposure, which was possibly a reason for the plasma ion imbalance.
A variety of enzymes have been confirmed to be involved in the regulation of ion transport in fish, such as Na+/K+-ATPase, H+/K+-ATPase, and Ca2+/Mg2+-ATPase [57]. Among these, Na+/K+-ATPase is one of the most studied enzymes in fish, which is a heterodimeric plasma-membrane-spanning protein including α and β subunits [58]. Its main function is pumping Na+ and K+ across the plasma membrane [59]. Many stimuli, such as ROS, can cause a specific change in Na+/K+-ATPase activity [60]. Numerous studies have found that an increase in ROS inhibits Na+/K+-ATPase activity via oxidizing the Na+/K+-ATPase α/β subunits and its independent regulator FXYD proteins in different types of cells [61]. H2O2, a stable ROS, activates the Na+/K+-ATPase at low concentrations (<1 μM) but inhibits its activity at high concentrations (>100 μM) in the brain synapses of rats [62]. In intestinal epithelial cells of C. carpio var. Jian, H2O2 treatment induced a significant decrease in Na+/K+-ATPase activity, indicating an underlying mechanism of ROS-induced modifications in ion transport [63]. Similar to previous studies, our study also found that the Na+/K+-ATPase activity was decreased after H2O2 exposure in the gills of common carp. Further, the nkaa gene expression was also downregulated in the gills by H2O2 exposure, which indicated that H2O2 inhibited the Na+/K+-ATPase activity at the transcriptional level. We speculate that the downregulation of nkaa may result from the damage to the gill structure under H2O2 exposure. The inhibition of Na+/K+-ATPase activity may further disturb ion transport in the gills, which was indirectly confirmed by the changes in Na+ and K+ concentrations in the gills and plasma.
The H+/ K+-ATPase is a proton pump that comprises theα1 and β subunits. It is mainly expressed in the stomach of vertebrates [64]. However, increasing evidence demonstrates that the H+/K+-ATPase has an established role in ion regulation in fish gills [65,66]. Barnawi et al. [65] suggested that H+/K+-ATPase regulated K+ absorption in the gill’s ionocytes in Oreochromis niloticus. Under acute air exposure, the activity of gill H+/K+-ATPase and plasma K+ was increased in Scyliorhinus canicula [67]. In the present study, the activity of H+/K+-ATPase in the gills was increased after H2O2 exposure, which may be an adaptive response to adverse stimuli. In addition, there was negative correction between the activity of H+/K+-ATPase and K+ concentration in the common carp. We speculate that the K+ transport might be regulated by other membrane proteins in addition to H+/K+-ATPase.
In teleost fish, the gill is a major site of ammonia elimination, a primary nitrogenous waste. Under adverse conditions, such as heightened pH, ammonia cannot efficiently diffuse across the gills, which may cause ammonia accumulation in the body [68]. Rh glycoproteins play a crucial role in NH3/NH4+ transport and excretion [69]. Three Rh genes including rhag, rhbg, and rhcg have been identified in fish [70]. The expression of various Rh genes was upregulated in the gills of O. mykiss in response to 12–48 h of high ammonia exposure [71]. Acute copper exposure downregulated Rh gene expression, which inhibited both the excretion and uptake of ammonia in O. mykiss [72]. Transcriptomic analyses indicated that Paramisgurnus dabryanus responded to high endogenous ammonia by regulating the expression of Rh genes under aerial exposure [73]. In the present study, the rhbg expression was downregulated in the gills after H2O2 exposure, which was not favorable to ammonia excretion across the gills. We suspect that the downregulation may be due to gill damage induced by H2O2 exposure.
Ca is a ubiquitous metal and a key intracellular signal involved in numerous physiological processes, such as cell proliferation, differentiation, and death. Its overload or disturbance can trigger apoptosis, endoplasmic reticulum stress, and autophagy in various types of cells [74]. A variety of proteins have evolved to regulate Ca concentration and sense Ca2+ signal [75]. Ca2+/Mg2+-ATPase is an important enzyme in regulating Ca2+ concentration [76,77]. Under cold stress, Ca2+/Mg2+-ATPase was activated to respond to adverse conditions in the gills of scylla serrata [78]. A low-salt-stress study determined that a moderate reduction in salinity can increase Ca2+/Mg2+-ATPase and Na+/K+-ATPase activities to maintain the osmotic pressure balance in Pampus argenteus [79]. Similarly, in this study, we also found that increased Ca2+/Mg2+-ATPase in the gills after H2O2 exposure was positively correlated with Ca2+ concentration. The activation may be an adaptive response to oxidative stress, which contributed to maintaining ion balance.
Cam is a well-known eukaryotic Ca2+ sensor that is activated after binding to Ca to regulate a diverse set of proteins, including CamK, a ubiquitous enzyme target of calcium signaling pathways [80,81]. The increase in the cytosolic Ca2+ level is a major feature of oxidative stress, which activates Ca2+-dependent degradative enzymes, such as phospholipases, proteases, and endonucleases, to regulate the onset of cell death [82]. Under oxidative stress, Ca2+ overload may excessively activate Cam and its downstream targets, leading to mitochondrial ROS generation [83]. In this study, the H2O2-induced oxidative stress caused the increase in Ca2+ concentration, which further led to the activation of Cam and CamK II, indicating that H2O2 exposure activated the Ca/CamK II signaling pathway. The activated pathway may participate in the regulation of apoptosis, endoplasmic reticulum stress, and autophagy [28,30].
5. Conclusions
In the present study, we used common carp as a model to evaluate, for the first time in fish, the toxic effects of chronic H2O2 exposure on ion transport in gills. The data showed that H2O2 exposure damaged gill structure and further caused ionic imbalance in the plasma and gills. H2O2 exposure downregulated nkaa expression and then inhibited the activity of Na+/K+-ATPase, which affected the concentrations of Na+ and K+. Conversely, the activities H+/ K+-ATPase and Ca2+/Mg2+-ATPase were increased in the gills after H2O2 exposure, which may be an adaptive response to oxidative stress. In addition, downregulated rhbg expression was not favorable to ammonia excretion across the gills under H2O2 exposure. The activated Ca/CamK II signaling pathway may be involved in other physiological responses to H2O2 exposure. These results provide a valuable reference for the application of H2O2 in aquaculture and its toxicity evaluation in fish.
Author Contributions
Conceptualization, methodology, writing—original draft preparation, software, Y.M.; validation, formal analysis, B.L.; resources, data curation, Y.H.; investigation, writing—review and editing, visualization, R.J.; supervision, project administration, funding acquisition, J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by earmarked fund for CARS (CARS-45), Central Public-Interest Scientific Institution Basal Research Fund, CAFS (2020TD60), and Young Science-Technology Talents Support Project of Jiangsu Association Science and Technology (TJ-2021-076).
Institutional Review Board Statement
All animals in this study were approved by the Animal Care and Use Ethics Committee of the Freshwater Fisheries Research Center (2020TD60, 3 April 2022), and all procedures were performed according to Jiangsu Laboratory’s Animal Management Guidelines (014000319/2008-00079).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ueki, R.; Imaizumi, Y.; Iwamoto, Y.; Sakugawa, H.; Takeda, K. Factors controlling the degradation of hydrogen peroxide in river water, and the role of riverbed sand. Sci. Total Environ. 2020, 716, 136971. [Google Scholar] [CrossRef]
- Cooper, W.J.; Zika, R.G.; Petasne, R.G.; Plane, J.M.C. Photochemical formation of hydrogen peroxide in natural waters exposed to sunlight. Environ. Sci. Technol. 1988, 22, 1156–1160. [Google Scholar] [CrossRef]
- Kang, C.-M.; Han, J.-S.; Sunwoo, Y. Hydrogen peroxide concentrations in the ambient air of Seoul, Korea. Atmos. Environ. 2002, 36, 5509–5516. [Google Scholar] [CrossRef]
- Cory, R.M.; Davis, T.W.; Dick, G.J.; Johengen, T.H.; Denef, V.J.; Berry, M.A.; Page, S.E.; Watson, S.B.; Yuhas, K.; Kling, G.W. Seasonal dynamics in dissolved organic matter, hydrogen peroxide, and cyanobacterial blooms in Lake Erie. Front. Mar. Sci. 2016, 3, 54. [Google Scholar] [CrossRef]
- Overton, K.; Samsing, F.; Oppedal, F.; Dalvin, S.; Stien, L.H.; Dempster, T. The use and effects of hydrogen peroxide on salmon lice and post-smolt Atlantic salmon. Aquaculture 2018, 486, 246–252. [Google Scholar] [CrossRef]
- Avendaño-Herrera, R.; Magariños, B.; Irgang, R.; Toranzo, A.E. Use of hydrogen peroxide against the fish pathogen Tenacibaculum maritimum and its effect on infected turbot (Scophthalmus maximus). Aquaculture 2006, 257, 104–110. [Google Scholar] [CrossRef]
- Yanong, R.P.E. Use of Hydrogen Peroxide in Finfish Aquaculture; Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 2008. [Google Scholar]
- Bögner, D.; Bögner, M.; Schmachtl, F.; Bill, N.; Halfer, J.; Slater, M.J. Hydrogen peroxide oxygenation and disinfection capacity in recirculating aquaculture systems. Aquac. Eng. 2021, 92, 102140. [Google Scholar] [CrossRef]
- Adams, M.B.; Crosbie, P.B.B.; Nowak, B.F. Preliminary success using hydrogen peroxide to treat Atlantic salmon, Salmo salar L., affected with experimentally induced amoebic gill disease (AGD). J. Fish Dis. 2012, 35, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Helgesen, K.O.; Romstad, H.; Aaen, S.M.R.; Horsberg, T.E. First report of reduced sensitivity towards hydrogen peroxide found in the salmon louse Lepeophtheirus salmonis in Norway. Aquac. Rep. 2015, 1, 37–42. [Google Scholar] [CrossRef]
- Bechmann, R.K.; Arnberg, M.; Gomiero, A.; Westerlund, S.; Lyng, E.; Berry, M.; Agustsson, T.; Jager, T.; Burridge, L.E. Gill damage and delayed mortality of Northern shrimp (Pandalus borealis) after short time exposure to anti-parasitic veterinary medicine containing hydrogen peroxide. Ecotoxicol. Environ. Saf. 2019, 180, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Z.; Chen, H.; Wen, Y. Effect of hydrogen peroxide on Microcystic aeruginosa: Role of cytochromes P450. Sci. Total Environ. 2018, 626, 211–218. [Google Scholar] [CrossRef]
- Sinha, A.K.; Eggleton, M.A.; Lochmann, R.T. An environmentally friendly approach for mitigating cyanobacterial bloom and their toxins in hypereutrophic ponds: Potentiality of a newly developed granular hydrogen peroxide-based compound. Sci. Total Environ. 2018, 637–638, 524–537. [Google Scholar] [CrossRef]
- Abele-Oeschger, D.; Tug, H.; Rottgers, R. Dynamics of UV-Driven Hydrogen Peroxide Formation on an Intertidal Sandflat. Limnol. Oceanogr. 1997, 42, 1406–1415. [Google Scholar] [CrossRef]
- Cooper, W.J.; Zika, R.G. Photochemical formation of hydrogen peroxide in surface and ground waters exposed to sunlight. Science 1983, 220, 711–712. [Google Scholar] [CrossRef] [PubMed]
- Sinel’nikov, V.E. Hydrogen peroxide level in river water, and methods for detecting it. Gidrobiol. Zh. 1971, 7, 115–119. [Google Scholar]
- Ndungu, L.K.; Steele, J.H.; Hancock, T.L.; Bartleson, R.D.; Milbrandt, E.C.; Parsons, M.L.; Urakawa, H. Hydrogen peroxide measurements in subtropical aquatic systems and their implications for cyanobacterial blooms. Ecol. Eng. 2019, 138, 444–453. [Google Scholar] [CrossRef]
- Fujiwara, K.; Ushiroda, T.; Takeda, K.; Kumamoto, Y.; Tsubota, H. Diurnal and seasonal distribution of hydrogen peroxide in seawater of the Seto Inland Sea. Geochem. J. 1993, 27, 103–115. [Google Scholar] [CrossRef]
- Sakugawa, H.; Kaplan, I.R.; Tsai, W.; Cohen, Y. Atmospheric hydrogen peroxide. Environ. Sci. Technol. 1990, 24, 1452–1462. [Google Scholar] [CrossRef]
- Schmidt, L.J.; Gaikowski, M.P.; Gingerich, W.H. Environmental Assessment for the Use of Hydrogen Peroxide in Aquaculture for Treating External Fungal and Bacterial Diseases of Cultured Fish and Fish Eggs; USGS: Washington DC, USA, 2006.
- Rach, J.J.; Schreier, T.M.; Howe, G.E.; Redman, S.D. Effect of Species, Life Stage, and Water Temperature on the Toxicity of Hydrogen Peroxide to Fish. Progress. Fish-Cult. 1997, 59, 41–46. [Google Scholar] [CrossRef]
- Tort, M.J.; Hurley, D.; Fernandez-Cobas, C.; Wooster, G.A.; Bowser, P.R. Effects of hydrogen peroxide treatments on catalase and glutathione activity in Walleye Sander vitreus. J. World Aquac. Soc. 2005, 36, 577–586. [Google Scholar] [CrossRef]
- Ana, R.; Hijranyavuzcan, Y.; Ignacio, C.; Neil, D. Physiological stress responses of sea bass (Dicentrarchus labrax) to hydrogen peroxide (H₂O₂) exposure. Aquaculture 2010, 304, 104–107. [Google Scholar]
- Mansour, A.T.; Espinosa, C.; García-Beltrán, J.M.; Miao, L.; Ceballos Francisco, D.C.; Alsaqufi, A.S.; Esteban, M.Á. Dietary supplementation of drumstick tree, Moringa oleifera, improves mucosal immune response in skin and gills of seabream, Sparus aurata, and attenuates the effect of hydrogen peroxide exposure. Fish Physiol. Biochem. 2020, 46, 981–996. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.O.; Kim, Y.K.; Nam, Y.K. Effect of hydrogen peroxide exposures on mucous cells and lysozymes of gill tissues of olive flounder Paralichthys olivaeceus. Aquac. Res. 2016, 47, 433–444. [Google Scholar] [CrossRef]
- Liu, Y.; Chi, L.; Feng, L.; Jiang, J.; Zhou, X. Protective Effect of Vitamin C on Oxidative Damage in Intestinal Epithelial Cells of Jian Carp (Cyprinus carpio var. Jian). Chin. J. Anim. Nutr. 2012, 24, 1503–1511. [Google Scholar]
- Jia, R.; Du, J.; Cao, L.; Li, Y.; Johnson, O.; Gu, Z.; Jeney, G.; Xu, P.; Yin, G. Antioxidative, inflammatory and immune responses in hydrogen peroxide-induced liver injury of tilapia (GIFT, Oreochromis niloticus). Fish Shellfish. Immunol. 2019, 84, 894–905. [Google Scholar] [CrossRef]
- Jia, R.; Du, J.; Cao, L.; Feng, W.; He, Q.; Xu, P.; Yin, G. Chronic exposure of hydrogen peroxide alters redox state, apoptosis and endoplasmic reticulum stress in common carp (Cyprinus carpio). Aquat. Toxicol. 2020, 229, 105657. [Google Scholar] [CrossRef]
- Jia, R.; Du, J.; Cao, L.; Feng, W.; He, Q.; Xu, P.; Yin, G. Application of transcriptome analysis to understand the adverse effects of hydrogen peroxide exposure on brain function in common carp (Cyprinus carpio). Environ. Pollut. 2021, 286, 117240. [Google Scholar] [CrossRef]
- Jia, R.; Du, J.; Cao, L.; Feng, W.; He, Q.; Xu, P.; Yin, G. Immune, inflammatory, autophagic and DNA damage responses to long-term H2O2 exposure in different tissues of common carp (Cyprinus carpio). Sci. Total Environ. 2021, 757, 143831. [Google Scholar] [CrossRef]
- Chen, X.; Liu, S.; Ding, Q.; Teame, T.; Yang, Y.; Ran, C.; Zhang, Z.; Zhou, Z. Research advances in the structure, function, and regulation of the gill barrier in teleost fish. Water Biol. Secur. 2023, 100139. [Google Scholar] [CrossRef]
- Wu, P.; Pan, F.-Y.; Feng, L.; Jiang, W.-D.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Tang, W.-N.; Zhang, Y.-A.; Zhou, X.-Q.; et al. Methionine hydroxy analogue supplementation modulates gill immunological and barrier health status of grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2018, 74, 637–648. [Google Scholar] [CrossRef]
- Seker, E.; Ispir, U.; Yonar, S.M.; Yonar, M.E.; Turk, C. Antioxidant responses of rainbow trout (Oncorhynchus mykiss) gills after exposure to hydrogen peroxide. Fresenius Environ. Bull. 2015, 24, 1837–1840. [Google Scholar]
- Tort, M.J.; Jenningsbashore, C.; Wilson, D.; Wooster, G.A.; Bowser, P.R. Assessing the Effects of Increasing Hydrogen Peroxide Dosage on Rainbow Trout Gills Utilizing a Digitized Scoring Methodology. J. Aquat. Anim. Health 2002, 14, 95–103. [Google Scholar] [CrossRef]
- Fernandez-Senac, C.; Monaghan, S.J.; Mascolo, D.; Baily, J.L.; Betancor, M.; Chalmers, L.; Paladini, G.; Adams, A.; Fridman, S.; Bron, J.E. Investigating the impacts of H2O2 treatment on gills of healthy Atlantic salmon reveals potential changes to mucus production with implications on immune activity. Fish Shellfish Immunol. 2022, 128, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Fujikata, A. Variation of carp serum constituent levels with time for storage of whole blood. Nsugaf 1984, 50, 1337–1340. [Google Scholar] [CrossRef]
- Spears, J.; Kamunde, C.; Stevens, E.D. Effect of TRIS and bicarbonate as buffers on anesthetic efficacy of tricaine methane sulfonate in zebrafish (Danio rerio). Zebrafish 2014, 11, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Bernet, D.; Schmidt, H.; Meier, W.; Burkhardt-Holm, P.; Wahli, T. Histopathology in fish: Proposal for a protocol to assess aquatic pollution. J. Fish Dis. 1999, 22, 25–34. [Google Scholar] [CrossRef]
- Nunes, B.; Campos, J.C.; Gomes, R.; Braga, M.R.; Ramos, A.S.; Antunes, S.C.; Correia, A.T. Ecotoxicological effects of salicylic acid in the freshwater fish Salmo trutta fario: Antioxidant mechanisms and histological alterations. Environ. Sci. Pollut. Res. 2015, 22, 667–678. [Google Scholar] [CrossRef]
- Li, D. The Invention Relates to a Method and a Kit for Detecting Sodium Ions. Patent CN 201110132290.1, 20 May 2011. [Google Scholar]
- Pan, Y.; Li, W. Clinical application of serum potassium ion determination. Contemp. Med. 2008, 14, 54. [Google Scholar]
- Liu, D.; Zhang, E.; Pu, Y.; Dong, X. Blockage role of self-designed multiple orgen preservation solution on mitochondrial calcium influx during cryopreservation of rabbit kidney. J. Med. Sci. Yanbian Univ. 2013, 36, 88–91. [Google Scholar]
- Tang, Y. Determination of the structure and stability constant of the final product of serum mercuric chlorothiocyanate method. Chin. J. Lab. Med. 2003, 4, 30–31. [Google Scholar]
- Zhang, Y.; Sun, H.J.; Zhang, J.Y.; Ndayambaje, E.; Lin, H.; Chen, J.; Hong, H. Chronic exposure to dichloroacetamide induces biochemical and histopathological changes in the gills of zebrafish. Environ. Toxicol. 2019, 34, 781–787. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Malll, A.K.; Klenk, D.C. Measurement of protein using BCA. Anal. Biochem. 1985, 150, 76–185. [Google Scholar] [CrossRef]
- Jia, R.; Cao, L.-P.; Du, J.-L.; He, Q.; Gu, Z.-Y.; Jeney, G.; Xu, P.; Yin, G.-J. Effects of High-Fat Diet on Steatosis, Endoplasmic Reticulum Stress and Autophagy in Liver of Tilapia (Oreochromis niloticus). Front. Mar. Sci. 2020, 7, 363. [Google Scholar] [CrossRef]
- Jia, R.; Gu, Z.; He, Q.; Du, J.; Cao, L.; Jeney, G.; Xu, P.; Yin, G. Anti-oxidative, anti-inflammatory and hepatoprotective effects of Radix Bupleuri extract against oxidative damage in tilapia (Oreochromis niloticus) via Nrf2 and TLRs signaling pathway. Fish Shellfish Immunol. 2019, 93, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods-A Companion Methods Enzymol. 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.K.; Kapotwe, M.; Dabi, S.B.; Montes, C.d.S.; Shrivastava, J.; Blust, R.; Boeck, G.D. Differential modulation of ammonia excretion, Rhesus glycoproteins and ion-regulation in common carp (Cyprinus carpio) following individual and combined exposure to waterborne copper and ammonia. Aquat. Toxicol. 2016, 170, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yu, J.; Xu, P.; Li, J.; Li, H.; Ren, H. Identification of housekeeping genes suitable for gene expression analysis in Jian carp (Cyprinus carpio var. Jian). Fish Shellfish Immunol. 2012, 33, 775–779. [Google Scholar] [CrossRef]
- Ribeiro, C.; Schreiner, M.; Iannini, C.; Gomes, A.D.; Moreira, R.G. Acute and chronic effects of temperature on membrane adjustments in the gills of a neotropical catfish. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2021, 256, 110625. [Google Scholar] [CrossRef]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef]
- Evans, D.H. The fish gill: Site of action and model for toxic effects of environmental pollutants. Environ. Health Perspect. 1987, 71, 47–58. [Google Scholar] [CrossRef]
- Pritchard, J.B. The gill and homeostasis: Transport under stress. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2003, 285, R1269–R1271. [Google Scholar] [CrossRef]
- Bowers, J.; Speare, D.J.; Burka, J.F. The effects of hydrogen peroxide on the stress response of Atlantic Salmon (Salmo salar). J. Vet. Pharmacol. Ther. 2002, 25, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Tort, M.J.; Wooster, G.A.; Bowser, P.R. Effects of hydrogen peroxide on hematology and blood chemistry parameters of walleye Stizostedion vitreum. J. World Aquac. Soc. 2003, 34, 236–242. [Google Scholar] [CrossRef]
- Hwang, P.-P.; Lee, T.-H.; Lin, L.-Y. Ion regulation in fish gills: Recent progress in the cellular and molecular mechanisms. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2011, 301, R28–R47. [Google Scholar] [CrossRef] [PubMed]
- Doğanli, C.; Oxvig, C.; Lykke-Hartmann, K. Zebrafish as a novel model to assess Na+/K+-ATPase-related neurological disorders. Neurosci. Biobehav. Rev. 2013, 37, 2774–2787. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ye, Q.; Liu, C.; Xie, J.X.; Yan, Y.; Lai, F.; Duan, Q.; Li, X.; Tian, J.; Xie, Z. Involvement of Na/K-ATPase in hydrogen peroxide-induced activation of the Src/ERK pathway in LLC-PK1 cells. Free. Radic. Biol. Med. 2014, 71, 415–426. [Google Scholar] [CrossRef]
- Dobrota, D.; Matejovicova, M.; Kurella, E.G.; Boldyrev, A.A. Na/K-ATPase under oxidative stress: Molecular mechanisms of injury. Cell. Mol. Neurobiol. 1999, 19, 141–149. [Google Scholar] [CrossRef]
- Liu, J.; Kennedy, D.J.; Yan, Y.; Shapiro, J.I. Reactive Oxygen Species Modulation of Na/K-ATPase Regulates Fibrosis and Renal Proximal Tubular Sodium Handling. Int. J. Nephrol. 2012, 2012, 381320. [Google Scholar] [CrossRef]
- Chkadua, G.; Nozadze, E.; Tsakadze, L.; Shioshvili, L.; Arutinova, N.; Leladze, M.; Dzneladze, S.; Javakhishvili, M. Effect of H2O2 on Na,K-ATPase. J. Bioenerg. Biomembr. 2022, 54, 241–249. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, X.Q.; Feng, L.; Liu, Y.; Jiang, J. Effects of glutamine on hydrogen peroxide-induced oxidative damage in intestinal epithelial cells of Jian carp (Cyprinus carpio var. Jian). Aquaculture 2009, 288, 285–289. [Google Scholar] [CrossRef]
- Deng, X.-s.; Meng, X.; Fullerton, D.; Stone, M.; Iguidbashian, J.; Jaggers, J. Complement Cross Talks With H-K-ATPase to Upregulate Runx2 in Human Aortic Valve Interstitial Cells. J. Surg. Res. 2023, 286, 118–126. [Google Scholar] [CrossRef]
- Barnawi, E.A.; Doherty, J.E.; Ferreira, P.G.; Wilson, J.M. Extra-gastric expression of the proton pump H+/K+-ATPase in the gills and kidney of the teleost Oreochromis niloticus. J. Exp. Biol. 2020, 223, jeb214890. [Google Scholar] [CrossRef] [PubMed]
- Choe, K.P.; Verlander, J.W.; Wingo, C.S.; Evans, D.H. A putative H+-K+-ATPase in the Atlantic stingray, Dasyatis sabina: Primary sequence and expression in gills. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2004, 287, R981–R991. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Jarabo, I.; Paullada-Salmeron, J.A.; Jerez-Cepa, I.; Neto, J.B.G.; Bystriansky, J.S.; Mancera, J.M. Acute Stress in Lesser-Spotted Catshark (Scyliorhinus canicula Linnaeus, 1758) Promotes Amino Acid Catabolism and Osmoregulatory Imbalances. Animals 2022, 12, 1192. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.; Wood, C. An analysis of branchial ammonia excretion in the freshwater rainbow trout: Effects of environmental pH change and sodium uptake blockade. J. Exp. Biol. 1985, 114, 329–353. [Google Scholar] [CrossRef]
- Nakada, T.; Westhoff, C.M.; Kato, A.; Hirose, S. Ammonia secretion from fish gill depends on a set of Rh glycoproteins. FASEB J. 2007, 21, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, A.M.; Wright, P.A.; Wood, C.M. Ammonia and urea handling by early life stages of fishes. J. Exp. Biol. 2017, 220, 3843–3855. [Google Scholar] [CrossRef]
- Nawata, C.M.; Hung, C.C.; Tsui, T.K.; Wilson, J.M.; Wright, P.A.; Wood, C.M. Ammonia excretion in rainbow trout (Oncorhynchus mykiss): Evidence for Rh glycoprotein and H+-ATPase involvement. Physiol. Genom. 2007, 31, 463–474. [Google Scholar] [CrossRef]
- Lim, M.Y.-T.; Zimmer, A.M.; Wood, C.M. Acute exposure to waterborne copper inhibits both the excretion and uptake of ammonia in freshwater rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2015, 168, 48–54. [Google Scholar] [CrossRef]
- Shang, Z.-H.; Huang, M.; Wu, M.-X.; Mi, D.; You, K.; Zhang, Y.-L. Transcriptomic analyses of the acute aerial and ammonia stress response in the gill and liver of large-scale loach (Paramisgurnus dabryanus). Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2021, 250, 109185. [Google Scholar] [CrossRef]
- Islam, M.S. Calcium Signaling: From Basic to Bedside. In Calcium Signaling, 2nd ed.; Islam, M.S., Ed.; Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2020; Volume 1131, pp. 1–6. [Google Scholar]
- Verkhratsky, A. Calcium and cell death. In Calcium Signalling and Disease: Molecular Pathology of Calcium; Carafoli, E., Brini, M., Eds.; Subcellular Biochemistry; Springer: Berlin/Heidelberg, Germany, 2007; Volume 45, pp. 465–480. [Google Scholar]
- Ogi, M.; Yokomori, H.; Inao, M.; Oda, M.; Ishii, H. Hepatic stellate cells express Ca^ pump-ATPase and Ca^-Mg^-ATPase in plasma membrane of caveolae. J. Gastroenterol. 2000, 35, 912–918. [Google Scholar] [CrossRef]
- Sugasini, D.; Lokesh, B.R. Rats fed linseed oil in microemulsion forms enriches the cardiac sarcoplasmic reticulum lipids with docosahexaenoic acid and lower calcium transport. J. Funct. Foods 2013, 5, 1863–1872. [Google Scholar] [CrossRef]
- Kong, X.H.; Wang, G.Z.; Li, S.J. Changes of antioxidant defenses, atpase activity and cell membrane fatty acid composition in gill of scylla serrata under low temperature acclimation. Acta Hydrobiol. Sin. 2007, 12, 708–713. [Google Scholar]
- Fei, Y.; Peng, S.; Peng, S.; Shi, Z. Effects of low salinity stress on the antioxidant enzyme activities in juvenile Pampus argenteus liver and the APTase activities in its gill and kidney. Yingyong Shengtai Xuebao 2011, 22, 1059–1066. [Google Scholar]
- Putney Jr, J.W. Calcium signaling: Up, down, up, down.... What’s the point? Science 1998, 279, 191–192. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, C.; Jana, B. Role of Calcium in Modulating the Conformational Landscape and Peptide Binding Induced Closing of Calmodulin. J. Phys. Chem. B 2021, 125, 2317–2327. [Google Scholar] [CrossRef] [PubMed]
- Orrenius, S.; Burkitt, M.J.; Kass, G.E.; Dypbukt, J.M.; Nicotera, P. Calcium ions and oxidative cell injury. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1992, 32, S33–S42. [Google Scholar] [CrossRef]
- Toledo, F.D.; Pérez, L.M.; Basiglio, C.L.; Ochoa, J.E.; Sanchez Pozzi, E.J.; Roma, M.G. The Ca(2+)-calmodulin-Ca(2+)/calmodulin-dependent protein kinase II signaling pathway is involved in oxidative stress-induced mitochondrial permeability transition and apoptosis in isolated rat hepatocytes. Arch. Toxicol. 2014, 88, 1695–1709. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).