Abstract
Since 2017, a fishing moratorium has been enforced in Lake Honghu as part of Chang–Jiang’s biodiversity conservation strategy. However, given that the lake is a semi-closed aquatic ecosystem and no longer serves as a habitat for certain fishes of the mid–lower Chang–Jiang basin, the efficacy of this moratorium remains uncertain. To address the question from a trophic ecology perspective, a stomach content analysis was performed on captured anchovies of C. brachygnathus, a current predominant species in Lake Honghu, from November 2020 to October 2021. The results showed that copepods, shrimps, and macrophytes were the main components of this anchovy’s diet, and there were size-related shifts in diets. The dominance of C. brachygnathus, a pelagic–littoral omnivore in this lake, implies a lacustrine environment shift triggered by continued human disturbances. The utilization of macrophytes as the main food items by large-sized anchovies of Lake Honghu has partially contributed to the rapid degradation of submerged vegetation. This highlights the need to remove large-sized individuals of C. brachygnathus and Carassius auratus, another benthic-omnivorous dominant fish, in order to effectively restore the aquatic vegetation and ecosystem of Lake Honghu. The current implementation of fishing moratoriums in subtropical shallow floodplain lakes such as Lake Honghu should be reviewed critically.
Key Contribution:
1. In Lake Honghu, C. brachygnathus feeds on zooplankton (copepods, cladocerans, and rotifers), shrimp, macrophytes, and other unrecognized species, and exhibited size-related dietary shifts. 2. Food items of plant origin, like aquatic macrophytes, have never been reported for C. brachygnathus, but this study confirms that macrophytes are a major food source for adults of this species. 3. The utilization of macrophytes as the main food items by large-sized anchovies of Lake Honghu has partially contributed to the rapid degradation of submerged vegetation. 4. This highlights the need to remove large-sized individuals of C. brachygnathus and other benthic–omnivorous dominant fish to effectively restore the aquatic vegetation and ecosystem of Lake Honghu.
1. Introduction
Lake Honghu is the seventh-largest freshwater lake in China, located in the middle Yangtze River (or Chang–Jiang in Chinese) basin of southern Hubei Province [1]. As a water-carrying system, it performs a crucial function in flood regulation, providing water for industry and domestic use, agricultural irrigation, transportation, and fisheries. It also serves as an important breeding and wintering habitat for East Asian migrating birds, such as Mergus squamates and Ciconia boyciana [2,3]. Lake Honghu has been listed in the Ramsar wetlands of international importance, and it is a key biodiversity region and one of the priority conservation areas set up by the Chinese Government.
Nevertheless, Lake Honghu’s wetland has undergone severe human perturbations since the mid-1950s, disrupting its connectivity to the Chang–Jiang mainstem [2,3]. Prior to this, it served as nurseries, spawning, and feeding grounds for many potamodromous (e.g., silver, grass, bighead, and blunt carps) and diadromous (e.g., Japanese eel) fishes. However, due to the construction of sluice gates from 1955 to 1975, it has transformed into a river-isolated lake under artificial water level regulation, resulting in the decline of its aquatic environment and ecology. As a consequence, there has been a decrease in the species diversity of fish and fishery resources [1,4]. The number of fish species has drastically decreased from 73 in the 1950s–1960s to 49 in the 1990s [5]. Furthermore, fish assemblages have shown a trend toward early sexual maturation and smaller body sizes [5]. Fish farming (pen culture) on a large scale from the 1990s to 2010s has caused environmental and ecological problems, including reduced water quality and aquatic vegetation [6]. These issues have been worsened by exogenous wastewater discharge into the lake, causing a continuous degradation of water quality and aquatic habitat. The lake’s altered environment has had significant impacts on its fish assemblages.
Under the current biodiversity conservation strategy in the Chang–Jiang basin, Lake Honghu is designated as a non-fishing zone at both the national and provincial levels. To restore its aquatic biodiversity and fish resources, strict regulations have been implemented by the national and local governments, including a fishing ban enforced since 2017. However, as a semi-closed or river-isolated aquatic ecosystem, Lake Honghu no longer serves as nursing, feeding, or breeding ground for many diadromous and potamodromous fish species. Therefore, the question of whether a fishing moratorium remains necessary in Lake Honghu remains debatable.
Coilia brachygnathus is a significant freshwater economic fish species in China, due to its substantial biomass, especially in affiliated lakes of the mid–lower Chang–Jiang [7,8,9]. The anchovy, previously regarded as the landlocked form of the anadromous species C. nasus, has been recently confirmed as a distinct species via molecular evidence, with its occurrence extending to Lakes Taihu, Poyang, and Dongting [10,11]. While the anchovy was reported to inhabit Lake Honghu, according to local fishermen, it was not the primary catch in the lake prior to 2015. Recent field surveys in Lake Honghu conducted between 2019 and 2021 revealed that C. brachygnathus, followed by benthic omnivorous Carassius auratus, were the most abundant fish species in the lake [12]. The anchovy’s flourishing in Lake Honghu may be due to its life history characteristics such as a short life span, early maturity, fast growth rate, availability of ample food, low predator pressure, and the fishing moratorium [12,13]. Understanding the trophic ecology of C. brachygnathus in Lake Honghu would aid in the rational management and utilization of fisheries resources.
Several studies have investigated the diet of C. brachygnathus in China. For instance, stomach content analyses of 52 individuals caught between March and June 1958 from the middle Chang–Jiang revealed that copepods and insect larvae, followed by small fish and shrimps, were the anchovy’s primary food items [14]. Cladocerans and oligochaetes were only occasionally consumed. Similarly, stomach content analyses of 146 individuals collected from Lake Taihu between 1982 and 1984, 87 individuals fished from Meiliang Bay from September to October 2004, and 43 individuals landed from the entire lake from September to December 2004 demonstrated that the anchovy mainly fed on animal diets such as copepods, cladocerans, aquatic insects, shrimps, and small fish [15,16,17]. The same findings were observed for the fish in Lake Chaohu [18]. Nonetheless, these studies mainly used snapshot surveys, and the diet composition of the anchovy was shown to be influenced by habitat trophic status [19], with the fish displaying a temporal dietary shift linked to hydrological fluctuation levels [9].
Hence, the present study aims to (1) conduct a stomach content analysis of a large number of C. brachygnathus caught from Lake Honghu in different sampling seasons, (2) to provide insight into the optimal utilization and management of the lake’s fisheries resources, taking into account the dietary preferences of this omnivorous fish, and (3) address the issue of the necessity for fishing in this semi-closed aquatic environment.
2. Materials and Methods
2.1. Study Site
Lake Honghu, located in the southeast region of Hubei province (29°41′–29°58′ N, 113°12′–113°28′ E) and at an altitude of 22.75 m above sea level (Figure 1), is the largest shallow lake in the Jianghan Plain. It spans 23.4 km from east to west, 20.8 km from north to south, and has a mean water depth of 1.91 m. The lake has a water surface area of 348.2 km2 and a watershed area of 8265 km2 [2,20]. The climate in Lake Honghu is subtropical monsoon, characterized by an average annual precipitation ranging from 1000 to 1300 mm and an annual water temperature of 15.9 to 16.6 °C [2].
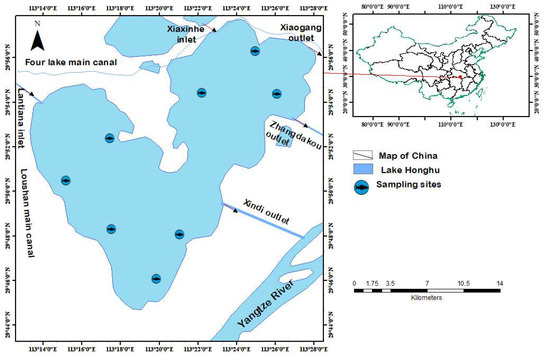
Figure 1.
Location of Lake Honghu with the spatial distribution of the eight sampling sites.
2.2. Fish Sampling and Gut Content Analysis
From November 2020 to December 2021, samples of C. brachygnathus were collected from Lake Honghu seasonally. To collect these samples, eight different sites were used and 30- and 60-mm-stretched mesh-size monofilament gill nets and <20 mm hoop nets were employed. The nets were set up between 14:00 and 16:00 h and were collected between 8:00 and 11:00 h the following morning. Individual fish were measured for total length to the nearest 0.1 mm, and the total weight was determined to the nearest 0.1 g using an electronic scale. Of the 800 fish sampled, their stomachs were removed, and the degree of gut fullness was recorded. Each fish was preserved individually in a buffered 4% formaldehyde solution and then transported for further diet analysis to the College of Fisheries Laboratory at Huazhong Agricultural University.
At the laboratory, the entire stomach contents were emptied onto a Petri dish, and subsamples were taken to scrutinize under a microscope [21]. For large-sized food items, such as shrimp and macrophyte fragments, visual identification was performed. The inverted compound microscope (magnification: ×400, Carl Zeiss; Germany) was utilized to count and identify small food items up to the lowest possible taxonomic level [22,23]. Any prey items that were partially digested and consequently unidentifiable were reported as unidentified [24].
The diet composition was quantified as the percentage frequency of occurrence (%O, the proportion of stomachs containing a particular prey item irrespective of amount) and percentage composition by number (%N; the proportion of the number of a particular prey item to the total number of all prey items in the entire stomach contents [25]. Given the difficult task of quantifying macrophytes, the frequency of occurrence in each fish stomach was computed. The diet diversity was expressed by the Shannon–Wiener diversity index (H’). Furthermore, Pielou’s evenness index (J’) was calculated to measure how evenly fish rely on food resources in different seasons.
Hierarchical cluster analysis based on the Euclidean distance and prey occurrence (%O) and abundance (%N) were used for petitioning size classes into different length groups. The matrix similarity was constructed using the Bray–Curtis distance after the abundance data were log (x + 1) transformed. Canonical analysis of principal coordinates (CAP) [26] was used to distinguish variations in the diet compositions among sampling seasons and fish size classes. In addition, nonparametric permutational multivariate analysis of variance (PERMANOVA) was run to test whether the variations in diet composition among the sampling periods and fish size classes were significant at 999 permutations [26]. All the statistical analyses were performed using OriginPro®2017 software (version 94E), and PAST software, Past4Project (version 1.0).
3. Results
3.1. Food Items in Gut Contents
A total of 800 stomachs from anchovies measuring between 50- and 359-mm TL (mean 184.12 ± 6.32 mm) were dissected for diet analyses, which were seasonally caught from November 2020 to October 2021. Of the examined stomachs, 480 (60%) contained food, while the remaining 320 (40%) were empty. The study revealed twelve different food items, including macrophyte fragments (one item), copepods (two items), cladocerans (two items), rotifers (five items), shrimp (one item), and the unrecognized (one item). Table 1 presents the frequency of occurrence (%O) and abundance (%N) of these food items. Zooplankton groups were dominated by copepods, followed by rotifers. The most frequently observed prey item in copepods remained consistent, with Calanus sp., making up 51.16% to 76.66% of observations. The most commonly observed prey item in cladocerans was Bosmina sp. The dominant prey item among rotifers was Brachionus calyciflorus, representing between 30.59% and 59.67% of observations throughout the sampling period. Macrophytes and shrimp were also among the most important food items throughout the entire duration of the study (Table 1, Figure 2). The study demonstrated a commonality of food items across all seasons, with Calanus sp., nauplii, B. calyciflorus, macrophytes, and shrimps appearing consistently as main food items.

Table 1.
Frequency of occurrence (%O) and number (%N) of food items found in the stomach of C. brachygnathus between November 2020 and October 2021 in Lake Honghu.
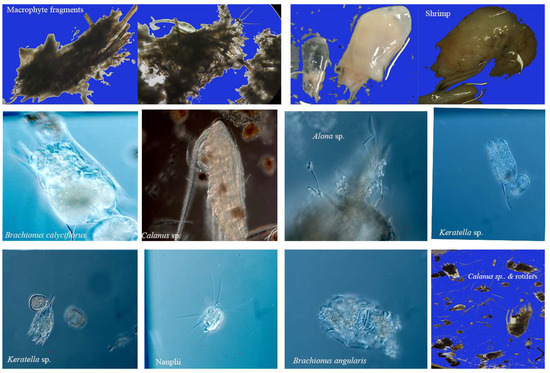
Figure 2.
Major food items identified in the stomach of C. brachygnathus from Lake Honghu.
3.2. Size-Related Dietary Variation
The cluster analysis conducted on collated anchovies, based on the occurrence of food items, showed size-related differences in the diet of the fish. As a result, three sizes of fish classes were identified and are outlined in Table 2 and Figure 3. The first group of individuals (<125 mm TL) subsisted exclusively on Calanus sp., Nauplii, Brachionus spp., Keratella sp., Tricocerca sp., Bosmina sp., and Alona sp. (Table 2). The second group (125–235 mm TL) fed mainly on Calanus sp., Nauplii, Keratella sp., Brachionus spp., Tricocerca sp., Bosmina sp., Alona sp., macrophytes, and shrimps. The third group (>235 mm TL) foraged on larger food items such as large-sized Calanus sp., a few amounts of nauplii, macrophytes, and shrimps (Table 2, Figure 2).

Table 2.
Frequency of occurrence (%O) and number (%N) of food items of the three size groups of C. brachygnathus in Lake Honghu.
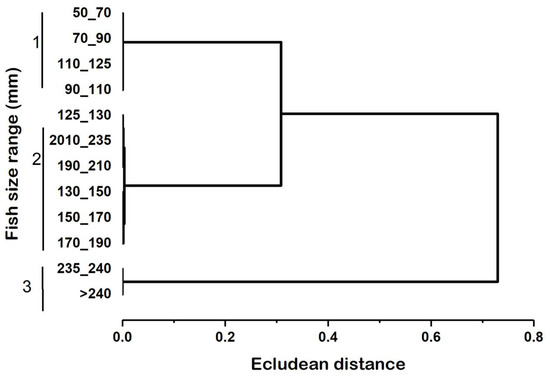
Figure 3.
Cluster analysis of the fish size groups in terms of prey occurrence based on the Euclidean distance.
In larger-sized anchovies, it was found that only a single food source, either macrophytes or shrimps, constituted the entirety of their diet (Table 3). Out of the more than 235 mm TL individuals of C. brachygnathus studied, only 20 were found to feed exclusively on macrophytes, another 3 individuals of more than 248 mm TL relied entirely on shrimps, and 14 individuals with sizes ranging from 181 to 358 mm TL (with an average of 206 mm TL) consumed both macrophytes and shrimps. However, individuals with a smaller size (125 mm TL) were observed to have a higher rate of feeding on mixed diets consisting of macrophytes, zooplankton, and shrimps (Table 3).

Table 3.
Food item contributions among different size groups of C. brachygnathus of Lake Honghu.
The findings from this study demonstrate that there are significant differences in the food composition between the three size groups (p < 0.05), as confirmed by the PERMANOVA analysis (Table 4). It was also affirmed by the CAP ordination plot that size differences existed among the sampled individuals (Figure 4). As shown in Figure 5, the proportion of food groups varied with the fish size. In the smaller fish class, copepods and rotifers were the predominant food groups. For the medium-sized fish class, copepods, rotifers, macrophytes, and shrimps were the top food groups. Finally, macrophytes and shrimp were the two most proportioned food groups for the large-sized fish classes.

Table 4.
Results of the permutational multivariate analysis of variance (PERMANOVA) applied to diet composition.
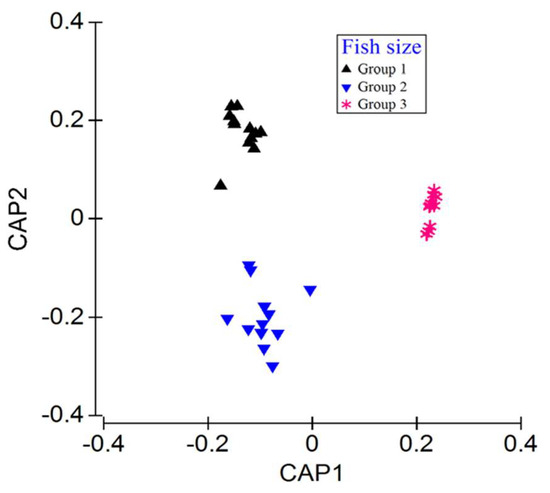
Figure 4.
Canonical analysis of principal coordinates (CAP) ordination plots based on the proportion of the feed compositions of the three length classes of fish.
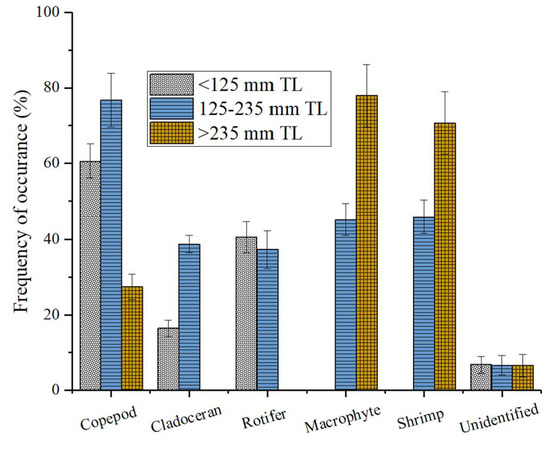
Figure 5.
The percentage contribution of different food items consumed by C. brachygnathus in different length classes in Lake Honghu.
4. Discussion
4.1. Dietary Variation of Anchovies in Lake Honghu
This study provides evidence supporting the omnivorous feeding habit and size-related dietary variation of C. brachygnathus in Lake Honghu. Based on this, the stomach content analysis of our study, which examined a large number of anchovies (800) fished from Lake Honghu in four sampling seasons, proved that small-sized anchovies (less than 125 mm TL) were juveniles with a generalized feeding habit of zooplanktivory, while large-sized anchovies (more than 235 mm TL) were adults with a specialized feeding habit of omni-invertivory, with a greater reliance on aquatic invertebrates (shrimps) and food items of plant origin (macrophytes). Medium-sized anchovies adopted a mixed feeding strategy with varying degrees of specialization of different food items. The transition from zooplanktivory to omni-invertivory in their diet occurred in anchovies reaching 125 to 235 mm TL. This range is consistent with the previous report for C. brachygnathus of the middle Chang–Jiang mainstem [14]. The minimum transition length of 131 mm TL reported by Tang [15] for Lake Taihu’s anchovies was consistent with our study, but the maximum (170 mm TL) was much shorter than the 235 mm TL reported here. This variation could be explained by the smaller body size of the anchovies in Lake Taihu compared to Lake Honghu, where the anchovy has long been a dominant capture fish [15]. The trend toward body miniaturization in Lake Taihu’s anchovies is likely due to high fishing pressure [17].
The study also revealed that the size-related dietary shift of C. brachygnathus in Lake Honghu was accompanied by a shift in spatial habitat utilization from the pelagic to the littoral zone. While small-sized fish preferred feeding on zooplankton in the pelagic zone of the lake, large-sized fish showed a preference for shrimps and/or macrophytes, indicating a preference for the well-vegetated littoral zone (Table 1 and Table 2). The density and distribution of shrimps in Lake Bao’an, located in the middle Chang–Jiang basin of Hubei Province, was known to depend on water depth and aquatic vegetation [27]. The shrimps were found to routinely use macrophytes as a refuge under pressure from predators such as Culter spp. and fed on attached algae. It is, therefore, likely that large-sized C. brachygnathus preferentially use the well-vegetated littoral zone of Lake Honghu. Unlike in previous studies [17,19,28], zoobenthos and demersal small fish were not observed in the stomachs of anchovies from Lake Honghu (Table 1 and Table 2). This clearly indicates that anchovies of the lake were not benthic feeders and should be referred to as pelagic or littoral omnivores instead.
In this study, no seasonal dietary difference was observed for C. brachygnathus of Lake Honghu (Table 4). This is in contrast to the findings of Zhang et al. [9] from Lake Poyang, where stable isotope analysis indicated a seasonal variation in the diet of this anchovy. The difference can be attributed to the fact that, unlike Lake Poyang, Lake Honghu is a semi-closed aquatic ecosystem where the natural sources of food are not regulated by the season. Being a shallow eutrophic floodplain lake with high productivity, Lake Honghu provides abundant food sources throughout the year for C. brachygnathus.
4.2. Plant-Origin Food Items
Food items of plant origin, like aquatic macrophytes, have never been reported for C. brachygnathus of Lakes Chaohu [18], Lake Dongting [9], and Taihu [19], and the congeneric species C. nasus of the Qingcaosha reservoir located near the estuary of the Chang–Jiang [6]. However, there have been some reports of phytoplankton and algae being found in the stomachs of anchovies. Diatoms and spirogyra have been sporadically observed in the diet of C. brachygnathus from the middle Chang–Jiang basin [14], while algae and a significant amount of Microcystis spp. were found in the diet of C. ectenes taihuensis (currently rendered synonymic to C. brachygnathus) from Lake Taihu [15,17]. Variations in feeding habits within the same species may be due to variations in prey availability as a response to the existence of differing hydrographic conditions among the surveyed areas [29]. In Lake Honghu, the presence of macrophytes in the guts of medium- to large-sized individuals of C. brachygnathus may be attributed to a restricting of feeding habits in response to a modified environment and available resources [29]. This shift in feeding strategy may also be driven by energetic demands, as larger-sized individuals need more energy to survive [30].
It cannot be ruled out that C. brachygnathus accidentally ingests macrophytes when chasing after shrimps that are attached to them. However, the majority of anchovies found with macrophytes in their guts had mixed diets consisting of macrophytes, zooplankton, and shrimps. The number of anchovies feeding only on shrimps or a combination of macrophytes and shrimps was lower than the number of anchovies that solely fed on macrophytes (Table 3). This implies that C. brachygnathus intentionally prefers macrophytes as a part of its diet instead of incorporating them accidentally or at random.
4.3. No Need for a Fishing Ban
Omnivores are more adaptable to varying conditions than specialists [31], and they are expected to be prevalent in ecosystems affected by anthropogenic and natural factors [32]. Lake Honghu exemplifies this trend, as it has undergone significant perturbations since the 1950s, resulting in the disappearance of aquatic vegetation and the deterioration of water quality. The construction of some sluice gates in its outlets during the 1950s–1980s resulted in the hydrological disconnection from the middle Chang–Jiang mainstem, which had great influences on its fish assemblage. Following this disconnection, Carassius auratus, a benthic omnivore, has become one of the three dominant capture fish of Lake Honghu. So far, C. brachygnathus and Carassius auratus, two omnivorous fish species, have become the dominant capture fish of the lake [33]. The rise of C. brachygnathus reflects further anthropogenic disturbances [12], indicating intensified fish stocking, water pollution, and over-exploitation of aquatic vegetation during the past two decades or more [6]. As a result, Lake Honghu has already shifted from a macrophyte-dominated to an algae-dominated eutrophic lake.
Omnivorous fishes can accelerate the eutrophication process in shallow lakes, and their biomass tends to increase with intensified natural and anthropogenic disturbances, such as fish stocking, water pollution, and global warming [34]. There was a positive relation between the capture of C. brachygnathus and the concentration of TN, TP, and DOC in Taihu Lake [17]. The removal of omnivorous fish from shallow lakes could be beneficial for improving water clarity and promoting benthic primary production [2]. In this context, the harvest of economically important omnivorous fish, such as C. brachygnathus and C. auratus, in Lake Honghu could be a viable restoration approach for this shallow eutrophic lake.
As shown here, C. brachygnathus of Lake Honghu can utilize macrophytes as its food items. Whether the anchovy assimilates this food item of plant origin or not, the persistence of medium- or large-sized individuals has adverse impacts on submerged macrophytes and affects the aquatic vegetation’s rehabilitation and habitat promotion of the lake. The current dominance of this anchovy in the lake is partially attributed to the fishing ban implemented in 2017, which has led to a decrease in competition among fish species. This ban may have unintentionally created a breeding ground for C. brachygnathus, resulting in the degradation of the aquatic ecosystem [12]. Therefore, it is crucial to reevaluate the fishing ban and implement a rational approach to managing the resources of this semi-closed aquatic ecosystem. A sustainable management plan for the dominant omnivorous fish is urgently needed to promote the rehabilitation and sustainable development of Lake Honghu.
Moreover, predation by planktivorous fish on zooplankton has an adverse impact on the abundance, size, and species composition of the zooplankton community [35,36]. This, in turn, indirectly affects the phytoplankton community and physicochemical conditions, leading to increases in phytoplankton abundance [37]. Due to the weak “top-down” control exerted by zooplankton on phytoplankton communities, this can result in the flourishing of phytoplankton and the deterioration of water quality [38]. In Lake Honghu, most of the population is dominated by C. brachygnathus, with 70% of the population being composed of older individuals aged one to two years (or attaining 154 ± 1.02−199 ± 1.51 mm TL) [12]. These individuals preferentially forage on zooplankton, like copepods and rotifers. When zooplankton abundance is low, phytoplankton can thrive, and water quality can suffer [38,39]. Hence, fishing of big-sized anchovy in Lake Honghu is predicted to increase the high zooplankton abundance, thereby providing a selective constraint on phytoplankton, ultimately facilitating the improvement of water quality and the promotion of aquatic habitats.
5. Conclusions
The study concluded that C. brachygnathus in Lake Honghu was generalized (omnivorous) and its feeding habits varied with fish size. Macrophytes are one of the main food items used by adult C. brachygnathus fish as their main source of nutrition. However, food items like macrophytes have never been reported for C. brachygnathus. Macrophytes are the main food items for the large-sized groups of this species. This contributes to the loss of aquatic macrophytes, and the lake has shifted from a macrophyte- to an algae-dominated one. This highlights the need to remove large-sized individuals of C. brachygnathus and another benthic–omnivorous dominant fish to effectively restore the aquatic vegetation and ecosystem of Lake Honghu. A critical rethink should be given to the current measures in subtropical shallow floodplain lakes like Lake Honghu. Stomach content-based diet analysis is crucial to provide valuable information for the fisheries management and feeding strategy of this commercially important fish species and also to provide essential baseline information for future studies.
Author Contributions
A.Y.M., E.Z. and L.C. designed the study, A.Y.M. and L.C. collected the survey data, E.Z. and J.-Z.S. provided the equipment, logistic support, and edited the manuscript, A.Y.M. analysed the data and elaborated the statistical analysis and figures, A.Y.M. and E.Z. wrote and modified the initial draft of the manuscript with the contribution of all the authors. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the scientific investigation program of Lake Honghu.
Institutional Review Board Statement
The fish used for this study were collected in accordance with the Chinese Laboratory Animal Welfare Laws (GB/T 35892–2018). The collection of fish was approved by the Department of Agriculture and Rural Affairs of Hubei Province, approval code: 2020 (000019). Ethical approval was waived because dead fish were used in the experiment.
Data Availability Statement
Data will be made available on request.
Acknowledgments
We thank the Lake Honghu Wetland Protection and Restoration Demonstration Office for their cooperation during our sampling period. We would like to thank all the Huazhong Agricultural University College of Fisheries laboratory students for their invaluable support during the field data collection and laboratory analysis. We are also grateful to the Institute of Hydrobiology (IHB) fish taxonomy and evolution research group for their immense support during data collection and laboratory analysis.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Han, M.; Dsouza, M.; Zhou, C.; Li, H.; Zhang, J.; Chen, C.; Yao, Q.; Zhong, C.; Zhou, H.; Gilbert, J.A.; et al. Agricultural Risk Factors Influence Microbial Ecology in Honghu Lake. Genom. Proteom. Bioinform. 2019, 17, 76–90. [Google Scholar]
- Zhang, T.; Ban, X.; Wang, X.; Cai, X.; Li, E.; Wang, Z.; Yang, C.; Lu, X. Analysis of nutrient transport and ecological response in Honghu Lake, China by using a mathematical model. Sci. Total Environ. 2017, 575, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, X.; Ning, L. Ecological impacts of water conservancy activity on fish resources in Honghu Lake. J. Water Resour. Water Eng. 2008, 19, 2007–2018. [Google Scholar]
- Zhao, S.; Fang, J.; Ji, W.; Tang, Z. Lake restoration from impoldering: Impact of land conversion on the riparian landscape in Honghu Lake area, Central Yangtze. Agric. Ecosyst. Environ. 2003, 95, 111–118. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Y.; Yang, H.; Ni, X. Hydrobiology and Resources Exploitation in Lake Honghu; Science Press: Beijing, China, 1995. [Google Scholar]
- Li, L.; Du, R.; Liu, Q.; Fang, S.; Chen, L.; Sun, S.; Hu, Z. The ontogenetic dietary shift of Japanese grenadier anchovy (Coilia nasus) in the Qingcaosha reservoir near the Yangtze River estuary. J. Fish. Sci. China 2019, 26, 765–773. [Google Scholar]
- Qin, X.; Wang, T.; Lin, P.; Wang, X.; Liu, H. Age, growth, mortality and movement patterns of short jaw tapertail anchovy, Coilia brachygnathus, in the channel connecting Dongting Lake and the Yangtze River in central China. J. Aquat. Living Resour. 2018, 31, 2–9. [Google Scholar]
- Xue, D.; Yang, Q.; Li, Y.; Zong, S.; Gao, T. Comprehensive assessment of population genetic structure of the overexploited Japanese grenadier anchovy (Coilia nasus): Implications for fisheries management and conservation. Fish. Res. 2019, 213, 113–120. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, G.; Xie, P.; Xu, J.; Zhou, Q. Role of body size and temporal hydrology in the dietary shifts of short jaw tapertail anchovy. Hydrobiologia 2013, 703, 247–256. [Google Scholar] [CrossRef]
- Cheng, F.; Wang, Q.; Maisano Delser, P.; Li, C. Multiple freshwater invasions of the tapertail anchovy (Clupeiformes: Engraulidae) of the Yangtze River. Ecol. Evol. 2019, 9, 12202–12215. [Google Scholar] [CrossRef]
- Xuan, Z.; Jiang, T.; Liu, H.; Chen, X.; Yang, J. Mitochondrial DNA and microsatellite analyses reveal strong genetic differentiation between two types of estuarine tapertail anchovies (Coilia) in Yangtze River Basin, China. Hydrobiologia 2021, 848, 1409–1431. [Google Scholar]
- Yimer, M.A.; Cao, L.; Shen, J.; Zhang, E. Age, growth, maturity and mortality of the tapetail anchovy Coilia brachygnathus (Engraulidae) in Lake Honghu, China. J. Fish Biol. 2023, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E. Fishes. In Comprehensive Scientific Report on the National Natural Reserve of Lake Honghu, Hubei Province; Li, E.H., Li, F.Y., Zhu, J.H., Eds.; Science Press: Beijing, China, 2023. [Google Scholar]
- Anonymous. Fishes of the Yangtze River; Science Press: Beijing, China, 1976. [Google Scholar]
- Tang, Y. On the population dynamics of lake anchovy in Taihu Lake and its rational exploitation. J. Fish. China 1987, 11, 62–72. [Google Scholar]
- Ye, J.; Liu, Z.; Wang, W. Comparative study on the feeding habits of Coilia ectenes and Hyporhamphus intermidius in the Meilliang Bay of Lake Taihu. J. Lake Sci. 2007, 19, 218–222. [Google Scholar]
- Liu, E. A study on diet composition of dominant fishes in Lake Taihu. J. Fish. China 2008, 32, 396–400. [Google Scholar]
- Diao, Z.; Wu, X. Dynamic of Lake Anchovy Resource and Suggestion of Fishery Utilization. In Research Report on the Enhancement of Fisheries Resources in Lake Chaohu; Anhui Chaohu Development Company: Hefei, China, 1982; Volume 1, pp. 62–73. [Google Scholar]
- Sha, Y.C.; Su, G.H.; Zhang, P.Y.; Zhang, H.; Xu, J. Diverse dietary strategy of lake anchovy Coilia ectenes taihuensis in lakes with different trophic status. J. Ichthyol. 2015, 55, 866–873. [Google Scholar] [CrossRef]
- Yao, S.; Xue, B.; Xia, W.; Zhu, Y.; Li, S. Lead pollution recorded in sediments of three lakes located at the middle and lower Yangtze River basin, China. Quat. Int. J. 2009, 208, 145–150. [Google Scholar] [CrossRef]
- Costalago, D.; Palomera, I.; Tirelli, V. Seasonal comparison of the diets of juvenile European anchovy Engraulis encrasicolus and sardine Sardina pilchardus in the Gulf of Lions. J. Sea Res. 2014, 89, 64–72. [Google Scholar]
- Botes, L. Phytoplankton Identification Catalogue Saldanha Bay, South Africa; Marine and Coastal Management: Rogge Bay, South Africa, 2001. [Google Scholar]
- MRC. Identification Handbook of Freshwater Zooplankton of the Mekong River and Its Tributaries Identification Handbook of Freshwater Zooplankton of the Mekong River and Its Tributaries; Technical Paper, Vientiane, Lao PDR in April 2015; Mekong River Commission: Vientiane, Laos, 2015. [Google Scholar]
- Bacha, M.; Amara, R. Spatial, temporal and ontogenetic variation in diet of anchovy (Engraulis encrasicolus) on the Algerian coast (SW Mediterranean). Estuary Coast Shelf Sci. 2009, 85, 257–264. [Google Scholar] [CrossRef]
- Hyslop, E.J. Stomach contents analysis—A review of methods and their application. J. Fish Biol. 1980, 17, 411–429. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ Primer V7: User Manual; Primer-E Ltd.: Plymouth, UK, 2008. [Google Scholar]
- Qin, H.M.; Zhang, T.; Li, Z.; Hong, Y.J. Species composition, spatial distribution, and biomass of shrimp community in the Biandangtang Lake. Acta Hydrobiol. Sin. 2005, 29, 379–384. [Google Scholar]
- Li, Y.; Bordinhon, A.M.; Allen, D.; Zhang, W.; Zhu, X. Protein: Energy ratio in practical diets for Nile tilapia Oreochromis niloticus. Aquac. Int. 2013, 21, 1109–1119. [Google Scholar] [CrossRef]
- Mustać, B.; Hure, M. The diet of the anchovy Engraulis encrasicolus (Linnaeus, 1758) during the spawning season in the Eastern Adriatic Sea. Acta Adriat. 2020, 61, 57–66. [Google Scholar] [CrossRef]
- Zorica, B.; Čikeš, K.V.; Vidjak, O.; Mladineo, I.; Ezgeta, B.D. Feeding habits and helminth parasites of sardine (S. pilchardus) and anchovy (E. encrasicolus) in the Adriatic Sea. Mediterr. Marnie Sci. 2016, 17, 216–229. [Google Scholar] [CrossRef]
- Fagan, W.F. Omnivory as a stabilizing feature of natural communities. Am. Nat. 1997, 150, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Wootton, K.L. Omnivory and stability in freshwater habitats: Does theory match reality? Freshw. Biol. 2017, 62, 821–832. [Google Scholar] [CrossRef]
- He, H.; Hu, E.; Yu, J.; Luo, X.; Li, K.; Jeppesen, E.; Liu, Z. Does turbidity induce by Carassius carassius limit phytoplankton growth? A mesocosm study. Environ. Sci. Pollut. Res. 2017, 24, 5012–5018. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Vladimir, R.; Lars, G.R.; Erik, J.; Tang, Y.; Zhang, X.; Liu, Z. Effects of omnivorous fish on benthic-pelagic habitats coupling in shallow aquatic ecosystems: A minireview. J. Lake Sci. 2021, 33, 667–674. [Google Scholar]
- Brooks, J.L.; Dodson, S.I. Predation, body size, and composition of zooplankton. Science 1965, 150, 28–35. [Google Scholar] [CrossRef]
- Jeppesen, E.; Jensen, J.P.; Sondergaard, M.; Lauridsen, T.; Landkildehus, F. Trophic structure, species richness and biodiversity in Danish lakes: Changes along a phosphorus gradient. Freshw. Biol. 2000, 45, 201–218. [Google Scholar]
- Jeppesen, E.; Søndergaard, M.; Lauridsen, T.L.; Davidson, T.A.; Liu, Z.; Mazzeo, N.; Trochine, C.; Özkan, K.; Jensen, H.S.; Trolle, D.; et al. Biomanipulation as a Restoration Tool to Combat Eutrophication. Recent Advances and Future Challenges. Adv. Ecol. Res. 2012, 47, 411–488. [Google Scholar]
- Liu, Z.; Hu, J.; Zhong, P.; Zhang, X.; Ning, J.; Larsen, S.E.; Chen, D.; Gao, Y.; He, H.; Jeppesen, E. Successful restoration of a tropical shallow eutrophic lake: Strong bottom-up but weak top-down effects recorded. Water Res. 2018, 146, 88–97. [Google Scholar] [PubMed]
- Yu, J.; Xia, M.; Kong, M.; He, H.; Guan, B.; Liu, Z.; Jeppesen, E. A small omnivorous bitterling fish (Acheilognathus macropterus) facilitates dominance of cyanobacteria, rotifers, and Limnodrilus in an outdoor mesocosm experiment. Environ. Sci. Pollut. Res. 2020, 27, 23862–23870. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).