Abstract
There are a wide variety of fishes that occur exclusively on coral reefs, though it is unclear to what extent these species (especially larger-bodied fisheries target species) are reliant on the specific reef habitat provided by corals. This study explored variation in the physiological condition of common coral trout (Plecropomus leopardus) on Australia’s Great Barrier Reef, explicitly testing whether fish condition varied with local coral cover in the aftermath of severe mass bleaching and coral loss. Both the physiological condition (specifically, the length–weight relationships, hepatocyte vacuolation, and electrical phase angle) of P. leopardus and the live cover of habitat-forming corals varied greatly among the sites considered in this study, but there was little correspondence between these factors. Fish condition was largely influenced by fish size and varied with latitude. While there was no apparent effect of recent coral bleaching and coral loss on the physiological condition of P. leopardus, this does not mean that these key fisheries species will be unaffected by further changes to the environmental conditions and reef habitat. It is important, therefore, that fisheries managers remain vigilant to apparent effects of climate change and other anthropogenic pressures on fisheries stocks.
Keywords:
coral trout (Plectropomus); coral reefs; disturbance; Great Barrier Reef; habitat degradation; physiological condition Key Contribution:
Mass coral bleaching on Australia’s Great Barrier Reef in 2016–2017 and the corresponding degradation of coral reef habitats was expected to adversely affect piscivorous fishes. Contrary to expectations, this study showed no apparent effect of local coral cover on the physiological condition of common coral trout (Plectropomus leopardus).
1. Introduction
Recent and ongoing environmental change is likely to have direct physiological effects on a wide range of fishes, thereby affecting the individual demographics, population dynamics, and/or geographical ranges of fisheries target species [,], with potentially major consequences for fisheries production and sustainability. On coral reefs, however, habitat degradation appears to be the foremost impact of climate change on fish populations and fisheries production [,,,,], especially given the increasing extent and severity of climatic disturbances []. Critically, severe marine heatwaves are causing extensive mass bleaching and mortality of habitat-forming corals [], compounding other anthropogenic pressures and leading to marked shifts in the biological and physical structure of coral reef habitats [,]. Aside from climate change, the most critical anthropogenic pressures impacting coral reef ecosystems relate to declining water quality, associated with increased sedimentation, eutrophication, and other pollutants from land-based run off, which undermine the resilience of reef corals []. The degradation of coral reef habitats and, in particular, extensive coral loss and corresponding declines in the topographic complexity of reef habitats has been linked to significant declines in the abundance of reef fishes across a broad range of functional groups []. There has also been a corresponding decline in catches of reef-associated fishes [], though the mechanisms underlying declines in reef fishes and fisheries productivity with sustained habitat degradation remain unclear.
Coral trout (Plectropomus spp.) are a conspicuous group of reef-associated fishes and among the most important tropical fisheries species throughout the Indo-West Pacific region [,]. Plectropomus spp. are at risk of over-exploitation, owing to escalating fishing pressure [], which may be further compounded by escalating environmental change and habitat degradation [,]. In experimental studies, common coral trout (or leopard coral grouper (Plectropomus leopardus)) have been shown to be sensitive to elevated temperature [,,,], which may exacerbate the effects of fishing [,]. In the wild, coral trout may moderate feeding and activity patterns when exposed to seasonal temperature extremes [], which may affect catchability, if not individual condition and long-term population viability []. Changes in environmental conditions may also be compounded by habitat degradation.
While some studies have shown that the abundance of coral trout is affected by reductions in coral cover and habitat degradation [,,], the extent to which coral trout are reliant on live coral or coral-rich habitats is unclear []. Given their dietary flexibility [], adult P. leopardus may be resilient to moderate changes in habitat condition and associated declines in the availability of reef-associated prey. However, shifts in diet composition may nonetheless lead to declines in individual condition, with longer-term consequences for population replenishment, if not survivorship []. For longer-lived fishes, such as coral trout, the effects of coral loss or habitat degradation may not become apparent for several years []. Extensive coral loss and habitat degradation on Australia’s Great Barrier Reef (GBR) due to unprecedented coral bleaching in 2016–2017 [] provides an unparalleled opportunity to explore the effects of coral cover and habitat condition on wild stocks of coral trout.
The purpose of this study was to explore spatial variation in the physiological condition of common coral trout (Plectropomus leopardus) across a broad range of locations on the GBR, explicitly testing whether fish condition varies in response to local coral cover. Any differences detected in physiological condition may forewarn of longer-term impacts due to coral loss and reef degradation on individual survival and population viability of coral trout and clarify the extent to which these are or are not reliant on coral-rich habitats []. Variation in the physiological condition of P. leopardus was measured using a combination of routine and contemporary methods, including length–weight relationships, hepatocyte vacuolation (HV), and electrical phase angle (PA), thereby, providing an opportunity to assess the covariance in these condition metrics for P. leopardus.
2. Materials and Methods
To assess the spatiotemporal variation in the condition of common coral trout (P. leopardus), replicate fish were collected across a broad range of sites and reefs (Figure 1) in February–March 2020 and 2021. Sampling was mostly undertaken at reefs in the northern part of the GBR, where effects of the 2016 and 2017 mass bleaching were most pronounced [,] and we had prior knowledge of the bleaching severity and corresponding coral loss. The fish were predominantly collected using pneumatic spearguns (on SCUBA) to avoid by-catch, though these samples were supplemented with line caught fish in some locations. The fish that were speared were mostly caught on the reef slope (4–10 m depths) while attempting to capture all coral trout that were observed while moving in a consistent direction along the reef edge. All fish were euthanised immediately post-capture, using pithing for larger individuals and cervical dislocation for smaller fishes. It was both necessary and unavoidable to sacrifice fish to derive critical information on their physiological condition, and only a very small sample (a maximum of 12 fish per site) were taken from each location. The option of simply analysing the fish caught by the commercial fisheries sector was considered, but it was not possible or viable to segregate fish and thereby keep track of fish caught in specific sites, whereas a critical component of this study involved relating the condition of individual fish to the specific habitat condition in which they were caught.
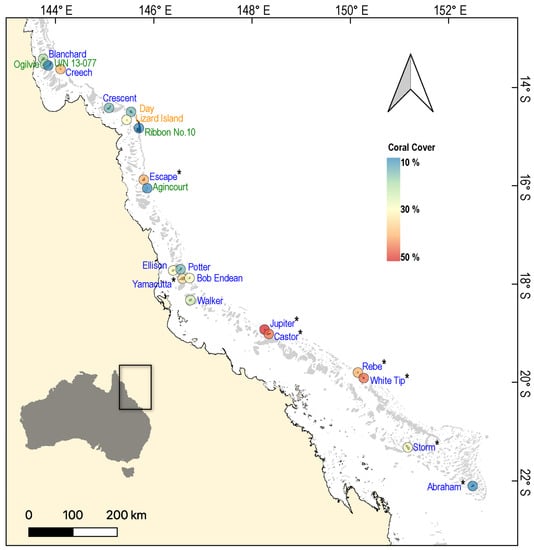
Figure 1.
Map of the Great Barrier Reef, showing the location of reefs (n = 21) surveyed from 2019–2021 to test for variation in the physiological condition of common coral trout (Plectropomus leopardus). Each reef is colour-coded according to the average cover of hard coral (order Scleractinia) recorded during surveys. The font colours of reef names correspond to the Great Barrier Reef Marine Park Authority (GBRMPA) zoning categories: blue (Habitat Protection Zone), green (Marine National Park Zone), and orange (Scientific Research Zone). Sites where phase angle data from bioelectrical impedance analysis were collected (n = 8 reefs) are marked with an asterisk (*).
Individual sampling sites (and the scale at which we compared the physiological condition of fishes to the local coral cover) were approximately 50,000 m2 (500 × 100 m) and separated by >1 km from other sites sampled on the same reef. Based on documented home ranges of P. leopardus [], it is unlikely that fish would travel between sites. Moreover, these fish largely utilise small reef areas (0.048–0.067 km2), such that our measurements of habitat condition should generally reflect the habitats that these fish utilise. Coral cover was surveyed along three replicate 50 m point intercept transects at each of two distinct depths (2–4 m and 6–8 m) within each site.
For each individual fish retained during this study, we recorded fork length (FL, in mm), standard length (SL, in mm) and overall body mass (Wt, in g). Otoliths were also extracted from individual fish, and age (years) was estimated by using transverse sections of sagittal otoliths, following Taylor et al. []. The liver was removed in its entirety, snap-frozen, and stored at −20 °C for assessing hepatocyte vacuolation (HV: the percent of intracellular vacuoles in cross sections of the liver), following Pratchett et al. []. Hepatocyte vacuolation is increasingly used as an indicator of physiological condition in a range of different fishes and has been shown to be very sensitive to changes in the quality (if not quantity) of food intake []. Hepatocyte vacuolation is also easily measured, albeit requiring lethal sampling of the fishes.
The livers removed from individual coral trout were partially thawed, weighed, and then sectioned (where necessary) prior to histological preparation. Liver samples from each fish were placed in individual histology cassettes and fixed in 10% calcium-buffered formalin (FAACC) for a minimum of one week. After fixing, liver samples were dehydrated in a graded ethanol series and embedded in paraffin wax before being sectioned (5 µm thick) and mounted on glass slides. Mounted sections were then stained using Mayer’s hematoxylin and eosin to emphasise hepatocyte vacuoles. Stained sections were viewed using a HD Lite capture camera (Scientific Instrument and Optical Sales, Kelvin Grove, QLD, Australia) attached to a high-power microscope (×40 magnification), and the proportion of vacuoles were quantified using an 8 × 8 grid projected onto the image displayed on a HD lite retina display using IsCapture software (Informer Technologies, Inc., Roseau, Dominica). The number of points (out of 64) that intersected hepatocyte vacuoles were counted and scaled to provide a percentage. Three estimates of hepatocyte vacuolation were recorded for each section (in haphazardly selected, non-overlapping areas of the liver that that were fully enclosed by the recording grid) by each of two different observers, giving a total of six estimates for each fish. Replicate sections were also prepared for a sub-sample (n = 6) of fish, which were independently scored to test for consistency.
In addition to HV (described above), the physiological conditions of P. leopardus were measured for a sub-sample of individuals (collected in 2021) using bioelectrical impedance analysis (BIA) and, more specifically, phase angle, following Champion et al. []. Phase angle (°) is a measure of resistance and reactance determined using electrical conductivity methods which scales with body condition and has been linked to variation in the nutritional status of individual fishes []. All BIA measurements were taken using the Seafood Analytics Certified Quality Reader (CQ Foods, Inc., Juneau, AK, USA; https://www.certifiedqualityfoods.com). Measurements were taken using large stainless probes along the dorsal musculature within 90 min of capture. All fishes were placed in a commercial chiller box filled with >30 L of seawater and set to 4.0 °C for a minimum of 30 min to standardise measurements of BIA, which can vary with temperature [].
A scatterplot matrix was initially constructed in R version 4.0.2 (R Core Team 2021) using the ggpairs function in the GGally package (Schloerke et al. 2022) to check for possible collinearity among the predictor variables: ‘Latitude’, ‘Coral Cover’, ‘Standard Length’, and ‘Age’. There was evidence for collinearity between ‘Standard Length’ and ‘Age’, such that ‘Age’ was removed from the model. Predictor variables were also centred using the scale function in R to avoid further issues associated with multicollinearity. Proportional HV data were arcsine–square-root-transformed to improve normality. Variation in HV and PA were modelled as a function of ‘Coral Cover’, ‘Latitude’, and ‘Standard Length’ using linear models. Given that the primary intention of the study was to test for variation in the physiological condition of coral trout with respect to the current condition of reef habitats, all models included ‘Coral Cover’, but alternative models were compared to assess whether variation in physiological condition was further explained by the location of reefs (‘Latitude’) and the ‘Standard Length’ or ‘Age’ of individual fish. Alternative models were compared using the Akaike information criterion (AIC), while individual p-values were used to infer which factors were most informative in the final models. To explore the utility of the condition metrics presented herein, we compared the values obtained for individual fish with Fulton’s condition factor (Fulton’s K). Fulton’s K was calculated using the following formula: (105 × Weight)/(Standard Length3). Pearson’s correlation coefficient was used to assess the correlation between the different measures of physiological condition: HV, PA, and Fulton’s K.
3. Results
Hepatocyte vacuolation (HV) of P. leopardus varied among individual fish mostly in accordance with their size. The average HV recorded across all P. leopardus (n = 407) was 3.98% (±0.17 SE) and ranged from 0.0% to 23.89%. The average HV was generally higher but also more variable among larger fishes; for fish that were <200 mm (SL), the average HV was 2.43% (0.53–7.36%), compared to 3.79% (0.00–23.89%) for fish that were 200–400 mm (SL) and 4.50% (0.00–18.24%) for fish that were >400 mm (SL). Hepatocyte vacuolation also varied spatially (among reefs) but did not relate to local coral cover. Notably, the average HV of P. leopardus was lowest on reefs in the northern GBR and tended to increase with increasing latitude, though the average HV was also low at reefs in the southern GBR where coral cover had been depleted due to localized population irruptions of Pacific crown-of-thorns starfish (Acanthaster cf. solaris). While there were insufficient data to test whether HV varied among the fishes sampled from areas that were open versus closed to fishing, it was not apparent that HV was any higher for fishes sampled from reefs closed to fishing (Figure 2B). Overall, the best model to account for variation in HV among P. leopardus only included the size of fish (standard length), though this model was not substantially better (AIC difference < 2) than models that included size, latitude, and coral cover (Table 1). Notably, spatial variation in HV (among sites and reefs) did not correspond to local coral cover (Figure 2B).
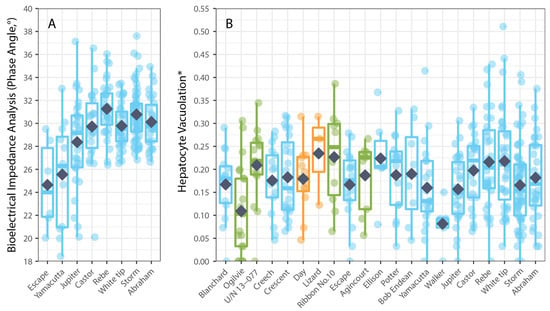
Figure 2.
Spatial variation in physiological condition measured using: (A) bioelectrical impedance analysis, phase angle (n = 224), and (B) hepatocyte vacuolation (n = 404) for common coral trout (Plectropomus leopardus) relative to the location (reefs arranged in increasing latitude (° S)) of the reef where the fish were caught. Colours of boxplots correspond to GBRMPA zoning (see Figure 1). Box-and-whisker plots show the minimum and maximum non-outlier values (whiskers) and the first quartiles, the medians, and the third quartiles (boxes), while dark blue diamond’s show means. (* Proportional HV is arcsine–square-root-transformed.).

Table 1.
Summary of linear model results for phase angle from bioelectrical impedance analysis, and hepatocyte vacuolation, predicted as a function of ‘Latitude’ (reef), Coral Cover, ‘Size’ (standard length (SL)), and their interactions. Significant effects (p < 0.05) are shown in bold.
Physiological condition was also measured using phase angel (PA) from BIA for a subset of P. leopardus (n = 233) sampled in 2020–2021. The average PA was 29.64° (±0.22 SE) and ranged from 13.20–37.60°. The best model to explain individual variation in PA included the size of fish (specifically, standard length) and the latitude of sampling locations (Table 1). As for HV, PA increased with increasing size (standard length) of fish (Figure 3), but spatial variation in PA (among sites and reefs) did not correspond to local coral cover (Figure 4).
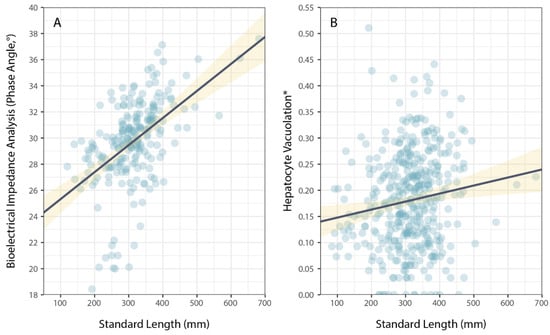
Figure 3.
Variation in (A) bioelectrical impedance analysis, phase angle, and (B) hepatocyte vacuolation as a function of the size (standard length) of Plectropomus leopardus. Size had a significant effect on PA and HV. (* Proportional HV is arcsine–square-root-transformed). Lines of best fit (black) and confidence intervals (yellow) are shown for linear models.
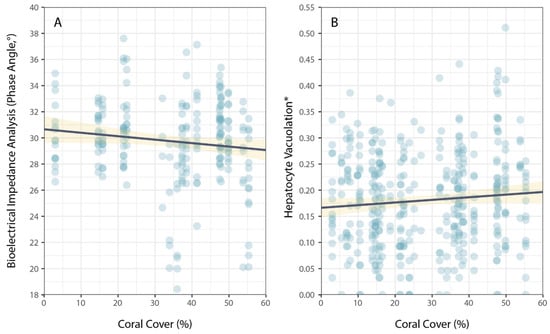
Figure 4.
Relationship between coral cover and measures of physiological condition for common coral trout (Plectropomus leopardus): (A) bioelectrical impedance analysis, phase angle, and (B) hepatocyte vacuolation. (* Proportional HV is arcsine–square-root-transformed.) Lines of best fit (black) and confidence intervals (yellow) are shown for linear models.
While PA measures different aspects of individual condition (overall impedance between fat and fat-free tissues []) relative to HV (liver lipid stores), there was a positive but very weak relationship between these two independent measures of physiological condition (Figure 5). Notably, there was considerable variation in PA among fish with low HV. Phase angle of P. leopardus was, however, positively correlated with Fulton’s K (a morphometric measure of overall body condition), though there was considerable variation around the fitted relationship (Figure 5).
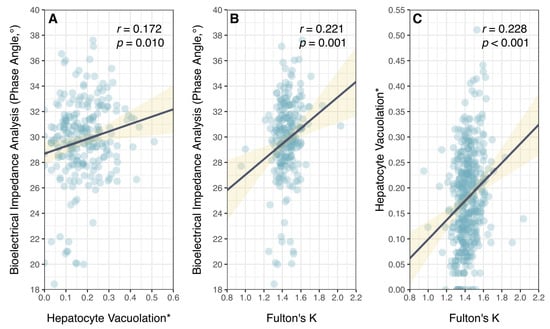
Figure 5.
Relationship between different measures of physiological condition in common coral trout (Plectropomus leopardus): (A) bioelectrical impedance analysis, phase angle; (B) hepatocyte vacuolation; and (C) Fulton’s condition factor (K). (* Proportional HV is arcsine–square-root-transformed.) Lines of best fit (black) and confidence intervals (yellow) are shown for linear models.
4. Discussion
Mass bleaching of corals has occurred at fairly regular intervals on the GBR since 1980 [], but the extent and severity of mass bleaching that occurred in 2016 and 2017 were unprecedented [,]. Extensive coral loss and marked shifts in coral composition were, however, largely restricted to the northern GBR []. Accordingly, coral cover ranged from 3.00–48.33% across the sites surveyed in 2020–2021 and was generally lower on reefs in the northern GBR. Spatial variation in coral cover was at least partly attributable to the 2016–2017 mass bleaching, though there are many other factors that are causing coral mortality and contributing to coral loss across the GBR [,]. Reefs in the southern GBR (Swains), for example, had very low coral cover (Figure 1), which was attributable to localised infestations of crown-of-thorns starfish [].
In this study, the physiological condition of P. leopardus and especially HV, which is expected to vary in response to changes in prey quality and quantity [], varied with the size of fish and, among reefs, mostly with respect to latitude. Variation in the physiological condition of P. leopardus was also apparent based on PA, which may provide a useful method for measuring the overall condition of coral trout without having to sacrifice fishes []. However, neither the HV nor PA of P. leopardus varied consistently in relation to the local coral cover at sites where individual fish were captured. These data suggest that adult P. leopardus may be resilient to changes in coral cover, at least over periods of 2–5 years following acute coral loss caused by climate-induced coral bleaching. It is also possible that the extent of sampling was insufficient or that the levels of coral loss that occurred on the reefs sampled were too low to effectively reveal sub-lethal effects of changing habitat conditions on P. leopardus.
Extensive coral loss and low levels of coral cover (<10%) undoubtedly affect the productivity and function of coral reef ecosystems [,,], but differentially affect reef fishes, depending on the type and breadth of their resource use []. While adult coral trout often feed on small reef fishes (Pomacentridae) that typically decline in abundance [] or change in composition [] following extensive coral loss and reef degradation, P. leopardus is a highly versatile piscivore [], capable of feeding on a wide diversity of prey, including pelagic species (Clupeidae and Engraulididae). Data from this study suggest that P. leopardus have relatively consistent food intake and physiological condition regardless of variation in coral cover and habitat condition among reefs, which might be explained by their versatility in prey use. Dietary shifts in response to reef degradation have been documented previously for the barcheek coral trout (Plectropomus maculatus), coinciding with localised bleaching and extensive coral loss []. For P. maculatus, these dietary shifts were also not sufficient to ameliorate effects of habitat degradation, and their local abundance declined in accordance with coral loss [,]. It is unknown whether P. leopardus has greater dietary flexibility than P. maculatus, but there still might be inherent constraints in their ability to cope with more devastating effects of environmental change and habitat degradation. Notably, P. leopardus have been shown to reduce both their feeding and activity in response to elevated temperature [,,], which might ultimately make them even more vulnerable to changing environmental and habitat conditions.
Most coral reef fishes, including carnivorous fishes (e.g., coral trout), are adversely affected by extensive coral loss and reef degradation [,,]. Following extensive coral loss and topographic collapse of reef habitats, the abundance and species richness of fishes have been shown to decline by > 60% [,]. While adult stocks of common coral trout appear to be somewhat resilient to changing environmental and habitat conditions, at least in the short term, further research is needed to test whether inevitable shifts in coral reef environments and habitats will have longer-term or progressive effects on wild stocks. Most notably, changes in environmental and habitat conditions may undermine the settlement and survival of juvenile coral trout []. If so, there may be protracted declines in the abundance of coral trout [], especially in areas subject to ongoing exploitation, which will undermine fisheries sustainability. The results of this study build upon experimental, laboratory-based studies on the potential responses of coral trout (especially common coral trout P. leopardus) to changing environmental conditions [,,,,,]. However, the specific effects of changing environmental and habitat conditions on wild stocks of coral trout are still unclear []. This is not to say that changing environmental and habitat conditions, as well as increasing effects of other major disturbances and anthropogenic pressures, do not or will not have substantive effects on coral trout (let alone other major fisheries target species). Importantly, the apparent stability of adult stocks may belie critical changes in the demography and population viability of coral trout, which are difficult to discern in the wild. Future research needs to better resolve the role of live coral and habitat structure in the settlement and survival of P. leopardus, as these factors might underpin their vulnerability to habitat degradation []. Most critical for fisheries management, however, is the need to test for changes in catch rates and/or the size and abundance of coral trout that may occur due to progressive shifts in environmental and habitat conditions. This will also provide the opportunity to reconcile independent changes in catch rates versus the abundance or biomass of coral trout, which is important for assessing how catchability (and thereby fisheries viability) might change with changing environmental and habitat conditions [].
There are a variety of different metrics used to explore individual variation in health or condition among fish [] that largely aim to assess energetic or fat reserves that can be mobilised to enhance future growth, survival, and/or reproductive output. Apparent increases in both HV and PA with increased size of coral trout in this study likely represent increased access to prey resources and/or reduced investment in key energetic processes (e.g., growth), which may provide increased resilience to habitat degradation. Given the variation in rates of fat storage and mobilisation across different organs, however, it is not surprising that contrasting metrics of physiological condition do not directly correspond. Contrasting metrics of physiological condition also vary in the extent to which they reflect the individual differences in recent feeding history or subsequent demography [,], but there is increasing evidence that PA is informative for understanding the variation in the condition of marine fishes, especially larger-bodied species [,,]. Further research will be required to understand the causes and consequences of individual variation in PA, but this study suggests that this is a promising non-lethal method for measuring the physiological condition of these important fisheries species.
5. Conclusions
Direct effects of increasing temperature on coral trout [,,,] will likely be compounded by climate-induced shifts in the structure of coral reef habitats [,,]. Most notably, extensive coral loss and corresponding declines in habitat complexity may lead to overall declines in the abundance of prey or otherwise moderate predator–prey interactions []. While there was no apparent effect of local coral cover on the physiological condition of common coral trout (P. leopardus) in the few years following the major coral bleaching and coral loss on the GBR, which occurred in 2016–2017 [,], negative effects of habitat degradation may ultimately become apparent with ongoing and escalating changes in habitat and environmental conditions. The most effective and immediate way to mitigate effects of environmental change on fisheries stocks is to restrict exploitation to levels below those that are otherwise considered sustainable, while also minimizing anthropogenic disturbances to natural ecosystems and reversing widespread habitat degradation []. The required changes in fisheries management do, however, directly contradict economic, social, and political imperatives. Critically, fisheries and environmental managers will need to balance the increasing demand for fishes against the need to maximise the resilience of species and maintain ecosystem processes [], which will become increasingly difficult within degraded reef environments and especially in areas where reef fisheries are critical to food security and livelihoods [].
Author Contributions
Conceptualisation, M.S.P., A.S.H. and J.-P.A.H.; methodology, M.S.P. and A.S.H.; data collection and collation, M.S.P., J.-P.A.H., B.B., J.D.D., P.W. and C.C.; formal analysis, C.F.C. and S.P.M.C.; writing—original draft preparation, M.S.P.; funding acquisition, M.S.P. and A.S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Australian Government Fisheries Research and Development Corporation (FRDC project no. 2018-034).
Institutional Review Board Statement
The animal study protocol was approved by the James Cook University Animal Ethics Committee (approval no. 2755).
Data Availability Statement
All new data arising from this study are available upon request via the JCU Data Hub.
Acknowledgments
This study benefited from significant logistic support from the skipper (Rob Benn) and crew of the MV Iron Joy. Assistance with benthic (coral cover) sampling was provided by D. Burn and C. Thompson.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cheung, W.W.; Sarmiento, J.L.; Dunne, J.; Frölicher, T.L.; Lam, V.W.; Deng Palomares, M.L.; Watson, R.; Pauly, D. Shrinking of fishes exacerbates impacts of global ocean changes on marine ecosystems. Nat. Clim. Change 2013, 3, 254–258. [Google Scholar] [CrossRef]
- Brown, C.J.; Mellin, C.; Edgar, G.J.; Campbell, M.D.; Stuart-Smith, R.D. Direct and indirect effects of heatwaves on a coral reef fishery. Glob. Change Biol. 2021, 27, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Pratchett, M.S.; Munday, P.L.; Wilson, S.K.; Graham, N.A.; Cinner, J.E.; Bellwood, D.R.; Jones, G.P.; Polunin, N.V.; McClanahan, T.R. Effects of climate-induced coral bleaching on coral-reef fishes—Ecological and economic consequences. Oceanogr. Mar. Biol. Annu. Rev. 2008, 46, 257–302. [Google Scholar]
- Bell, J.D.; Ganachaud, A.; Gehrke, P.C.; Griffiths, S.P.; Hobday, A.J.; Hoegh-Guldberg, O.; Johnson, J.E.; Le Borgne, R.; Lehodey, P.; Lough, J.M.; et al. Mixed responses of tropical Pacific fisheries and aquaculture to climate change. Nat. Clim. Change 2013, 3, 591–599. [Google Scholar] [CrossRef]
- Rogers, A.; Blanchard, J.L.; Mumby, P.J. Fisheries productivity under progressive coral reef degradation. J. Appl. Ecol. 2018, 55, 1041–1049. [Google Scholar] [CrossRef]
- Russ, G.R.; Rizzari, J.R.; Abesamis, R.A.; Alcala, A.C. Coral cover a stronger driver of reef fish trophic biomass than fishing. Ecol. Appl. 2021, 31, e02224. [Google Scholar] [CrossRef]
- Hughes, T.P.; Anderson, K.D.; Connolly, S.R.; Heron, S.F.; Kerry, J.T.; Lough, J.M.; Baird, A.H.; Baum, J.K.; Berumen, M.L.; Bridge, T.C.; et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 2018, 359, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Bellwood, D.R.; Pratchett, M.S.; Morrison, T.H.; Gurney, G.G.; Hughes, T.P.; Álvarez-Romero, J.G.; Day, J.C.; Grantham, R.; Grech, A.; Hoey, A.S.; et al. Coral reef conservation in the Anthropocene: Confronting spatial mismatches and prioritizing functions. Biol. Conserv. 2019, 236, 604–615. [Google Scholar] [CrossRef]
- Eddy, T.D.; Lam, V.W.; Reygondeau, G.; Cisneros-Montemayor, A.M.; Greer, K.; Palomares, M.L.D.; Bruno, J.F.; Ota, Y.; Cheung, W.W. Global decline in capacity of coral reefs to provide ecosystem services. One Earth 2021, 4, 1278–1285. [Google Scholar] [CrossRef]
- Ortiz, J.C.; Wolff, N.H.; Anthony, K.R.; Devlin, M.; Lewis, S.; Mumby, P.J. Impaired recovery of the Great Barrier Reef under cumulative stress. Sci. Adv. 2018, 4, eaar6127. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Thompson, C.A.; Hoey, A.S.; Cowman, P.F.; Wilson, S.K. Effects of coral bleaching and coral loss on the structure and function of reef fish assemblages. In Coral Bleaching: Patterns, Processes, Causes and Consequences, 2nd ed.; van Oppen, M.J.H., Lough, J.M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 265–293. [Google Scholar]
- Sadovy de Mitcheson, Y.; Craig, M.T.; Bertoncini, A.A.; Carpenter, K.E.; Cheung, W.W.; Choat, J.H.; Cornish, A.S.; Fennessy, S.T.; Ferreira, B.P.; Heemstra, P.C.; et al. Fishing groupers towards extinction: A global assessment of threats and extinction risks in a billion dollar fishery. Fish Fish. 2013, 14, 119–136. [Google Scholar] [CrossRef]
- Frisch, A.J.; Cameron, D.S.; Pratchett, M.S.; Williamson, D.H.; Williams, A.J.; Reynolds, A.D.; Hoey, A.S.; Rizzari, J.R.; Evans, L.; Kerrigan, B.; et al. Key aspects of the biology, fisheries and management of Coral grouper. Rev. Fish Biol. Fish. 2016, 26, 303–325. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Cameron, D.S.; Donelson, J.; Evans, L.; Frisch, A.J.; Hobday, A.J.; Hoey, A.S.; Marshall, N.A.; Messmer, V.; Munday, P.L.; et al. Effects of climate change on coral grouper (Plectropomus spp.) and possible adaptation options. Rev. Fish Biol. Fish. 2017, 27, 297–316. [Google Scholar] [CrossRef]
- Fox, A.R.; Campbell, A.B.; Zieth, J.D. Stock Assessment of Queensland East Coast Common Coral Trout (Plectropomus leopardus), Australia, with Data to December 2021; Queensland Government: Brisbane, Australia, 2022; p. 83. Available online: https://era.daf.qld.gov.au/id/eprint/8618/ (accessed on 1 May 2023).
- Johansen, J.L.; Messmer, V.; Coker, D.J.; Hoey, A.S.; Pratchett, M.S. Increasing ocean temperatures reduce activity patterns of a large commercially important coral reef fish. Glob. Change Biol. 2014, 20, 1067–1074. [Google Scholar] [CrossRef]
- Johansen, J.L.; Pratchett, M.S.; Messmer, V.; Coker, D.J.; Tobin, A.J.; Hoey, A.S. Large predatory coral trout species unlikely to meet increasing energetic demands in a warming ocean. Sci. Rep. 2015, 5, 13830. [Google Scholar] [CrossRef]
- Sun, Z.; Xia, S.; Feng, S.; Zhang, Z.; Rahman, M.M.; Rajkumar, M.; Jiang, S. Effects of water temperature on survival, growth, digestive enzyme activities, and body composition of the leopard coral grouper Plectropomus leopardus. Fish. Sci. 2015, 81, 107–112. [Google Scholar] [CrossRef]
- Messmer, V.; Pratchett, M.S.; Hoey, A.S.; Tobin, A.J.; Coker, D.J.; Cooke, S.J.; Clark, T.D. Global warming may disproportionately affect larger adults in a predatory coral reef fish. Glob. Change Biol. 2017, 23, 2230–2240. [Google Scholar] [CrossRef]
- Clark, T.D.; Messmer, V.; Tobin, A.J.; Hoey, A.S.; Pratchett, M.S. Rising temperatures may drive fishing-induced selection of low-performance phenotypes. Sci. Rep. 2017, 7, 40571. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.; Heupel, M.; Tobin, A.; Pratchett, M.S. A large predatory reef fish species moderates feeding and activity patterns in response to seasonal and latitudinal temperature variation. Sci. Rep. 2017, 7, 12966. [Google Scholar] [CrossRef]
- Payet, S.D.; Lowe, J.R.; Mapstone, B.D.; Pratchett, M.S.; Sinclair-Taylor, T.H.; Taylor, B.M.; Waldie, P.A.; Harrison, H.B. Comparative demography of commercially important species of coral grouper, Plectropomus leopardus and P. laevis, from Australia’s great barrier reef and Coral Sea marine parks. J. Fish Biol. 2020, 97, 1165–1176. [Google Scholar] [CrossRef]
- Williamson, D.H.; Ceccarelli, D.M.; Evans, R.D.; Jones, G.P.; Russ, G.R. Habitat dynamics, marine reserve status, and the decline and recovery of coral reef fish communities. Ecol. Evol. 2014, 4, 337–354. [Google Scholar] [CrossRef]
- Emslie, M.J.; Cheal, A.J.; Logan, M. The distribution and abundance of reef-associated predatory fishes on the Great Barrier Reef. Coral Reefs 2017, 36, 829–846. [Google Scholar] [CrossRef]
- Hempson, T.N.; Graham, N.A.; MacNeil, M.A.; Williamson, D.H.; Jones, G.P.; Almany, G.R. Coral reef mesopredators switch prey, shortening food chains, in response to habitat degradation. Ecol. Evol. 2017, 7, 2626–2635. [Google Scholar] [CrossRef]
- Kingsford, M.J. Spatial and temporal variation in predation on reef fishes by coral trout (Plectropomus leopardus, Serranidae). Coral Reefs 1992, 11, 193–198. [Google Scholar] [CrossRef]
- Graham, N.A.; Wilson, S.K.; Jennings, S.; Polunin, N.V.; Robinson, J.A.N.; Bijoux, J.P.; Daw, T.M. Lag effects in the impacts of mass coral bleaching on coral reef fish, fisheries, and ecosystems. Conserv. Biol. 2007, 21, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Pratchett, M.S.; Heron, S.F.; Mellin, C.; Cumming, G.S. Recurrent mass-bleaching and the potential for ecosystem collapse on Australia’s Great Barrier Reef. In Ecosystem Collapse and Climate Change; Canadell, J.G., Jacksonn, R.B., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 265–289. [Google Scholar]
- Scott, M.E.; Heupel, M.R.; Simpfendorfer, C.A.; Matley, J.K.; Pratchett, M.S. Latitudinal and seasonal variation in space use by a large, predatory reef fish, Plectropomus leopardus. Funct. Ecol. 2017, 33, 670–680. [Google Scholar] [CrossRef]
- Taylor, B.M.; Gourley, J.; Trianni, M.S. Age, growth, reproductive biology and spawning periodicity of the forktail rabbitfish (Siganus argenteus) from the Mariana Islands. Mar. Freshw. Res. 2016, 68, 1088–1097. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Wilson, S.K.; Berumen, M.L.; McCormick, M.I. Sublethal effects of coral bleaching on an obligate coral feeding butterflyfish. Coral Reefs 2004, 23, 352–356. [Google Scholar] [CrossRef]
- Crowe, B.H.; Harris, J.O.; Bansemer, M.S.; Stone, D.A. Restricted feeding and dietary energy levels affect liver structure in cultured Yellowtail Kingfish (Seriola lalandi, Valenciennes) at summer water temperatures. Aquac. Res. 2021, 52, 6074–6086. [Google Scholar] [CrossRef]
- Champion, C.; Hobday, A.J.; Pecl, G.T.; Tracey, S.R. Maximising the utility of bioelectrical impedance analysis for measuring fish condition requires identifying and controlling for sources of error. Fish. Res. 2020, 229, 105575. [Google Scholar] [CrossRef]
- Cox, M.K.; Hartman, K.J. Nonlethal estimation of proximate composition in fish. Can. J. Fish. Aquat. Sci. 2015, 62, 269–275. [Google Scholar] [CrossRef]
- Mellin, C.; Matthews, S.; Anthony, K.R.; Brown, S.C.; Caley, M.J.; Johns, K.A.; Osborne, K.; Puotinen, M.; Thompson, A.; Wolff, N.H.; et al. Spatial resilience of the Great Barrier Reef under cumulative disturbance impacts. Glob. Change Biol. 2019, 25, 2431–2445. [Google Scholar] [CrossRef]
- Ling, S.D.; Cowan, Z.L.; Boada, J.; Flukes, E.B.; Pratchett, M.S. Homing behaviour by destructive crown-of-thorns starfish is triggered by local availability of coral prey. Proc. R. Soc. B 2020, 287, 20201341. [Google Scholar] [CrossRef]
- Wilson, S.K.; Graham, N.A.J.; Pratchett, M.S.; Jones, G.P.; Polunin, N.V. Multiple disturbances and the global degradation of coral reefs: Are reef fishes at risk or resilient? Glob. Change Biol. 2006, 12, 2220–2234. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Hoey, A.S.; Wilson, S.K. Reef degradation and the loss of critical ecosystem goods and services provided by coral reef fishes. Curr. Opin. Environ. Sustain. 2014, 7, 37–43. [Google Scholar] [CrossRef]
- Morais, R.A.; Depczynski, M.; Fulton, C.; Marnane, M.; Narvaez, P.; Huertas, V.; Brandl, S.J.; Bellwood, D.R. Severe coral loss shifts energetic dynamics on a coral reef. Funct. Ecol. 2020, 34, 1507–1518. [Google Scholar] [CrossRef]
- Emslie, M.J.; Logan, M.; Cheal, A.J. The distribution of planktivorous damselfishes (Pomacentridae) on the Great Barrier Reef and the relative influences of habitat and predation. Diversity 2019, 11, 33. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Hoey, A.S.; Ackerman, J.L.; Depczynski, M. Coral bleaching, reef fish community phase shifts and the resilience of coral reefs. Glob. Change Biol. 2006, 12, 1587–1594. [Google Scholar] [CrossRef]
- Wilson, S.K.; Fisher, R.; Pratchett, M.S.; Graham, N.A.J.; Dulvy, N.K.; Turner, R.A.; Cakacaka, A.; Polunin, N.V.; Rushton, S.P. Exploitation and habitat degradation as agents of change within coral reef fish communities. Glob. Change Biol. 2008, 14, 2796–2809. [Google Scholar] [CrossRef]
- Hoey, A.S.; Howells, E.; Johansen, J.L.; Hobbs, J.P.A.; Messmer, V.; McCowan, D.M.; Wilson, S.K.; Pratchett, M.S. Recent advances in understanding the effects of climate change on coral reefs. Diversity 2016, 8, 12. [Google Scholar] [CrossRef]
- Clark, T.D.; Scheuffele, H.; Pratchett, M.S.; Skeeles, M.R. Behavioural temperature regulation is a low priority in a coral reef fish (Plectropomus leopardus): Insights from a novel behavioural thermoregulation system. J. Exp. Biol. 2022, 225, 244212. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.E.; Tebbett, S.B.; Whitman, K.L.; Thompson, C.A.; Mancini, F.B.; Heupel, M.R.; Pratchett, M.S. Variation in abundance, diversity and composition of coral reef fishes with increasing depth at a submerged shoal in the northern Great Barrier Reef. Rev. Fish Biol. Fish. 2022, 32, 941–962. [Google Scholar] [CrossRef]
- Wen, C.K.; Pratchett, M.S.; Almany, G.; Jones, G.P. Patterns of recruitment and microhabitat associations for three predatory coral reef fishes on the southern Great Barrier Reef, Australia. Coral Reefs 2013, 32, 389–398. [Google Scholar] [CrossRef]
- Caldarone, E.M.; MacLean, S.A.; Sharack, B. Evaluation of bioelectrical impedance analysis and Fulton’s condition factor as nonlethal techniques for estimating short-term responses in postsmolt Atlantic salmon (Salmo salar) to food availability. Fish. Bull. 2012, 110, 257–270. [Google Scholar]
- Donelson, J.M.; McCormick, M.I.; Munday, P.L. Parental condition affects early life-history of a coral reef fish. J. Exp. Mar. Biol. Ecol. 2008, 360, 109–116. [Google Scholar] [CrossRef]
- Willis, J.; Hobday, A.J. Application of bioelectrical impedance analysis as a method for estimating composition and metabolic condition of southern bluefin tuna (Thunnus maccoyii) during conventional tagging. Fish. Res. 2008, 93, 64–71. [Google Scholar] [CrossRef]
- Fitzhugh, G.R.; Wuenschel, M.J.; McBride, R.S. Evaluation of bioelectrical impedance analysis (BIA) to measure condition and energy allocated to reproduction in marine fishes. J. Phys. Conf. Ser. 2010, 224, 012137. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).