Abstract
Biofloc technology (BFT) represents a promising approach among sustainable options for the sustainable intensification of shrimp aquaculture, helping to mitigate environmental impacts while maintaining production yields. This study evaluated the effects of stocking density (200, 400, 600, and 800 ind/m3) on the water quality, nitrogen dynamics, and production performance of Penaeus vannamei in BFT systems with limited water exchange (<10%). During an eight-week production-scale trial, water quality exhibited density-dependent deterioration, with TAN and NO2−-N peaks increasing from 0.4 to 2.3 mg/L and 1.0 to 4.2 mg/L, respectively, as density rose from 200 to 800 ind/m3. Concurrently, DO and pH declined significantly from 6.7 to 5.1 mg/L and 7.6 to 7.3, respectively. Production performance revealed critical trade-offs: while yield rose from 3.62 to 9.09 kg/m3, individual growth metrics declined, including harvest body weight (19.14 to 14.12 g), size variation (14.03% to 23.90%), and survival rate (94.6% to 79.8%). Quadratic regression analysis and response surface analysis identified 400~600 ind/m3 as the optimal density range, achieving balanced outcomes: yield (6.74~8.43 kg/m3), harvest body weight (16.72~18.03 g), survival rate (84.0%~93.5%), and feed conversion ratio (1.14~1.22). These findings provide actionable guidelines for optimizing stocking density in commercial BFT systems, highlighting the importance of balancing productivity with environmental sustainability under limited water exchange.
Keywords:
Penaeus vannamei; biofloc technology; stocking density; harmful nitrogen; production performance Key Contribution:
An optimal stocking density of 400~600 ind/m3 for Penaeus vannamei intensive cultures in biofloc systems balances high shrimp yield with manageable water quality and good growth performance.
1. Introduction
The global aquaculture industry faces mounting pressure to meet the rapidly growing demand for shrimp while addressing serious environmental concerns associated with conventional farming practices [,]. As the dominant farmed shrimp species, Penaeus vannamei accounts for over 50% of global production [], yet traditional intensive culture methods relying on high water exchange rates contribute to eutrophication, disease transmission, and coastal ecosystem degradation [,,]. In response, biofloc technology (BFT) has emerged as a promising sustainable alternative that utilizes microbial communities to convert toxic nitrogenous wastes into microbial protein while simultaneously improving water quality and providing supplemental nutrition [,,]. This approach offers significant advantages including dramatically reduced water exchange rate (<10%), improved nutrient utilization efficiency, and enhanced biosecurity through competitive exclusion of pathogens [,,].
Despite these benefits, critical knowledge gaps remain regarding the optimization of stocking density in BFT systems—a key parameter that directly affects productivity, water quality, and economic viability [,,]. Previous studies have been limited by small-scale experimental designs that do not reflect commercial conditions, short trial durations insufficient to capture full grow-out cycles, narrow density gradients (typically ≤ 600 ind/m3), and incomplete assessments of nitrogen dynamics and their management implications [,,]. These limitations have hindered the development of practical guidelines for commercial-scale BFT implementation, particularly regarding the density-dependent trade-offs between production yield and harmful nitrogen control [,].
This study addresses these gaps through a comprehensive, production-scale evaluation of four stocking densities (200, 400, 600, and 800 ind/m3) over a complete 56-day grow-out cycle, with three specific objectives: (1) to quantify the temporal dynamics of harmful nitrogen compounds under different densities, (2) to evaluate management requirements for system stability, and (3) to determine optimal density ranges balancing yield and shrimp performance. Our findings provide critical, evidence-based guidelines for commercial BFT operations while advancing fundamental understanding of density effects in biofloc systems. The results establish quantitative relationships between stocking density, water quality parameters, and production performance, while identifying practical thresholds for sustainable intensification of shrimp aquaculture.
2. Materials and Methods
2.1. Biofloc-Based Systems Preparation
The study was conducted in twelve biofloc systems with identical physical conditions, housed in a temperature-controlled greenhouse with semi-shaded plastic film cover (50% light transmission). Each system consisted of a square concrete tank (6 m × 6 m × 1.0 m, 6 m side length × 1.0 m depth, 30 m3 water volume) equipped with nine water injectors and a 750 W circulating pump []. The water source was natural seawater that underwent sand filtration and chlorination prior to use. To accelerate biofloc development, each system was inoculated with 5% (v/v) mature biofloc water from a shared source of the previous production cycle (see [] for inoculum composition details).
2.2. Trial Design and Shrimp Stocking
The trial employed a completely randomized design to evaluate four stocking density treatments (200, 400, 600, and 800 individuals per cubic meter, denoted as D200, D400, D600, and D800, respectively). Each treatment was replicated in three independent biofloc-based systems, resulting in a total of twelve experimental units.
Healthy P. vannamei juveniles at post-larval stage 27 (PL27) were sourced from a single nursery pond at the base, and individuals with a mean weight of 0.36 ± 0.14 g (mean ± S.D.) were selected and stocked into the trial systems according to the four stocking densities. Density-specific biomass was precisely weighed and distributed to achieve target loadings (2.16, 4.32, 6.48, and 8.64 kg/tank for D200, D400, D600, and D800, respectively), with <2% variation from targets based on weight measurement. The 56-day culture period encompassed the critical grow-out phase from juvenile to marketable size. Water temperature was maintained at 28.0 ± 2.0 °C using greenhouse climate control systems, with continuous aeration ensuring dissolved oxygen (DO) concentration above 5.0 mg/L throughout the trial period.
2.3. Feeding and Aquaculture Management
During the trial, the shrimp were fed a commercial diet (40.1% protein, 8.4% lipid, 3.5% fiber, and 13.5% ash) using automatic feeders programmed to deliver feed twelve times daily. The feeding rate was progressively reduced from 12% to 3% of estimated biomass based on continuous monitoring of feed consumption using submerged feeding trays [].
Water exchange was rigorously maintained below 10% of the total volume to simulate limited-exchange conditions, and meanwhile to control target total suspended solids (TSS) concentrations corresponding to stocking densities: 200, 300, 400, and 500 mg/L for D200, D400, D600, and D800 treatments, respectively. Agricultural molasses (38.5% organic carbon) was added into the culture water at an input C/N ratio of 12 when total ammonia nitrogen (TAN) concentrations exceeded 1.0 mg/L during the early and middle phases of the trial, with addition discontinued when both TAN and nitrite nitrogen (NO2−-N) concentrations stabilized below 1.0 mg/L during the later phase of the trial []. The pH was maintained above 7.0 through periodic additions of food-grade sodium carbonate (>95% purity). All management inputs including offered feed, exchanged water, and added molasses and sodium carbonate were recorded daily.
2.4. Water Quality Monitoring and Analysis
Water quality monitoring was performed throughout the trial period. Daily measurements of salinity, temperature, DO, and pH were conducted using a hand-held YSI-650 multi-parameter instrument (Yellow Springs Instruments Inc., Yellow Springs, OH, USA). Every two days, TSS were quantified using a TSS portable hand-held system (Hach Company, Loveland, CO, USA). Weekly water samples were analyzed for TAN, NO2−-N, nitrate nitrogen (NO3−-N), and total nitrogen (TN) following Standard Methods for the Examination of Water and Wastewater []. All measurements were taken between 08:00 and 09:00 h to minimize diurnal variation effects.
2.5. Shrimp Production Performance Analysis
At trial termination (day 56), shrimp were harvested and evaluated for production performance. The total biomass per tank was recorded, and a random sample of 200 shrimp from each tank was individually weighed to determine mean harvest body weight and size variation. The size variation is expressed as the coefficient of variation in harvest body weight, which was calculated as the ratio of the standard deviation to the mean and expressed as a percentage. The indicators of shrimp production performance were calculated using the following equations []:
Specific growth rate (SGR, %/day) = 100 × [Ln (harvest body weight) − Ln (initial body weight)]/trial days,
Survival rate (SR, %) = 100 × (total harvest biomass/mean harvest body weight)/stocking count,
Yield (kg/m3) = harvest biomass/tank water volume,
Feed conversion ratio (FCR) = total offered feed weight/harvest biomass.
2.6. Statistical Analysis
Data are presented as mean ± standard deviation (S.D.); inferential statistics are reported separately. Statistical analyses were performed using IBM SPSS Statistics 26.0 (IBM Corp., Armonk, NY, USA) with a significance threshold of p < 0.05 for all tests. Model assumptions were verified through examination of residuals using Shapiro–Wilk tests for normality and Levene’s tests for homogeneity of variance; non-normal residuals from percentage data analyses were subjected to arcsine square-root transformation. One-way ANOVA with Tukey’s HSD post hoc tests was employed to assess treatment effects on dependent variables for system management inputs, water quality parameters, and shrimp production performance. Further, linear mixed models were applied to analyze biofloc and nitrogen dynamics, incorporating stocking density and time as fixed effects and tank as a random effect to account for repeated measures []. Quadratic polynomial regression (Y = β0 + β1X + β2X2 + ε) was employed to characterize nonlinear relationships between stocking density and production performance indicators, enabling identification of inflection points and optimal stocking densities for each indicator. Response surface methodology integrated with a desirability function approach was employed for multi-objective optimization of stocking density, balancing key performance indicators weighted by their production significance: yield (60%), harvest body weight (20%), survival rate (20%), and feed conversion rate (20%). The analysis calculated comprehensive desirability as the geometric mean of individual desirability functions to determine the optimal stocking density, and rigorously validated models through residual diagnostics with all procedures conforming to CONSORT guidelines for mixed models.
3. Results
3.1. System Management Inputs and Water Quality
A significant density-dependent pattern was observed in management inputs (p < 0.05, Table 1). Water exchange rates increased proportionally with stocking density, ranging from 0.6%/d at 200 ind/m3 (D200) to 7.9%/d at 800 ind/m3 (D800) (p < 0.05). Similarly, molasses and sodium carbonate usage showed density-dependent increases, with the highest inputs recorded in D800 (41.8 kg/tank and 57.3 kg/tank, respectively) (p < 0.05). During the trial, as the stocking density of shrimp was increased from 200 to 800 ind/m3, the required water exchange rate, molasses usage, and sodium carbonate usage were found to increase by 1217%, 160%, and 321%, respectively.

Table 1.
System management inputs in the biofloc-based system for 56-day culture of P. vannamei at four stocking densities (means ± S.D., n = 3).
DO, pH, TAN, and NO2−-N of the system were significantly affected by stocking density (Table 2). DO and pH levels declined from 6.7 mg/L to 5.1 mg/L and 7.6 to 7.3, respectively, with a density increase from 200 to 800 ind/m3 (p < 0.05), while TAN and NO2−-N concentrations increased from 0.23 mg/L to 0.96 mg/L and 0.47 mg/L to 1.68 mg/L, respectively, with a density increase from 200 to 800 ind/m3 (p < 0.05). Notably, threshold effects for TAN and NO2−-N were observed with significant deteriorations occurring above 600 ind/m3 (p < 0.05). During the trial, as the stocking density of shrimp was increased from 200 to 800 ind/m3, DO and pH levels were observed to decrease by 24% and 4%, respectively, while TAN and NO2−-N concentrations increased by 317% and 257%, respectively.

Table 2.
Water quality parameters in the biofloc-based system for 56-day culture of P. vannamei at four stocking densities (means ± S.D., n = 3).
3.2. Biofloc Control and Nitrogen Dynamics
TSS showed similar increase trends initially and then stabilized across different stocking densities (Figure 1). Significantly higher TSS were maintained as stocking density increased from 200 to 800 ind/m3 (p < 0.05). To maintain TSS around target ranges of 200, 300, 400, and 500 mg/L for D200, D400, D600, and D800, respectively, water exchange rates were adjusted proportionally to stocking density (Table 1).
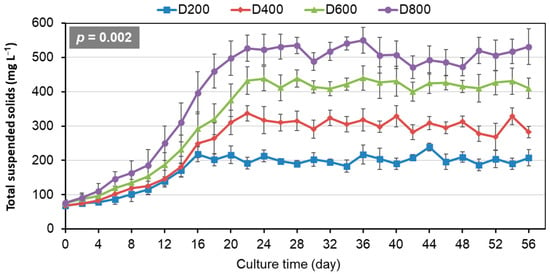
Figure 1.
Temporal dynamics of total suspended solids in the biofloc-based system for 56-day culture of Penaeus vannamei at four stocking densities (means ± S.D., n = 3).
The dynamics of nitrogen compounds displayed pronounced density-dependent fluctuations (Figure 2). The concentrations of both TAN and NO2−-N first increased and then decreased during the early to middle phase of the trial, respectively, and then were maintained at almost below 1.0 mg/L until the end of the trial in all systems (Figure 2a,b). During the peaking stage, the concentrations of TAN and NO2−-N were significantly higher and their duration days lasted longer when the stocking densities of the system were higher (p < 0.05). Notably, in D800, TAN reached a peak of 2.3 mg/L at week 3, and its concentration above 1.0 mg/L lasted for more than three weeks, while NO2−-N peaked at 4.2 mg/L at week 4, and its concentration above 1.0 mg/L lasted for more than four weeks (Figure 2a,b). The concentrations of NO3−-N and TN increased in volatility during the early and middle phases of the trial and showed irregular fluctuation later on in all systems (Figure 2c,d). NO3−-N and TN accumulation was significantly higher when the stocking densities of the systems were higher (p < 0.05).
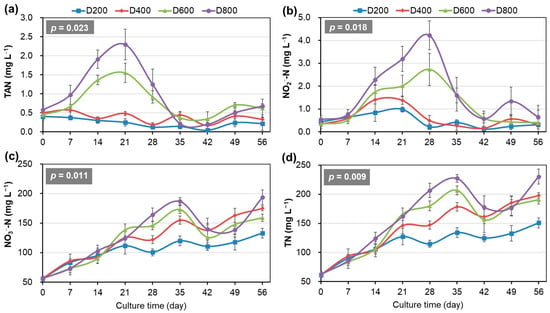
Figure 2.
Temporal dynamics of various nitrogen types in the biofloc-based system for 56-day culture of Penaeus vannamei at four stocking densities (means ± S.D., n = 3). (a) TAN: total ammonium nitrogen; (b) NO2−-N; (c) NO3−-N; (d) TN: total nitrogen.
3.3. Shrimp Production Performance and Optimal Density Identification
Shrimp production performance varied significantly across stocking densities (Table 3). While yield increased from 3.62 kg/m3 in D200 to 9.09 kg/m3 in D800 (p < 0.05), individual growth metrics declined. Harvest body weight decreased from 19.14 g in D200 to 14.12 g in D800, while size variation increased from 14.03% in D200 to 23.90% in D800 (p < 0.05). Specific growth rates dropped from 7.10%/d in D200 to 6.62%/d in D800 (p < 0.05), and survival rates decreased from 94.6% in D200 to 79.8% in D800 (p < 0.05). The feed conversion ratio worsened with density, rising from 1.12 ± 0.03 (D200) to 1.29 ± 0.05 (D800) (p < 0.05). During the trial, as the stocking density of shrimp was increased from 200 to 800 ind/m3, harvest body weight, specific growth rate and survival rate were observed to decrease by 36%, 7% and 19%, respectively, while size variation, yield and feed conversion rate increased by 70%, 151% and 15%, respectively.

Table 3.
Production performance of P. vannamei in the biofloc-based system for 56-day culture at four stocking densities (means ± S.D., n = 3).
The quadratic polynomial regression analysis effectively characterized the relationships between shrimp production indicators and stocking density (all R2 ≥ 0.88), with significant quadratic terms (β2, p < 0.05) confirming the nonlinear response patterns (Figure 3). The optimal stocking densities corresponding to peak performance of each indicator were determined as 724, 352, 387, 615, 421, and 563 ind/m3 for aquaculture yield, survival rate, harvest body weight, size variation, specific growth rate, and feed conversion rate, respectively. Using response surface analysis based on weighted geometric means with quadratic regression (Figure 4), the optimal stocking densities for multi-objective optimization were determined as follows: (1) 513 ind/m3 for composite performance considering yield (60%), harvest body weight (20%), and feed conversion rate (20%), and (2) 529 ind/m3 for composite performance considering yield (60%), harvest body weight (20%), and survival rate (20%). Overall, the optimal stocking densities were identified within the range of 400~600 ind/m3.
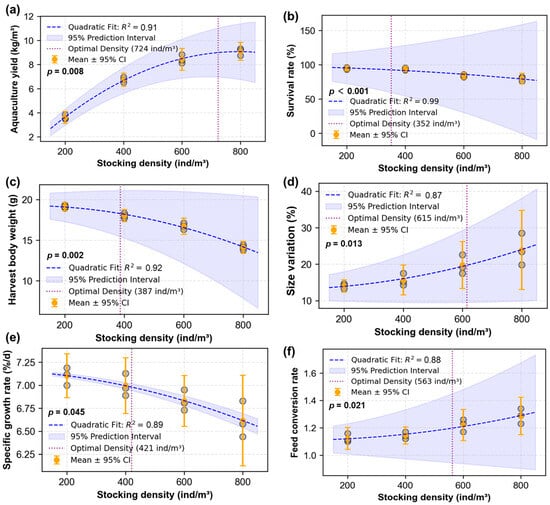
Figure 3.
Quadratic polynomial regression analysis of the relationships between stocking density and production performance in the biofloc-based system during a 56-day culture period of Penaeus vannamei (means ± S.D., n = 3). (a) Relationship between aquaculture yield and stocking density; (b) relationship between survival rate and stocking density; (c) relationship between harvest body weight and stocking density; (d) relationship between harvest size variation and stocking density; (e) relationship between specific growth rate and stocking density; (f) relationship between feed conversion rate and stocking density.
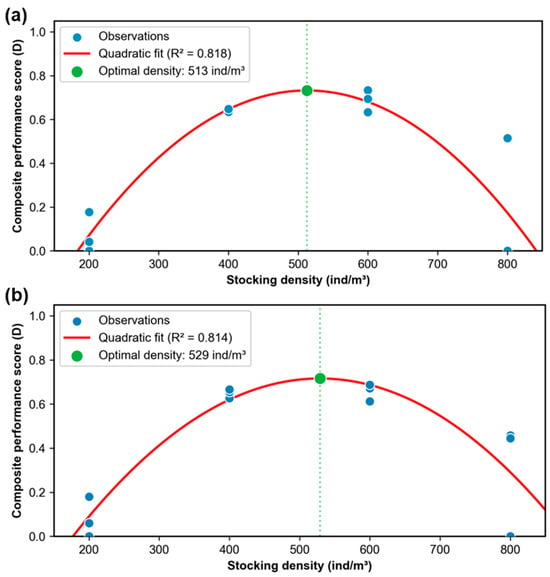
Figure 4.
Response surface analysis of composite performance score as a function of stocking density. (a) Multi-objective optimization using weighted geometric mean with quadratic regression (yield: 60%; harvest body weight: 20%; feed conversion rate: 20%); (b) multi-objective optimization using weighted geometric mean with quadratic regression (yield: 60%; harvest body weight: 20%; survival rate: 20%).
4. Discussion
4.1. High Density Increases System Management Demands and Water Quality Challenges
Significant increases in system management inputs were observed with higher stocking densities of Penaeus vannamei in biofloc-based systems during the 56-day culture period. Water exchange rates increased to maintain target TSS levels for four stocking densities, reflecting the substantial inputs of feed and accumulation of metabolic wastes at higher densities. Similarly, molasses and sodium carbonate addition increased significantly, indicating greater microbial demand for nitrogen transformation and pH regulation [,]. These findings align with Samocha et al. [] and Xu et al. [], who reported similar pH instability in systems exceeding 500 ind/m3, while extending their observations to commercial-scale operations.
Critical water quality parameters exhibited significant variations across stocking densities of shrimp. DO concentrations progressively declined across the density gradient, while pH maintenance became increasingly challenging despite continuous buffering. These results corroborate previous studies regarding oxygen consumption and pH stability in biofloc systems at high density of shrimp [,]. Notably, our improved TSS control protocol (maintained at 200~500 mg/L through regulated water exchange) represents a significant advancement over earlier studies [,].
The management intensity required to maintain water quality parameters within optimal ranges increased with the stocking density of shrimp. Our data suggest that operational complexity and resource requirements escalate disproportionately beyond 600 ind/m3, supporting the practical density thresholds proposed by previous studies [,,]. The results have important implications for commercial operations, as they demonstrate that marginal production gains at higher densities must be carefully weighed against exponentially increasing management demands [,,]. These insights are particularly valuable for farmers implementing biofloc technology at production scale.
4.2. High Density Exacerbates Harmful Nitrogen Fluctuations and Toxicity Risks
Significant density-dependent effects on TAN and NO2−-N fluctuations and TIN and TN accumulation were evident in the biofloc-based systems during the 56-day culture period. The systems with higher stocking densities showed higher TAN and NO2−-N concentrations, and their highest peaks and longest duration days were found in D800. These findings demonstrate that elevated stocking densities lead to prolonged exposure to harmful nitrogen compounds, which aligns with recent observations in intensive biofloc systems [,,]. The severity of harmful nitrogen fluctuations observed in our study exceeds those reported in conventional aquaculture systems, highlighting the unique challenges of high-density biofloc operations.
Harmful nitrogen processing efficiency showed clear density-dependent limitations, with the establishment of complete nitrification (TAN and NO2−-N < 1.0 mg/L) requiring more than three weeks in the 800 ind/m3 treatment compared to the 200 ind/m3 system []. This delayed processing capacity suggests that microbial communities in high-density systems need extended adaptation periods to effectively metabolize nitrogenous wastes, supporting the findings regarding microbial community dynamics under density stress [,]. The prolonged exposure to elevated nitrogen levels provides a mechanistic explanation for the observed growth reduction at higher densities, as chronic nitrogen stress is known to impair shrimp physiology and immune function [,]. These results emphasize the critical need for enhanced monitoring and intervention strategies in high-density biofloc culture.
The observed harmful nitrogen dynamics in this study have important implications for commercial biofloc operations. The increase in harmful nitrogen accumulation beyond 600 ind/m3 suggests a critical threshold for system stability. Our findings indicate that without proper intervention strategies, such as optimized carbon supplementation or microbial augmentation [,], high-density systems may face persistent harmful nitrogen control challenges [,]. These results demonstrated that the costs of harmful nitrogen management must be carefully considered when determining optimal stocking densities. Future research should focus on developing targeted approaches to enhance nitrogen processing efficiency in high-density biofloc systems while maintaining shrimp health and performance.
4.3. Density-Dependent Trade-Offs Between Production Yield and Shrimp Performance
The study revealed a clear trade-off between production yield and individual shrimp performance as stocking density increased. The increase in stocking density significantly increased yield, size variation, and feed conversion rate while it reduced harvest body weight, specific growth rate, and survival rate in the P. vannamei culture. These findings align with previous studies reporting density-dependent growth inhibition in biofloc systems, where physiological stress and resource competition intensify under crowded conditions [,,]. Notably, the observed decline in individual performance was accompanied by an increase in size variation, indicating heightened heterogeneity in shrimp growth at higher densities. Such size disparities may further exacerbate competition for feed and space, creating a feedback loop that amplifies stress and reduces overall system efficiency [,,,].
The deterioration in shrimp performance at higher densities can be attributed to a complex interplay of biological and environmental factors. First, elevated stocking densities intensify competition for feed, leading to unequal nutrient access and reduced feed utilization efficiency. This is evidenced by the increase in feed conversion ratio from 1.12 in D200 to 1.29 in D800, suggesting that shrimp in high-density systems expend more energy competing for resources rather than allocating it to growth [,]. Second, aggressive interactions, such as cannibalism and territorial disputes, become more frequent in crowded environments, further impairing growth and survival [,]. For example, studies have shown that P. vannamei exhibits increased aggression and reduced feeding activity at densities above 600 ind/m3, which correlates with our observations of higher size variation and lower specific growth rate in the D600 and D800 groups [,]. Third, chronic exposure to suboptimal water quality—particularly elevated TAN (peaking at 2.3 mg/L in D800) and NO2−-N (peaking at 4.2 mg/L in D800)—induces metabolic stress, suppressing immune function and growth rates [,]. The prolonged duration of harmful nitrogen exposure in high-density systems (e.g., >3 weeks for TAN > 1.0 mg/L and >4 weeks for NO2−-N > 1.0 mg/L in D800) likely exacerbated these effects, as shrimp subjected to prolonged stress exhibit reduced hepatopancreas function and impaired nutrient assimilation [,]. These specific physiological parameters (hepatopancreas health and nutrient assimilation) should be quantitatively measured in future trials to verify this mechanistic explanation.
Moreover, distinct optimal density ranges for different performance metrics (e.g., 352 ind/m3 for survival rate vs. 724 ind/m3 for yield) highlighted the need for balanced management strategies. For production purposes, yield and harvest body weight determine sale price and output value, and survival rate and feed conversion rate are related to input cost. Based on their importances for production, the optimal stocking densities were 513 ind/m3 and 529 ind/m3, which were within the range of 400~600 ind/m3. This range aligns with lab-scale findings [] and commercial observations [] where moderate densities optimize microbial nitrogen processing and minimize stress. Practical recommendations for farmers include (1) adopting densities of 400~600 ind/m3 to balance productivity and shrimp performance; (2) implementing real-time monitoring of water quality (especially TAN and NO2−-N) to mitigate nitrogen stress; and (3) adjusting feeding regimes to account for density-dependent competition, such as using multiple feeding trays to ensure equitable feed distribution [,]. Future research should explore microbial interventions (e.g., probiotic supplementation) to enhance nitrogen metabolism and shrimp resilience at higher densities.
The divergent optimal density ranges for key performance metrics—such as 352 ind/m3 for maximum survival rate versus 724 ind/m3 for peak yield—underscored the critical need for multi-objective management strategies in aquaculture, as yield and harvest body weight directly influence revenue while survival rate and feed conversion rate govern input costs. Through integrated weighting of these parameters, the study determined 513 ind/m3 (prioritizing yield: 60%; FCR: 20%; and harvest weight: 20%) and 529 ind/m3 (yield: 60%; survival rate: 20%; and harvest weight: 20%) as optimal stocking densities, both within the validated range of 400~600 ind/m3 that aligns with lab-scale evidence [] of enhanced microbial nitrogen processing and commercial observations [] of stress mitigation at moderate densities. Practical recommendations include (1) adopting 400~600 ind/m3 to balance shrimp productivity and system input, and (2) implementing real-time monitoring of TAN and NO2−-N to address density-dependent harmful nitrogen stress.
5. Conclusions
This study evaluated the effects of stocking density (200~800 ind/m3) on P. vannamei production performance in biofloc-based systems, demonstrating that higher densities significantly increased harmful nitrogen fluctuation and operational demands while compromising shrimp growth and feed efficiency. The optimal density range of 400~600 ind/m3 balanced yield (6.74~8.43 kg/m3) with manageable water quality, providing a practical guideline for farmers to optimize production sustainability. For commercial applications, we recommend adopting densities of 400~600 ind/m3, implementing real-time water quality monitoring, and adjusting carbon supplementation to mitigate nitrogen stress. Future research should focus on microbial community engineering to enhance nitrogen processing, long-term shrimp health impacts under density stress, and cost–benefit analyses for large-scale implementation.
Author Contributions
Conceptualization, W.X.; methodology, W.X.; validation, W.X. and Y.Z.; formal analysis, W.X. and Y.Z.; investigation, W.X., B.Z. and Y.Z.; resources, W.X. and B.Z.; data curation, W.X. and Y.C.; writing—original draft preparation, W.X.; writing—review and editing, W.X. and B.Z.; visualization, W.X.; supervision, W.X.; project administration, W.X., B.Z. and Y.Z.; funding acquisition, W.X., B.Z., Y.Z. and Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the open fund of China (Guangxi)-ASEAN Key Laboratory of Comprehensive Exploitation and Utilization of Aquatic Germplasm Resources, Ministry of Agriculture and Rural Affairs; and Key Laboratory of Aquaculture Genetic and Breeding and Healthy Aquaculture of Guangxi, Guangxi Academy of Fishery Sciences (GXKEYLA-2023-02-4); Guangxi Science and Technology Major Program (AA23062046, AA23062047); Natural Science Foundation of Guangdong Province (2023A1515011657); and the earmarked fund for CARS-48.
Institutional Review Board Statement
The shrimp experiment was conducted with the ethical guidelines established by the South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences. The study protocol received formal approval from the Institutional Animal Care and Use Committee (Approval No.: SCSFRI-2023024; Approval Date: 4 February 2023).
Data Availability Statement
Data will be made available on request.
Acknowledgments
We would like to thank the staff of station for their assistance in trial execution and sample collection during the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAO (Food and Agriculture Organization of the United Nations). The State of World Fisheries and Aquaculture 2024; FAO: Rome, Italy, 2024. [Google Scholar]
- Hatje, V.; de Souza, M.M.; Ribeiro, L.F.; Eca, G.F.; Barros, F. Detection of environmental impacts of shrimp farming through multiple lines of evidence. Environ. Pollut. 2016, 219, 672–684. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.F.M. Use of biofloc technology in shrimp aquaculture: A comprehensive review, with emphasis on the last decade. Rev. Aquac. 2021, 13, 676–705. [Google Scholar] [CrossRef]
- Yang, P.; Lai, D.Y.F.; Jin, B.S.; Bastviken, D.; Tan, L.S.; Tong, C. Dynamics of dissolved nutrients in the aquaculture shrimp ponds of the Min River estuary, China: Concentrations, fluxes and environmental loads. Sci. Total Environ. 2017, 603, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Thitamadee, S.; Prachumwat, A.; Srisala, J.; Jaroenlak, P.; Salachan, P.V.; Sritunyalucksana, K.; Itsathitphaisarn, O. Review of Current Disease Threats for Cultivated Penaeid Shrimp in Asia. Aquaculture 2016, 452, 69–87. [Google Scholar] [CrossRef]
- Nguyen, T.A.T.; Nguyen, K.A.T.; Jolly, C. Is super-intensification the solution to shrimp production and export sustainability? Sustainability 2019, 11, 5277. [Google Scholar] [CrossRef]
- Avnimelech, Y. Biofloc Technology: A Practical Guide Book, 3rd ed.; World Aquaculture Society: Baton Rouge, LA, USA, 2015. [Google Scholar]
- Martínez-Córdova, L.R.; Emerenciano, M.; Miranda-Baeza, A.; Martínez-Porchas, M. Microbial-Based Systems for Aquaculture of Fish and Shrimp: An Updated Review. Rev. Aquac. 2015, 7, 131–148. [Google Scholar] [CrossRef]
- Samocha, T.M.; Prangnell, D.I.; Hanson, T.R.; Treece, G.D.; Morris, T.C.; Castro, L.F.; Staresinic, N. Design and Operation of Super Intensive, Biofloc-Dominated Systems for Indoor Production of the Pacific White Shrimp, Litopenaeus vannamei—The Texas A&M AgriLife Research Experience; World Aquaculture Society: Baton Rouge, LA, USA, 2017. [Google Scholar]
- Emerenciano, M.G.; Miranda-Baeza, A.; Martínez-Porchas, M.; Poli, M.A.; Vieira, F.D. Biofloc Technology (BFT) in Shrimp Farming: Past, Present and Future. Front. Mar. Sci. 2021, 8, 813091. [Google Scholar] [CrossRef]
- Abakari, G.; Wu, X.; He, X.; Fan, L.P.; Luo, G.Z. Bacteria in biofloc technology aquaculture systems: Roles and mediating factors. Rev. Aquacult. 2022, 14, 1260–1284. [Google Scholar] [CrossRef]
- Xu, W.J.; Huang, F.; Zhao, Y.Z.; Su, H.C.; Hu, X.J.; Xu, Y.; Wen, G.L.; Cao, Y.C. Carbohydrate addition strategy affects nitrogen dynamics, budget and utilization, and its microbial mechanisms in biofloc-based Penaeus vannamei culture. Aquaculture 2024, 589, 740123. [Google Scholar] [CrossRef]
- Yu, Y.B.; Choi, J.H.; Lee, J.H.; Jo, A.H.; Lee, J.W.; Choi, H.J.; Kang, Y.J.; Choi, C.Y.; Kang, J.C.; Lee, K.M.; et al. The use, application and efficacy of biofloc technology (BFT) in shrimp aquaculture industry: A review. Environ. Technol. Innov. 2024, 33, 103345. [Google Scholar] [CrossRef]
- Liu, F.; Sun, J.; Long, J.; Sun, L.; Liu, C.; Wang, X.; Zhang, L.; Hao, P.; Wang, Z.; Cui, Y.; et al. Assessing the Interactive Effects of High Salinity and Stocking Density on the Growth and Stress Physiology of the Pacific White Shrimp Litopenaeus vannamei. Fishes 2024, 9, 62. [Google Scholar] [CrossRef]
- Krummenauer, D.; Peixoto, S.; Cavalli, R.O.; Poersch, L.H.; Wasielesky, W. Superintensive Culture of White Shrimp, Litopenaeus vannamei, in a Biofloc Technology System in Southern Brazil at Different Stocking Densities. J. World Aquac. Soc. 2011, 42, 726–733. [Google Scholar] [CrossRef]
- Da Silveira, L.G.P.; Krummenauer, D.; Poersch, L.H.; Rosas, V.T.; Wasielesky, W. Hyperintensive Stocking Densities for Litopenaeus vannamei Grow-out in Biofloc Technology Culture System. J. World Aquac. Soc. 2020, 51, 1290–1300. [Google Scholar] [CrossRef]
- Da Silveira, L.G.P.; Rosas, V.T.; Krummenauer, D.; Fróes, C.; Da Silva, A.; Poersch, L.H.; Fóes, G.; Wasielesky, W. Establishing the most productive stocking densities for each stage of a multi-phase shrimp culture in BFT system. Aquac. Int. 2022, 30, 1889–1903. [Google Scholar] [CrossRef]
- Irani, M.; Rajabi Islami, H.; Nafisi Bahabadi, M.; Hosseini Shekarabi, S.P. Production of Pacific white shrimp under different stocking density in a zero-water exchange biofloc system: Effects on water quality, zootechnical performance, and body composition. Aquac. Eng. 2023, 100, 102313. [Google Scholar] [CrossRef]
- Xu, W.J.; Xu, Y.; Su, H.C.; Hu, X.J.; Xu, Y.N.; Li, Z.J.; Wen, G.L.; Cao, Y.C. Production performance, inorganic nitrogen control and bacterial community characteristics in a controlled biofloc-based system for indoor and outdoor super-intensive culture of Litopenaeus vannamei. Aquaculture 2021, 531, 735876. [Google Scholar] [CrossRef]
- Said, M.M.; El-barbary, Y.A.; Ahmed, O.M. Assessment of Performance, Microbial Community, Bacterial Food Quality, and Gene Expression of Whiteleg Shrimp (Litopenaeus vannamei) Reared under Different Density Biofloc Systems. Aquac. Nutr. 2022, 2022, 3499061. [Google Scholar] [CrossRef]
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Wastewater, 20th ed.; APHA: Washington, DC, USA, 1998. [Google Scholar]
- Furtado, P.S.; Poersch, L.H.; Wasielesky, W., Jr. Effect of calcium hydroxide, carbonate and sodium bicarbonate on water quality and zootechnical performance of shrimp Litopenaeus vannamei reared in bio—Flocs technology (BFT) systems. Aquaculture 2012, 321, 130–135. [Google Scholar] [CrossRef]
- Schveitzer, R.; Baccarat, R.F.C.; Gaona, C.A.P.; Wasielesky, W., Jr.; Arantes, R. Concentration of suspended solids in superintensive culture of the Pacific white shrimp Litopenaeus vannamei with biofloc technology (BFT): A review. Rev. Aquac. 2024, 16, 785–795. [Google Scholar] [CrossRef]
- Ray, A.J.; Dillon, K.S.; Lotz, J.M. Water quality dynamics and shrimp (Litopenaeus vannamei) production in intensive, mesohaline culture systems with two levels of biofloc management. Aquac. Eng. 2011, 45, 127–136. [Google Scholar] [CrossRef]
- Luo, G.Z.; Xu, J.X.; Meng, H.Y. Nitrate accumulation in biofloc aquaculture systems. Aquaculture 2020, 520, 734675. [Google Scholar] [CrossRef]
- Raza, B.; Zheng, Z.M.; Zhu, J.Y.; Yang, W. A Review: Microbes and Their Effect on Growth Performance of Litopenaeus vannamei (White Leg Shrimps) during Culture in Biofloc Technology System. Microorganisms 2024, 12, 1013. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.J.; Wen, G.L.; Su, H.C.; Xu, Y.; Hu, X.J.; Cao, Y.C. Effect of Input C/N Ratio on Bacterial Community of Water Biofloc and Shrimp Gut in a Commercial Zero-Exchange System with Intensive Production of Penaeus vannamei. Microorganisms 2022, 10, 1060. [Google Scholar] [CrossRef]
- Said, M.M.; Abo-Al-Ela, H.G.; El-Barbary, Y.A.; Ahmed, O.M.; Dighiesh, H.S. Influence of Stocking Density on the Growth, Immune and Physiological Responses, and Cultivation Environment of White-Leg Shrimp (Litopenaeus vannamei) in Biofloc Systems. Sci. Rep. 2024, 14, 11147. [Google Scholar] [CrossRef]
- Sharawy, Z.Z.; Abbas, E.M.; Abdelkhalek, N.K.; Ashry, O.A.; Abd El-Fattah, L.S.; El-Sawy, M.A.; Helal, M.F.; El-Haroun, E. Effect of Organic Carbon Source and Stocking Densities on Growth Indices, Water Microflora, and Immune-Related Genes Expression of Litopenaeus vannamei Larvae in Intensive Culture. Aquaculture 2022, 546, 737397. [Google Scholar] [CrossRef]
- Abakari, G.; Luo, G.Z.; Kombat, E.O.; Alhassan, E.H. Supplemental carbon sources applied in biofloc technology aquaculture systems: Types, effects and future research. Rev. Aquac. 2021, 13, 1193–1222. [Google Scholar] [CrossRef]
- Xu, W.J.; Morris, T.C.; Samocha, T.M. Effects of C/N ratio on biofloc development, water quality, and performance of Litopenaeus vannamei juveniles in a biofloc-based, high-density, zero-exchange, outdoor tank system. Aquaculture 2016, 453, 169–175. [Google Scholar] [CrossRef]
- Kring, N.A.; Fleckenstein, L.J.; Tierney, T.W.; Fisk, J.C.; Lawson, B.C.; Ray, A.J. The effects of stocking density and artificial substrate on production of Pacific white shrimp Litopenaeus vannamei and water quality dynamics in greenhouse-based biofloc systems. Aquac. Eng. 2023, 101, 102322. [Google Scholar] [CrossRef]
- Bardera, G.; Owen, M.A.G.; Façanha, F.N.; Alcaraz-Calero, J.M.; Alexander, M.E.; Sloman, K.A. The influence of density and dominance on Pacific white shrimp (Litopenaeus vannamei) feeding behaviour. Aquaculture 2021, 531, 735949. [Google Scholar] [CrossRef]
- Hamilton, S.; Filho, F.C.; Silva, J.F.; Duarte-Neto, P.J.; Soares, R.; Peixoto, S. The loud crowd: Interactions between stocking density and acoustic feeding activity of different size classes of Litopenaeus vannamei. Aquaculture 2023, 563, 738904. [Google Scholar] [CrossRef]
- Da Costa, F.P.; Gomes, B.S.F.D.F.; Pereira, S.D.D.N.A.; De Fátima Arruda, M. Influence of stocking density on the behaviour of juvenile Litopenaeus vannamei (Boone, 1931). Aquac. Res. 2016, 47, 912–924. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).