Effects of Vertical Water Column Temperature on Distribution of Juvenile Tuna Species in the South China Sea
Abstract
1. Introduction
2. Materials and Methods
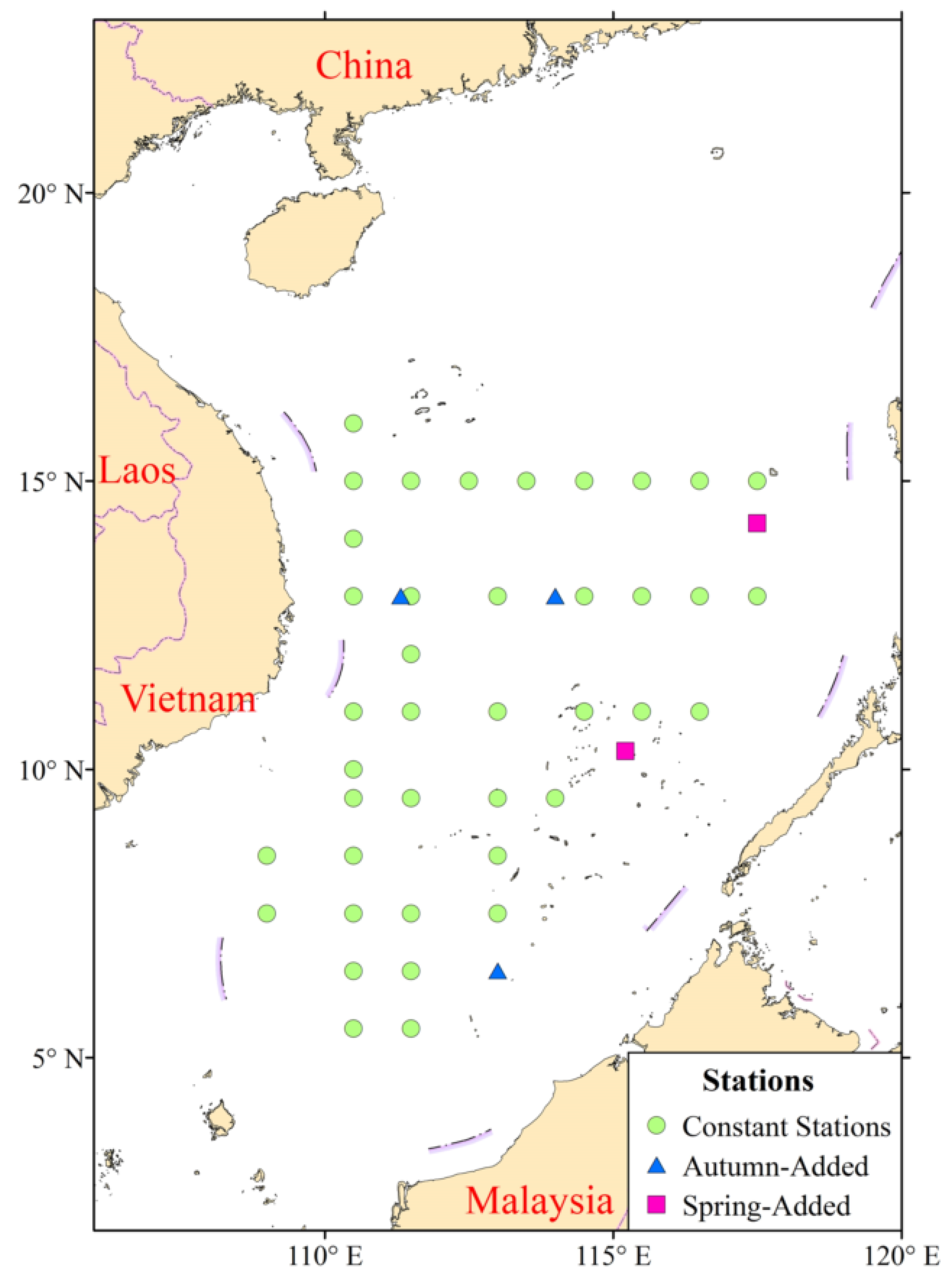
2.1. Fisheries Data
2.2. Environmental Data
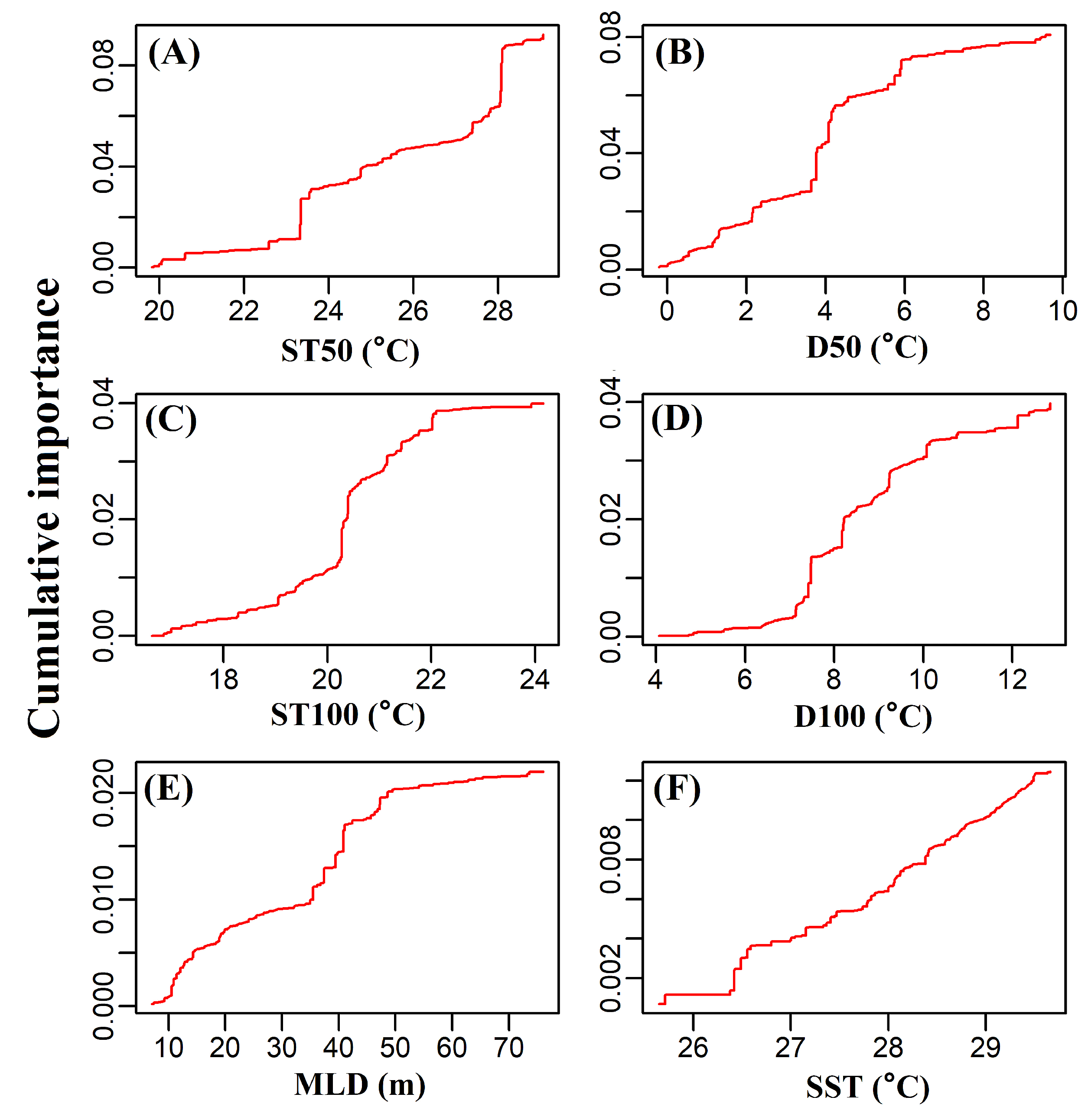
2.3. Gradient Forests Analysis
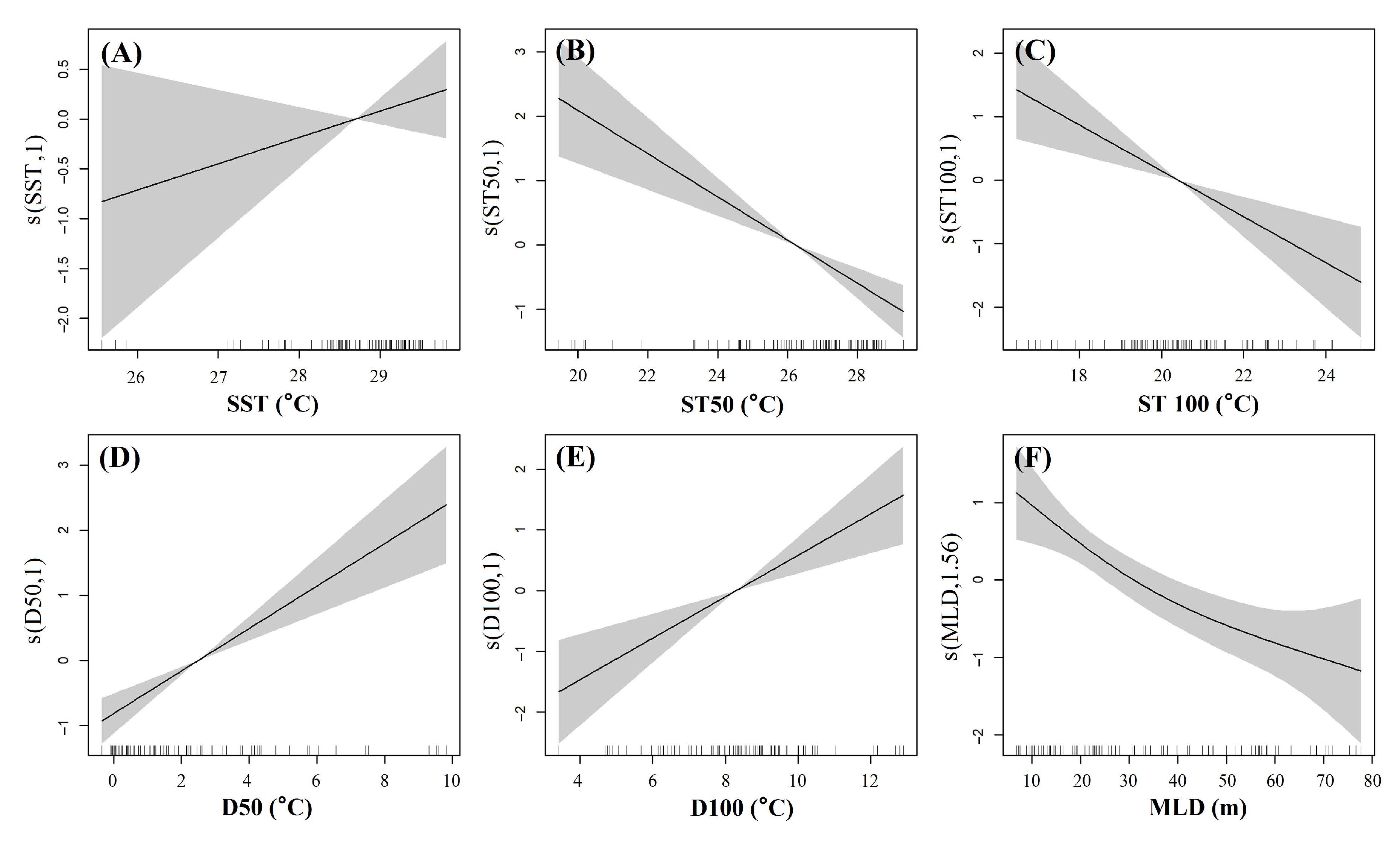
2.4. Generalized Additive Model (GAM)
3. Results
3.1. Composition and Distribution of Juvenile Tuna Species
3.2. Environmental Factors at Study Stations
3.3. Effects of Environmental Factors on the Distribution of Juvenile Tuna Species
4. Discussion
4.1. Occurrence of Tuna Species in the South China Sea
4.2. The Effect of Water Column Temperature on Juvenile Tuna
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Arrizabalaga, H.; Bruyn, P.; Diaz, G.A.; Murua, H.; Chavance, P.; Molina, A.D.; Gaertner, D.; Ariz, J.; Ruiz, J.; Kell, L.T. Productivity and susceptibility analysis for species caught in Atlantic tuna fisheries. Aquat. Living Resour. 2011, 24, 1–12. [Google Scholar] [CrossRef]
- Kaplan, D.M.; Chassot, E.; Amandé, J.M.; Dueri, S.; Demarcq, H.; Dagorn, L.; Fonteneau, A. Spatial management of Indian Ocean tropical tuna fisheries: Potential and perspectives. ICES J. Mar. Sci. 2014, 71, 1728–1749. [Google Scholar] [CrossRef]
- Evans, K.; Young, J.W.; Nicol, S.; Kolody, D.; Allain, V.; Bell, J.; Singh, A. Optimising fisheries management in relation to tuna catches in the western central Pacific Ocean: A review of research priorities and opportunities. Mar. Policy 2015, 59, 94–104. [Google Scholar] [CrossRef]
- Hallier, J.P.; Gaertner, D. Drifting fish aggregation devices could act as an ecological trap for tropical tuna species. Mar. Ecol. Prog. 2008, 353, 255–264. [Google Scholar] [CrossRef]
- Báez, J.C.; Czerwinski, I.A.; Ramos, M.L. Climatic oscillations effect on the yellowfin tuna (Thunnus albacares) Spanish captures in the Indian Ocean. Fish Oceanogr. 2020, 29, 572–583. [Google Scholar] [CrossRef]
- Mediodia, H. Effects of sea surface temperature on tuna catch: Evidence from countries in the Eastern Pacific Ocean. Ocean Coast Manag. 2021, 209, 105657. [Google Scholar] [CrossRef]
- Lehodey, P.; Senina, I.; Calmettes, B.; Hampton, J.; Nicol, S. Modelling the impact of climate change on pacific skipjack tuna population and fisheries. Clim. Chang. 2013, 119, 95–109. [Google Scholar] [CrossRef]
- Feng, B.; Li, Z.; Hou, G. Fish species and quantity in the South China Sea surveyed by deep longline. J. Trop. Oceanogr. 2015, 1, 64–70. [Google Scholar]
- Thanh, H. Ocean tuna fishing and marketing in Vietnam. Vietfish 2009, 6, 56–59. [Google Scholar]
- Li, Z.; Dong, A.; Wang, M.; Yan, Y.; Lu, H. History and trends of Vietnamese tuna fishery in the South China Sea. J. Modern. Fish Inform. 2014, 1, 60–64. [Google Scholar]
- Bakun, A. Ocean eddies, predator pits and bluefin tuna: Implications of an inferred ‘low risk–limited payoff’ reproductive scheme of a (former) archetypical top predator. Fish Fish 2013, 14, 424–438. [Google Scholar] [CrossRef]
- Tian, Y.; Uchikawa, K.; Ueda, Y.; Cheng, J. Comparison of fluctuations in fish communities and trophic structures of ecosystems from three currents around Japan: Synchronies and differences. ICES J. Mar. Sci. 2014, 71, 19–34. [Google Scholar] [CrossRef]
- Lan, K.W.; Lee, M.A.; Nishida, T.; Lu, H.J.; Weng, J.S.; Chang, Y. Environmental effects on yellowfin tuna catch by the Taiwan longline fishery in the Arabian Sea. Int. J. Remote Sens 2012, 33, 7491–7506. [Google Scholar] [CrossRef]
- Ma, S.; Cheng, J.; Li, J.; Liu, Y.; Wan, R.; Tian, Y. Interannual to decadal variability in the catches of small pelagic fishes from China Seas and its responses to climatic regime shifts. Deep-Sea Res II Top Stud. Oceanogr. 2018, 159, 112–129. [Google Scholar] [CrossRef]
- Checkley, D.; Alheit, J.; Oozeki, Y.; Roy, C. Climate Change and Small Pelagic Fish. New York, NY, USA, 2009. Available online: https://www.researchgate.net/profile/Juergen-Alheit/publication/266567677_Climate_Change_and_Small_Pelagic_Fish/links/54b5057c0cf26833efd05664/Climate-Change-and-Small-Pelagic-Fish.pdf (accessed on 2 January 2023).
- Hollowed, A.B.; Barange, M.; Beamish, R.J.; Brander, K.; Cochrane, K.L.; Drinkwater, K.F.; Foreman, M.G.G.; Hare, J.A.; Holt, J.; Ito, S.-I.; et al. Projected impacts of climate change on marine fish and fisheries. ICES J. Mar. Sci. 2013, 70, 1023–1037. [Google Scholar] [CrossRef]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.C.; Williams, S.E. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355, 9214. [Google Scholar] [CrossRef]
- Gou, A.; Chen, X. The relationship between ENSO and tuna purse-seine resource abundance and fishing grounds distribution in the Western and Central Pacific Ocean. Mar. Fish 2005, 27, 76–80. [Google Scholar]
- Mugo, R.; Saitoh, S.I.; Nihira, A.; Kuroyama, T. Habitat characteristics of skipjack tuna (Katsuwonus pelamis) in the western North Pacific: A remote sensing perspective. Fish Oceanogr. 2010, 19, 382–396. [Google Scholar] [CrossRef]
- Lan, K.W.; Lee, M.A.; Lu, H.J.; Shieh, W.J.; Lin, W.K.; Kao, S.C. Ocean variations associated with fishing conditions of yellowfin tuna (Thunnus albacares) in the equatorial Atlantic Ocean. ICES J. Mar. Sci. 2011, 68, 1063–1071. [Google Scholar] [CrossRef]
- Lan, K.W.; Evans, K.; Lee, M.A. Effects of climate variability on the distribution and fishing conditions of yellowfin tuna (Thunnus albacares) in the western Indian Ocean. Clim. Chang. 2013, 119, 63–77. [Google Scholar] [CrossRef]
- Lan, K.W.; Lee, M.A.; Chou, C.P.; Vayghan, A.H. Association between the interannual variation in the oceanic environment and catch rates of bigeye tuna (Thunnus obesus) in the Atlantic Ocean. Fish Oceanogr. 2018, 27, 395–407. [Google Scholar] [CrossRef]
- Kumar, P.S.; Pillai, G.N.; Manjusha, U. El Nino Southern Oscillation (ENSO) impact on tuna fisheries in Indian Ocean. SpringerPlus 2014, 3, 591. [Google Scholar] [CrossRef]
- Pikitch, E.; Santora, C.; Babcock, E.; Bakun, A.; Bonfil, R.; Conover, D.; Dayton, P.; Doukakis, P.; Fluharty, D.; Heneman, B. Ecosystem-Based Fishery Management. Policy Forum 2004, 350, 346–347. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.-M.; Tseng, H.-S. Scientific Research and Its Influence in Decision-Making of Tuna Regional Fisheries Management Organizations: Case Studies in the Atlantic Ocean and Indian Ocean. Fishes 2022, 7, 76. [Google Scholar] [CrossRef]
- Sissenwine, M.P.; Mace, P.M.; Powers, J.E.; Scott, G.P. A commentary on western Atlantic bluefin tuna assessments. Trans. Am. Fish. Soc. 1998, 127, 838–855. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Fu, C.; Yan, L.; Xu, Y.; Wan, R.; Li, J.; Tian, Y. Using novel spawning ground indices to analyze the effects of climate change on Pacifc saury abundance. J. Mar. Syst. 2019, 191, 13–23. [Google Scholar] [CrossRef]
- Wiryawan, B.; Loneragan, N.; Mardhiah, U.; Kleinertz, S.; Wahyuningrum, P.I.; Pingkan, J.; Wildan, T.; Duggan, D.; Yulianto, I. Catch per Unit Effort Dynamic of Yellowfin Tuna Related to Sea Surface Temperature and Chlorophyll in Southern Indonesia. Fishes 2020, 5, 28. [Google Scholar] [CrossRef]
- Brill, R.; Lutcavage, R.; Metzger, G.; Bushnell, P.; Lucy, M.; Foley, D. Horizontal and vertical movements of juvenile bluefin tuna (Thunnus thynnus), in relation to oceanographic conditions of the western North Atlantic, determined with ultrasonic telemetry. Fish Bull 2021, 100, 155–167. [Google Scholar]
- Blank, J.M.; Morrissette, J.M.; Farwell, C.J.; Price, M.; Schallert, R.J.; Block, B.A. Temperature effects on metabolic rate of juvenile Pacific bluefin tuna Thunnus orientalis. J. Exp. Biol. 2007, 210, 4254–4261. [Google Scholar] [CrossRef]
- Ji, S.; Zhou, W.; Xu, H.; Wang, X. A WebGIS application: Tuna fishing ground forecasting information service system for the open South China Sea. In Proceedings of the 2016 IEEE International Geoscience and Remote Sensing Symposium, Beijing, China, 10–15 July 2016; pp. 3628–3631. [Google Scholar]
- Zhou, W.; Li, A.; Ji, S.; Qiu, Y. Yellowfin Tuna (Thunnusalbacares) Fishing Ground Forecasting Model Based on Bayes Classifier in The South China Sea. Pol. Marit. Res. 2017, 24, 140–146. [Google Scholar] [CrossRef]
- Arrizabalaga, H.; Pereira, J.G.; Royer, F.; Galuardi, B.; Goni, N.; Artetxe, I.; Lutcavage, M. Bigeye tuna (Thunnus obesus) vertical movements in the Azores Islands determined with pop-up satellite archival tags. Fish Oceanogr. 2008, 17, 74–83. [Google Scholar] [CrossRef]
- Williams, A.J.; Allain, V.; Nicol, S.J.; Evans, K.J.; Hoyle, S.D.; Dupoux, C.; Vourey, E.; Dubosc, J. Vertical behavior and diet of albacore tuna (Thunnus alalunga) vary with latitude in the South Pacific Ocean. Deep-Sea Res. II Top Stud. Oceanogr. 2015, 113, 154–169. [Google Scholar] [CrossRef]
- Uchiyama, J.; Struhsaker, P. Age and growth of skipjack tuna, Katsuwonus pelamis, and yellowfin tuna, Thunnus albacares, as indicated by daily growth increments of sagittae. Fish Bull. 1981, 79, 151–162. [Google Scholar]
- Neilson, J.D.; Campana, S.E. A validated description of age and growth of western Atlantic bluefin tuna (Thunnus thynnus). Can. J. Fish Aquat. Sci. 2008, 65, 1523–1527. [Google Scholar] [CrossRef]
- Soares, J.B.; Monteiro-Neto, C.; Costa, M.R.D.; Martins, R.R.M.; Vieira, F.C.D.S.; Andrade-Tubino, M.F.D.; Bastos, A.L.; Tubino, R.D.A. Size structure, reproduction, and growth of skipjack tuna (Katsuwonus pelamis) caught by the pole-and-line fleet in the southwest Atlantic. Fish Res. 2019, 212, 136–145. [Google Scholar] [CrossRef]
- Shi, P.; Du, Y.; Wang, D.; Gan, Z. Annual cycle of mixed layer in South China Sea. J. Trop Oceanogr. 2001, 20, 10–17. [Google Scholar]
- Ellis, N.; Smith, S.J.; Pitcher, C.R. Gradient forests: Calculating importance gradients on physical predictors. Ecology 2012, 93, 156–168. [Google Scholar] [CrossRef]
- Hastie, T.J.; Tibshirani, R.J. Generalized Additive Models. Stat Sci. 1986, 1, 297–310. [Google Scholar] [CrossRef]
- Litzow, M.A.; Hobday, A.J.; Frusher, S.D.; Dann, P.; Tuck, G.N. Detecting regime shifts in marine systems with limited biological data: An example from southeast Australia. Prog. Oceanogr. 2016, 14, 96–108. [Google Scholar] [CrossRef]
- Pang, Y.; Tian, Y.; Fu, C.; Wang, B.; Li, J.; Ren, Y.; Wan, R. Variability of coastal cephalopods in overexploited China Seas under climate change with implications on fisheries management. Fish Res. 2018, 208, 22–33. [Google Scholar] [CrossRef]
- Tittensor, D.P.; Mora, C.; Jetz, W.; Lotze, H.K.; Ricard, D.; Berghe, E.V.; Worm, B. Global patterns and predictors of marine biodiversity across taxa. Nature 2010, 466, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.T.; Sun, C.L.; Belkin, I.M.; Yeh, S.Z.; Kuo, C.L.; Liu, D.C. Sea surface temperature fronts affect distribution of Pacific saury (Cololabis saira) in the Northwestern Pacific Ocean. Deep-Sea Res II Top Stud Oceanogr. 2014, 107, 15–21. [Google Scholar] [CrossRef]
- Dagorn, L.; Holland, K.N.; Hallier, J.-P.; Taquet, M.; Moreno, G.; Sancho, G.; Itano, D.G.; Aumeeruddy, R.; Girard, C.; Million, J.; et al. Deep diving behavior observed in yellowfin tuna (Thunnus albacares). Aquat. Living Res. 2006, 19, 85–88. [Google Scholar] [CrossRef]
- Schaefer, K.M.; Fuller, D.W. Movements, behavior, and habitat utilization of yellowfin tuna (Thunnus albacares) in the northeastern Pacific Ocean, ascertained through archival tag data. Mar. Biol. 2007, 152, 503–525. [Google Scholar] [CrossRef]
- Zainuddin, M.; Saitoh, K.; Saitoh, S.I. Albacore (Thunnus alalunga) fishing ground in relation to oceanographic conditions in the western North Pacific Ocean using remotely sensed satellite data. Fish Oceanogr. 2008, 17, 61–73. [Google Scholar] [CrossRef]
- Syah, A.F.; Saitoh, S.I.; Alabia, I.D.; Hirawake, T. Detection of potential fishing zone for Pacific saury (Cololabis saira) using generalized additive model and remotely sensed data. IOP Conf. Ser. Earth Env. Sci. 2017, 54, 012074. [Google Scholar] [CrossRef]
- Kuroda, H.; Yokouchi, K. Interdecadal decrease in potential fishing areas for Pacific saury off the southeastern coast of Hokkaido, Japan. Fish. Oceanogr. 2017, 26, 439–454. [Google Scholar] [CrossRef]
- Bakun, A. Fronts and eddies as key structures in the habitat of marine fish larvae: Opportunity, adaptive response and competitive advantage. Sci. Mar. 2006, 70, 105–122. [Google Scholar] [CrossRef]
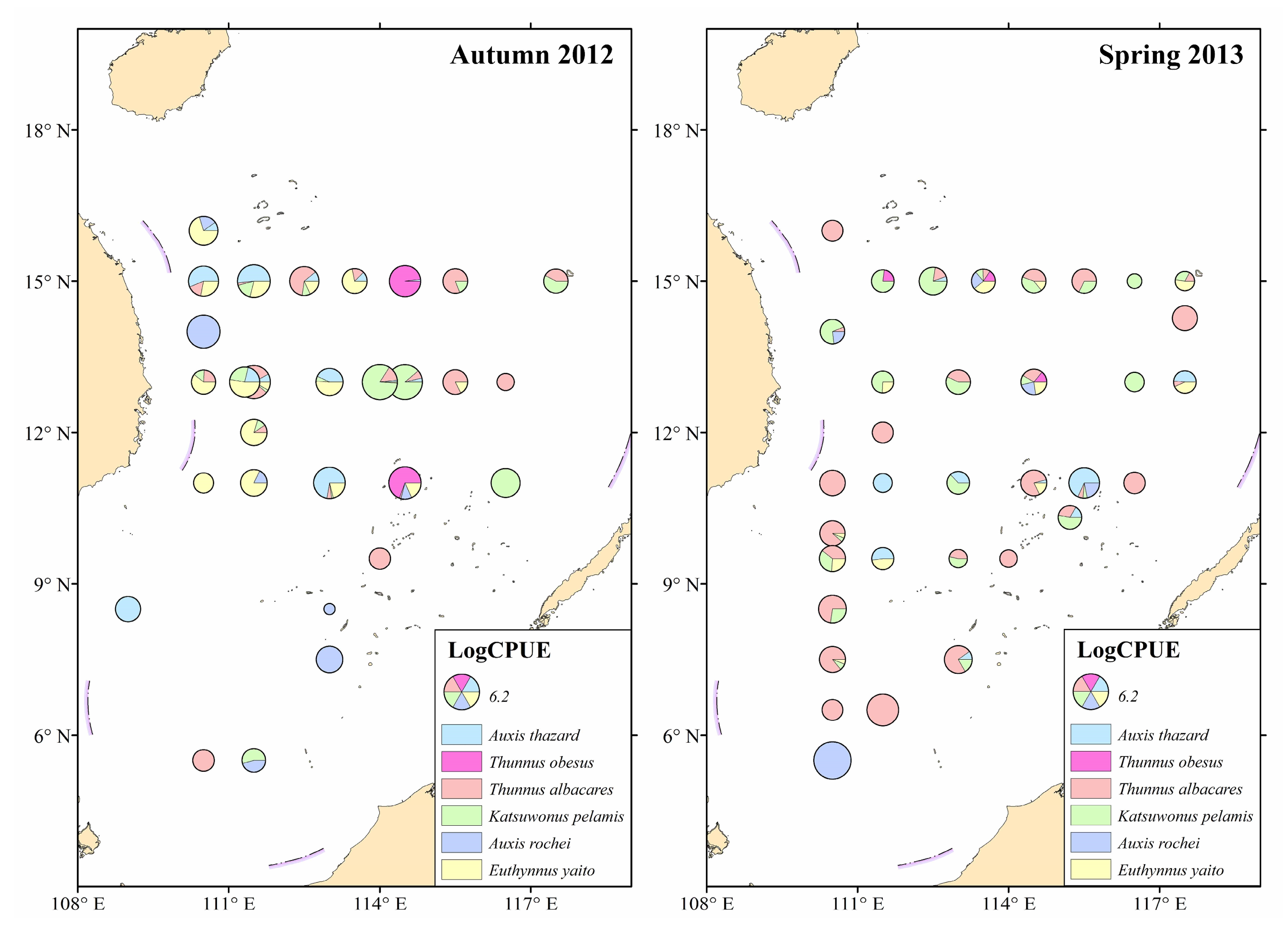


| Species | Autumn 2012 | Spring 2013 | ||
|---|---|---|---|---|
| Number of Present Stations | Total Catch (kg) | Number of Present Stations | Total Catch (kg) | |
| T. albacares | 19 | 446.82 | 26 | 103.03 |
| T. obesus | 2 | 145.00 | 3 | 0.40 |
| K. pelamis | 16 | 1810.80 | 22 | 12.95 |
| A. thazard | 13 | 245.95 | 10 | 26.79 |
| A. rochei | 8 | 223.13 | 5 | 6474.20 |
| E. yaito | 19 | 174.84 | 12 | 2.27 |
| Tuna species | 29 | 3046.53 | 34 | 6619.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Li, Y.; Wang, R.; Miao, X.; Zhang, R.; Chen, S.; Song, P.; Lin, L. Effects of Vertical Water Column Temperature on Distribution of Juvenile Tuna Species in the South China Sea. Fishes 2023, 8, 135. https://doi.org/10.3390/fishes8030135
Liu S, Li Y, Wang R, Miao X, Zhang R, Chen S, Song P, Lin L. Effects of Vertical Water Column Temperature on Distribution of Juvenile Tuna Species in the South China Sea. Fishes. 2023; 8(3):135. https://doi.org/10.3390/fishes8030135
Chicago/Turabian StyleLiu, Shigang, Yuan Li, Rui Wang, Xing Miao, Ran Zhang, Siyuan Chen, Puqing Song, and Longshan Lin. 2023. "Effects of Vertical Water Column Temperature on Distribution of Juvenile Tuna Species in the South China Sea" Fishes 8, no. 3: 135. https://doi.org/10.3390/fishes8030135
APA StyleLiu, S., Li, Y., Wang, R., Miao, X., Zhang, R., Chen, S., Song, P., & Lin, L. (2023). Effects of Vertical Water Column Temperature on Distribution of Juvenile Tuna Species in the South China Sea. Fishes, 8(3), 135. https://doi.org/10.3390/fishes8030135







