Bisphenols: Endocrine Disruptors and Their Impact on Fish: A Review
Abstract
1. Introduction
2. Characteristics of Bisphenols and Their Occurrence in the Aquatic Environment
| Bisphenol Type | Continent | Water | Fish (ng/g w.w.) | Sediment (ng/g d.w.) |
|---|---|---|---|---|
| Bisphenol A (BPA) | Asia | 5.26–76.6 ng/L [22,23]; mean 23 ng/L [24]; <810 pg/L [25] | Coilia mystus: ~4–6, Pseudorasbora parva: ~12–14, Cyprinus carpio: ~11–13, Silurus asotus: ~11–14, [26] | 0.56–5.22 [22]; mean 13.0 [24]; <0.6 [25]; 0.18–2010 [26] |
| North America | <90.0 ng/L | n/a | <25,300 [18] | |
| Bisphenol S (BPS) | Asia | 0.07–5.2 ng/L [22,23]; 2.2 ng/L [24] | Coilia mystus: ~2–3, Pseudorasbora parva: ~1–2, Cyprinus carpio: ~1–2, Silurus asotus: ~1–2, [26] | n.d. −0.19 [22]; 0.69 [24] |
| Bisphenol F (BPF) | Asia | n.d.–12.6 ng/L [23] | Coilia mystus: ~2–3, Pseudorasbora parva: ~1–2, Cyprinus carpio: ~1–2, Silurus asotus: ~1–2, [26] | 1.6 [24] |
| Bisphenol AF (BPAF) | Asia | 0.44–10.8 ng/L [22,23]; 0.9–246 ng/L [26] | Coilia mystus: ~21, Pseudorasbora parva: ~13–17, Cyprinus carpio: ~3–4, Silurus asotus: ~8, [26] | 0.08–0.66 [22]; 0.18–2010 [26] |
| Bisphenol B (BPB) | Asia | n.d.–14.3 ng/L [23] | Coilia mystus: ~2, Pseudorasbora parva: ~1–4, Cyprinus carpio: ~1–2, Silurus asotus: ~3, [26] | n/a |
| Bisphenol E (BPE) | Asia | n.d.–6.2 ng/L [23] | Coilia mystus: ~1–2, Pseudorasbora parva: ~1–2, Cyprinus carpio: ~1–2, Silurus asotus: ~1–2, [26] | n/a |
| Bisphenol Z (BPZ) | Asia | n/a | Coilia mystus: ~2–4, Pseudorasbora parva: ~2–3, Cyprinus carpio: ~1–3, Silurus asotus: ~1–3, [26] | n/a |
| Tetrabromo bisphenol A (TBBPA) | Asia | 2.3 ng/L [24]; <810 pg/L [25] | n/a | <0.6 [25] |
3. Regulatory Framework and Restriction of Bisphenol Usage
4. Toxicity of Bisphenols to Fish
4.1. Estrogenic Activity and Disruption
| Bisphenol Type | Fish Species | Exposure Duration | Concentration | Effects Observed | References |
|---|---|---|---|---|---|
| BPA | Danio rerio | 15 days | 0.01, 0.1, 1 mg/L | ↑ egg production, ↓ fertilisation rate, altered reproductive gene expression, ↓ DNA methylation in ovaries, impaired reproductive processes | [68] |
| 30 days | 1, 10, 100 µg/L | ↑ atretic follicles, altered estrogen receptor expression, impaired ovarian function, reduced reproductive fitness | [3] | ||
| Danio rerio, embryo–larvae | 96 hpf–6 dpf | 1–200 mg/L | ↑ VTG, induced estrogenic response in heart, liver, muscle, and fins (ER-dependent) | [50,69] | |
| Danio rerio, male | 6 weeks | 100 and 2000 µg/L | early reproductive feminisation, female-like lipid metabolism, gonad damage, feminisation | [70] | |
| Danio rerio, larvae | 5–14 dpf | up to 2500 µg/L | estrogenic response in the heart, altered heart rhythm, ↓ heart rate | [71] | |
| Pimephales promelas | 43, 71, 164 days | 1–1, 280 µg/L | ↑ VTG in males (≥160 μg/L), gonadal growth inhibition (≥640 μg/L), ↓ egg production | [72] | |
| 4 days | 16–1, 280 µg/L | ↓ gonadal growth, altered sex cell types, ↓ egg production at high concentrations | [69] | ||
| Oryzias melastigma | 70 days | 200 µg/L | follicular atresia, irregular oocytes, empty follicles, ↓ eggs laid, ↓ fertilisation rate | [73] | |
| Carassius auratus | 7–90 days | 0.2 and 20 µg/L | ↑ VTG (liver, 60 days), ↑ aromatase and ERs, estrogenic effects | [74] |
| Bisphenol Type | Fish Species | Exposure Duration | Concentration | Effects Observed | References |
|---|---|---|---|---|---|
| BPF | Danio rerio | 96 h (embryo–larva) | 1–100 µg/L | induced estrogenic response, ↑ ER and aromatase gene activity, ↑ VTG, altered reproductive neuroendocrine genes | [19,50,69] |
| long-term (duration not specified) | 1–100 µg/L | ↑ expression of reproductive neuroendocrine genes (kiss1, gnrh3, lhβ, fshβ), ↑ VTG, ER and aromatase activity | [19] | ||
| 96 hpf–6 dpf | 1–200 mg/L | ↑ VTG in heart, liver, muscle, and fins | [50,69] | ||
| 21 days | 0.1 and 1 mg/L | ↑ VTG (males) and estrogenic effects | [75] | ||
| Oryzias melastigma | 70 days | 200 µg/L | follicular atresia, irregular oocytes, empty follicles, ↓ eggs laid, ↓ fertilisation rate | [73] | |
| BPAF | Danio rerio | 4 hpf–120 dpf | 5, 25, 125 µg/l | ↑ 17β-estradiol in females, ↓ egg fertilisation | [61] |
| 96 h (embryo–larva) | 1–200 mg/L | most potent estrogenic response, ↑ ER and aromatase gene activity, ↑ VTG | [50,69] | ||
| 96 hpf | 100 and 200 µg/L | delayed gonadal migration, ↓ germ cell progenitors, altered hormone receptor expression | [76] | ||
| 96 hpf–6 dpf | 1–200 mg/L | most potent ↑ VTG in heart, liver, muscle, fins | [50,69] | ||
| Oryzias melastigma | 70 days | 200 µg/L | most pronounced ↓ eggs laid, follicular atresia, irregular oocytes, empty follicles, ↓ fertilisation | [73] | |
| BPB | Danio rerio | 21 days | 0.1 and 1 mg/L | ↓ egg production, estrogenic-like activity similar to or greater than BPA | [77] |
| 96 h (embryo–larva) | 1–100 µg/L | ↑ ER and aromatase gene activity, altered reproductive neuroendocrine genes | [78] | ||
| BPS | Danio rerio | 4 hpf–120 dpf | 1 and 100 µg/L | female-dominant sex ratio, impaired reproductive capacity | [61] |
| 75 days | 0.1, 1, 10, 100 μg/L | ↑ female to male sex ratio, ↑ estradiol and VTG (males, females), ↓ egg production | [79] | ||
| Danio rerio, male | 96 hpf–6 dpf | 1–200 mg/L | ↑ VTG at high concentrations, induced estrogenic response, less potent than BPA | [50,69] | |
| 21 days | 8, 40, 200 μg/mL | ↑ VTG, ↑ aromatase and estradiol | [52] |
4.2. Androgenic Activity and Disruption
| Bisphenol Type | Fish Species | Exposure Duration | Concentration | Effects Observed | References |
|---|---|---|---|---|---|
| BPA | Carassius auratus | 7–90 days | 0.2 and 20 µg/L | ↓ sperm quality (number, motility, volume), ↓ testosterone, antiandrogenic effects | [74] |
| BPS | Danio rerio | 21 days | 8, 40, 200 μg/mL | ↓ endogenous androgens (males) | [52] |
| 21 days | 0.5, 5, 50 μg/L | antiandrogenic effect, ↓ gonad weight (males and females) | [79] | ||
| 75 days | 0.1, 1, 10, 100 μg/L | ↓ testosterone (males), ↓ gonad weight, ↓ testosterone (males), ↓ sperm count | [79] | ||
| 4 hpf–120 dpf | 1 and 100 µg/L | altered steroid hormone levels | [61] | ||
| Gadus morhua, cells | EC50 25.0 µmol/L; tested in the range of 0.003–50 µmol/L | ↑ activity of androgen receptor gmAR | [15] | ||
| BPF | Danio rerio | 21 days | 0.1 and 1 mg/L | ↓ testosterone (males), antiandrogenic effects | [75] |
| BPAF | Gadus morhua, cells | EC50 25.0 µmol/L; tested in the range of 0.003–50 µmol/L | ↑ activation of the androgen receptor gmAR | [15] | |
| Danio rerio | 6 dpf | 0.1 and 1 µmol/L | ↓ locomotor activity, ↑ aromatase B (estrogenic/antiandrogenic marker) | [91] | |
| 4 hpf–120 dpf | 5, 25, 125 μg/L | ↓ testosterone (males), impaired parental sperm quality | [92] |
4.3. Thyroid Activity and Disruption
| Bisphenol Type | Fish Species | Exposure Duration | Concentration | Effects Observed | References |
|---|---|---|---|---|---|
| BPA | Danio rerio | 120 hpf | 0.4, 2, 10 mg/L | ↑ T3/T4, altered thyroid development/transport/metabolism genes, delayed hatching | [56] |
| Up to 8 dpf | 0.2, 0.6, 1.3, 2.8 mg/L | ↓ T4, ↓ 3,5-T2, disrupted THS homeostasis, altered retinal morphology | [121] | ||
| Up to 8 dpf | 0.25, 0.5, 1.2, 4 µg/L | TRβ antagonism, inhibition of deiodinases, and altered phase II enzyme transcripts | [122] | ||
| 96 hpf | 1 and 100 µg/L | ↓ heart rate, ↑ SV-BA distance, altered dio3b, thrβ, myh7 gene expression | [123] | ||
| 96 hpf–6 dpf | 1–200 mg/L | developmental deformities | [50,69] | ||
| Oryzias melastigma | 70 days | 200 µg/L | ↓ hatching rate, altered HPG axis gene expression, epigenetic changes in offspring | [73] | |
| Pimephales promelas | 4 days | 16–1280 µg/L | ↓ hatchability in F1 generation | [69] | |
| BPS | Danio rerio | 7 days (adult) | 100 µg/L | ↑ TTR protein (plasma, liver, brain), ↑ T3/T4, thyroid tissue damage, altered HPT axis gene expression | [124] |
| 75 days | 0.1, 1, 10, 100 μg/L | ↓ body length and weight, ↓ T3 and T4, ↑ liver weight, ↓ hatching rate, ↑ time to hatch | [79] | ||
| 96 h | 1 and 100 µg/L | Promoted heart pumping, altered thrβ, myh7 gene expression | [123] | ||
| 4 hpf–120 dpf | 1 and 100 µg/L | delayed hatching, ↓ offspring survival | [61] |
| Bisphenol Type | Fish Species | Exposure Duration | Concentration | Effects Observed | References |
|---|---|---|---|---|---|
| BPF | Danio rerio | 7 days (adult) | 10 and 100 µg/L | ↑ TTR protein (plasma, liver, brain), ↑ T3/T4, thyroid follicle pathology, altered HPT axis gene expression | [124] |
| 120 hpf (larvae) | 2 mg/L | ↑ T3/T4, altered thyroid development/transport/metabolism genes, delayed hatching | [56] | ||
| 96 h | 1 and 100 µg/L | promoted heart pumping, altered thrβ, myh7 gene expression | [123] | ||
| 96 hpf–6 dpf | 1–200 mg/L | developmental deformities | [50,69] | ||
| 21 days | 0.1 and 1 mg/L | disrupted HPG axis gene expression | [75] | ||
| Oryzias melastigma | 70 days | 200 µg/L | ↓ hatching rate, altered HPG axis gene expression, epigenetic changes in offspring | [73] | |
| BPAF | Danio rerio | 4 hpf–120 dpf | 5, 25, 125 µg/L | ↑ malformations and lower survival in offspring, delayed hatching, altered HPG axis and liver gene expression | [61] |
| 96 hpf–6 dpf | 1–200 mg/L | developmental deformities | [50,69] | ||
| 4 hpf–120 dpf | 5, 25, 125 μg/L | ↑ malformations, ↓ survival rate in offspring, ↓ hatching at 5 μg/L BPAF | [92] | ||
| Oryzias melastigma | 70 days | 200 µg/L | ↓ hatching rates, altered HPG axis gene expression, epigenetic changes in offspring | [73] | |
| BPB | Danio rerio | 21 days | 1 mg/L | ↓ hatching rate and viability | [77] |
| BPZ | Danio rerio | 120 hpf | 0.18 and 2.9 µg/L | disrupted T3/T4, altered thyroid-related gene expression, delayed hatching | [56] |
| TBBPA | Danio rerio | 5 dpf | 100, 200, 300, 400 µg/L | inhibition of deiodinases, altered gene expression | [125,126] |
4.4. Glucocorticoid Activity and Disruption
| Bisphenol Type | Fish Species | Exposure Duration | Concentration | Effects Observed | References |
|---|---|---|---|---|---|
| BPA | Danio rerio | 96 h | 1500 µg/L | ↑ anxiety-like behaviour, neurotoxic effects | [140] |
| 30 days | 1, 10, 100 µg/L | ↑ oxidative stress | [3] | ||
| Danio rerio, male | 6 weeks | 100 and 2000 µg/L | ↑ fat deposition, ↑ body weight, ↑ lipid synthesis, inflammation, antioxidant response | [70] | |
| Cyprinus carpio, juvenile | 30 days | 0.1, 1, 10, 100, 1000 µg/L | (≥0.1 μg/L) ↓ immune response, ↑ oxidative stress | [141] | |
| Ctenopharyngodon idella, ovary cells | 48 h (in vitro) | 30 μmol/L | ↑ oxidative stress, altered DNA methylation | [142] | |
| Aristichthys nobilis | 60 days | 0.1, 1, 10 µg/L | ↑ oxidative stress, behavioural changes, and physiological disturbances | [58] | |
| BPS | Danio rerio | 120 days (embryo to adult) | 1, 10, 100 µg/L | ↑ whole-body cortisol, altered HPI axis gene expression, ↑ anxiety-like behaviour | [62] |
| 75 days | 1, 10, 30 µg/L | impaired anxiety/fear responses, altered antioxidant gene expression | [143] | ||
| Danio rerio, adult female | 120 days | 1, 10, 30 µg/L | 1 μg/L improved cognitive behaviours, 10/30 μg/L impaired cognitive behaviours, altered glutamatergic signalling | [144] | |
| Cyprinus carpio, juvenile | 60 days | 1, 10, 100 µg/L | ↑ oxidative stress, chronic inflammatory stress in the liver | [141] | |
| BPAF | Danio rerio, embryo/adult | 120 hpf | 5, 50, 500 µg/L | altered anxiety-like/aggressive behaviour, ↑ oxidative stress | [92] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Li, S.; Wu, T.; Wu, Z.; Guo, J. The effect of androgen on wool follicles and keratin production in Hetian sheep. Braz. J. Biol. 2021, 81, 526–536. [Google Scholar] [CrossRef]
- De Coster, S.; van Larebeke, N. Endocrine-disrupting chemicals: Associated disorders and mechanisms of action. J. Environ. Public Health 2012, 2012, 713696. [Google Scholar] [CrossRef]
- Faheem, M.; Bhandari, R. Detrimental Effects of bisphenol compounds on physiology and reproduction in fish: A literature review. Environ. Toxicol. Pharmacol. 2021, 81, 103497. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2018, 94, 849–873. [Google Scholar] [CrossRef] [PubMed]
- Martyniuk, C.J.; Mehinto, A.C.; Denslow, N.D. Organochlorine pesticides: Agrochemicals with potent endocrine-disrupting properties in fish. Mol. Cell. Endocrinol. 2020, 507, 110764. [Google Scholar] [CrossRef] [PubMed]
- Alves da Silva, A.P.; Lins de Oliveira, C.D.; Siqueira Quirino, A.M.; Morais da Silva, F.D.; de Aquino Saraiva, R.; Santos Silva-Cavalcanti, J. Endocrine disruptors in aquatic environment: Effects and consequences on the biodiversity of fish and amphibian species. Adv. Sci. Technol. 2018, 6, 35. [Google Scholar] [CrossRef]
- Pinto, I.P.; Estêvão, D.M.; Power, M.D. Effects of estrogens and estrogenic disrupting compounds on fish mineralized tissues. Mar. Drugs 2014, 12, 4474–4494. [Google Scholar] [CrossRef]
- Molina-López, A.M.; Bujalance-Reyes, F.; Ayala-Soldado, N.; Mora-Medina, R.; Lora-Benítez, A.J.; Moyano-Salvago, R. An overview of the health effects of bisphenol A from a One Health perspective. Animals 2023, 13, 2439. [Google Scholar] [CrossRef]
- Kim, J.; Kumar, S.; Kumar, V.; Lee, Y.; Kim, Y.; Kumar, V. Bisphenols as a legacy pollutant, and their effects on organ vulnerability. Int. J. Environ. Res. Public Health 2019, 17, 112. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S.; Choi, J.; Soung, N.; Heo, J. Effects of bisphenol A and its alternatives, bisphenol F and tetramethyl bisphenol F on osteoclast differentiation. Molecules 2021, 26, 6100. [Google Scholar] [CrossRef]
- Ragavan, K.V.; Rastogi, N.K.; Thakur, M.S. Sensors and biosensors for analysis of bisphenol-A. TrAC Trends Anal. Chem. 2013, 52, 248–260. [Google Scholar] [CrossRef]
- Kim, M.; Park, Y. Bisphenols and thyroid hormone. Endocrinol. Metab. 2019, 34, 340. [Google Scholar] [CrossRef] [PubMed]
- Berto-Júnior, C.; Santos-Silva, A.P.; Ferreira, A.C.F.; Graceli, J.B.; Carvalho, D.P.; Soares, P.; Romeiro, N.C.; Miranda-Alves, L. Unraveling molecular targets of bisphenol A and S in the thyroid gland. Environ. Sci. Pollut. Res. 2018, 25, 26916–26926. [Google Scholar] [CrossRef] [PubMed]
- Fabrello, J.; Matozzo, V. Bisphenol analogs in aquatic environments and their effects on marine species—A review. J. Mar. Sci. Eng. 2022, 10, 1271. [Google Scholar] [CrossRef]
- Goksøyr, S.; Yadetie, F.; Johansen, C.; Jacobsen, R.; Lille-Langøy, R.; Goksøyr, A.; Karlsen, O. Interaction of bisphenol A and its analogs with estrogen and androgen receptor from Atlantic cod (Gadus morhua). Environ. Sci. Technol. 2024, 58, 14098–14109. [Google Scholar] [CrossRef] [PubMed]
- Zaborowska, M.; Wyszkowska, J.; Borowik, A.; Kucharski, J. Bisphenol A—A dangerous pollutant distorting the biological properties of soil. Int. J. Mol. Sci. 2021, 22, 12753. [Google Scholar] [CrossRef]
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.; Feng, Y.; Wu, Y.; Widelka, M. Bisphenol analogues other than BPA: Environmental occurrence, human exposure, and toxicity—A review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef]
- Liao, C.; Liu, F.; Moon, H.-B.; Yamashita, N.; Yun, S.; Kannan, K. Bisphenol analogues in sediments from industrialized areas in the nited States, Japan, and Korea: Spatial and temporal distributions. Environ. Sci. Technol. 2012, 46, 11558–11565. [Google Scholar] [CrossRef]
- Qiu, W.; Fang, M.; Liu, J.; Fu, C.; Zheng, C.; Chen, B.; Wang, K. In vivo actions of bisphenol F on the reproductive neuroendocrine system after long-term exposure in zebrafish. Sci. Total Environ. 2019, 665, 995–1002. [Google Scholar] [CrossRef]
- Meeker, J.D.; Sathyanarayana, S.; Swan, S.H. Phthalates and other additives in plastics: Human exposure and associated health outcomes. Philos. Trans. R. Soc. B 2009, 364, 2097–2113. [Google Scholar] [CrossRef]
- Arcand-Hoy, D.L.; Benson, H.W. Fish reproduction: An ecologically relevant indicator of endocrine disruption. Environ. Toxicol. Chem. 1998, 17, 49–57. [Google Scholar] [CrossRef]
- Gao, Y.; Xiao, S.; Wu, Q.; Pan, C. Bisphenol analogues in water and sediment from the Beibu Gulf, South China Sea: Occurrence, partitioning and risk assessment. Sci. Total Environ. 2022, 857, 159445. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Li, J.; Yang, M. Occurrence and exposure assessment of bisphenol analogues in source water and drinking water in China. Sci. Total Environ. 2019, 655, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhao, N.; Zhang, Y.; Hu, H.; Zhao, M.; Jin, H. Occurrence and partitioning of bisphenol analogues, triclocarban, and triclosan in seawater and sediment from East China Sea. Chemosphere 2021, 287 Pt 2, 132218. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Li, G.; An, T.; Zhang, C.; Wei, C. Emission patterns and risk assessment of polybrominated diphenyl ethers and bromophenols in water and sediments from the Beijiang River, South China. Environ. Pollut. 2016, 219, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, M.; Shan, G.; Chen, P.; Cui, S.; Yi, S.; Zhu, L. Bioaccumulation and biomagnification of emerging bisphenol analogues in aquatic organisms from Taihu Lake, China. Sci. Total Environ. 2017, 598, 814–820. [Google Scholar] [CrossRef]
- Akash, H.; Sajid, M.; Rasheed, S.; Rehman, K.; Imran, M.; Assiri, M.A. Toxicological evaluation of bisphenol analogues: Preventive measures and therapeutic interventions. RSC Adv. 2023, 13, 21613–21628. [Google Scholar] [CrossRef]
- Braun, M.; Joe, M.; Hauser, R. Bisphenol A and children’s health. Curr. Opin. Pediatr. 2011, 23, 233–239. [Google Scholar] [CrossRef]
- Maamar, B.; Lesné, M.; Lethimonier, C.; Coiffec, I.; Lassurguère, J.; Lavoué, V.; Deceuninck, Y.; Antignac, J.-P.; Le Bizec, B.; Perdu, E.; et al. An investigation of the endocrine-disruptive effects of bisphenol A in human and rat fetal testes. PLoS ONE 2015, 10, e0117226. [Google Scholar] [CrossRef]
- Lindeman, B.; Ritz, V. Regulation and Risk Management of Endocrine Disruptors: Current Status and Future Perspectives; Royal Society of Chemistry: London, UK, 2020; pp. 495–511. [Google Scholar] [CrossRef]
- Wheeler, R.J.; Coady, K.K. Are all chemicals endocrine disruptors? Integr. Environ. Assess. Manag. 2016, 12, 402–403. [Google Scholar] [CrossRef]
- Office of the Federal Register, National Archives and Records Administration. 77 FR 41899—Indirect Food Additives: Polymers; Office of the Federal Register, National Archives and Records Administration: Washington, DC, USA, 2012. Available online: https://www.govinfo.gov/app/details/FR-2012-07-17/2012-17366 (accessed on 1 February 2025).
- Office of the Federal Register, National Archives and Records Administration. 78 FR 41840—Indirect Food Additives: Adhesives and Components of Coatings; Office of the Federal Register, National Archives and Records Administration: Washington, DC, USA, 2013. Available online: https://www.govinfo.gov/app/details/FR-2013-07-12/2013-16684 (accessed on 12 February 2025).
- Grignard, E.; Jesus, D.K.; Hubert, P. Regulatory testing for endocrine disruptors; need for validated methods and integrated approaches. Front. Toxicol. 2022, 3, 821736. [Google Scholar] [CrossRef]
- Ministry of Health, People’s Republic of China. Bulletin No. 15 of 2011: Safety Evaluation of Bisphenol A in Food Contact Materials; Ministry of Health, People’s Republic of China: Beijing, China, 2011. [Google Scholar]
- Canada Consumer Product Safety Act (S.C. 2010, c. 21); Department of Justice Canada: Ottawa, ON, Canada, 2010.
- Ministry of Food and Drug Safety. Standards and Specifications for Utensils, Containers and Packages (2019-2, 20190109); MFDS: Seoul, Republic of Korea, 2019. [Google Scholar]
- EU. Commission Implementing Regulation (EU) No 321/2011 of 1 April 2011 Amending Regulation (EU) No 10/2011 as Regards the Restriction of Use of Bisphenol A in Plastic Infant Feeding Bottles. Available online: https://eur-lex.europa.eu/eli/reg_impl/2011/321/oj/eng (accessed on 20 January 2025).
- EU. Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), Establishing a European Chemicals Agency, Amending Directive 1999/45/EC and Repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. Available online: https://eur-lex.europa.eu/eli/reg/2006/1907/oj/eng (accessed on 20 January 2025).
- EU. Commission Regulation (EU) 2016/2235 of 12 December 2016 Amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as Regards Bisphenol A. Available online: https://eur-lex.europa.eu/eli/reg/2016/2235/oj/eng (accessed on 20 January 2025).
- EU. Commission Regulation (EU) 2024/3190 of 19 December 2024 on the Use of bisphenol A (BPA) and Other Bisphenols and Bisphenol Derivatives with Harmonised Classification for Specific Hazardous Properties in Certain Materials and Articles Intended to Come into Contact with Food, Amending Regulation (EU) No 10/2011 and Repealing Regulation (EU) 2018/213. Available online: https://eur-lex.europa.eu/eli/reg/2024/3190/oj/eng (accessed on 20 January 2025).
- Ji, Z.; Liu, J.; Sakkiah, S.; Guo, W.; Hong, H. BPA replacement compounds: Current status and perspectives. ACS Sustain. Chem. Eng. 2021, 9, 2433–2446. [Google Scholar] [CrossRef]
- Carnevali, O.; Morini, M.; Santi, A.; Volpi, N.; Neri, F.; Franchi, M.; Maggi, M. Endocrine-disrupting chemicals in aquatic environment: What are the risks for fish gametes? Fish Physiol. Biochem. 2018, 44, 1561–1576. [Google Scholar] [CrossRef] [PubMed]
- Crisp, M.; Clegg, E.D.; Cooper, R.L.; Wood, W.R.; Anderson, D.G.; Baetcke, K.P.; Hoffmann, J.; Morrow, M.S.; Rodier, D.J.; Schaeffer, J.E.; et al. Environmental endocrine disruption: An effects assessment and analysis. Natl. Inst. Environ. Health Sci. 1998, 106 (Suppl. S1), 11–56. [Google Scholar] [CrossRef]
- Gassman, N. Induction of oxidative stress by bisphenol A and its pleiotropic effects. Environ. Mol. Mutagen. 2017, 58, 60–71. [Google Scholar] [CrossRef]
- Kovacic, P. How safe is bisphenol A? Fundamentals of toxicity: Metabolism, electron transfer and oxidative stress. Med. Hypotheses 2010, 75, 1–4. [Google Scholar] [CrossRef]
- Silva, D.A.; Martins, M.; Xavier, L.L.F.; Gonçalves, C.F.L.; Santos-Silva, A.P.; Paiva-Melo, F.D.; de Freitas, M.L.; Fortunato, R.S.; Miranda-Alves, L.; Ferreira, A.C.F. Bisphenol A increases hydrogen peroxide generation by thyrocytes both in vivo and in vitro. Endocr. Connect. 2018, 7, 1196–1207. [Google Scholar] [CrossRef]
- Stroustrup, A.; Swan, S.H. Endocrine Disruptors. In Textbook of Children’s Environmental Health, 2nd ed.; Etzel, R.A., Landrigan, P.J., Eds.; Oxford Academic: New York, NY, USA, 2024. [Google Scholar] [CrossRef]
- Segovia-Mendoza, M.; Palacios-Arreola, M.I.; Monroy-Escamilla, L.M.; Soto-Piña, A.E.; Nava-Castro, K.E.; Becerril-Alarcón, Y.; Camacho-Beiza, R.; Aguirre-Quezada, D.E.; Cardoso-Peña, E.; Amador-Muñoz, O.; et al. Association of serum levels of plasticizers compounds, phthalates and bisphenols, in patients and survivors of breast cancer: A real connection? Int. J. Environ. Res. Public Health 2022, 19, 8040. [Google Scholar] [CrossRef]
- Moreman, J.; Lee, O.; Trznadel, M.; David, A.; Kudoh, T.; Tyler, C. Acute Toxicity, Teratogenic, and Estrogenic Effects of bisphenol A and its alternative replacements bisphenol S, bisphenol F, and bisphenol AF in zebrafish embryo-larvae. Environ. Sci. Technol. 2017, 51, 12796–12805. [Google Scholar] [CrossRef]
- Naderi, M.; Wong, M.; Gholami, F. Developmental exposure of zebrafish (Danio rerio) to bisphenol-S impairs subsequent reproduction potential and hormonal balance in adults. Aquat. Toxicol. 2014, 148, 195–203. [Google Scholar] [CrossRef]
- Park, C.; Kim, G.; On, J.; Pyo, H.; Park, J.; Cho, S. Sex-specific effects of bisphenol S with tissue-specific responsiveness in adult zebrafish: The antiandrogenic and antiestrogenic effects. Ecotoxicol. Environ. Saf. 2021, 229, 113102. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Tian, X.; Fang, X.; Ji, F. Waterborne exposure to bisphenol F causes thyroid endocrine disruption in zebrafish larvae. Chemosphere 2016, 147, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhou, E.; Yang, Z. Waterborne exposure to BPS causes thyroid endocrine disruption in zebrafish larvae. PLoS ONE 2017, 12, e0176927. [Google Scholar] [CrossRef] [PubMed]
- Frenzilli, G.; Martorell-Ribera, J.; Bernardeschi, M.; Scarcelli, V.; Jönsson, E.; Diano, N.; Moggio, M.; Guidi, P.; Sturve, J.; Asker, N. Bisphenol A and bisphenol S induce endocrine and chromosomal alterations in bown trout. Front. Endocrinol. 2021, 12, 645519. [Google Scholar] [CrossRef]
- Lee, S.; Kim, C.; Shin, H.; Kho, Y.; Choi, K. Comparison of thyroid hormone disruption potentials by bisphenols A, S, F, and Z in embryo-larval zebrafish. Chemosphere 2019, 221, 115–123. [Google Scholar] [CrossRef]
- Wei, P.; Zhao, F.; Zhang, X.; Liu, W.; Jiang, G.; Wang, H.; Ru, S. Transgenerational thyroid endocrine disruption induced by bisphenol S affects the early development of zebrafish offspring. Environ. Pollut. 2018, 243 Pt B, 800–808. [Google Scholar] [CrossRef]
- Akram, R.; Iqbal, R.; Hussain, R.; Jabeen, F.; Ali, M. Evaluation of oxidative stress, antioxidant enzymes and genotoxic potential of bisphenol A in fresh water bighead carp (Aristichthys nobilis) fish at low concentrations. Environ. Pollut. 2020, 268, 115896. [Google Scholar] [CrossRef]
- Anjali, V.; Remya, V.; Reshmi, S.; Mahim, S.; Devi, C. Impact of bisphenol S as an endocrine disruptor in a freshwater fish, Oreochromis mossambicus. J. Endocrinol. Reprod. 2021, 23, 49–63. [Google Scholar] [CrossRef]
- Birceanu, O.; Mai, T.; Vijayan, M. Maternal transfer of bisphenol A impacts the ontogeny of cortisol stress response in rainbow trout. Aquat. Toxicol. 2015, 168, 11–18. [Google Scholar] [CrossRef]
- Shi, J.; Jiao, Z.; Zheng, S.; Li, M.; Zhang, J.; Feng, Y.; Yin, J.; Shao, B. Long-term effects of bisphenol AF (BPAF) on hormonal balance and genes of hypothalamus-pituitary-gonad axis and liver of zebrafish (Danio rerio), and the impact on offspring. Chemosphere 2015, 128, 252–257. [Google Scholar] [CrossRef]
- Wei, P.; Zhao, F.; Zhang, X.; Ru, S. Long-term exposure of zebrafish to bisphenol S impairs stress function of hypothalamic-pituitary-interrenal axis and causes anxiety-like behavioral responses to novelty. Sci. Total Environ. 2020, 716, 137092. [Google Scholar] [CrossRef] [PubMed]
- Arslan, P.; Özeren, S.C.; Dikmen, B.Y. The effects of endocrine disruptors on fish. Environ. Res. Technol. 2021, 4, 145–151. [Google Scholar] [CrossRef]
- Wojnarowski, K.; Cholewińska, P.; Palić, D.; Bednarska, M.; Jarosz, M.; Wiśniewska, I. Estrogen receptors mediated negative effects of estrogens and xenoestrogens in teleost fishes—Review. Int. J. Mol. Sci. 2022, 23, 2605. [Google Scholar] [CrossRef] [PubMed]
- Wahab, M.; Tyler, R.C. Endocrine disrupting chemicals and sexual behaviors in fish—A critical review on effects and possible consequences. Crit. Rev. Toxicol. 2012, 42, 653–668. [Google Scholar] [CrossRef]
- Lombó, M.; Depincé, A.; Labbé, C.; Herráez, M.P. Embryonic exposure to bisphenol A impairs primordial germ cell migration without jeopardizing male breeding capacity. Biomolecules 2019, 9, 307. [Google Scholar] [CrossRef]
- Cai, M.; Liu, Y.; Zheng, C.; Zhao, L.; Liu, X.; Hu, M.; Zheng, X. Evaluation of the effects of low concentrations of bisphenol AF on gonadal development using the Xenopus laevis model: A finding of testicular differentiation inhibition coupled with feminization. Environ. Pollut. 2020, 260, 113980. [Google Scholar] [CrossRef]
- Laing, L.; Viana, J.; Dempster, E.; Trznadel, M.; Trunkfield, L.; Webster, T.; Van Aerle, R.; Paull, G.; Wilson, R.; Mill, J.; et al. Bisphenol A causes reproductive toxicity, decreases DNMT1 transcription, and reduces global DNA methylation in breeding zebrafish (Danio rerio). Epigenetics 2016, 11, 526–538. [Google Scholar] [CrossRef]
- Mu, X.; Huang, Y.; Li, X.; Lei, Y.; Teng, M.; Li, X.; Wang, C. Developmental effects and estrogenicity of bisphenol A alternatives in a zebrafish embryo model. Environ. Sci. Technol. 2018, 52, 3002–3010. [Google Scholar] [CrossRef]
- Sun, S.-X.; Wu, J.-L.; Lv, H.-B.; Zhang, H.-Y.; Zhang, J.; Limbu, S.M.; Qiao, F.; Chen, L.-Q.; Yang, Y.; Zhang, M.-L.; et al. Environmental estrogen exposure converts lipid metabolism in male fish to a female pattern mediated by AMPK and mTOR signaling pathways. J. Hazard. Mater. 2020, 394, 122537. [Google Scholar] [CrossRef]
- Moreman, J.; Takesono, A.; Trznadel, M.; Winter, M.; Perry, A.; Wood, M.; Rogers, N.; Kudoh, T.; Tyler, C. Estrogenic mechanisms and cardiac responses following early life exposure to bisphenol A (BPA) and its metabolite 4-methyl-2,4-bis(p-hydroxyphenyl)pent-1-ene (MBP) in zebrafish. Environ. Sci. Technol. 2018, 52, 6656–6665. [Google Scholar] [CrossRef]
- Sohoni, P.; Tyler, C.R.; Hurd, K.; Caunter, J.; Hetheridge, M.; Williams, T.; Woods, C.; Evans, M.; Toy, R.; Gargas, J.P.; et al. Reproductive effects of long-term exposure to bisphenol A in the fathead minnow (Pimephales promelas). Environ. Sci. Technol. 2001, 35, 2917–2925. [Google Scholar] [CrossRef]
- Chen, Z.; Li, X.; Gao, J.; Liu, Y.; Zhang, N.; Guo, Y.; Wang, Z.; Dong, Z. Reproductive toxic effects of chronic exposure to bisphenol A and its analogues in marine medaka (Oryzias melastigma). Aquat. Toxicol. 2024, 271, 106927. [Google Scholar] [CrossRef]
- Hatef, A.; Zare, A.; Alavi, S.; Habibi, H.; Linhart, O. Modulations in androgen and estrogen mediating genes and testicular response in male goldfish exposed to bisphenol A. Environ. Toxicol. Chem. 2012, 31, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Yang, X.; Liu, J.; Ren, W.; Chen, Y.; Shen, S. Effects of BPF on steroid hormone homeostasis and gene expression in the hypothalamic–pituitary–gonadal axis of zebrafish. Environ. Sci. Pollut. Res. 2017, 24, 21311–21322. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Su, S.; Wei, X.; Wang, S.; Guo, T.; Li, J.; Song, H.; Wang, M.; Wang, Z. Exposure to bisphenol A alternatives bisphenol AF and fluorene-9-bisphenol induces gonadal injuries in male zebrafish. Ecotoxicol. Environ. Saf. 2023, 253, 114634. [Google Scholar] [CrossRef] [PubMed]
- Serra, H.; Beausoleil, C.; Habert, R.; Minier, C.; Picard-Hagen, N.; Michel, C. Evidence for bisphenol B endocrine properties: Scientific and regulatory perspectives. Environ. Health Perspect. 2019, 127, 106001. [Google Scholar] [CrossRef]
- Qiu, W.; Liu, S.; Chen, H.; Luo, S.; Xiong, Y.; Wang, X.; Xu, B.; Zheng, C.; Wang, K. The comparative toxicities of BPA, BPB, BPS, BPF, and BPAF on the reproductive neuroendocrine system of zebrafish embryos and its mechanisms. J. Hazard. Mater. 2020, 406, 124303. [Google Scholar] [CrossRef]
- Rochester, J.; Bolden, A. Bisphenol S and F: A systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef]
- Lan, H.-C.; Wu, K.-Y.; Lin, I.-W.; Yang, Z.-J.; Chang, A.-A.; Hu, M.-C. Bisphenol A disrupts steroidogenesis and induces a sex hormone imbalance through c-Jun phosphorylation in Leydig cells. Chemosphere 2017, 185, 237–246. [Google Scholar] [CrossRef]
- Jorgensen, E.; Alderman, M.; Taylor, H. Preferential epigenetic programming of estrogen response after in utero xenoestrogen (bisphenol-A) exposure. FASEB J. 2016, 30, 3194–3201. [Google Scholar] [CrossRef]
- Jin, S.; Yang, F.; Xu, Y.; Dai, H.; Liu, W. Risk assessment of xenoestrogens in a typical domestic sewage-holding lake in China. Chemosphere 2013, 93, 892–898. [Google Scholar] [CrossRef]
- Kundakovic, M.; Gudsnuk, K.; Franks, B.; Madrid, J.; Miller, R.L.; Perera, F.P.; Champagne, F.A. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc. Natl. Acad. Sci. USA 2013, 110, 9956–9961. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, A.; Brunström, B. Effects of selective and combined activation of estrogen receptor α and β on reproductive organ development and sexual behaviour in Japanese quail (Coturnix japonica). PLoS ONE 2017, 12, e0180548. [Google Scholar] [CrossRef] [PubMed]
- Chelcea, I.; Örn, S.; Hamers, T.; Koekkoek, J.; Legradi, J.; Vogs, C.; Andersson, P. Physiologically based toxicokinetic modeling of bisphenols in zebrafish (Danio rerio) accounting for variations in metabolic rates, brain distribution, and liver accumulation. Environ. Sci. Technol. 2022, 56, 10216–10228. [Google Scholar] [CrossRef] [PubMed]
- Delfosse, V.; Grimaldi, M.; Pons, J.L.; Boulahtouf, A.; Le Maire, A.; Cavaillès, V.; Labesse, G.; Bourguet, W.; Balaguer, P. Structural and mechanistic insights into bisphenols action provide guidelines for risk assessment and discovery of bisphenol A substitutes. Proc. Natl. Acad. Sci. USA 2012, 109, 14930–14935. [Google Scholar] [CrossRef]
- Mesnage, R.; Phedonos, A.; Biserni, M.; Arno, M.; Balu, S.; Corton, J.C.; Ugarte, R.; Antoniou, M.N. Evaluation of estrogen receptor alpha activation by glyphosate-based herbicide constituents. Food Chem. Toxicol. 2017, 108 Pt A, 30–42. [Google Scholar] [CrossRef]
- Liu, Y.H.; Huang, J.W.; Huang, Z.; Mei, Y.X.; Zhao, J.L.; Ying, G.G. Tissue-specific bioaccumulation and health risks of bisphenols in wild fish from West and North Rivers, South China. J. Environ. Expo. Assess. 2023, 2, 21. [Google Scholar] [CrossRef]
- Cano-Nicolau, J.; Vaillant, C.; Pellegrini, E.; Charlier, T.; Kah, O.; Coumailleau, P. Estrogenic effects of several BPA analogs in the developing zebrafish brain. Front. Neurosci. 2016, 10, 112. [Google Scholar] [CrossRef]
- Nakamura, D.; Yanagiba, Y.; Duan, Z.; Ito, Y.; Okamura, A.; Asaeda, N.; Tagawa, Y.; Li, C.; Taya, K.; Zhang, S.; et al. Bisphenol A may cause testosterone reduction by adversely affecting both testis and pituitary systems similar to estradiol. Toxicol. Lett. 2010, 194, 16–25. [Google Scholar] [CrossRef]
- Coumailleau, P.; Trempont, S.; Pellegrini, E.; Charlier, T. Impacts of bisphenol A analogues on zebrafish post-embryonic brain. J. Neuroendocrinol. 2020, 32, e12879. [Google Scholar] [CrossRef]
- Rao, R.; Cao, X.; Li, L.; Zhou, J.; Sun, D.; Li, B.; Guo, S.; Yuan, R.; Cui, H.; Chen, J. Bisphenol AF induces multiple behavioral and biochemical changes in zebrafish (Danio rerio) at different life stages. Aquat. Toxicol. 2022, 253, 106345. [Google Scholar] [CrossRef]
- Ahn, C.; Jeung, E. Endocrine-disrupting chemicals and disease endpoints. Int. J. Mol. Sci. 2023, 24, 5342. [Google Scholar] [CrossRef] [PubMed]
- Kinch, C.; Ibhazehiebo, K.; Jeong, J.; Habibi, H.; Kurrasch, D. Low-dose exposure to bisphenol A and replacement bisphenol S induces precocious hypothalamic neurogenesis in embryonic zebrafish. Proc. Natl. Acad. Sci. USA 2015, 112, 1475–1480. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, X.; Tan, H.; Shi, W.; Zhang, X.; Wei, S.; Yu, H. Molecular initiating events of bisphenols on androgen receptor-mediated pathways provide guidelines for in silico screening and design of substitute compounds. Environ. Sci. Technol. Lett. 2019, 6, 205–210. [Google Scholar] [CrossRef]
- Li, X.; Wen, Z.; Wang, Y.; Mo, J.; Zhong, Y.; Ge, R. Bisphenols and Leydig cell development and function. Front. Endocrinol. 2020, 11, 447. [Google Scholar] [CrossRef] [PubMed]
- Werner, J.; Ouellet, J.; Cheng, C.; Ju, Y.; Law, R. Pulp and paper mill effluents induce distinct gene expression changes linked to androgenic and estrogenic responses in the fathead minnow (Pimephales promelas). Environ. Toxicol. Chem. 2009, 29, 430–439. [Google Scholar] [CrossRef]
- Larsen, M.; Baatrup, E. Functional behavior and reproduction in androgenic sex reversed zebrafish (Danio rerio). Environ. Toxicol. Chem. 2010, 29, 1828–1833. [Google Scholar] [CrossRef]
- Brander, S.; Connon, R.; He, G.; Hobbs, J.; Smalling, K.; Teh, S.; Cherr, G. From ‘omics to otoliths: Responses of an estuarine fish to endocrine disrupting compounds across biological scales. PLoS ONE 2013, 8, e74251. [Google Scholar] [CrossRef]
- Huang, G.; Liu, Y.; Chen, X.; Liang, Y.; Liu, S.; Yang, Y.; Ying, G. Feminization and masculinization of western mosquitofish (Gambusia affinis) observed in rivers impacted by municipal wastewaters. Sci. Rep. 2016, 6, 20884. [Google Scholar] [CrossRef]
- Brockmeier, E.; Jayasinghe, B.; Pine, W.; Wilkinson, K.; Denslow, N. Exposure to paper mill effluent at a site in North Central Florida elicits molecular-level changes in gene expression indicative of progesterone and androgen exposure. PLoS ONE 2014, 9, e106644. [Google Scholar] [CrossRef]
- Narita, Y.; Tsutiya, A.; Nakano, Y.; Ashitomi, M.; Sato, K.; Hosono, K.; Ohtani-Kaneko, R. Androgen-induced cellular proliferation, neurogenesis, and generation of GnRH3 neurons in the brain of mature female Mozambique tilapia. Sci. Rep. 2018, 8, 35303. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jiang, X.; Feng, H.; Shi, H.; Sun, L.; Tao, W.; Wang, D. Simultaneous exposure to estrogen and androgen resulted in feminization and endocrine disruption. J. Endocrinol. 2016, 228, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Rolland, A.; Lardenois, A.; Goupil, A.; Lareyre, J.; Houlgatte, R.; Chalmel, F.; Gac, F. Profiling of androgen response in rainbow trout pubertal testis: Relevance to male gonad development and spermatogenesis. PLoS ONE 2013, 8, e53302. [Google Scholar] [CrossRef] [PubMed]
- Orlando, E.; Davis, W.; Guillette, L. Aromatase activity in the ovary and brain of the eastern mosquitofish (Gambusia holbrooki) exposed to paper mill effluent. Environ. Health Perspect. 2002, 110 (Suppl. S3), 429–433. [Google Scholar] [CrossRef]
- León, A.; Wu, P.; Hall, L.; Johnson, M.; Teh, S. Global gene expression profiling of androgen disruption in QURT strain medaka. Environ. Sci. Technol. 2007, 42, 962–969. [Google Scholar] [CrossRef]
- Schooley, D.J.; Geik, A.; Scarnecchia, L.D. First observations of intersex development in paddlefish Polyodon spathula. J. Fish Biol. 2020, 97, 919–925. [Google Scholar] [CrossRef]
- Cortés-Ramírez, S.; Salazar, A.; Sordo, M.; Ostrosky-Wegman, P.; Morales-Pacheco, M.; Cruz-Burgos, M.; Rodríguez-Dorantes, M. Transcriptome-wide analysis of low-concentration exposure to bisphenol A, S, and F in prostate cancer cells. Int. J. Mol. Sci. 2023, 24, 9462. [Google Scholar] [CrossRef]
- Conroy-Ben, O.; García, I.; Teske, S. In silico binding of 4,4′-bisphenols predicts in vitro estrogenic and antiandrogenic activity. Environ. Toxicol. 2018, 33, 569–578. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, B.; Jin, Y.; Huang, S.; Niu, X.; Mao, Z.; Xin, D. Pttg1, a novel androgen responsive gene is required for androgen-induced prostate cancer cell growth and invasion. Exp. Cell Res. 2017, 350, 1–8. [Google Scholar] [CrossRef]
- Sen, A.; Prizant, H.; Light, A.; Biswas, A.; Hayes, E.; Lee, H.; Hammes, S. Androgens regulate ovarian follicular development by increasing follicle stimulating hormone receptor and microrna-125b expression. Proc. Natl. Acad. Sci. USA 2014, 111, 3008–3013. [Google Scholar] [CrossRef]
- Bu, H.; Narisu, N.; Schlick, B.; Rainer, J.; Manke, T.; Schäfer, G.; Klocker, H. Putative prostate cancer risk SNP in an androgen receptor-binding site of the melanophilin gene illustrates enrichment of risk SNPs in androgen receptor target sites. Hum. Mutat. 2015, 37, 52–64. [Google Scholar] [CrossRef]
- Martinez, H.; Hsiao, J.; Jasavala, R.; Hinkson, I.; Eng, J.; Wright, M. Androgen-sensitive microsomal signaling networks coupled to the proliferation and differentiation of human prostate cancer cells. Genes Cancer 2011, 2, 956–978. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Wu, G.; Wang, S.; Lawless, J.; Sinn, A.; Chen, D.; Zheng, Z. Prenatal exposure to atrazine induces cryptorchidism and hypospadias in F1 male mouse offspring. Birth Defects Res. 2021, 113, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Beg, M.; Sheikh, I. Endocrine disruption: Molecular interactions of environmental bisphenol contaminants with thyroid hormone receptor and thyroxine-binding globulin. Toxicol. Ind. Health 2020, 36, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, K.; Tagami, T.; Akamizu, T.; Usui, T.; Saijo, M.; Kanamoto, N.; Hataya, Y.; Shimatsu, A.; Kuzuya, H.; Nakao, K. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J. Clin. Endocrinol. Metab. 2002, 87, 5185–5190. [Google Scholar] [CrossRef]
- Wu, Y.; Beland, F.; Fang, J. Effect of triclosan, triclocarban, 2,2′,4,4′-tetrabromodiphenyl ether, and bisphenol A on the iodide uptake, thyroid peroxidase activity, and expression of genes involved in thyroid hormone synthesis. Toxicol. Vitr. 2016, 32, 310–319. [Google Scholar] [CrossRef]
- Andrianou, X.; Gängler, S.; Piciu, A.; Charisiadis, P.; Zira, C.; Aristidou, K.; Makris, K. Human exposures to bisphenol A, bisphenol F and chlorinated bisphenol A derivatives and thyroid function. PLoS ONE 2016, 11, e0155237. [Google Scholar] [CrossRef]
- Choi, J.; Choi, E.; Lee, S.; Park, B.; Lee, H.; Hong, Y.; Park, H. Relationship of urinary bisphenol A in childhood on thyroid hormone function in adolescents: A cohort study. PLoS ONE 2023, 20, e0322658. [Google Scholar] [CrossRef]
- Kitamura, S. Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol. Sci. 2005, 84, 249–259. [Google Scholar] [CrossRef]
- Volz, S.; Poulsen, R.; Hansen, M.; Holbech, H. Bisphenol A alters retinal morphology, visually guided behavior, and thyroid hormone levels in zebrafish larvae. Chemosphere 2023, 340, 140776. [Google Scholar] [CrossRef]
- Yang, J.; Chan, K. Evaluation of the toxic effects of brominated compounds (BDE-47, 99, 209, TBBPA) and bisphenol A (BPA) using a zebrafish liver cell line, ZFL. Aquat. Toxicol. 2015, 159, 138–147. [Google Scholar] [CrossRef]
- Qin, J.; Jia, W.; Ru, S.; Xiong, J.; Wang, J.; Wang, W.; Hao, L.; Zhang, X. Bisphenols induce cardiotoxicity in zebrafish embryos: Role of the thyroid hormone receptor pathway. Aquat. Toxicol. 2022, 254, 106354. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Zhang, Z.; Liu, M.; Cui, X.; Wang, J. Evaluation of thyroid-disrupting effects of bisphenol F and bisphenol S on zebrafish (Danio rerio) using anti-transthyretin monoclonal antibody-based immunoassays. Aquat. Toxicol. 2024, 273, 106968. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L.; Ros, A.; Rehberger, K.; Neuhauss, S.; Segner, H. Thyroid disruption in zebrafish (Danio rerio) larvae: Different molecular response patterns lead to impaired eye development and visual functions. Aquat. Toxicol. 2016, 172, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L.; Segner, H.; Ros, A.; Knapen, D.; Vergauwen, L. Thyroid hormone disruptors interfere with molecular pathways of eye development and function in zebrafish. Int. J. Mol. Sci. 2019, 20, 1543. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tian, M.; Qin, H.; Chen, D.; Mzava, S.; Wang, X.; Bigambo, F. Maternal bisphenols exposure and thyroid function in children: A systematic review and meta-analysis. Front. Endocrinol. 2024, 15, 1420540. [Google Scholar] [CrossRef]
- Guignard, D.; Gayrard, V.; Lacroix, M.; Puel, S.; Picard-Hagen, N.; Viguié, C. Evidence for bisphenol A-induced disruption of maternal thyroid homeostasis in the pregnant ewe at low level representative of human exposure. Chemosphere 2017, 182, 458–467. [Google Scholar] [CrossRef]
- Gorini, F.; Bustaffa, E.; Coi, A.; Iervasi, G.; Bianchi, F. Bisphenols as environmental triggers of thyroid dysfunction: Clues and evidence. Int. J. Environ. Res. Public Health 2020, 17, 2654. [Google Scholar] [CrossRef]
- Lü, W.; Zhuo, S.; Wang, Z.; Qu, M.; Shi, Z.; Song, Q.; Zang, J. The joint effects of bisphenols and iodine exposure on thyroid during pregnancy. Nutrients 2023, 15, 3422. [Google Scholar] [CrossRef]
- Liu, X.; Lu, X.; Hong, J.; Zhang, J.; Lin, J.; Jiang, M.; Zhang, J. Effects of long-term exposure to TDCPP in zebrafish (Danio rerio)—Alternations of hormone balance and gene transcriptions along hypothalamus-pituitary axes. Anim. Models Exp. Med. 2022, 5, 239–247. [Google Scholar] [CrossRef]
- Kraft, M.; Gölz, L.; Rinderknecht, M.; Koegst, J.; Braunbeck, T.; Baumann, L. Developmental exposure to triclosan and benzophenone-2 causes morphological alterations in zebrafish (Danio rerio) thyroid follicles and eyes. Environ. Sci. Pollut. Res. 2022, 30, 33711–33724. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Yang, Y.; Chen, Y.; Tang, W.; Wang, F.; Diao, X. Thyroid disruption in zebrafish larvae by short-term exposure to bisphenol AF. Int. J. Environ. Res. Public Health 2015, 12, 13069–13084. [Google Scholar] [CrossRef] [PubMed]
- Špirhanzlová, P.; Leemans, M.; Demeneix, B.; Fini, J. Following endocrine-disrupting effects on gene expression in Xenopus laevis. Cold Spring Harb. Protoc. 2019, 2019, pdb-prot098301. [Google Scholar] [CrossRef] [PubMed]
- Knapen, D.; Stinckens, E.; Cavallin, J.; Ankley, G.; Holbech, H.; Villeneuve, D.; Vergauwen, L. Toward an AOP network-based tiered testing strategy for the assessment of thyroid hormone disruption. Environ. Sci. Technol. 2020, 54, 8491–8499. [Google Scholar] [CrossRef]
- Holbech, H.; Matthiessen, P.; Hansen, M.; Schüürmann, G.; Knapen, D.; Reuver, M.; Baumann, L. ERGO: Breaking down the wall between human health and environmental testing of endocrine disrupters. Int. J. Mol. Sci. 2020, 21, 2954. [Google Scholar] [CrossRef]
- Franchimont, D.; Kino, T.; Galon, J.; Meduri, G.U.; Chrousos, G. Glucocorticoids and inflammation revisited: The state of the art. NIH clinical staff conference. Neuroimmunomodulation 2002, 10, 247–260. [Google Scholar] [CrossRef]
- Barton, B.; Iwama, K.G. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu. Rev. Fish Dis. 1991, 1, 3–26. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, X.; Li, Y.; Yao, X.; Li, C.; Qin, Z.; Guo, L. Bisphenol A alternatives bisphenol S and bisphenol F interfere with thyroid hormone signaling pathway in vitro and in vivo. Environ. Int. 2018, 237, 1072–1079. [Google Scholar] [CrossRef]
- Heredia-García, G.; Elizalde-Velázquez, G.; Gómez-Oliván, L.; Islas-Flores, H.; García-Medina, S.; Galar-Martínez, M.; Dublán-García, O. Realistic concentrations of bisphenol A trigger a neurotoxic response in the brain of zebrafish: Oxidative stress, behavioral impairment, acetylcholinesterase inhibition, and gene expression disruption. Chemosphere 2023, 330, 138729. [Google Scholar] [CrossRef]
- Qiu, W.; Chen, J.; Li, Y.; Chen, Z.; Jiang, L.; Yang, M.; Wu, M. Oxidative stress and immune disturbance after long-term exposure to bisphenol A in juvenile common carp (Cyprinus carpio). Ecotoxicol. Environ. Saf. 2016, 130, 93–102. [Google Scholar] [CrossRef]
- Fan, X.; Hou, T.; Jia, J.; Tang, K.; Wei, X.; Wang, Z. Discrepant dose responses of bisphenol A on oxidative stress and DNA methylation in grass carp ovary cells. Chemosphere 2020, 248, 126110. [Google Scholar] [CrossRef]
- Salahinejad, A.; Attaran, A.; Naderi, M.; Meuthen, D.; Niyogi, S.; Chivers, D. Chronic exposure to bisphenol S induces oxidative stress, abnormal anxiety, and fear responses in adult zebrafish (Danio rerio). Sci. Total Environ. 2020, 750, 141633. [Google Scholar] [CrossRef]
- Naderi, M.; Salahinejad, A.; Attaran, A.; Chivers, D.; Niyogi, S. Chronic exposure to environmentally relevant concentrations of bisphenol S differentially affects cognitive behaviors in adult female zebrafish. Environ. Pollut. 2020, 261, 114060. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Harris, C.; Wang, J. Glucocorticoid receptor and adipocyte biology. Nucl. Recept. Res. 2018, 5, 101373. [Google Scholar] [CrossRef] [PubMed]
- Kouche, S.; Halvick, S.; Morel, C.; Duca, R.; Nieuwenhuyse, A.; Turner, J.; Meyre, D. Pollution, stress response, and obesity: A systematic review. Obes. Rev. 2025, 26, e13895. [Google Scholar] [CrossRef] [PubMed]
- Macíková, P.; Groh, K.; Ammann, A.; Schirmer, K.; Suter, M. Endocrine disrupting compounds affecting corticosteroid signaling pathways in Czech and Swiss waters: Potential impact on fish. Environ. Sci. Technol. 2014, 48, 12902–12911. [Google Scholar] [CrossRef]
- Biddie, S.; Conway-Campbell, B.; Lightman, S. Dynamic regulation of glucocorticoid signalling in health and disease. Rheumatology 2011, 51, 403–412. [Google Scholar] [CrossRef]
- Darbre, P. Chemical components of plastics as endocrine disruptors: Overview and commentary. Birth Defects Res. 2020, 112, 1300–1307. [Google Scholar] [CrossRef]
- Liang, W.; Chen, Y.; Huang, M.; Song, Z.; Li, C.; Zheng, Y.; Yi, Z. Interaction between halogenated phenols and thyroxine-binding protein: A multispectral and computational approach. ChemistrySelect 2024, 9, e202401288. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, X.; Liu, W.-B.; Zhang, D.-D.; Li, X.-F.; Zhang, C.-N.; Chen, W.-H.; Abasubong, K.P.; Jiang, G.-Z. Corticosterone Can Be an Essential Stress Index in Channel Catfish (Ictalurus punctatus). Front. Mar. Sci. 2021, 8, 692726. [Google Scholar] [CrossRef]
- Meling, H.; Berge, K.; Knudsen, D.L.; Rønning, P.O.; Brede, C. Monitoring farmed fish welfare by measurement of cortisol as stress marker in fish feces by liquid chromatography coupled with tandem mass spectrometry. Molecules 2022, 27, 2481. [Google Scholar] [CrossRef]
- Carbajal, A.; Soler, P.; Tallo-Parra, O.; Isasa, M.; Echevarria, C.; Lopez-Bejar, M.; Vinyoles, D. Towards non-invasive methods in measuring fish welfare: The measurement of cortisol concentrations in fish skin mucus as a biomarker of habitat quality. Animals 2019, 9, 839. [Google Scholar] [CrossRef]
- Yeh, P.; Glöck, M.; Ryu, S. An optimized whole-body cortisol quantification method for assessing stress levels in larval zebrafish. PLoS ONE 2013, 8, e79406. [Google Scholar] [CrossRef]
- Cao, Y.; Tveten, A.-K.; Stene, A. Establishment of a non-invasive method for stress evaluation in farmed salmon based on direct fecal corticoid metabolites measurement. Fish Shellfish. Immunol. 2017, 68, 317–324. [Google Scholar] [CrossRef]
- Wu, Y.; Ohnuki, H.; Ren, H.; Endo, H. Carbon-nanotube-enhanced label-free immunosensor for highly sensitive detection of plasma cortisol level in fish. Sens. Mater. 2015, 27, 793–803. [Google Scholar] [CrossRef]
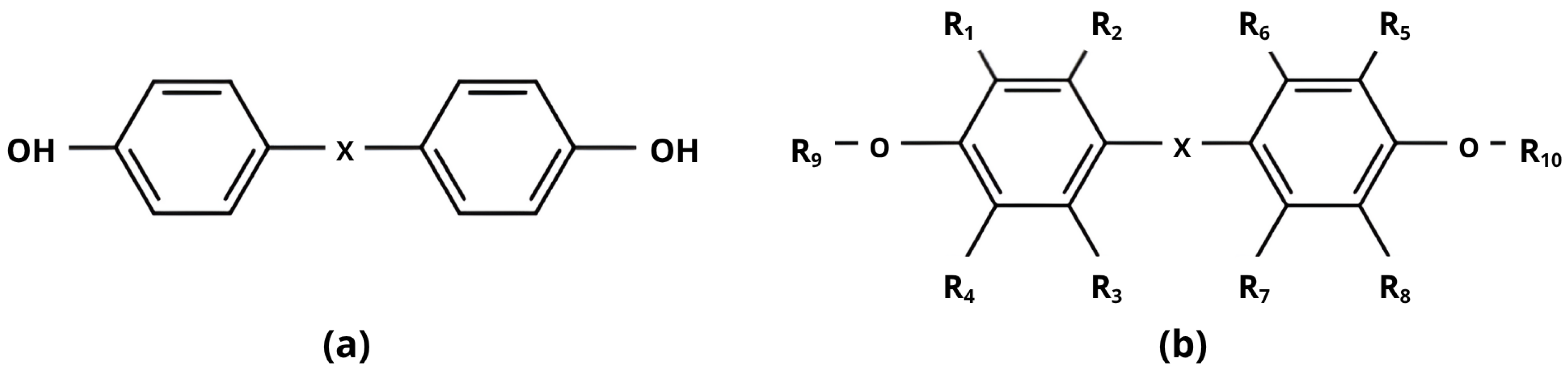
| Bisphenol Type | Chemical Formula | Structural formula |
|---|---|---|
| Bisphenol A (BPA) | C15H16O2 | 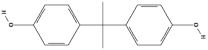 |
| Bisphenol S (BPS) | C12H10O4S | 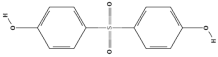 |
| Bisphenol F (BPF) | C13H12O2 | 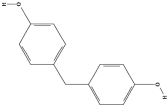 |
| Bisphenol AF (BPAF) | C15H10F6O2 | 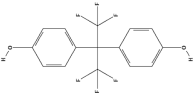 |
| Bisphenol B (BPB) | C16H18O2 | 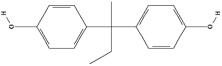 |
| Bisphenol E (BPE) | C14H14O2 | 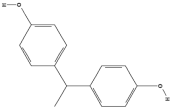 |
| Bisphenol Z (BPZ) | C18H22O2 | 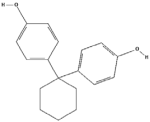 |
| Tetrabromobisphenol A (TBBPA) | C15H12Br4O2 | 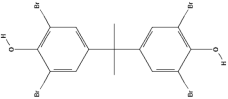 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peskova, N.; Blahova, J. Bisphenols: Endocrine Disruptors and Their Impact on Fish: A Review. Fishes 2025, 10, 365. https://doi.org/10.3390/fishes10080365
Peskova N, Blahova J. Bisphenols: Endocrine Disruptors and Their Impact on Fish: A Review. Fishes. 2025; 10(8):365. https://doi.org/10.3390/fishes10080365
Chicago/Turabian StylePeskova, Nikola, and Jana Blahova. 2025. "Bisphenols: Endocrine Disruptors and Their Impact on Fish: A Review" Fishes 10, no. 8: 365. https://doi.org/10.3390/fishes10080365
APA StylePeskova, N., & Blahova, J. (2025). Bisphenols: Endocrine Disruptors and Their Impact on Fish: A Review. Fishes, 10(8), 365. https://doi.org/10.3390/fishes10080365








