Abstract
The freshwater bivalve Anodonta woodiana is native to China and widely distributed in Asia, Europe, and North America. However, natural populations of A. woodiana in China have dramatically declined recently. Several fish species have been used as potential hosts in breeding programs; nonetheless, the optimal host species is yet to be identified. In this study, we examined the suitability of five potential host fish species (bighead carp, common carp, crucian carp, yellow catfish, and tilapia) for A. woodiana under laboratory conditions. No significant difference was found in the number of glochidia attached to the five fish species; however, tilapia hosted more transformed juveniles than bighead carp, common carp, or yellow catfish (p < 0.05), with bighead carp hosting the least (p < 0.05). Yellow catfish had the highest host capacity index (the number of successfully transformed juveniles/the weight of the host fish × the survival rate of the host fish), 133 times higher than bighead carp and 1.3–2.9 times higher than the other species. The shell length and height of freshly transformed juveniles from yellow catfish were significantly larger than those of other host fish (p < 0.05). The juvenile index (shell length × shell height) of yellow catfish was significantly higher than that of other fish species (p < 0.05). In conclusion, yellow catfish appears to be the most suitable host fish out of the five species tested here.
1. Introduction
Freshwater bivalves have both important ecological and economic value. In freshwater ecosystems, they purify water, improve substrate structure, provide substrates/habitats for other biota, and are recognized as ecosystem engineers []. Freshwater bivalves are also ideal bioindicators for monitoring the pollution status of aquatic environments due to their sedentary lifestyle, high bioaccumulation, and poor metabolic capacity for pollutants, and they correlate pollutant content between organisms and habitats [,]. Freshwater bivalves are also used as food, for pearl cultivation, and as a source of antitumor medicines [,]. However, freshwater bivalves are one of the most threatened animal groups globally, with 40% of species being near threatened, threatened, or extinct [].
The freshwater bivalve Anodonta woodiana, commonly known as the Chinese pond mussel, originates from the Yangtze and Heilongjiang Rivers of China []. It has invaded many countries in Asia, Europe, and North America, through the spread of host fish [,]. It is noteworthy that the natural populations of A. woodiana in its native country have dramatically declined recently. For example, in Poyang Lake, the largest freshwater lake in China, the populations of this species plummeted by 70% over the past 26 years from 1981 to 2007 []. A key factor contributing to this decline has been the limited efforts to establish the suitability of host fish species [].
The life cycle of A. woodiana is typical among unionid bivalves including an obligatory parasitic stage [,]. The glochidia of A. woodiana are broad host generalists, parasitizing barb (Puntius semifasciolatus), bighead carp (Aristichthys nobilis), brown trout (Salmo trutta), Chinese bitterling (Rhodeus sinensis), chub (Leuciscus cephalus), common carp (Cyprinus carpio), crucian carp (Carassius auratus), European barbel (Barbus barbus), European bitterling (Rhodeus amarus), Gibel carp (Carassius gibelio), grass carp (Ctenopharyngodon idella), gudgeon (Gobio gobio), ide (Leuciscus idus), moderlieschen (Leucaspius delineatus), mosquitofish (Gambusia affinis), perch (Perca fluviatilis), roach (Rutilus rutilus), stickleback (Gasterosteus aculeatus), tilapia (Oreochromis nilotica), topmouth gudgeon (Pseudorasbora parva), and yellow catfish (Pelteobagrus fulvidraco) [,,,]. However, the effects of A. woodiana parasitism vary significantly among host fishes. For example, on average, the metamorphosis of 2045 glochidia can be completed on a single tilapia of 15 cm body length [], whereas just 39 glochidia can metamorphose on a single perch of similar body length (12 cm) []. Multiple attempts to restore the natural population of this mussel have been made in China, taking into account practicality and the efficacy of host fishes, with bighead carp, common carp, crucian carp, yellow catfish, and tilapia having been investigated as potential hosts for A. woodiana glochidia in large-scale breeding programs. However, it remains unclear as to which fish species would be the most appropriate hosts for the restoration of this bivalve species.
In this study, we evaluated the suitability of yellow catfish, crucian carp, common carp, tilapia, and bighead carp as hosts for A. woodiana under laboratory conditions. We hypothesized that the efficiency and success of glochidia transformation would be species-dependent. The main objectives included: (1) quantifying the host capacity of the five fishes to glochidia; and (2) delineating the morphologies of freshly transformed juvenile A. woodiana from the five fish species. The findings of this study will contribute to identifying optimal host fish species for A. woodiana breeding programs, thereby enabling more effective conservation of these imperiled freshwater bivalves.
2. Materials and Methods
2.1. Sources of Glochidia and Host Fishes
Anodonta woodiana were collected from the Freshwater Fisheries Research Center, Chinese Academy of Fisheries Sciences (Wuxi, China). To avoid inter-individual variation, a single gravid mussel (3 years old, shell length 13.9 cm, weight 271 g) was used to provide glochidia. A random subsample of 100 glochidia was tested for viability with sodium chloride []. The survival rate of glochidia prior to encysted fish was 95.4% ± 3.0%, thereby indicating good infection potential.
One-year-old bighead carp, common carp, crucian carp, yellow catfish, and tilapia were collected from the Nanquan experimental site of the Freshwater Fisheries Research Centre, Chinese Academy of Fisheries Sciences. Six fish of each species that were free of disease and injury were selected (Table 1). None of the fish had any previous contact with freshwater bivalves to exclude possible pre-immunization. Prior to parasitism, all host fish were initially cultured for 1 week, to enable adaptation to the experimental environment.

Table 1.
Specifications of host fishes (mean ± SD; n = 6).
2.2. Parasitism of Glochidia
Glochidia parasitized fish following the method of Chen et al. [], with slight modifications. First, the glochidia were placed in a plastic container with 30 L pond water (filtered through a 75 μm mesh filter), and were mixed well. Three suspensions of 1 mL each were taken randomly and used to detect glochidia density. The density of alive glochidia was 11,448 ± 2524 glochidia/L. The six fish from the five host fish species (30 individuals in total) were simultaneously placed in the plastic container and were left to be parasitized for 20 min. Afterward, each host fish was placed in a mesh bag (90 cm length × 35 cm width × 35 cm height; mesh size 5 mm) suspended in the center of a single glass aquarium (100 cm length × 45 cm width × 40 cm height) filled with 100 L filtered pond water. Each fish had its own aquarium. This method ensured that any glochidia/juveniles dropping from the host fish fell to the bottom of the glass aquarium, and prevented the host fish from eating any glochidia/juveniles. Host fish were not fed during the period of parasitism. The temperature, pH, and dissolved oxygen (DO) of the culture water were 27.4 ± 0.8 °C, 7.4 ± 0.1, and 8.2 ± 0.2 mg/L, respectively. Previous studies confirmed this water environment condition, including the temperature, pH, and DO, was suitable for glochidia parasitism [,].
2.3. Comparison of Host Capacity
Host fish survival was checked daily, and any dead fish were immediately removed. At 09:00–10:00 daily, untransformed glochidia/transformed juveniles were collected by siphoning the bottom of the glass aquarium. Untransformed glochidia and transformed juveniles were isolated from debris in the siphoned water using nylon screens (mesh size 150 and 550 μm). A stereo microscope with 10–40 × magnification (NV10; Shanghai Precision Instruments Co., Ltd., Shanghai, China) was used to count the number of untransformed glochidia and transformed juveniles. All collected individuals were identified. If foot activity was observed, individuals were classified as live juveniles []. Filtered pond water (approximately 50 L) was then added to 100 L in the aquarium.
The host capacity index (HCI; mussels/g fish weight) was first attempted to calculate to compare the host capacity of different host fishes for the glochidia of A. woodiana, comprehensively.
where j is the number of successfully transformed juveniles, w is the weight of the host fish (g), and s is the survival rate of the host fish.
HCl = j/w × s
2.4. Morphometry of Freshly Transformed Juveniles
Thirty freshly transformed juveniles were randomly collected from each host fish species at 09:00–10:00 during the peak release period (day 6 after parasitism). In contrast to the glochidium, the freshly transformed juvenile had a free-moving foot (Figure 1a) and formed new transparent shells along its ventrolateral edge (Figure 1b). The juveniles were immediately fixed in 95% ethanol to avoid morphometry being disturbed by movement. The fixed samples were photographed using an Olympus BX51 microscope (Olympus Co., Tokyo, Japan) at 200× magnification. Shell length and height (Figure 1b) were measured using built-in Olympus measurement software. With reference to the method used to calculate the glochidia index [], the juvenile index (JI, mm2) was attempted to calculate to compare the size of freshly transformed juveniles released from different host fish species:
JI = shell length × shell height
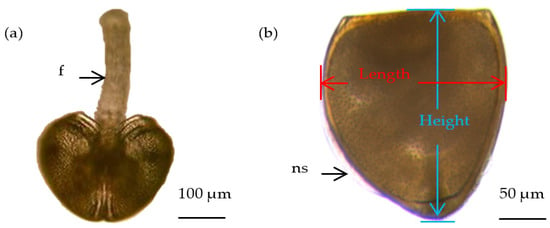
Figure 1.
Morphology of freshly transformed juveniles. (a) front view; (b) side view; f, foot; ns, new shell.
2.5. Statistical Analysis
All data are expressed as the mean ± SD. SPSS 25.0 for Windows (SPSS, Inc., Chicago, IL, USA) was used to perform statistical analysis. All data from the five fish groups were checked for normality. One-way ANOVA was used to compare differences in the number of attached glochidia, numbers of transformed juveniles, transformation success, HCI of host fish, shell length, shell height, and JI of freshly transformed juveniles from different host fish species. p < 0.05 indicates a significant difference.
3. Results
3.1. Host Capacity of the Five Fish Species
There was no significant difference in the number of A. woodiana glochidia attached to any of the five fish species (p > 0.05; Table 2). Glochidia developed successfully on all five host species (Figure 2). The survival rate of four of the parasitized host fish species was 100%, with that of bighead carp being 16.7% (Table 2). The mean duration of juvenile release from the five host species ranged from 5.5 to 7.0 days, with the peak release period of transformed juvenile mussels generally on day 6 after infection (Figure 2). Significantly more juveniles transformed on tilapia than on yellow catfish, common carp, and bighead carp (p < 0.05; Table 2). No significant differences were obtained between common carp, crucian carp, and yellow catfish (p > 0.05; Table 2). However, significantly fewer juveniles transformed on bighead carp than on crucian carp, yellow catfish, and tilapia (p < 0.05; Table 2). The transformation success of glochidia was similar on crucian carp, yellow catfish, and tilapia (p > 0.05), which was significantly higher than that on common carp and bighead carp (p < 0.05; Table 2). The HCI of yellow catfish was non-significantly (p > 0.05) higher than that of tilapia, but significantly higher than that of the other fish species (p < 0.05; Table 2). The HCI was 1.3, 1.4, 2.9, and 133 times higher in yellow catfish than in tilapia, crucian carp, common carp, and bighead carp, respectively (Table 2).

Table 2.
Results of host capacity tests (mean ± SD).
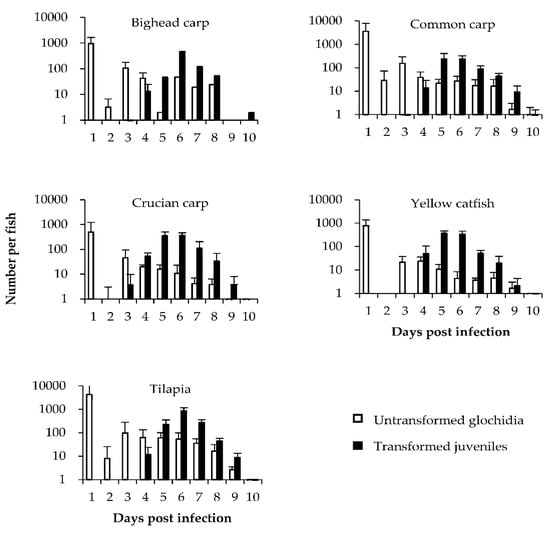
Figure 2.
Developmental dynamics of Anodonta woodiana glochidia on the five host fish species. Bars represent the mean ± SD of the number of untransformed (white bars) and transformed (black bars) juveniles recovered per host fish on each day after infection.
3.2. Morphometry of Freshly Transformed Juveniles
The morphometry of the freshly transformed juveniles from the five host fish species is shown in Table 3. The shell length and height of juveniles from yellow catfish were significantly larger than those from the other four host species (p < 0.05). The shell length and height of juveniles from bighead carp, common carp, crucian carp, and tilapia were not significantly different (p > 0.05). The JI of yellow catfish was significantly higher than that of other fish species (p < 0.05; Table 3).

Table 3.
Morphometry of freshly transformed juveniles from the five host species (mean ± SD; n = 30).
4. Discussion
4.1. Host Capacity of the Five Fish Species
The glochidia of freshwater bivalves mainly infect the gills and fins of host fish, from which they absorb nutrients to complete metamorphosis [,]. The current study revealed that bighead carp, common carp, crucian carp, yellow catfish, and tilapia acquired similar numbers of A. woodiana glochidia, but that the numbers of transformed juveniles and rates of transformation success differed significantly among host species. Similar findings were obtained for other freshwater bivalves, including Anodonta anatina, Anodonta cygnea, and Unio tumidiformis [,]. Thus, host suitability substantially influences the development of glochidia. The suitability of host fish might be associated with their nutritional composition. For example, Douda [] found that the transformation success rate of Unio crassus glochidia was positively associated with lipid reserves absorbed from host fish. The immune response of host fish might also affect the metamorphosis of glochidia. O’Connell and Neves [] demonstrated that fish humoral defense factors react with glochidia antigens to combat infection. Furthermore, host-acquired resistance significantly reduces the transformation success rate of glochidia from the same mussel genera and subfamilies [].
Although parasitism by glochidia generally has only minor effects on their host fish [,], a high-density infestation might reduce host osmotic potential and growth, and in extreme cases, cause host mortality [,]. However, the death of a host fish inevitably results in the failure of glochidia to complete metamorphosis [], thereby substantially reducing the parasitic efficiency of fish and increasing the cost of breeding. Notably, previous studies on the host capacity of fish quantified the number of transformed juvenile mussels but did not consider host fish mortality [,]. For example, Huber and Geist [] classified the host fishes of A. woodiana as “good” (grass carp, stickleback, ide, and gudgeon) and “poor” (brown trout, moderlieschen, roach, perch, topmouth gudgeon, and European bitterling) hosts based on the number of transformed juveniles. Yet, the mortality rates of grass carp, stickleback, ide, and gudgeon parasitized by glochidia were as high as 47%, 46%, 14%, and 14%, respectively []. In the present study, we similarly observed high mortality among bighead carp individuals, with only a 16.7% survival being recorded. Therefore, the survival rate of host fish should be an indispensable factor in evaluating the host capacity of fish against glochidia parasitism. Based on the weight normalization of host fish [], we innovatively attempted to introduce the survival rate of host fish and propose the HCI as a comprehensive index to evaluate host capability. In the present study, we only compared HCI in the five host fish species of the same age under laboratory conditions. In future studies, we will further examine HCI in host fish of different ages as well as in wild fish populations to further confirm its validity.
The HCI indicated that yellow catfish had the strongest host capability for A. woodiana glochidia, followed by tilapia, crucian carp, and common carp, with bighead carp having the lowest capability. On the basis of the HCI values of “good hosts” calculated from data in the published literature [], we found that compared with the “good host” approach, the HCI of yellow catfish was similar to that of grass carp and ide, but was higher than that of stickleback and gudgeon. Thus, yellow catfish might be the most suitable host fish for breeding A. woodiana.
In natural waters, it has been established that populations of freshwater bivalves are correlated with species and quantity of host fish [,]. An appropriate host fish could increase the likelihood of glochidia parasitization and subsequent successful transformation [,]. Consequently, it is reasonable to speculate that the restoration of imperiled freshwater bivalve populations might be achieved by increasing the quantity of host fish with a strong host capability.
4.2. Morphological Characteristics of Freshly Transformed Juveniles from the Five Fish Species
The size of freshly transformed juveniles could be influenced by a combination of internal factors (e.g., source of glochidia and duration of parasitism) and external factors (e.g., water environment and host fish species). The glochidia used in this study originated from the same gravid mussel, and all parasitized host fish for 6 days (peak release period). The water environment was the same for all five host fish species. Therefore, morphological differences among the freshly transformed juveniles could be attributed to specific host fish species.
Notably, only freshly transformed juveniles released from yellow catfish had significantly different morphometry. Compared to a single shell length or shell height as the morphological measure, JI may provide a more complete picture of the size of freshly transformed juveniles. The shell length, shell height, and JI of the juveniles obtained from yellow catfish were clearly greater than those of the juveniles released from other host fish species. During the period from initial parasitism to the metamorphosis of glochidia to juveniles, the nutrients of freshly transformed juveniles were derived exclusively from the host fish [,]. We can thus speculate that the nutritional composition of yellow catfish might be more conducive to meeting the growth and developmental requirements of A. woodiana glochidia. This suggestion is supported by the fact that Douda [] reported a positive association between the growth rate of freshly transformed juvenile mussels of U. crassus and A. anatina and the amount of lipid reserves absorbed from host fish during the parasitic stage. In addition, freshly transformed juveniles were larger at the start of their benthic developmental phase, enhancing the chances of survival during the first winter [], and helping to increase their populations. Although glochidia used in this study originated from a single brood of one gravid female mussel, the mussel produced more than 300,000 individual glochidia. Such a large sample size of glochidia should be able to cover glochidia intra-variation across A. woodiana populations. Nevertheless, we will further validate the morphological characteristics of freshly transformed juveniles from the five fish species in wild in the next step of our research.
5. Conclusions
In conclusion, yellow catfish had the strongest host capability for A. woodiana glochidia, followed by tilapia, crucian carp, common carp, and bighead carp. Freshly transformed juveniles released from yellow catfish were larger than those from the other host species. Overall, we confirmed yellow catfish were the optimal host for A. woodiana. Accordingly, we propose that this species should be preferentially used as host fish for the breeding of A. woodiana. These results would be useful for better conserving imperiled freshwater bivalves.
Author Contributions
Conceptualization, J.Y., X.C. and G.D.; methodology, G.D., M.Y., H.L., T.J., X.C. and J.Y.; investigation, G.D., M.Y., X.C. and J.Y.; funding acquisition, X.C.; writing—original draft, G.D., M.Y., H.L. and T.J.; writing—review and editing, X.C. and J.Y.; supervision, X.C. and J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Central Public-Interest Scientific Institution Basal Research Fund, CAFS (2022XT0504), the Young Scientists Fund of the National Natural Science Foundation of China (31502166), and the Guangdong Provincial Key Laboratory of Fishery Ecology and Environment fund (FEEL-2019-7).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of the Freshwater Fisheries Research Center, Chinese Academy of Fisheries Sciences (protocol code 2011AA1004020012).
Data Availability Statement
Relevant information has been included in the article.
Acknowledgments
We thank Kai Liu, Yahua Zhu, Junren Xue, and Yuhai Hu (Nanjing Agricultural University) for their support in the breeding of mussels.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vaughn, C.C. Ecosystem services provided by freshwater mussels. Hydrobiologia 2018, 810, 15–27. [Google Scholar] [CrossRef]
- Van Hassel, J.H.; Farris, J.L. A review of the use of unionid mussels as biological indicators of ecosystem health. In Freshwater Bivalve Ecotoxicology; CRC Press: Webster, NY, USA, 2007; pp. 19–49. [Google Scholar]
- Yang, J.; Harino, H.; Liu, H.; Miyazaki, N. Monitoring the organotin contamination in the Taihu Lake of China by bivalve mussel. Anodonta woodiana. B. Environ. Contam. Tox. 2008, 81, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gu, B.; Bian, J.; Hu, S.; Cheng, X.; Ke, Q.; Yan, H. Antitumor activities of liposome-incorporated aqueous extracts of Anodonta woodiana (Lea, 1834). Eur. Food Res. Technol. 2008, 227, 919–924. [Google Scholar] [CrossRef]
- Lopes-Lima, M.; Burlakova, L.E.; Karatayev, A.Y.; Mehler, K.; Seddon, M.; Sousa, R. Conservation of freshwater bivalves at the global scale: Diversity, threats and research needs. Hydrobiologia 2018, 810, 1–14. [Google Scholar] [CrossRef]
- Donrovich, S.W.; Douda, K.; Plechingerová, V.; Rylková, K.; Horký, P.; Slavík, O.; Liu, H.Z.; Reichard, M.; Lopes-Lima, M.; Sousa, R. Invasive Chinese pond mussel Sinanodonta woodiana threatens native mussel reproduction by inducing cross-resistance of host fish. Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 27, 1325–1333. [Google Scholar] [CrossRef]
- Watters, G.T. A synthesis and review of the expanding range of the Asian freshwater mussel Anodonta woodiana (Lea, 1834) (Bivalvia: Unionidae). Veliger 1997, 40, 152–156. [Google Scholar]
- Dobler, A.H.; Hoos, P.; Geist, J. Distribution and potential impacts of non-native Chinese pond mussels Sinanodonta woodiana (Lea, 1834) in Bavaria, Germany. Biol. Invas. 2022, 24, 1689–1706. [Google Scholar] [CrossRef]
- Liu, Y.J. Resource status and reproductive traits of freshwater bivalves in the Poyang Lake. Master’s Thesis, Nanchang University, Nanchang, China, 2008. (In Chinese with English abstract). [Google Scholar]
- Liu, X.; Liu, Y.; Wu, R.; Zanatta, D.T.; Lopes-Lima, M.; Gonçalves, D.V.; Bogan, A.E.; Ouyang, S.; Wu, X. Systematics, distribution, biology, and conservation of freshwater mussels (Bivalvia: Unionida) in China. Aquat. Conserv. Mar. Freshw. Ecosyst. 2022, 32, 859–895. [Google Scholar] [CrossRef]
- Dudgeon, D.; Morton, B. Site selection and attachment duration of Anodonta woodiana (Bivalvia: Unionacea) glochidia on fish hosts. J. Zool. 1984, 204, 355–362. [Google Scholar] [CrossRef]
- Hua, D.; Gu, R.; Xu, G.; Wen, H. Propagation and juvenile culture of the round mussel (Anodonta woodiana pacifica). Tentacle 2006, 14, 23. [Google Scholar]
- Douda, K.; Vrtílek, M.; Slavík, O.; Reichard, M. The role of host specificity in explaining the invasion success of the freshwater mussel Anodonta woodiana in Europe. Biol. Invas. 2012, 14, 127–137. [Google Scholar] [CrossRef]
- Huber, V.; Geist, J. Reproduction success of the invasive Sinanodonta woodiana (Lea 1834) in relation to native mussel species. Biol. Invasions 2019, 21, 3451–3465. [Google Scholar] [CrossRef]
- Wang, N.; Ingersoll, C.G.; Ivey, C.D.; Hardesty, D.K.; May, T.W.; Augspurger, T.; Roberts, A.D.; Van Genderen, E.; Barnhart, M.C. Sensitivity of early life stages of freshwater mussels (Unionidae) to acute and chronic toxicity of lead, cadmium, and zinc in water. Environ. Toxicol. Chem. 2010, 29, 2053–2063. [Google Scholar] [CrossRef]
- Chen, X.; Liu, H.; Su, Y.; Yang, J. Morphological development and growth of the freshwater mussel Anodonta woodiana from early juvenile to adult. Invert. Reprod. Dev. 2015, 59, 131–140. [Google Scholar] [CrossRef]
- Davis, G.M.; Fuller, S.L.H. Genetic relationships among recent unionacea (bivalvia) of North America. Malacologia 1981, 20, 217–253. [Google Scholar]
- Denic, M.; Taeubert, J.E.; Geist, J. Trophic relationships between the larvae of two freshwater mussels and their fish hosts. Invert. Biol. 2015, 134, 129–135. [Google Scholar] [CrossRef]
- Douda, K. Host-dependent vitality of juvenile freshwater mussels: Implications for breeding programs and host evaluation. Aquaculture 2015, 445, 5–10. [Google Scholar] [CrossRef]
- Reis, J.; Collares-Pereira, M.J.; Araujo, R. Host specificity and metamorphosis of the glochidium of the freshwater mussel Unio tumidiformis (Bivalvia: Unionidae). Folia Parasit. 2014, 61, 81–89. [Google Scholar] [CrossRef]
- O’Connell, M.T.; Neves, R.J. Evidence of immunological responses by a host fish (Ambloplites rupestris) and two non-host fishes (Cyprinus carpio and Carassius auratus) to glochidia of a freshwater mussel (Villosa iris). J. Freshw. Ecol. 1999, 14, 71–78. [Google Scholar] [CrossRef]
- Dodd, B.J.; Barnhart, M.C.; Rogers-Lowery, C.L.; Fobian, T.B.; Dimock, R.V. Cross-resistance of largemouth bass to glochidia of unionid mussels. J. Parasitol. 2005, 91, 1064–1072. [Google Scholar] [CrossRef]
- Rock, S.L.; Watz, J.; Nilsson, P.A.; Österling, M. Effects of parasitic freshwater mussels on their host fishes: A review. Parasitology 2022, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.M.R.; Marjomäki, T.J.; Taskinen, J. Effect of glochidia infection on growth of fish: Freshwater pearl mussel Margaritifera margaritifera and brown trout Salmo trutta. Hydrobiologia 2021, 848, 3179–3189. [Google Scholar] [CrossRef]
- Douda, K.; Velíšek, J.; Kolářová, J.; Rylková, K.; Slavík, O.; Horký, P.; Langrová, I. Direct impact of invasive bivalve (Sinanodonta woodiana) parasitism on freshwater fish physiology: Evidence and implications. Biol. Invas. 2017, 19, 989–999. [Google Scholar] [CrossRef]
- Holliman, F.M.; Kwak, T.J.; Cope, W.G.; Levine, J.F. Exposure of unionid mussels to electric current: Assessing risks associated with electrofishing. Trans. Am. Fish. Soc. 2007, 136, 1593–1606. [Google Scholar] [CrossRef]
- Khym, J.R.; Layzer, J.B. Host fish suitability for glochidia of Ligumia recta. Am. Midl. Nat. 2000, 143, 178–184. [Google Scholar] [CrossRef]
- Hart, M.A.; Haag, W.R.; Bringolf, R.; Stoeckel, J.A. Novel technique to identify large river host fish for freshwater mussel propagation and conservation. Aquacult. Rep. 2018, 9, 10–17. [Google Scholar] [CrossRef]
- Fritts, M.W.; Fritts, A.K.; Carleton, S.A.; Bringolf, R.B. Shifts in stable-isotope signatures confirm parasitic relationship of freshwater mussel glochidia attached to host fish. J. Molluscan Stud. 2013, 79, 163–167. [Google Scholar] [CrossRef][Green Version]
- Marwaha, J.; Jensen, K.H.; Jakobsen, P.J.; Geist, J. Duration of the parasitic phase determines subsequent performance in juvenile freshwater pearl mussels (Margaritifera margaritifera). Ecol. Evol. 2017, 7, 1375–1383. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).